Abstract
Objectives
Sober living houses are alcohol- and drug-free recovery residences that help individuals with substance use disorders maintain long-term abstinence. Given the prevalence of co-occurring mental disorders among individuals entering substance use treatment, it is likely that many residents entering sober living houses are also contending with psychiatric symptoms, and it is unclear how these symptoms may affect their sobriety. This study sought to describe the prevalence and trajectories of different types of symptoms among sober living house residents and examine how these symptoms affect substance use outcomes.
Methods
300 residents (241 men and 59 women, with a mean age of 38.5 years) were interviewed upon entry and re-interviewed at 6-, 12-, and 18-month follow-ups. Psychiatric symptoms were assessed using the Brief Symptom Inventory (BSI). General estimating equations were used to test changes in BSI global psychological distress and clinical symptom scales (depression, hostility, somatization, and phobic anxiety) over time and to test the relationship between scale scores and substance use in longitudinal models controlling for demographics, length of stay, and psychiatric service utilization.
Results
Psychiatric symptoms were common. At baseline, the majority (51%) of participants endorsed 20 or more symptoms. Overall psychological distress and symptoms of depression and phobic anxiety significantly improved over time. Rates of abstinence and days of use among those who reported using substances also improved over time. Overall distress and all symptoms dimensions measured were associated with a decreased likelihood of abstinence. Symptoms of somatization were associated with an increase in the number of days substances were used among those who reported use.
Conclusions
Psychological symptoms among sober living house residents improve over time, but they are risk factors for relapse, suggesting that additional support provided to residents with psychiatric symptoms could improve substance use outcomes.
Keywords: sober living houses, recovery residences, psychiatric symptoms, Brief Symptom Inventory, sobriety
Introduction
In the general population, the problem of co-occurring substance use and mental disorders is common. The largest study of co-occurring disorders to date, the National Epidemiologic Survey on Alcohol and Related Conditions (Grant & Dawson, 2006), found that approximately 20% of persons with a current substance use disorder had at least one independent (i.e., nonsubstance-induced) mood disorder, and 18% had at least one independent anxiety disorder (Grant et al., 2004). Co-occurring mental disorders are even more prevalent among individuals entering substance use treatment (Compton, Cottler, Jacobs, Ben-Abdallah, & Spitznagel, 2003; Havassy, Alvidrez, & Owen, 2004; Watkins et al., 2004), and a number of studies have found that overall psychiatric severity as well as certain psychiatric symptoms are associated with worse retention in and outcomes of substance use treatment (Burns, Teesson, & O'Neill, 2005; Lipsky, Krupski, Roy-Byrne, Lucenko, Mancuso, & Huber, 2010; Ray, Mertens, & Weisner, 2005; Shane, Jasiukaitis, & Green, 2003).
As science on substance use and mental disorders has advanced, both are increasingly recognized as chronic, often relapsing brain diseases (Duckworth, 2013; National Institute on Drug Abuse, 2014). Concomitantly, the substance use treatment and mental health fields (particularly regarding treatment of severe mental illness) have increasingly shifted in focus from acute-care models toward recovery-oriented approaches (Anthony, 1993; Dennis & Scott, 2007; Gagne, White, & Anthony, 2007). According to the Substance Abuse and Mental Health Services Administration (Substance Abuse and Mental Health Services Administration, 2012), recovery is defined as “a process of change through which individuals improve their health and wellness, live a self-directed life, and strive to reach their full potential.” In both fields, a variety of services have emerged to help individuals in this process (Laudet & Humphreys, 2013; Whitley, Strickler, & Drake, 2012). One type of support service that has received renewed attention in the substance use treatment field is the recovery residence, generally defined as a sober, safe, and healthy living environment that promotes recovery from alcohol and drugs and associated problems (Jason, Mericle, Polcin, & White, 2013).
Sober living houses are one type of recovery residence that has been studied in California. Unlike residential treatment settings, sober living houses generally do not provide group counseling, case management, treatment planning, or a structure of daily activities. However, residents are either encouraged or required to attend 12-step meetings. Residents can stay as long as they wish, provided they abide by house rules (such as maintaining abstinence from alcohol and drugs) and pay fees for rent, utilities, etc.(Polcin, 2001; Polcin & Henderson, 2008). Although these houses have a manager living with the residents, a social model philosophy of recovery (Borkman, 1998; Polcin, Mericle, Howell, Sheridan, & Christensen, 2014) is promoted that emphasizes resident input into house operations and management, peer support for recovery, financial self-sufficiency, and resident participation in the household. Sober living houses can serve persons in recovery at various stages in their recovery, including after residential treatment, during outpatient treatment, and after release from incarceration (Polcin, 2006a, 2006b).
Studies of sober living houses provide support for their role in promoting recovery from alcohol and drug addiction. A longitudinal study tracking 300 individuals in Northern California recruited upon entry showed that residents made significant improvements on a wide variety of outcomes including alcohol and drug use, alcohol- and drug-related problems, employment, and arrests (Polcin, Korcha, Bond, & Galloway, 2010a; Polcin, Korcha, Bond, & Galloway, 2010b). Importantly all improvements between baseline and 6-month follow-up were maintained at 12- and 18-month follow-up even though the vast majority of residents left the houses by 18 months. However, assessments of psychiatric symptoms among residents have been limited to baseline assessments or global measures of severity. For example, Polcin and colleagues (2012) used the Psychiatric Diagnostic Screening Questionnaire (Zimmerman & Mattia, 2001), a measure designed to screen for the most common DSM-IV Axis I disorders encountered in outpatient mental health settings, to assess psychiatric symptoms among residents entering sober living houses who had past year methamphetamine dependence. They found over 70% of the sample met the screening criteria for at least one type of anxiety disorder (i.e. PTSD, OCD, panic, agoraphobia, and social phobia) and nearly half (48%) met the criteria for one of the two somatoform disorders (somatization and hypochondriasis). Symptoms were particularly prevalent among women, especially somatoform disorders. Using the Global Severity Index from the Brief Symptom Inventory (Derogatis, 1993), Polcin, Korcha, and Bond (2015) found overall psychiatric severity among residents of decreased over time and motivation for maintaining sobriety measured with the Alcohol and Drug Consequences Questionnaire (Cunningham, 1997) differed among high versus low psychiatric severity groups. However, neither of the previous studies assessed changes in different types of psychiatric symptoms over time or how different types of symptoms affected resident outcomes.
By examining overall severity and a number of different types of psychiatric symptoms as well as how these are associated with substance use outcomes, the present study extends prior work on residents in sober living houses. Further, although housing is recognized in the substance use (Laudet & White, 2010) and mental health fields (Drake & Whitley, 2014) as critical to recovery, little attention has been paid to housing for individuals in recovery from substance use and mental health disorders to help inform best practices. Using data from the largest study of residents in sober living houses to date, “An Evaluation of Sober Living Houses” (Polcin et al. 2004), the aims of this exploratory study are twofold: (1) To describe the prevalence and course of different types of psychiatric symptoms among sober living house residents over 6, 12, and 18-month follow-up interviews; (2) To examine how these symptoms are associated with substance use.
Methods
The data used for this study were collected as part of a larger study on the outcome of sober living house residents in Northern California (see Polcin et al., 2010a; Polcin et al., 2010b) for additional details on study methods).
Participants
The analytic sample for this study consists of 300 residents recruited from two groups of sober living houses. One group of houses, Clean and Sober Transitional Living is located near Sacramento and consists of 16 houses with a 136-bed capacity (Polcin et al., 2010b). The other group consists of four different houses operated by Options Recovery Services, an outpatient addiction treatment program in Berkeley which opened its houses in response to the large number of clients in their treatment program who needed housing (Polcin et al., 2010a).
Table 1 lists baseline characteristics of the study participants recruited from the Sacramento houses (N=245) and the Berkeley houses (N=55). The majority of participants were male, Caucasian, age 38 or older, and had at least a high school/GED degree. Roughly half the sample was never married and reported working in the past 6 months, and many reported being arrested in the past 6 months. The majority met DSM IV criteria for past year alcohol or drug dependence. Reports of spending time in an inpatient or residential psychiatric treatment program in the past 6 months and attending an outpatient treatment program were relatively infrequent, but close to a third reported taking psychiatric medication. Compared to those in the Berkeley houses, participants recruited from the Sacramento houses were younger (37.5 vs. 42.8, t(298)=3.63, p<0.001), more likely to be female, (OR=5.1, p=0.008) and Caucasian (OR=6.6, p<0.001), and less likely to have attended outpatient psychiatric treatment in the past 6 months (OR=0.4, p=0.038).
Table 1. Baseline Sample Characteristics (N=300).
| n | % | |
|---|---|---|
| Gender | ||
| Male | 241 | 80.3 |
| Female | ||
| Race | ||
| White | 193 | 64.8 |
| Black | 58 | 19.5 |
| Hispanic | 23 | 7.7 |
| Other | 24 | 8.1 |
| Age (M, SD) | 38.5 | 10.1 |
| Education | ||
| Less than High school | 66 | 22.0 |
| High school/GED | 234 | 78.0 |
| Marital Status | ||
| Never Married | 148 | 49.3 |
| Other (e.g., married, cohabitating, separated, divorced, widowed) | 152 | 50.7 |
| Worked in the Past 6 Months (N=298) | 149 | 50.0 |
| Arrested in the Past 6 Months (N=299) | 125 | 41.8 |
| DSM IV Substance Use Disorders | ||
| Alcohol Dependence | 150 | 50.3 |
| Drug Dependence | 223 | 74.8 |
| Alcohol or Drug Dependence | 260 | 87.3 |
| Inpatient Psychiatric Stay (in the past 6 months) | 25 | 8.3 |
| Outpatient Psychiatric Services (in the past 6 months; N=299) | 35 | 11.7 |
| Psychiatric Medications (in the past 6 months) | 93 | 31.0 |
Note. Valid percentages presented.
Recruitment and Data Collection Procedures
Study participants were recruited consecutively between January 2004 and July 2006 and interviewed within their first week of entering the houses and again at 6-, 12-, and 18-month follow-ups. To maximize generalizability of study findings, few exclusionary criteria were specified. Participants needed to be able to provide informed consent and contact information for follow-up interviews. These exclusionary criteria were rarely implemented (n=1) and the vast majority of participants invited to participate in the study were enrolled. Interviews were completed in an onsite office by trained research interviewers and lasted about 2 hours. Participants were paid $30 for the baseline interview and $50 for each follow-up. All participants signed an informed consent form to take part in the study, and study procedures were approved by the Public Health Institute Institutional Review Board.
Among the sample of 300, 90% (n = 269) participated in at least one follow-up interview. Follow-up rates for each time point included 76% (n=227) at 6 months, 73% (n=218) at 12 months, and 75% (n=224) at 18 months. To assess potential sample bias due to attrition, we compared baseline demographic characteristics, overall psychiatric distress, and substance use among those who completed interviews and those who did not at each follow-up. No demographic differences were found (at any time point) between individuals who completed follow-ups and those who did not. Length of stay in the house was associated with completion of the 6- and 12-month follow-up interviews (but not with completion of the 18-month interview). Significantly fewer individuals who reported an inpatient psychiatric stay or use of psychiatric medications in the past 6 months at baseline completed the 18-month follow-up.
Instruments and Measures
Brief Symptom Inventory (BSI)
As a brief form of the Symptom Checklist-90 (SCL-90; Derogatis, 1983), the BSI (Derogatis & Melisaratos, 1983) is a 53-item self-report inventory which asks participants to rate on a 5-point scale ranging from 0 (Not at all) to 4 (Extremely) the extent to which they have been bothered during the past week by various symptoms in nine different dimensions (e.g., somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism). In addition to producing counts of the number of symptoms endorsed with a non-zero response, raw scores (ranging from 0-4) for symptom subscales can be calculated by summing the items in each dimension and dividing by the number of items endorsed in that dimension. A measure of global psychological distress, the Global Severity Index (GSI) can be similarly calculated by summing all items (Derogatis, 1993). Normed scores for the BSI are available, however we used raw scores (rather than converted T scores) in our analyses because our sample differed from the patient and non-patient populations on which the BSI has been normed.
Although the BSI is generally regarded as an appropriate measure of general psychological distress and has been found to be sensitive to change (Carscaddon, George, & Wells, 1990; Holden, Starzyk, McLeod, & Edwards, 2000; Piersma, Reaume, & Boes, 1994), several studies have questioned the discriminant validity of the BSI subscales (Benishek, Hayes, Bieschke, & Stöffelmayr, 1998; Boulet & Boss, 1991; Hayes, 1997; Piersma, Boes, & Reaume, 1994; Skeem et al., 2006). Given concerns about the validity of the subscales, we conducted our own psychometric analyses to determine whether there was evidence to support using them as the developers intended. Exploratory factor analyses of the BSI data collected from participants in this study at each interview resulted in the identification of anywhere from five to six factors with Eigenvalues greater than 1.0 (Kaiser, 1960), with the majority of the items loading onto the first factor which accounted for anywhere from 59% to 66% of the variance across administrations. Review of the items that loaded onto these factors (varimax rotated factor loadings > 0.4) revealed that many of the items from the original subscales did indeed cluster together, and we retained all the items in these subscales to ensure comparability of our subscale scores.
For example, the majority of the hostility items (reflecting thoughts, feelings or actions characteristic of negative affect or anger) consistently loaded onto one factor, the majority of the phobic anxiety items (reflecting a persistent fear response that is irrational, disproportionate and leads to avoidance behavior) consistently loaded onto another factor, and all of the somatization items (reflecting distress arising from perceptions of bodily dysfunction) consistently loaded onto another. The largest factor contained all the depression items (reflecting a representative range of indicators of clinical depression) as well as many items from several other subscales. Among the various symptom dimensions represented in this factor, we decided to use only the depression items because these items generally had higher loadings on this factor than items from other subscales. Further, depression is a more readily understood clinical syndrome, and it is common among individuals with substance use disorders (Grant et al., 2004; Kessler, Chiu, Demler, & Walters, 2005; Regier et al., 1990). The overall GSI and the items in the four retained subscales demonstrated good internal consistency across administrations (α=.75–.98; see Appendix A).
Substance Use
To assess substance use at each interview, we used a measure of substance use peak density (Gerstein et al., 1994) which represents the number of days of any substance use (i.e., any alcohol or drug) during the month of highest use over the past 6 months. Values for substance use peak density can range from 0 to 31. Because peak density is a count variable and because many respondents at each time time-point reported 0 days of substance use, we created two measures so that substance use data could be analyzed in a two-step or hurdle process (Mullahy, 1986) We created an indicator variable reflecting abstinence, meaning 0 days of substance use in the past 6 months and another count variable (responses following a negative binomial distribution) reflecting the number of days used during the month of highest use among those who used substances in the past 6 months.
Control Variables
In addition to adjusting for demographic characteristics that differed between groups of participants from the Sacramento and Berkeley houses—gender (female indicator), race (Caucasian indicator), and age (continuous measure), we also included measures of length of stay and psychiatric service use. Length of stay in the house is based on the house manager's report of when the participant entered and left the house. Services used in the past six months were assessed at each time point using questions developed for this study that asked respondents to report how many days over the past six months they had spent in an inpatient psychiatric treatment program and how many days they attended outpatient psychiatric treatment. Because the distribution of the number of days was highly skewed and the majority of respondents reported 0 days, responses were dichotomized to create indicators of any inpatient stays and any outpatient treatment in the past 6 months. Service use questions also assessed whether the respondent had taken any psychiatric medications in the past 6 months.
Data Analyses
To assess changes in BSI scale scores and the relationship between BSI scale scores and substance use outcomes over time, we used general estimating equations (Diggle, Heagerty, Liang, & Zeger, 2002; Liang & Zeger, 1986; Zeger, Liang, & Albert, 1988). General estimating equations are used to estimate population average models (also called marginal models) and are commonly used in longitudinal data analysis because they can account for within-subject non-independence of observations across multiple waves of data collection. To test changes in psychiatric symptoms, separate models were run for each BSI scale score that included a categorical interview time point variable to test differences in scores at baseline to each follow-up time point (i.e., 6, 12 and 18 months). To examine the relationship between BSI scale scores and substance use outcomes over time, separate models were run for each scale score predicting changes in abstinence rates and predicting changes in the number of days substances were used among those who used substances in the past 6 months. All analyses were conducted in Stata v13.1 using xtgee commands specifying robust variance estimation (Huber, 1967; White, 1980, 1982) to adjust for clustering of residents within houses and controlled for demographics, length of stay, and service use.
Results
Prevalence and Trajectories of Clinical Symptoms
At baseline, the average number of symptoms endorsed with a non-zero response in the past week was 21.02 (SD=14.24; a full listing of the percent of respondents who provided a non-zero response to each symptom is available from the corresponding author); the majority (51%) of participants endorsed 20 or more symptoms. The average level of distress at baseline reflected by the GSI was 0.80 (SD=0.74).
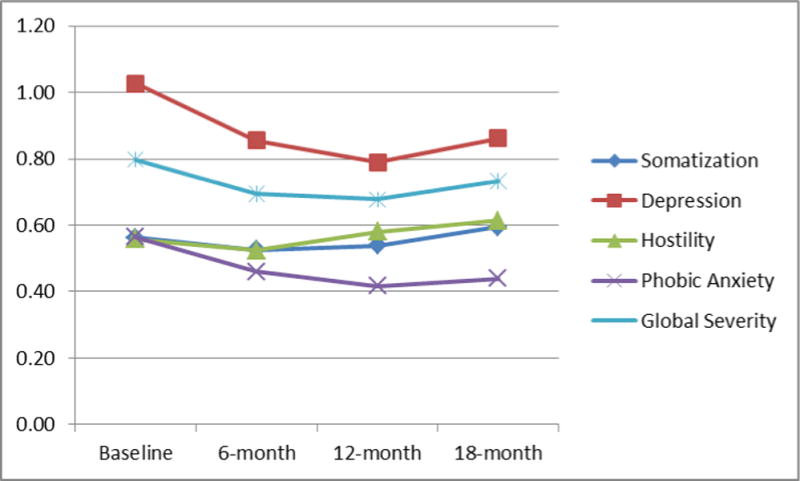
Table 2 displays mean scores for the GSI and the subscale scores for respondents at each interview time point, and Figure 1 graphically depicts these scores. Table 2 also displays the unstandardized coefficients for categories of time in the models testing changes in BSI scores over time controlling for demographics, length of stay, and service use. A negative coefficient represents a decrease in a score from baseline. The joint test represents the Wald test of all follow-up coefficients compared to baseline and thereby represents the overall test of time. As Table 2 shows, overall GSI scores (Wald Chi-square=7.99, df=3, p=0.046) as well as subscale scores for depression (Wald Chi-square=13.57, df=3, p=0.004) and phobic anxiety (Wald Chi-square=7.89, df=3, p=0.048) significantly decreased from baseline.
Table 2. Trajectories of BSI Scale Scores.
| Mean BSI Scores by Interview | Time Coefficients from General Estimating Equations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Baseline (N=300) | 6-month (N=227) | 12-month (N=217) | 18-Month (N=224) | 6-month Coefficient | 12-month Coefficient | 18-month Coefficient | Joint Effect Wald Test | ||||||
|
|
|||||||||||||
| M | SE | M | SE | M | SE | M | SE | B (SE) | B(SE) | B(SE) | χ2 | p | |
| GSI | 0.80 | 0.04 | 0.70 | 0.04 | 0.68 | 0.05 | 0.73 | 0.05 | -0.12(0.05)** | -0.11(0.05)* | -0.05(0.05) | 7.99 | 0.046 |
| Somatization | 0.56 | 0.04 | 0.53 | 0.04 | 0.54 | 0.05 | 0.59 | 0.05 | -0.02(0.04) | 0.00(0.05) | 0.07(0.05) | 3.55 | 0.315 |
| Depression | 1.03 | 0.06 | 0.85 | 0.06 | 0.79 | 0.06 | 0.86 | 0.07 | -0.18(0.07)** | -0.25(0.07)*** | -0.15(0.07)* | 13.57 | 0.004 |
| Hostility | 0.56 | 0.04 | 0.52 | 0.04 | 0.58 | 0.05 | 0.61 | 0.05 | -0.05(0.06) | 0.02(0.06) | 0.06(0.05) | 4.95 | 0.175 |
| Phobic Anxiety | 0.56 | 0.05 | 0.46 | 0.05 | 0.42 | 0.05 | 0.44 | 0.05 | -0.13(0.05)* | -0.14(0.06)* | -0.11(0.06)* | 7.89 | 0.048 |
Note. Means (M) and standard errors (SE) are presented for individuals who had BSI data at a particular time point. General estimating equations test changes in BSI scores across various time points (entered into the model as a categorical predictor) and control for gender (female), race (white), service use (receipt of inpatient, outpatient, and psychiatric medication), and length of stay. Unstandardized coefficients (B) and standard errors for each time category are presented as well as the Wald test (χ2, df=3) for combined effect of time across follow-up interviews.
p<0.05;
p<0.01;
p<0.001.
Figure 1. Mean BSI Scale Scores by Interview Time Point CAPTION: Scores can range from 0 to 4.
Clinical Symptoms as Predictors of Substance Use
Table 3 displays the results of separate models using each of the BSI scores to predict changes in abstinence rates. Higher GSI scores (OR=0.48, p<0.001) as well as higher scores on the somatization (OR=0.56, p<0.001), depression (OR=0.53, p<0.001), hostility (OR=0.71, p=0.006), and phobic anxiety (OR=0.74, p=0.012) subscales scores were significantly associated with a decreased likelihood of abstinence even after adjusting for changes in abstinence over time and other control variables.
Table 3. Models Predicting Abstinence.
| GSI | Somatization | Depression | Hostility | Phobic Anxiety | |
|---|---|---|---|---|---|
|
|
|||||
| Abstinence | OR[95% CI] | OR[95% CI] | OR[95% CI] | OR[95% CI] | OR[95% CI] |
| BSI Score (GSI, Somatization, etc.) | 0.48[0.37-0.62]*** | 0.56[0.44-0.71] *** | 0.53[0.43-0.65]*** | 0.71[0.55-0.90] ** | 0.74[0.58-0.93] * |
| Time Point | |||||
| Baseline (Reference) | |||||
| 6-month | 4.02[2.57-6.30] *** | 4.32[2.76-6.74] *** | 4.01[2.54-6.32]*** | 4.14[2.66-6.46]*** | 4.04[2.59-6.30]*** |
| 12-month | 4.72[3.05-7.28] *** | 5.11[3.30-7.92] *** | 4.53[2.91-7.04]*** | 4.95[3.21-7.63]*** | 4.70[3.06-7.22]*** |
| 18-month | 4.06[2.58-6.38] *** | 4.39[2.80-6.91] *** | 3.95[2.50-6.24]*** | 4.19[2.68-6.56]*** | 3.94[2.52-6.15]*** |
| Female | 2.03[1.23-3.36] ** | 2.08[1.24-3.49] ** | 1.92[1.16-3.17] * | 1.92[1.17-3.16]* | 1.78[1.09-2.90] * |
| White | 0.76[0.51-1.13] | 0.80[0.54-1.19] | 0.83[0.56-1.23] | 0.77[0.52-1.14] | 0.76[0.52-1.11] |
| Age | 1.02[1.00-1.04] * | 1.03[1.01-1.05] ** | 1.02[1.00-1.04] * | 1.02[1.00-1.04] | 1.02[1.00-1.04] * |
| Length of Stay | 1.00[1.00-1.00] *** | 1.00[1.00-1.00] *** | 1.00[1.00-1.00]*** | 1.00[1.00-1.00]*** | 1.00[1.00-1.00]*** |
| Inpatient Psychiatric Stay | 0.62[0.25-1.53] | 0.60[0.24-1.46] | 0.63[0.25-1.58] | 0.55[0.23-1.33] | 0.57[0.24-1.40] |
| Outpatient Psychiatric Services | 1.13[0.73-1.73] | 1.03[0.67-1.58] | 1.11[0.72-1.70] | 1.03[0.69-1.56] | 1.10[0.72-1.69] |
| Psychiatric Medication | 1.08[0.74-1.59] | 0.97[0.66-1.42] | 1.13[0.77-1.66] | 0.94[0.65-1.35] | 0.98[0.68-1.42] |
| Wald Test for Joint Effect of Time | χ2=52.0, p<0.001 | χ2=57.0, p<0.001 | χ2=48.2, p<0.001 | χ2=55.5, p<0.001 | χ2=52.7, p<0.001 |
Note. This table displays odd ratios (OR) and 95% confidence intervals (CI) for variables entered into models predicting abstinence in the past 6 months. Separate models were run to test the relationship between abstinence and different BSI scores (GSI and subscale scores for somatization, depression, hostility, and phobic anxiety). All models included interview time point and controlled for gender, race, age, length of stay and services use. Wald tests (χ2 with 3 degrees of freedom) were conducted to test the combined effect of time across follow-up interviews.
p<0.05;
p<0.01
p<0.001.
Table 4 displays the results of models using each of the BSI scores to predict changes in days of use among those who reported substance use. In these models, negative coefficients (unstandardized) for categories of the time variable indicate that days of use decreased at a subsequent time points compared to baseline, with the Wald test indicating a significant decrease across follow-ups. The positive coefficients for BSI scores indicate that higher scores were associated with increased use, however, this was only statistically significant with respect to symptoms of somatization (0.092, SE=0.029, p=0.002).
Table 4. Models Predicting Days of Use Among Those Reporting Use.
| GSI | Somatization | Depression | Hostility | Phobic Anxiety | |
|---|---|---|---|---|---|
|
|
|||||
| Abstinence | B(SE) | B(SE) | B(SE) | B(SE) | B(SE) |
| BSI Score (GSI, Somatization, etc.) | 0.060(0.031) | 0.092(0.029)** | 0.047(0.025) | 0.021(0.034) | 0.009(0.033) |
| Time Point | |||||
| Baseline (Reference) | |||||
| 6-month | -0.358(0.074)*** | -0.358(0.074)*** | -0.359(0.074)*** | -0.359(0.073)*** | -0.358(0.074)*** |
| 12-month | -0.305(0.071)*** | -0.309(0.072)*** | -0.305(0.071)*** | -0.309(0.071)*** | -0.306(0.071)*** |
| 18-month | -0.177(0.059)** | -0.189(0.059)** | -0.177(0.059)** | -0.173(0.059)** | -0.170(0.059)** |
| Female | -0.007(0.083) | -0.016(0.083) | -0.003(0.083) | 0.005(0.083) | 0.009(0.083) |
| White | 0.107(0.067) | 0.098(0.067) | 0.105(0.067) | 0.108(0.066) | 0.108(0.066) |
| Age | -0.003(0.003) | -0.004(0.003) | -0.003(0.003) | -0.003(0.003) | -0.003(0.003) |
| Length of Stay | 0.000(0.000) | 0.000(0.000) | 0.000(0.000) | 0.000(0.000) | 0.000(0.000) |
| Inpatient Psychiatric Stay | 0.193(0.086)* | 0.189(0.086)* | 0.200(0.088)* | 0.205(0.086)* | 0.207(0.086)* |
| Outpatient Psychiatric Services | -0.255(0.109)* | -0.254(0.109)* | -0.254(0.109)* | -0.243(0.108)* | -0.240(0.111)* |
| Psychiatric Medication | -0.006(0.067) | -0.001(0.067) | -0.013(0.068) | 0.001(0.066) | 0.000(0.066) |
| Wald Test for Joint Effect of Time | χ2=33.3, p<0.001 | χ2=33.7, p<0.001 | χ2=33.3, p<0.001 | χ2=33.9, p<0.001 | χ2=33.3, p<0.001 |
Note. This table displays unstandardized coefficients (B) and standard errors (SE) for variables entered into models predicting days of use during the month of highest use among those reporting use in the past 6 months. Separate models were run to test the relationship between days of use and different BSI scores (GSI and subscale scores for somatization, depression, hostility, and phobic anxiety). All models included interview time point and controlled for gender, race, age, length of stay and services use. Wald tests (χ2 with 3 degrees of freedom) were conducted to test the combined effect of time across follow-up interviews.
p<0.05;
p<0.01
p<0.001.
Discussion
The aims of this study were to describe the prevalence and course of different types of psychiatric symptoms among sober living house residents over 6, 12, and 18-month follow-up interviews and to examine how these symptoms are associated with substance use outcomes. We found that many individuals enter sober living houses with current psychiatric symptoms—51% endorsed 20 or more of the 53 symptoms assessed in the BSI. Normative data is available for adult psychiatric outpatient samples and for adult non-patient samples (Derogatis, 1993). At the item-level and based on measures of overall distress, the symptomology of residents in this sample fell somewhere between these two populations. Adult psychiatric outpatients in the norming samples positively endorsed an average of 30.80 (SD=11.63) symptoms with an average GSI score of 1.32 (SD=0.72); adult non-patients positively endorsed an average of 11.45 (SD=9.20) symptoms with an average GSI score of 0.30 (SD=0.31). Although it is difficult to compare findings from this study to studies of other individuals living in recovery residences due to how psychiatric symptoms have been assessed, studies of Oxford House residents using the Addiction Severity Index have also found that average scores are lower than those found in treatment samples, but approximately a third could be classified as exhibiting high psychiatric severity (Majer et al., 2008).
We were particularly interested in examining changes in symptoms over time, and we found that overall psychological distress and symptoms of depression and phobic anxiety significantly improved, even after controlling for length of stay and receipt of psychiatric services. To get a sense of how clinically meaningful gains might be for overall distress, we looked at the average number of symptoms endorsed among residents at each follow-up interview. At baseline, residents endorsed an average of 21 symptoms, at the 6-month follow-up 15, at the 12-month follow-up 19, and at the 18-month follow-up 19. Although one could argue that the reduction in symptoms between baseline and the 6-month follow-up interview is likely meaningful, it is harder to discern the importance of a reduction in just a few symptoms at later follow-ups. To get a sense of how clinically meaningful gains in depression and phobic anxiety might be, we consulted the BSI norming data. At each interview, average raw scores for sober living house residents were still higher than average raw scores for those in the non-patient norming sample. Data collected on functioning and quality of life might provide additional context to interpret the clinical significance of observed statistically significant differences from baseline to follow-up.
This study also sought to examine how psychiatric symptoms were related to substance use outcomes. We found that overall psychological distress as well as symptoms of somatization, depression, hostility, and phobic anxiety were each associated with decreased rates of abstinence. These findings suggest that even though substance use and psychiatric symptoms improve among sober living house residents, psychiatric symptoms represent a risk factor for relapse among individuals entering these environments. We also found that symptoms of somatization were associated with increased days of use among those who reported substance use. Items assessing somatization query physical complaints such as weakness, nausea, hot or cold spells, dizziness, chest pains, and numbness—sensations that may result from substance use or that may trigger someone to self-medicate to relieve these symptoms. The association between somatic complaints and substance use has been noted in a variety of other studies, and additional work is needed to clarify the complex nature of this association (Hassan & Ali, 2011; Yoshimasu, 2012).
A number of limitations to this study should be highlighted. Data for this study were collected close to a decade ago. Although we have no reason to suspect that the nature of sober living houses or the residents who live in them have appreciably changed, only replication of this study could definitively answer this question. Replication of this study could also address other limitations. For example, this study lacked a comparison group which limits conclusions that can be drawn regarding the specific role that sober living houses may play in improving psychiatric symptoms among residents. Future studies should also include more robust measures of substance use as well as psychiatric symptoms and quality of life. The peak density measure used in this study only captured days of use in the month of heaviest use in the past six months rather than days of use in the past six months. Also, while the BSI is an appropriate and commonly used multidimensional measure of psychological distress, it does not produce psychiatric diagnoses, and it may have limited utility as a comprehensive assessment of diverse psychiatric symptoms among populations on which norms have yet to be established. Further, it captures information about symptoms in the past week and may not be an ideal predictor of behaviors assessed within a larger recall timeframe (e.g., within the past month). Finally future studies should formally model the effects of clustering of residents within as well as the characteristic of the sober living houses studied.
Co-occurring mental disorders are common among individuals with substance use disorders. This study found that many sober living house residents report experiencing a number of psychiatric symptoms upon entry. Although some of these symptoms may improve over time, they are risk factors for relapse. More research is needed to understand how psychiatric symptoms are addressed in sober living houses as well as to understand how individuals with co-occurring psychiatric symptoms experience this environment. This work may lead to the development and testing of specific interventions and strategies targeted to improve outcomes such as training for house managers on the unique needs of residents with psychiatric symptoms and formal linkages with mental health service providers to deliver in-house services to residents. Important advances have been made in the articulation of essential elements of a recovery-oriented environment for individuals with mental disorders (Farkas, Gagne, Anthony, & Chamberlin, 2005; Whitley et al., 2012) and in the understanding of the challenges faced by individuals in recovery from substance use and mental disorders (MacDonald et al., 2004; Padgett, Henwood, Abrams, & Drake, 2008), including the development of mutual aid fellowships for them (Magura, 2008). Research investigating the integration of these elements into the sober living house environment is needed.
Acknowledgments
The authors of this manuscript would like to thank Jane Witbrodt for preliminary work assessing properties of measures used in this study.
Funding: This work was completed with the support of R03DA035175.
Appendix A
Measures of Internal Consistency (Cronbach's Alpha) for BSI Scales Across Interviews.
| Items | Baseline | 6-month | 12-month | 18-month | |
|---|---|---|---|---|---|
| GSI | 53 | 0.97 | 0.97 | 0.97 | 0.98 |
| Somatization | 7 | 0.87 | 0.77 | 0.82 | 0.85 |
| Depression | 6 | 0.89 | 0.90 | 0.88 | 0.90 |
| Hostility | 5 | 0.82 | 0.75 | 0.79 | 0.83 |
| Phobic Anxiety | 5 | 0.85 | 0.82 | 0.82 | 0.85 |
Footnotes
Disclosures: The authors report no financial relationships with commercial interests.
Contributor Information
Doug Polcin, Email: dpolcin@arg.org, Alcohol Research Group at the Public Health Institute.
Rachael Korcha, Email: rkorcha@arg.org, Alcohol Research Group at the Public Health Institute.
Shalika Gupta, Email: sgupta@arg.org, Alcohol Research Group at the Public Health Institute.
Meenakshi Sabina Subbaraman, Email: msubbaraman@arg.org, Alcohol Research Group at the Public Health Institute.
Amy A. Mericle, Email: americle@arg.org, Alcohol Research Group at the Public Health Institute.
References
- Anthony WA. Recovery from mental illness: the guiding vision of the mental health service system in the 1990s. Psychosocial Rehabilitation Journal. 1993;16(4):11–23. doi: 10.1037/h0095655. [DOI] [Google Scholar]
- Benishek LA, Hayes CM, Bieschke KJ, Stöffelmayr BE. Exploratory and confirmatory factor analyses of the Brief Symptom Inventory among substance abusers. Journal of Substance Abuse. 1998;10(2):103–114. doi: 10.1016/S0899-3289(99)80127-8. [DOI] [PubMed] [Google Scholar]
- Borkman T. Resident self-governance in social model recovery programs. Contemporary Drug Problems. 1998;25(4):741–771. [Google Scholar]
- Boulet J, Boss MW. Reliability and validity of the Brief Symptom Inventory. Psychological Assessment. 1991;3(3):433–437. doi: 10.1037/1040-3590.3.3.433. [DOI] [Google Scholar]
- Burns L, Teesson M, O'Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100(6):787–796. doi: 10.1111/j.1360-0443.2005.001069.x. [DOI] [PubMed] [Google Scholar]
- Carscaddon DM, George M, Wells G. Rural community mental health consumer satisfaction and psychiatric symptoms. Community Mental Health Journal. 1990;26(4):309–318. doi: 10.1007/BF00752722. [DOI] [PubMed] [Google Scholar]
- Compton WM, III, Cottler LB, Jacobs JL, Ben-Abdallah A, Spitznagel EL. The role of psychiatric disorders in predicting drug dependence treatment outcomes. The American Journal of Psychiatry. 2003;160(5):890–895. doi: 10.1176/appi.ajp.160.5.890. [DOI] [PubMed] [Google Scholar]
- Cunningham JA, Sobell LC, Gavin DR, Sobell MB, Breslin FC. Assessing motivation for change: Preliminary development and evaluation of a scale measuring the costs and benefits of changing alcohol and drug use. Psychology of Addictive Behaviors. 1997;11(2):107–114. doi: 10.1037/0893-164X.11.2.107. [DOI] [Google Scholar]
- Dennis ML, Scott CK. Managing addiction as a chronic condition. Addiction Science and Clinical Practice. 2007;4(1):45–55. doi: 10.1151/ascp074145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R: Administration, scoring and procedures manual-11. Towson, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- Derogatis LR. BSI Brief Symptom Inventory: Administration, scoring, and procedure manual. 4th. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13(3):595–605. doi: 10.1017/S0033291700048017. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- Drake RE, Whitley R. Recovery and severe mental illness: description and analysis. Canadian Journal of Psychiatry [La Revue canadienne de psychiatrie] 2014;59(5):236–242. doi: 10.1177/070674371405900502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth K. Arlington, VA: National Alliance on Mental Illness; 2013. [Accessed: 2015-07-31]. Mental Illness: What you need to know; p. 19. Archived by WebCite® at http://www.webcitation.org/6aR8iUNyG. [Google Scholar]
- Farkas M, Gagne C, Anthony W, Chamberlin J. Implementing recovery oriented evidence based programs: identifying the critical dimensions. Community Mental Health Journal. 2005;41(2):141–158. doi: 10.1007/s10597-005-2649-6. [DOI] [PubMed] [Google Scholar]
- Gagne C, White W, Anthony WA. Recovery: a common vision for the fields of mental health and addictions. Psychiatric Rehabilitation Journal. 2007;31(1):32–37. doi: 10.2975/31.1.2007.32.37. [DOI] [PubMed] [Google Scholar]
- Gerstein DR, Johnson RA, Foote M, Suter N, Jack K, Merker G, Fountain D. Evaluating Recovery Services: The California Drug and Alcohol Treatment Assessment (CALDATA): Methodology Report. Sacramento, CA: State of California Department of Alcohol and Drug Programs; 1994. [Google Scholar]
- Grant BF, Dawson DA. Introduction to the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research and Health. 2006;29(2):74–78. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton WM, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hassan I, Ali R. The association between somatic symptoms, anxiety disorders and substance use. A literature review. Psychiatric Quarterly. 2011;82(4):315–328. doi: 10.1007/s11126-011-9174-2. [DOI] [PubMed] [Google Scholar]
- Havassy BE, Alvidrez J, Owen KK. Comparisons of patients with comorbid psychiatric and substance use disorders: implications for treatment and service delivery. American Journal of Psychiatry. 2004;161(1):139–145. doi: 10.1176/appi.ajp.161.1.139. [DOI] [PubMed] [Google Scholar]
- Hayes JA. What does the Brief Symptom Inventory measure in college and university counseling center clients? Journal of Counseling Psychology. 1997;44(4):360–367. doi: 10.1037/0022-0167.44.4.360. [DOI] [Google Scholar]
- Holden RR, Starzyk KB, McLeod LD, Edwards MJ. Comparisons among the Holden Psychological Screening Inventory (HPSI), the Brief Symptom Inventory (BSI), and the Balanced Inventory of Desirable Responding (BIDR) Assessment. 2000;7(2):163–175. doi: 10.1177/107319110000700208. [DOI] [PubMed] [Google Scholar]
- Huber PJ. The behavior of maximum likelihood estimates under nonstandard. In: Le Cam LM, Neyman J, editors. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1. Berkeley, CA: University of California Press; 1967. pp. 221–233. [Google Scholar]
- Jason LA, Mericle AA, Polcin DL, White WL. The role of recovery residences in promoting long-term addiction recovery. American Journal of Community Psychology. 2013;52(3-4):406–411. doi: 10.1007/s10464-013-9602-6. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educational and Psychological Measurement. 1960;20(1):141–151. doi: 10.1177/001316446002000116. [DOI] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet AB, Humphreys K. Promoting recovery in an evolving context: what do we know and what do we need to know about recovery support services? Journal of Substance Abuse Treatment. 2013;45(1):126–133. doi: 10.1016/j.jsat.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet AB, White W. What are your priorities right now? Identifying service needs across recovery stages to inform service development. Journal of Substance Abuse Treatment. 2010;38(1):51–59. doi: 10.1016/j.jsat.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- Lipsky S, Krupski A, Roy-Byrne P, Lucenko B, Mancuso D, Huber A. Effect of co-occurring disorders and intimate partner violence on substance abuse treatment outcomes. Journal Of Substance Abuse Treatment. 2010;38(3):231–244. doi: 10.1016/j.jsat.2009.12.005. [DOI] [PubMed] [Google Scholar]
- MacDonald EM, Luxmoore M, Pica S, Tanti C, Blackman JM, Catford N, Stockton P. Social networks of people with dual diagnosis: the quantity and quality of relationships at different stages of substance use treatment. Community Mental Health Journal. 2004;40(5):451–464. doi: 10.1023/B:COMH.0000040658.41548.b2. [DOI] [PubMed] [Google Scholar]
- Magura S. Effectiveness of dual focus mutual aid for co-occurring substance use and mental health disorders: A review and synthesis of the ‘double trouble’ in recovery evaluation. Substance Use and Misuse. 2008;43(12-13):1904–1926. doi: 10.1080/10826080802297005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer JM, Jason LA, North CS, Ferrari JR, Porter NS, Olson B, Molloy JP. A longitudinal analysis of psychiatric severity upon outcomes among substance abusers residing in self-help settings. American Journal Of Community Psychology. 2008;42(1-2):145–153. doi: 10.1007/s10464-008-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullahy J. Specification and testing of some modified count data models. Journal of Econometrics. 1986;33(3):341–365. doi: 10.1016/0304-4076(86)90002-3. [DOI] [Google Scholar]
- National Institute on Drug Abuse. Rockville, MD: 2014. [Accessed: 2015-07-31]. Drugs, Brains, and Behavior: The science of addiction; p. 31. Archived by WebCite® at http://www.webcitation.org/6aRGKr3mu. [Google Scholar]
- Padgett DK, Henwood B, Abrams C, Drake RE. Social relationships among persons who have experienced serious mental illness, substance abuse, and homelessness: implications for recovery. American Journal of Orthopsychiatry. 2008;78(3):333–339. doi: 10.1037/a0014155. [DOI] [PubMed] [Google Scholar]
- Piersma HL, Boes J, Reaume WM. Unidimensionality of the Brief Symptom Inventory (BSI) in adult and adolescent inpatients. Journal of Personality Assessment. 1994;63(2):338–344. doi: 10.1207/s15327752jpa6302_12. [DOI] [PubMed] [Google Scholar]
- Piersma HL, Reaume WM, Boes JL. The Brief Symptom Inventory (BSI) as an outcome measure for adult psychiatric inpatients. Journal of Clinical Psychology. 1994;50(4):555–563. doi: 10.1002/1097-4679(199407)50:4<555::AID-JCLP2270500410>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Polcin DL. Sober Living Houses: Potential roles in substance abuse services and suggestions for research. Substance Use and Misuse. 2001;36(3):301–311. doi: 10.1081/JA-100102627. [DOI] [PubMed] [Google Scholar]
- Polcin DL. Addiction Health Services Research Conference. Little Rock, AK: 2006a. Oct, Sober Living Houses After, During, and as an Alternative to Treatment; pp. 23–25. [Google Scholar]
- Polcin DL. What about Sober Living Houses for parolees? Criminal Justice Studies. 2006b;19(3):291–300. doi: 10.1080/14786010600921712. [DOI] [Google Scholar]
- Polcin DL, Buscemi R, Nayak M, Korcha R, Galloway G. Sex differences in psychiatric symptoms among methamphetamine-dependent residents in sober living houses. Addictive Disorders and Their Treatment. 2012;11(2):53–63. doi: 10.1097/ADT.0b013e3182213ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin DL, Galloway GP, Taylor KD, Benowitz-Fredericks A. Where are they going to live? Why we need to study Sober Living Houses. Counselor. 2004;5(5):36–45. [Google Scholar]
- Polcin DL, Henderson DM. A clean and sober place to live: philosophy, structure, and purported therapeutic factors in sober living houses. Journal of Psychoactive Drugs. 2008;40(2):153–159. doi: 10.1080/02791072.2008.10400625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin DL, Korcha R, Bond J. Interaction of motivation and psychiatric symptoms on substance abuse outcomes in sober living houses Substance Use and Misuse. 2015;50(2):195–204. doi: 10.3109/10826084.2014.962055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin DL, Korcha R, Bond J, Galloway G. Eighteen-month outcomes for clients receiving combined outpatient treatment and sober living houses. Journal of Substance Abuse. 2010a;15(5):352–366. doi: 10.3109/14659890903531279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin DL, Korcha R, Bond J, Galloway GP. Sober living houses for alcohol and drug dependence: 18-month outcomes. Journal of Substance Abuse Treatment. 2010b;38(4):356–365. doi: 10.1016/j.jsat.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin D, Mericle A, Howell J, Sheridan D, Christensen J. Maximizing social model principles in residential recovery settings. Journal Of Psychoactive Drugs. 2014;46(5):436–443. doi: 10.1080/02791072.2014.960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray GT, Mertens J, Weisner C. The relationship of psychiatric severity and services to five-year alcohol and drug treatment outcomes. Psychiatric Services. 2005;56(2):164–171. doi: 10.1176/appi.ps.56.2.164. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) study. The Journal of the American Medical Association. 1990;264(19):2511–2518. doi: 10.1001/jama.1990.03450190043026. [DOI] [PubMed] [Google Scholar]
- Shane PA, Jasiukaitis P, Green RS. Treatment outcomes among adolescents with substance abuse problems: The relationship between comorbidities and post-treatment substance involvement. Evaluation And Program Planning. 2003;26(4):393–402. doi: 10.1016/S0149-7189(03)00055-7. [DOI] [Google Scholar]
- Skeem JL, Schubert C, Odgers C, Mulvey EP, Gardner W, Lidz C. Psychiatric symptoms and community violence among high-risk patients: a test of the relationship at the weekly level. Journal of Consulting and Clinical Psychology. 2006;74(5):967–979. doi: 10.1037/0022-006X.74.5.967. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: 2012. [Accessed: 2015-07-31]. SAMHSA's Working Definition of Recovery; p. 7. Archived by WebCite® at http://www.webcitation.org/6aRHz0R8X. [Google Scholar]
- Watkins KE, Hunter SB, Wenzel SL, Tu W, Paddock SM, Griffin A, Ebener P. Prevalence and characteristics of clients with co-occurring disorders in outpatient substance abuse treatment. American Journal Of Drug and Alcohol Abuse. 2004;30(4):749–764. doi: 10.1081/ADA-200037538. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–838. doi: 10.2307/1912934. [DOI] [Google Scholar]
- White H. Maximum likelihood estimation of misspecified models. Econometrica. 1982;50(1):1–25. [Google Scholar]
- Whitley R, Strickler D, Drake RE. Recovery centers for people with severe mental illness: a survey of programs. Community Mental Health Journal. 2012;48(5):547–556. doi: 10.1007/s10597-011-9427-4. [DOI] [PubMed] [Google Scholar]
- Yoshimasu K. Substance-related disorders and somatic symptoms: how should clinicians understand the associations? Current Drug Abuse Reviews. 2012;5(4):291–303. doi: 10.2174/1874473711205040004. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. doi: 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI. A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Archives of General Psychiatry. 2001;58(8):787–794. doi: 10.1001/archpsyc.58.8.787. [DOI] [PubMed] [Google Scholar]


