Abstract
Introduction
Far too few smokers receive recommended interventions at their healthcare visits, highlighting the importance of identifying effective, low-cost smoking interventions that can be readily delivered. Self-help interventions (e.g., written materials) would meet this need, but they have shown low efficacy. The purpose of this RCT was to determine the efficacy of a self-help intervention with increased duration and intensity.
Design
Randomized parallel trial design involving enrollment between April 2010 and August 2011 with follow-up data for 24 months.
Setting/participants
U.S. national sample of daily smokers (N=1,874).
Intervention
Participants were randomized to one of three arms of a parallel trial design: Traditional Self-Help (TSH, n=638), Standard Repeated Mailings (SRM, n=614), or Intensive Repeated Mailings (IRM, n=622). TSH received an existing self-help booklet for quitting smoking. SRM received eight different cessation booklets mailed over a 12-month period. IRM received monthly mailings of ten booklets and additional material designed to enhance social support over 18 months.
Main outcome measures
The primary outcome was 7-day point-prevalence abstinence collected at 6, 12, 18, and 24 months.
Results
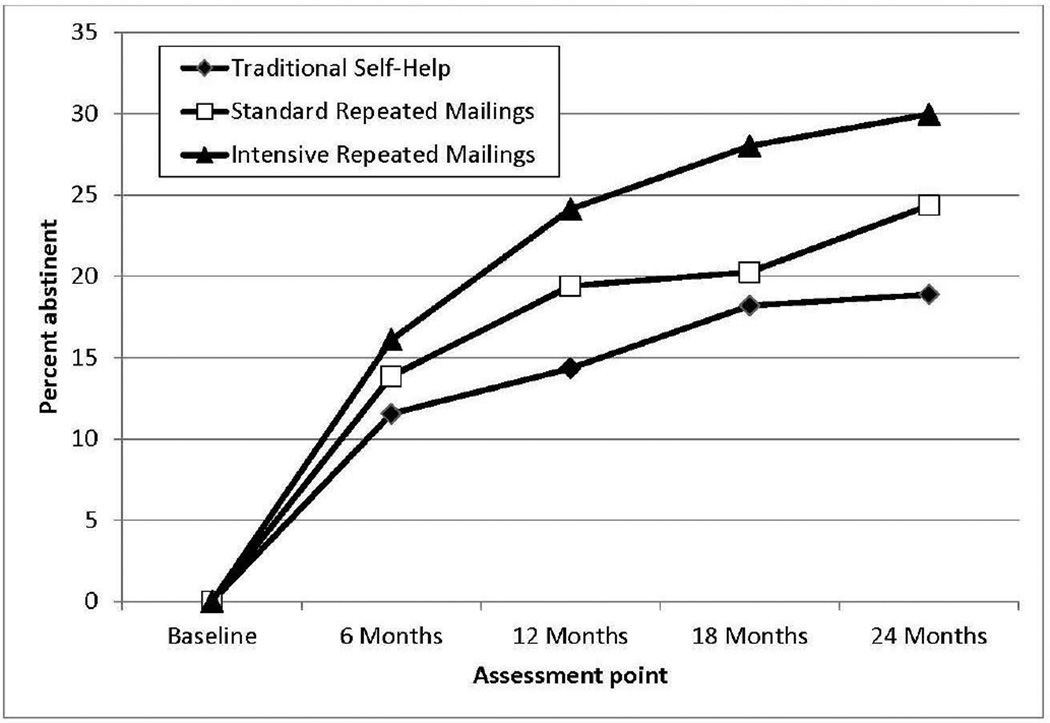
Data were analyzed between 2013 and 2015. A dose–response effect was found across all four follow-up points. For example, by 24 months, IRM produced the highest abstinence rate (30.0%), followed by SRM (24.4%), and TSH (18.9%). The difference in 24-month abstinence rates between IRM and TSH was 11.0% (95% CI=5.7%, 16.3%). Cost analyses indicated that, compared with TSH, the incremental cost per quitter who received SRM and IRM was $560 and $361, respectively.
Conclusions
Self-help interventions with increased intensity and duration resulted in significantly improved abstinence rates that extended 6 months beyond the end of the intervention. Despite the greater intensity, the interventions were highly cost effective, suggesting that widespread dissemination in healthcare settings could greatly enhance quitting. This study is registered with ClinicalTrials.gov (NCT01352195).
Introduction
Tobacco smoking continues to be a pressing healthcare problem, responsible for nearly 6 million worldwide deaths annually. The average smoker loses 10 years of life, but can regain 6–10 years by quitting smoking by age 50 years.1 Although most smokers report wanting to quit smoking, cessation rates continue to be low and the population-level public health impact of the most prevalent interventions has been questioned.2 Indeed, despite tremendous efforts to encourage integration of smoking-cessation interventions into healthcare settings, the results have been modest at best,3 perhaps because of lack of provider training, time constraints, insurance limitations, conflicting clinical roles, and suboptimal clinical practices. This highlights the need for low-cost, easily implemented, effective interventions for smoking that can be readily used in healthcare settings (e.g., disseminated in hospitals and clinics) and elsewhere. Self-help interventions, defined as written materials “used to assist a quit attempt not aided by health professionals, counselors, or group support,”4 have high potential in this regard, but their efficacy to date has been unacceptably low. Both the U.S. Public Health Service’s Clinical Practice Guidelines for Treating Tobacco Use and Dependence and a Cochrane Review concluded that self-help materials have at best marginal efficacy, improving cessation rates by less than 2% compared with no-treatment controls and finding no difference when compared to less structured written materials.4,5 These rates compare unfavorably to other interventions, such as counseling or medication.5,6 However, if more-effective self-help materials could be developed, the potential public health impact is substantial.
One of the most obvious differences between prior self-help materials and smoking-cessation counseling is the intensity of the interventions themselves. The counseling programs that have demonstrated the greatest efficacy involve multiple sessions typically delivered over several weeks, totaling 5–12 hours of face-to-face interaction.5 The most important factor predictive of efficacy is the intensity of the intervention.5 Yet, self-help interventions have been low intensity and short term.4 This leads to the empirical question of whether the efficacy of self-help could be improved by enhancing its contact intensity (number of written contacts) and duration (period of time over which written materials are sent); that is, “extended self-help.”
The hypothesis that extended self-help would be efficacious is supported by findings that a series of eight booklets (currently named Forever Free®) mailed to participants over 12 months substantially reduced relapse rates for recent quitters in two RCTs.7,8 Moreover, an adaptation of these booklets reduced postpartum smoking relapse among low-income women.9 Indeed, a recent meta-analysis found that written self-help materials were the only type of relapse-prevention intervention for unaided quitters with established efficacy.10 These interventions are also highly cost effective.11 An extended self-help cessation intervention would greatly expand the impact of this approach, because it would not be restricted to recent quitters.
Based on these positive results for smoking relapse prevention, a similar extended self-help intervention for smoking cessation was developed and tested in the current study. Moreover, it examined whether cessation rates could be improved by extending the intensity and duration of the intervention mailings, while enhancing the perception of social support.12 The goal from a public health perspective was to maximize efficacy without sacrificing the advantages of self-help in terms of cost, reach, and ease of dissemination, thereby continuing to produce favorable cost effectiveness compared with traditional smoking-cessation interventions. Thus, the primary hypothesis was that extended self-help would be more effective than traditional self-help and that increasing the intensity and duration of the intervention would produce enhanced efficacy in a dose–response manner. It was also hypothesized that extended self-help would continue to produce favorable cost effectiveness compared with traditional smoking-cessation interventions.
Methods
Study Design
This RCT utilized a three-arm parallel trial design (1:1:1 allocation) that varied the duration and intensity of mailed self-help interventions.13 The intervention was administered by postal mail to participants throughout the U.S. The protocol was approved by the IRB of the University of South Florida.
Study Participants
Participants were recruited nationally via multimedia advertisements, including newspapers, radio, cable TV, public transit, public service announcements, and direct community engagement. Smokers called a toll-free telephone number in response to the recruitment efforts that advertised quit smoking materials/informational booklets for current smokers interested in quitting and were screened for the minimal inclusion criteria:
smoke at least five cigarettes per day over the past year;
desire to quit smoking, indicated by a score of 5 (Think I should quit, but not quite ready) or higher on the Contemplation Ladder14;
able to speak and read English;
not currently enrolled in a face-to-face smoking-cessation program; and
aged >18 years.
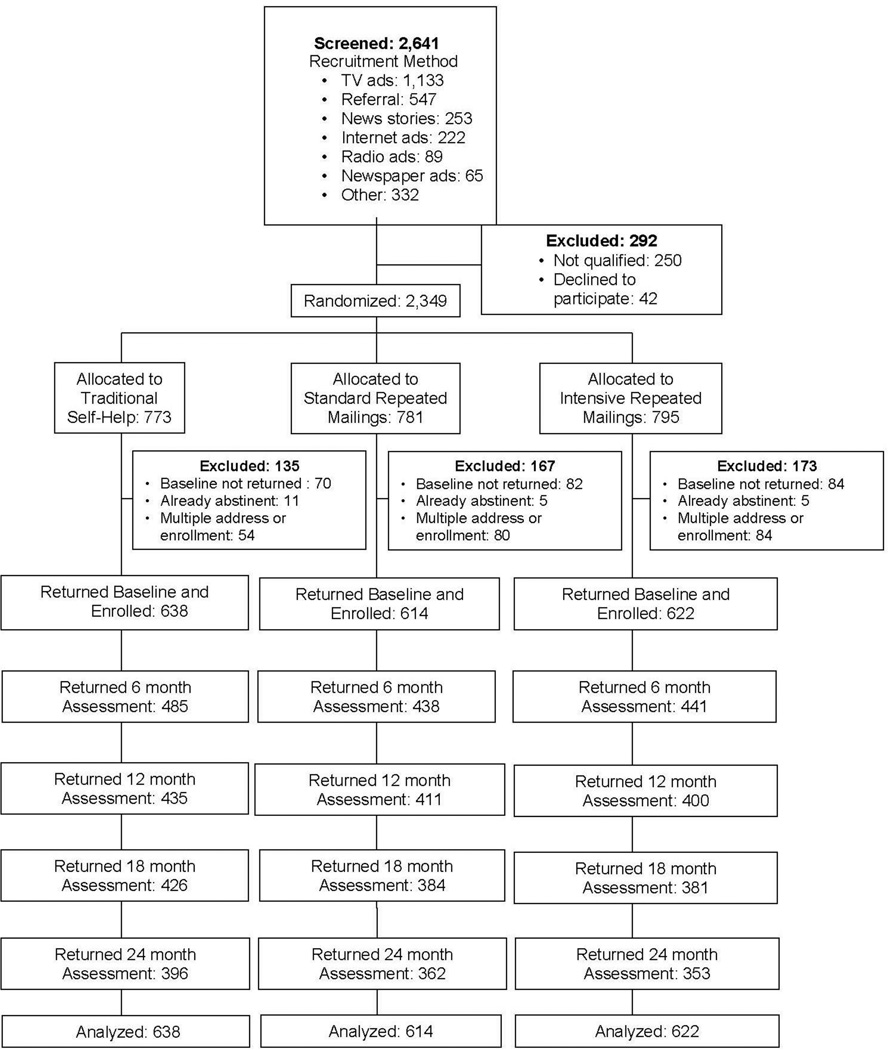
There was no racial or gender bias in the selection of participants. After initial telephone screening, baseline assessments were sent to 2,349 smokers. Of these, 1,874 returned the baseline questionnaires, continued to meet inclusion criteria, and were enrolled in the study. Recruitment and study flow are shown in Figure 1 and described in more detail elsewhere.13 Individuals were allocated to the three intervention arms using simple randomization without stratification. Intervention assignment was generated by computer upon entry of screening data into a relational database.
Figure 1.
CONSORT diagram.
Procedures
Eligible participants were randomized to one of three treatment arms and mailed the appropriate intervention materials after return of the baseline assessment. Participants were enrolled between April 9, 2010 and August 18, 2011 until the target sample size was reached.
The Traditional Self-Help (TSH) arm comprised a single self-help booklet that was in dissemination at the time of the study: Clearing the Air: Quit Smoking Today,15 a 37-page booklet with high-quality visual presentation and content published by the National Cancer Institute and disseminated by the Cancer Information Service. It was selected as a credible comparison arm representing a traditional, single-contact, self-help intervention.
The two extended self-help interventions tested in this study were based on the original Forever Free relapse prevention booklets.7,8 Booklet content was originally derived from cognitive– behavioral theory as well as empirical evidence on tobacco dependence, cessation, and relapse.16,17 They were designed as a means of translating the cognitive–behavioral counseling that typically occurs in a clinic into a written format that would be more accessible to smokers. The booklets were updated and revised to alter any language that explicitly assumed that the reader had already achieved abstinence. For example, information was added about preparing to quit smoking and increased the emphasis on use of pharmacotherapy. The cessation booklet series was titled, Forever Free®: Stop Smoking for Good.
The revised eight booklets comprised the Standard Repeated Mailings (SRM)) treatment. The booklets were written at a fifth to sixth grade reading level (U.S.) so as to maximize their accessibility to a wide range of literacy levels.18 The first booklet was mailed to participants immediately after receipt of their baseline assessment, and the rest were mailed at the following time points: 1, 2, 3, 5, 7, 9, and 12 months. The first of the eight booklets provides a general summary of the process of quitting smoking, and each of the subsequent seven booklets include more extensive information on a topic related to cessation and relapse prevention: Smoking Urges; Smoking and Weight; What if You Have a Cigarette?; Your Health; Smoking, Stress, and Mood; Lifestyle Balance; and Life Without Cigarettes.
To maximize the frequency and duration of contact, the Intensive Repeated Mailings (IRM) intervention expanded on SRM by:
extending treatment an additional 6 months;
maintaining monthly contact throughout the intervention period; and
enhancing perceived social support.
Thus, this arm sent two additional booklets (at 15 and 18 months) and nine brief pamphlets (every month without a booklet). These two additional booklets, The Benefits of Quitting Smoking and The Road Ahead, offered the opportunity to reinforce key points conveyed in earlier booklets by reemphasizing the health and other benefits of long-term tobacco abstinence, stress management, anticipation of potential high-risk situations, and the use of cognitive and behavioral coping responses.
The brief pamphlets mailed in non-booklet months were designed to enhance the perception of social support—a benefit of face-to-face counseling often lost in self-help approaches. These tri-fold color pamphlets reinforced key messages about quitting smoking (e.g., dealing with stress, keeping weight gain in perspective, finding other forms of positive reinforcement). In addition, the intention in developing the brief pamphlets was to provide smokers with a sense of social support by communicating the messages via a first-person narrative from a former smoker, and accompanied by a supportive cover letter. Given the evidence that social support benefits smoking cessation,19,20 these pamphlets served as unique method for incorporating social support constructs in a written intervention modality.
Assessments
The baseline questionnaire assessed demographic and smoking variables, including the Fagerström Test for Nicotine Dependence.21 In addition, current and past use of pharmacotherapy and other smoking-cessation aids as well as e-cigarette use was assessed. Follow-up assessment packets were mailed at 6-month intervals for all conditions, beginning with 6 months after study entry through 30 months. The 24-month assessment reflects 6, 12, and 24 months since the last receipt of intervention materials for the IRM, SRM, and TSH conditions, respectively. These assessments were brief to minimize participant burden and reactance. Each follow-up assessed participants’ use of pharmacotherapy and other smoking-cessation aids since the last contact, and included the 8-item Client Satisfaction Questionnaire22 to assess participants’ use and evaluation of the self-help materials. Self-reported 7-day point-prevalence abstinence (i.e., no tobacco cigarettes smoked in the previous 7 days) served as the primary outcome variable and the focus of the follow-up assessments. Because there was no specified target quit date and the interventions continuously encouraged both initial cessation and recovery from relapse at any point, continuous abstinence was not an appropriate outcome metric. Biochemical verification of self-reported smoking abstinence was not included, based on evidence of little benefit derived from inclusion of biochemical verification measures in low-intensity interventions such as these that have no face-to-face contact, and there is little incentive to falsely report abstinence.23 Further, the assessments included a motivational measure of readiness to quit (Contemplation Ladder14) and a single-item assessing confidence in their ability to not smoke in the next 6 months.
Statistical Analysis
Data were analyzed between 2013 and 2015. Sample size was selected to detect minimum differences of 5% in the primary outcome measure (abstinence rates between pairs of arms) using generalized estimating equation (GEE) analyses. A priori sample size calculation indicated that a final sample of ≥527 smokers per arm would provide 80% power, with α=0.05, to detect differences under conservative assumptions about the possible range of abstinence rates of the TSH arm.
Outcomes are reported here through 24 months with a focus on longitudinal point prevalence. Analyses were “intent to treat” based on the sample at randomization and study enrollment. Treatment effects across the four follow-ups were first assessed with the logistic framework of the GEEs using an autoregressive working correlation structure with the main effects of arm, month, and their interaction. GEE was also used to conduct paired comparisons of the three treatment arms over time. For these paired comparisons, p-values were adjusted using the Benjamini–Hochberg method with a false discovery rate of 0.05. Potential moderators were tested individually in separate GEE models, and incorporated in the main effects model. Logistic regression was used to test treatment effects at each follow-up point.
To manage missing data, multiple imputation24 was conducted under the Missing at Random assumption using a Markov Chain Monte Carlo method via the procedure of PROC MI in SAS, version 9.3.25 In addition to treatment arm and 7-day point prevalence at all four follow-ups, the model included:
ten baseline measures (and their interaction with arm) that were either directly related to smoking status during follow-up (e.g., Fagerström Test for Nicotine Dependence) or were considered prospective moderators of the treatment effect (e.g., gender); and
follow-up reports of Contemplation Ladder level and abstinence confidence ratings.
Twenty sets of complete data were generated. A post hoc approach was then used to address the influence of Missing Not at Random on smoking status (i.e., missing implies smoking)26 using a small to medium effect size (Cohen’s d=0.35). A sensitivity analysis was conducted on this post hoc adjustment by retesting the primary outcome findings using the extreme assumptions of either:
no adjustment for missing implies smoking; or
imputing smoking for all missing outcome data.
To determine cost effectiveness, intervention costs were first calculated by recording the printing, postage, and handling costs per intervention condition, excluding all research-specific expenses. Then, cost-effectiveness ratios were calculated for each arm by dividing the difference in intervention costs between the conditions (TSH versus SRM and TSH versus IRM) by the difference in 24-month abstinence rates. An intervention was deemed to be more cost effective if the resulting value were lower than its comparisons.
Descriptive statistics and group comparisons were used in exploratory analyses to describe pharmacotherapy use and satisfaction.
Results
The majority of the sample was aged 36–60 years, female, non-Hispanic, and Caucasian with an annual household income <$20,000 (Table 1). Participants tended to be moderately nicotine dependent and smoked an average of about a pack of cigarettes per day.
Table 1.
Baseline Sample Characteristics by Treatment Arm
| Traditional self-help N=638 |
Standard repeated mailings N=614 |
Intensive repeated mailings N=622 |
|
|---|---|---|---|
| Age, years: M(SD) | 48.4 (12.0) | 46.5 (12.5) | 47.5 (11.5) |
| Sex, N(%) | |||
| Men | 208 (33%) | 219 (36%) | 214 (34%) |
| Women | 430 (67%) | 395 (64%) | 408 (66%) |
| Race, N(%) | |||
| White | 409 (65%) | 410 (67%) | 393 (64%) |
| Black/African-American | 193 (31%) | 175 (29%) | 189 (31%) |
| Other | 30 (4%) | 28 (4%) | 36 (5%) |
| Hispanic/Latino ethnicity N(%) | 32 (5%) | 35 (6%) | 36 (6%) |
| Income, N(%) | |||
| Under $10,000 | 209 (34%) | 205 (34%) | 198 (32%) |
| $10,000-$19,999 | 141 (23%) | 145 (24%) | 151 (25%) |
| $20,000-$29,999 | 80 (13%) | 75 (13%) | 75 (12%) |
| $30,000+ | 192 (30%) | 171 (29%) | 191 (31%) |
| Cigarettes per day, M(SD) | 20.4 (11.2) | 20.9 (13.0) | 20.3 (11.2) |
| Fagerström Test of Nicotine Dependence, M(SD) | 5.7 (2.3) | 5.6 (2.3) | 5.7 (2.2) |
| Baseline pharmacotherapy use | |||
| Nicotine gum | 20 (3%) | 25 (4%) | 26 (4%) |
| Nicotine patch | 35 (5%) | 29 (5%) | 34 (5%) |
| Nicotine lozenge | 8 (1%) | 11 (2%) | 8 (1%) |
| bupropion | 8 (1%) | 19 (3%) | 21 (3%) |
| varenicline | 12 (2%) | 8 (1%) | 20 (3%) |
| Baseline e-cigarette use | 31 (5%) | 24 (4%) | 22 (4%) |
The GEE analyses tested the effect of self-help dose by testing for a linear trend in abstinence rates of the TSH, SRM, and IRM, respectively, across the 6, 12, 18, and 24-month assessments. Analyses revealed an increase in abstinence over time across all three arms (p=0.024, Figure 2). As predicted, there was an overall group difference with abstinence highest in IRM, followed by SRM, and then TSH (p<0.0001). No time-by-arm interaction emerged (p=0.302), indicating that differences between arms were relatively consistent across the four assessments. Paired comparison results indicated that IRM was superior to both TSH (p<0.0001) and SRM (p=0.039), and SRM was superior to TSH (p=0.045), further confirming the dose–response effect.
Figure 2.
Abstinence rates of the three intervention arms through 24-month assessment.
Paired comparisons at each assessment point are shown in Table 2. Most notably, the IRM arm produced significantly greater abstinence than TSH at each follow-up, with ORs ranging from 1.47 to 1.90. Abstinence rates of the SRM arm were consistently between the rates of TSH and IRM, but these differences reached statistical significance only twice at individual follow-ups. Both demographic (sex, age, minority status, marital status, income) and smoking-related variables (nicotine dependence, motivation to quit, quitting confidence, and baseline use of cessation medication) were tested as treatment moderators, but no effects emerged. Thus, the observed treatment outcome differences were consistent across participant subgroups.
Table 2.
7-Day Point-Prevalence Abstinence Rates and Paired Comparisons Between Treatment Arms
| Paired comparisons |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Abstinence rate | SRM versus TSH |
IRM versus TSH |
IRM versus SRM |
||||||
| Assessment | TSH | SRM | IRM | OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|||
| point | (638) | (614) | (622) | AD (95% CI) |
p- value |
AD (95% CI) |
p- value |
AD (95% CI) |
p- value |
| 6 months | 11.54 | 13.85 | 16.09 | 1.23 (0.85 – 1.79) 2.32 (− 1.80 – 6.43) |
0.269 | 1.47 (1.03 – 2.10) 4.55 (0.35 – 8.75) |
0.033 | 1.19 (0.84 – 1.69) 2.23 (− 2.14 – 6.60) |
0.319 |
| 12 months | 14.34 | 19.40 | 24.12 | 1.44 (1.03 – 2.01) 5.06 (0.37 – 9.74) |
0.035 | 1.90 (1.38 – 2.62) 9.78 (4.86 – 14.71) |
0.0001 | 1.32 (0.97 – 1.81) 4.73 (− 0.54 – 10·00) |
0.081 |
| 18 months | 18.19 | 20.25 | 28.01 | 1.14 (0.84 – 1.56) 2.06 (− 2.75 – 6.87) |
0.399 | 1.75 (1.30 – 2.36) 9.82 (4.66 – 14.99) |
0.0002 | 1.53 (1.13 – 2.09) 7.76 (2.22 – 13.30) |
0.007 |
| 24 months | 18.88 | 24.35 | 29.96 | 1.38 (1.00 – 1.91) 5.47 (− 0.01 – 10.95) |
0.051 | 1.84 (1.37 – 2.47) 10.99 (5.74 – 16.25) |
<0.0001 | 1.33 (0.98 – 1.80) 5.61 (− 0.27 – 11.49) |
0.065 |
Notes: Boldface indicates statistical significance (p < 0.05).
Abstinence rates are averaged across the 20 imputed data sets. Analysis results also based on all 20 imputed data sets.
TSH, Traditional Self Help; SRM, Standard Repeated Mailings; IRM, Intensive Repeated Mailings; AD, Absolute Difference
Sensitivity analyses were conducted to test the extreme approaches to missingness implies smoking via post hoc adjustment strategy. When no post hoc adjustment was made (i.e., retaining the results of multiple imputation alone), both the GEE and paired comparisons at specific assessment points via logistic regression showed similar patterns of significant differences as reported above and in Table 2, except that every paired comparison at 12 months reached significance. At the other extreme, when the post hoc adjustment converted all missing point-prevalence data to smoking, the main effect of the self-help dose remained significant in the GEE analyses (p=0.0004), as did the paired comparison between IRM and TSH (p=0.0004), but SRM no longer differed significantly from IRM (p=0.063) or THS (p=0.097). At specific assessment points, the only changes in the paired comparisons were that IRM versus TSH differences no longer reached significance at 6 months (p=0.064) or 12 months (p=0.080), but they remained significant at 18 months (p<0.0001) and 24 months (p=0.0032). Thus, even under this extreme approach, IRM showed significantly higher abstinence rates than TSH at the most important long-term assessment points.
From a payer’s perspective, these interventions are rather inexpensive. The printing, postage, and handling costs were $5.46 (TSH), $36.12 (SRM), and $45.50 (IRM) per intervention. Note that this estimate does not include research-only expenses, the resources needed to identify smokers, or the indirect costs of smoking (e.g., cigarettes, medical care, productivity, transfer payments). Compared with a traditional self-help booklet, SRM and IRM increased both the costs (by $30.66 and $40.04, respectively) and the 24-month abstinence rates (by 5.5% and 11.1%). In terms of cost per quitter, results indicate that the incremental cost-effectiveness ratios (i.e., the difference in cost divided by difference in abstinence at 24 months, compared with TSH) of the SRM and IRM arms were $560 and $361, respectively. Because the IRM cost less per quitter than SRM, it was deemed more cost effective than SRM.
At baseline, 68.5% reported past use of a smoking-cessation medication and 11.7% reported current use, with no group differences. Medication use at the four follow-ups initially increased to 22.7% at 6 months and then decreased over subsequent follow-ups to 19.1% at 24 months. Group differences occurred only at 6 months when medication use was greater for the IRM arm (28.4%) than for both the TSH arm (18.2%) and the SRM arm (21.9%) (p<0.03 for both).
The authors collected participants’ evaluations of treatment materials at all follow-ups. Separate GEE analyses of whether or not participants read, saved, or shared the materials revealed group differences. Both the IRM and SRM arms were more likely to report reading the materials than the TSH arm (97%, 98%, and 94%, respectively, all p <0.0005), were more likely to report saving the materials than the TSH arm (80%, 81%, and 73%, all p <0.0001), and were more likely to report sharing the materials than the TSH arm (45%, 47%, and 35%, all p <0.0001). There were no significant differences between the IRM and SRM arms. GEE analyses of the Client Satisfaction Questionnaire scores (possible range, 8–32) revealed similar results. Both the IRM (24.9) and SRM (24.8) arms reported higher satisfaction ratings (“Good” to “Excellent” range) than the TSH arm (22.7, p <0.0001, “Fair” to “Good” range), with no significant difference between the IRM and SRM arms.
Discussion
This large RCT varied the intensity and duration of self-help intervention for smoking cessation. Intensity was defined by the number of mailings (i.e., one, eight, and 19 mailings for TSH, SRM, and IRM, respectively) and duration reflects the time period over which the self-help materials were delivered (i.e., a single time point, over 12 months, and over 18 months for the TSH, SRM, and IRM conditions, respectively). Results provide evidence that when a low-cost, smoking-cessation, self-help intervention is delivered over an extended period of time, abstinence rates greatly exceed those for a traditional “one-shot” self-help intervention. Group differences remained a full 6 months beyond the end of the longest intervention. The 11-point difference in abstinence rates between IRM and TSH at the end of the trial far exceeded the typical 1%–2% differential abstinence rates achieved by traditional self-help.4,5,27 Also, consistent with most studies of smoking cessation, abstinence rates increased with the number of contacts (i.e., contact intensity; in this case, written contacts) and duration. Indeed, the abstinence rates achieved by the most intensive arm (IRM) are within the typical range achieved by the combination of medication and counseling.5
These findings demonstrate the utility of a low-cost self-help approach in addressing a notoriously intractable behavior with enormous personal and economic costs. For healthcare systems, self-help represents the “holy grail” of behavioral interventions because its implementation does not rely upon changing the behavior of healthcare providers. Because the decision can be made to target all smokers in a system, the potential reach of self-help is vast; consequently, its public health impact (and impact on healthcare costs) could be great if even moderate efficacy were achieved. Unfortunately, efficacy levels for self-help in tobacco abstinence to date have been quite low. This study demonstrates that much higher abstinence rates can be achieved by increasing contact intensity and extending self-help over a longer period. Doing so, however, adds labor, printing, and mailing costs that are higher than a traditional self-help program. Costs per patient would decrease if a self-help program were disseminated widely to all smokers in a large healthcare system. Even at the scale implemented in the present study, the cost analyses indicate that the cost per quitter ($361 for IRM) is similar to a highly cost-effective public health campaign28 and far lower than most other smoking-cessation interventions, which tend to yield cost per quitter estimates between $1,000 and $10,000.29–35 The cost of the intervention is likely to be recovered by a medical system within 3 years through reduced smoking-related medical expenses.36
As with other population-based cessation trials, the abstinence rates increased over time in all arms as smoking-cessation attempts and successes accumulated over the passage of time.37,38 This pattern contrasts with a typical counseling or medication intervention study with a uniform target quit date, for which the usual pattern includes high initial cessation rates followed by a prolonged period of smoking relapse. Nevertheless, because the interventions differed in their duration, the length of time after treatment ended also differed across arms. An assumption underlying extended self-help is that the longer treatment duration reduces relapse during the period of greatest risk, and that there will be little residual relapse thereafter (after 18 months, in the case of IRM). These results indicate that the treatment effects endured for at least another 6 months, but verification of maintenance will require longer follow-up.
This is the first study to test the effect of an extended-duration self-help intervention for tobacco smoking cessation. The study has several strengths: randomized design, adequate power to detect expected differences, multimodal recruitment of a national sample to enhance generalizability, and follow-up that was much longer than typical. Moreover, the experimental interventions were compared with a credible, high quality, self-help intervention created and disseminated by the National Cancer Institute. Thus, outcome differences represent improvements over traditional self-help and likely underestimate both the efficacy and cost effectiveness compared with no treatment.
Limitations
There were, nevertheless, some limitations to the study design. Because of the national recruitment, it was not feasible to include biochemical verification of self-reported smoking abstinence. However, as noted above, research demonstrates there is little benefit derived from inclusion of biochemical verification measures in low-intensity interventions such as these.23 Another limitation is that the intensive intervention included multiple components that differed from both the standard intervention and traditional self-help (intensity and duration). In addition, the IRM condition included social support pamphlets that do not constitute typical social support per se. Instead, they were designed to provide an analog of social support via the self-help modality. That is, they provided additional “contact” with other smokers that may be perceived as supportive. Future studies will need to examine if the pamphlets were perceived as they were conceived. Additionally, with the exception of pharmacotherapy use, there was no assessment of the recommended self-help strategies (e.g., cognitive–behavioral coping strategies) that were used by participants. Thus, it was not possible to identify the most active components. Also, although the long-term follow up was a strength, it had a cost in terms of relatively high attrition. However, the multiple imputation strategy allowed for a conservative, yet realistic estimate of abstinence rates across the three study arms. Finally, materials were delivered by mail. Distribution via the Internet would have some advantages, such as greater reach and lower cost. Now that efficacy of this traditional distribution method has been established, future research can test different dissemination modalities (e.g., Internet, mobile).
Conclusions
The differential abstinence rates achieved by the IRM intervention in this study lend strong support for intensive self-help approaches to treating tobacco dependence. Compared with more traditional counseling, self-help offers low cost and easy dissemination, and is likely to appeal to the majority of smokers who do not want to commit to formal, in-person treatments. In addition to population-based dissemination via multiple outreach channels, enrollment in an extended self-help program could be standard and automatic for all medical patients who are current smokers, and future studies should focus on cost effectiveness of dissemination in the context of larger accountable healthcare systems.
Acknowledgments
The authors thank Timothy B. Baker, PhD and James D. Sargent, MD for feedback on earlier drafts of this manuscript.
This work was supported by grant R01CA137357 from the National Cancer Institute. This work has also been supported in part by the Biostatistics and Survey Methods Core Facility at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute–designated Comprehensive Cancer Center (P30CA76292). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. ClinicalTrials.gov registration: NCT01352195.
THB, VNS, MU, CDM, BMC, and JHL contributed to the study design. THB and SKS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SKS conducted the statistical analyses. BMC and PTH conducted the cost analyses. MU and LRM recruited participants and administered the intervention. THB wrote the manuscript with assistance from PTH and input and approval from all the other authors.
Thomas Brandon has consulted for and received tobacco-related research support from Pfizer, Inc. The rights to the intervention materials used in this study are owned by Moffitt Cancer Center. In the event that future revenue derives from these products, Moffitt has a revenue-sharing plan with investigators. No other financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jha P, Peto R. Global Health: Global Effects of Smoking, of Quitting, and of Taxing Tobacco. N Engl J Med. 2014;370(1):60–68. doi: 10.1056/NEJMra1308383. http://dx.doi.org/10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 2.Pierce JP, Cummins SE, White MM, Humphrey A, Messer K. Quitlines and nicotine replacement for smoking cessation: Do we need to change policy? Annu Rev Public Health. 2012;33:341–356. doi: 10.1146/annurev-publhealth-031811-124624. http://dx.doi.org/10.1146/annurev-publhealth-031811-124624. [DOI] [PubMed] [Google Scholar]
- 3.Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A. Strategies to increase the delivery of smoking cessation treatments in primary care settings: A systematic review and meta-analysis. Prev Med. 2010;51(3–4):199–213. doi: 10.1016/j.ypmed.2010.06.007. http://dx.doi.org/10.1016/j.ypmed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann-Boyce J, Lancaster T, Stead L. Print-based self-help interventions for smoking cessation. Cochrane Database Syst Rev. 2014;6:CD001118. doi: 10.1002/14651858.CD001118.pub3. http://dx.doi.org/10.1002/14651858.cd001118.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. DHHS, Public Health Service; 2008. [Google Scholar]
- 6.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;2:CD001292. doi: 10.1002/14651858.CD001292.pub2. http://dx.doi.org/10.1002/14651858.cd001292.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Brandon TH, Collins BN, Juliano LM, Lazev AB. Preventing relapse among former smokers: a comparison of minimal interventions through telephone and mail. J Consul Clin Psychol. 2000;68(1):103–113. doi: 10.1037//0022-006x.68.1.103. http://dx.doi.org/10.1037/0022-006X.68.1.103. [DOI] [PubMed] [Google Scholar]
- 8.Brandon TH, Meade CD, Herzog TA, Chirikos TN, Webb MS, Cantor AB. Efficacy and cost-effectiveness of a minimal intervention to prevent smoking relapse: dismantling the effects of amount of content versus contact. J Consul Clin Psychol. 2004;72(5):797–808. doi: 10.1037/0022-006X.72.5.797. http://dx.doi.org/10.1037/0022-006X.72.5.797. [DOI] [PubMed] [Google Scholar]
- 9.Brandon TH, Simmons VN, Meade CD, et al. Self-help booklets for preventing postpartum smoking relapse: a randomized trial. Am J Public Health. 2012;102(11):2109–2115. doi: 10.2105/AJPH.2012.300653. http://dx.doi.org/10.2105/AJPH.2012.300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agboola S, McNeill A, Coleman T, Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction. 2010;105(8):1362–1380. doi: 10.1111/j.1360-0443.2010.02996.x. http://dx.doi.org/10.1111/j.1360-0443.2010.02996.x. [DOI] [PubMed] [Google Scholar]
- 11.Chirikos TN, Herzog TA, Meade CD, Webb MS, Brandon TH. Cost-effectiveness analysis of a complementary health intervention: the case of smoking relapse prevention. Int J Technol Assess Health Care. 2004;20(4):475–480. doi: 10.1017/s0266462304001382. http://dx.doi.org/10.1017/S0266462304001382. [DOI] [PubMed] [Google Scholar]
- 12.Westmaas JL, Bontemps-Jones J, Bauer JE. Social support in smoking cessation: reconciling theory and evidence. Nicotine Tob Res. 2010;12(7):695–707. doi: 10.1093/ntr/ntq077. http://dx.doi.org/10.1093/ntr/ntq077. [DOI] [PubMed] [Google Scholar]
- 13.Unrod M, Simmons VN, Sutton SK, et al. A randomized clinical trial of self-help intervention for smoking cessation: Research design, interventions, and baseline data. Contem Clin Trials. 2014;38(2):284–290. doi: 10.1016/j.cct.2014.05.010. http://dx.doi.org/10.1016/j.cct.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. http://dx.doi.org/10.1037/0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 15.NCI. Clearing the air: quit smoking today. 2003 [Google Scholar]
- 16.Marlatt GA. Relapse prevention: Theoretical rationale and overview of the model. Relapse prevention. 1985:3–70. [Google Scholar]
- 17.Shiffman S, Shumaker SA, Abrams DB, et al. Models of smoking relapse. Health Psychol. 1986;(5 Suppl):13–27. http://dx.doi.org/10.1037/0278-6133.5.Suppl.13. [PubMed]
- 18.Meade CD, Byrd JC. Patient literacy and the readability of smoking education literature. Am J Public Health. 1989;79(2):204–206. doi: 10.2105/ajph.79.2.204. http://dx.doi.org/10.2105/AJPH.79.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creswell KG, Cheng Y, Levine MD. A test of the stress-buffering model of social support in smoking cessation: is the relationship between social support and time to relapse mediated by reduced withdrawal symptoms? Nicotine Tob Res. 2015;17(5):566–571. doi: 10.1093/ntr/ntu192. http://dx.doi.org/10.1093/ntr/ntu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz U, Stadler G, Ochsner S, Rackow P, Hornung R, Knoll N. Examining the relationship between daily changes in support and smoking around a self-set quit date. Health Psychol. 2015 doi: 10.1037/hea0000286. Epub ahead of print. http://dx.doi.org/10.1037/hea0000286. [DOI] [PubMed]
- 21.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br L Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 22.Attkisson CC, Greenfield TK. Client satisfaction questionnaire-8 and service satisfaction scale-30. In: Maruish M, editor. The use of psychological testing for treatment planning and outcome assessment. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1994. pp. 402–420. [Google Scholar]
- 23.Benowitz NL, Jacob P, Hall S, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. http://dx.doi.org/10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Stuart EA, Allison DB. Multiple imputation: A flexible tool for handling missing data. JAMA. 2015;314(18):1966–1967. doi: 10.1001/jama.2015.15281. http://dx.doi.org/10.1001/jama.2015.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman & Hall; 1997. http://dx.doi.org/10.1201/9781439821862. [Google Scholar]
- 26.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. http://dx.doi.org/10.1002/9780470316696. [Google Scholar]
- 27.Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev. 2005;3:CD001118. doi: 10.1002/14651858.CD001118.pub2. http://dx.doi.org/10.1002/14651858.cd001118.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Alexander RL, Jr, Simpson SA, et al. A Cost-Effectiveness Analysis of the First Federally Funded Antismoking Campaign. Am J Prev Med. 2015;48(3):318–325. doi: 10.1016/j.amepre.2014.10.011. http://dx.doi.org/10.1016/j.amepre.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronckers ET, Groot W, Ament AJ. Systematic review of economic evaluations of smoking cessation: standardizing the cost-effectiveness. Med Decis Making. 2005;25(4):437–448. doi: 10.1177/0272989X05278431. http://dx.doi.org/10.1177/0272989×05278431. [DOI] [PubMed] [Google Scholar]
- 30.Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. JAMA. 1997;278(21):1759–1766. http://dx.doi.org/10.1001/jama.1997.03550210057039. [PubMed] [Google Scholar]
- 31.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med. 1998;339(10):673–679. doi: 10.1056/NEJM199809033391006. http://dx.doi.org/10.1056/NEJM199809033391006. [DOI] [PubMed] [Google Scholar]
- 32.Smit ES, Evers SM, de Vries H, Hoving C. Cost-effectiveness and cost-utility of Internet-based computer tailoring for smoking cessation. J Med Internet Res. 2013;15(3):e57. doi: 10.2196/jmir.2059. http://dx.doi.org/10.2196/jmir.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nohlert E, Helgason ÁR, Tillgren P, Tegelberg Å, Johansson P. Comparison of the cost-effectiveness of a high-and a low-intensity smoking cessation intervention in Sweden: a randomized trial. Nicotine Tob Res. 2013;15(9):1519–1527. doi: 10.1093/ntr/ntt009. http://dx.doi.org/10.1093/ntr/ntt009. [DOI] [PubMed] [Google Scholar]
- 34.Smith MY, Cromwell J, DePue J, Spring B, Redd W, Unrod M. Determining the cost-effectiveness of a computer-based smoking cessation intervention in primary care. Manag Care. 2007;16(7):48–55. [PubMed] [Google Scholar]
- 35.Song F, Raftery J, Aveyard P, Hyde C, Barton P, Woolacott N. Cost-effectiveness of pharmacological interventions for smoking cessation: a literature review and a decision analytic analysis. Med Decis Making. 2002;22(5 suppl):S26–S37. doi: 10.1177/027298902237708. http://dx.doi.org/10.1177/027298902237708. [DOI] [PubMed] [Google Scholar]
- 36.Leif Associates. [Accessed December 16, 2015];Report: The Business Case for Coverage of Tobacco Cessation-2012 Update. www.naquitline.org/news/86973/The-Business-Case-for-Coverage-of-Tobacco-Cessation.htm.
- 37.Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population-based interventions for smoking cessation: a MOST trial. Transl Behav Med. 2014;4(4):382–390. doi: 10.1007/s13142-014-0278-8. http://dx.doi.org/10.1007/s13142-014-0278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham AL, Cobb NK, Papandonatos GD, et al. A randomized trial of Internet and telephone treatment for smoking cessation. Arch Intern Med. 2011;171(1):46–53. doi: 10.1001/archinternmed.2010.451. http://dx.doi.org/10.1001/archinternmed.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]