Abstract
Objective
To investigate whether mitochondrial function is altered in circulating immune cells from calcium oxalate (CaOx) stone formers compared to healthy subjects.
Methods
Adult healthy subjects (n=18) and CaOx stone formers (n=12) were included in a pilot study. Data collection included demographic and clinical values from electronic medical records. Bioenergetic function was assessed in monocytes, lymphocytes, and platelets isolated from blood samples using the Seahorse XF96 Analyzer. Plasma Interleukin-6 (IL-6) was measured using ELISA.
Results
All participants were age matched (healthy subjects 44.5 ± 3.0 vs CaOx stone formers 42.3 ± 4.8 years, p=0.6905). CaOx stone formers did not have urinary tract infection, ureteral stones or obstructing renal stones. Monocyte mitochondrial function was decreased in CaOx stone formers compared to healthy subjects. Specifically, mitochondrial maximal respiration (p=0.0011) and reserve capacity (p<0.0001) were significantly lower. In contrast, lymphocyte and platelet mitochondrial function was similar between the two groups. The bioenergetic health index (BHI), an integrated value of mitochondrial function, was significantly lower in monocytes from CaOx stone formers compared to healthy subjects (p=0.0041). Lastly, plasma IL-6 levels were significantly increased (p=0.0324).
Conclusions
The present pilot study shows that CaOx stone formers have decreased monocyte mitochondrial function. Plasma IL-6 was also increased in this cohort. These data suggest that impaired monocyte mitochondrial function and inflammation may be linked to CaOx kidney stone formation. Further studies are needed to confirm these findings in a larger cohort of patients.
Keywords: Mitochondria, monocytes, kidney stone disease, calcium oxalate
INTRODUCTION
Kidney stones are prevalent in the United States (8.8%) and impart a significant economic burden.1 In addition, patients with kidney stones have an increased risk of developing recurrent stones, chronic kidney disease (CKD), diabetes, and cardiovascular diseases.2,3 The most common type of kidney stone is the calcium oxalate (CaOx) kidney stone. The etiology of CaOx stone formation and the development of recurrent stones in patients are not well understood. Several lines of evidence have identified dietary and lifestyle factors,1,4 and genetics5 as contributors to the pathogenesis of CaOx stones. In addition, CaOx stone formation appears to be linked to oxidative stress and inflammation.6
Recent experiments have indicated that there is an association between renal CaOx crystal formation and macrophages in a mouse kidney stone model.7 Suppression of anti-inflammatory (M2) macrophages have also been shown reduce crystal clearance.8 In addition, human macrophages have been suggested to play a role in renal interstitial crystal clearance.9 Monocytes, the precursors of renal macrophages, can be readily obtained from CaOx stone formers and could provide insight regarding the role of macrophages in stone disease. The differentiation of monocytes to macrophages is influenced by mitochondrial function.10 Mitochondria play a critical role in the innate immune system by stimulating cellular effector responses and reactive oxygen species (ROS) generation to assist in monocyte/macrophage immune activation.11 Mitochondrial ROS are essential for regulating cellular metabolism; however, chronic over-production of mitochondrial ROS can be harmful and cause oxidative stress.12 Imbalance of this system can lower the ability of monocytes/macrophages to attenuate inflammation caused by tissue injury or infection.10 As a result, this can lead to increased inflammation locally within tissues and/or the circulation.
We previously reported that mitochondrial function differs substantially between monocytes, lymphocytes, and platelets in healthy subjects13,14 and that these cells could be used to evaluate an individual’s bioenergetic health for translational purposes.15 Other groups have shown that mitochondrial dysfunction, oxidative stress, and cell death occur in circulating cells in systemic pathologies such as sepsis and fibromyalgia.16,17 Both inflammation and oxidative stress have been suggested to play an integral role in kidney stone pathogenesis in both experimental models and clinical studies.18–20 In particular, clinical studies have shown elevated levels of C-reactive protein in serum21 and pro-inflammatory cytokines and chemokines including IL-6 in urine23 and plasma of hypercalciuria kidney stone patients.22,23 Thus, it appears that inflammation and oxidative stress could affect immune cell function during kidney stone formation.
The purpose of this pilot study was to determine whether mitochondrial function is suppressed in monocytes, lymphocytes, and platelets isolated from CaOx stone formers and if inflammation is elevated in these patients compared to healthy subjects. We determined that 1) monocytes but not lymphocytes or platelets from CaOx stone formers have decreased mitochondrial function compared to healthy subjects and 2) Interleukin-6 (IL-6) plasma levels are elevated in CaOx stone formers. These data suggest that impaired monocyte mitochondrial function and inflammation may be linked to CaOx kidney stone formation.
MATERIALS AND METHODS
Materials
The following antibodies were purchased from Miltenyi Biotec Inc. (San Diego, CA) for cell isolation: anti-CD14, anti-CD65, and anti-CD235a. RPMI medium 1640 was from Life Technologies (Grand Island, NY). Histopaque density gradients (1.077 and 1.119), BSA, oligomycin, FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone), and antimycin A were all bought from Sigma-Aldrich (St. Louis, MO). Materials for the mitochondrial assays were purchased from Seahorse Biosciences (North Billerica, MA). All other reagents or kits used are noted elsewhere.
Human Subjects, Clinical Procedures, and Cell Isolation
This study was conducted with adherence to the Declaration of Helsinki and was approved by the University of Alabama at Birmingham Institutional Review Board. This study was designed as a pilot study as none of the mitochondrial parameters have ever been measured in a kidney stone population. All blood samples were collected after receiving written informed consent from participants. Blood was drawn during an outpatient clinic appointment for CaOx stone formers and at the UAB Center for Clinical and Translational Science Clinical Research Unit for healthy subjects. We recruited 12 CaOx stone formers and 18 age-matched healthy subjects. Idiopathic CaOx stone formers were included in the study if they had a CaOx content greater than 50% by stone analysis. The patients did not have renal obstruction, active urinary tract infection, chronic kidney disease, diabetes or hyperparathyroidism. This was based on history and evaluation with serum electrolytes, BUN, creatinine, calcium and intact parathyroid hormone testing. They had not been subjected to recent stone removing surgery and they did not have infections. The patient cohort was comprised of both first time stone formers and recurrent stone formers as we did not know whether mitochondrial function would differ in the two groups.
All blood samples were prepared for bioenergetic analysis within 1 hour of collection. Blood leukocytes were isolated using density gradient centrifugation and magnetic bead selection as previously described.13,14 In brief, the participant’s blood was centrifuged to separate the platelet rich plasma (PRP) and the buffy coat/red blood cells. The PRP was centrifuged and washed with prostaglandin I2 to collect platelets. The platelet cell count was then measured by turbidimetry using a spectrophotometer. The buffy coat/red blood cells were diluted with RPMI media, mixed, and added to a ficoll density gradient prior to centrifuging. Following centrifugation, the mononuclear cell fraction was obtained to positively select for anti-CD14+ monocytes. The flow through was collected to negatively select for lymphocytes using magnetic bead antibody selection (anti-CD65 and CD235a). Monocytes and lymphocytes were counted using the Bio-Rad TC20 Automated Cell Counter (Bio-Rad, Hercules, CA).
Cellular Bioenergetic Function Analysis
Mitochondrial function of monocytes, lymphocytes, and platelets was determined using the Seahorse XF96e Analyzer (Seahorse Biosciences, North Billerica, MA). Cells were seeded on Cell-Tak treated Seahorse plates: monocytes (150,000 cells/well), lymphocytes (150,000 cells/well), and platelets (10×106 cells/well) as described.13,14 Following plating, cells were equilibrated in XF media (DMEM with 1 mM pyruvate, 5.5 mM D-glucose, 4 mM L-glutamine, pH 7.4) for approximately 1 hour prior to measuring mitochondrial oxygen consumption rate (OCR) using the mitochondrial stress test inhibitors: oligomycin (0.5 μg/mL), FCCP (0.6 μM), and antimycin A (10 μM). In addition, the extracellular acidification (ECAR) was measured simultaneously.
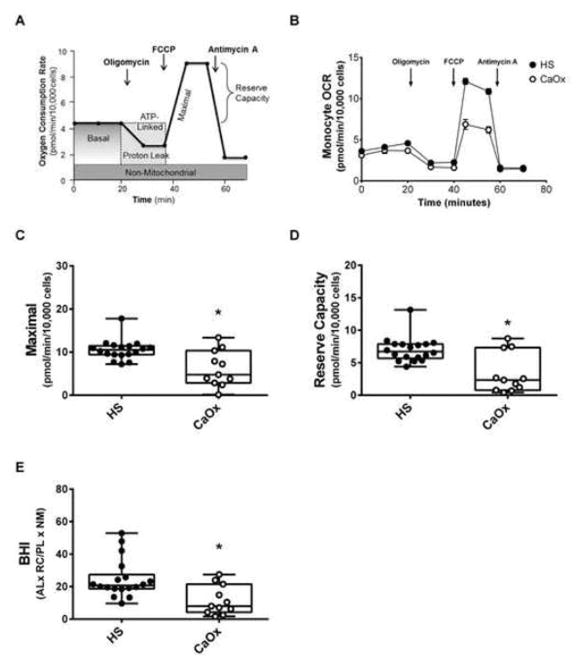
Each of the inhibitors generated a mitochondrial profile (Figure 1A) consisting of the following six parameters: basal respiration, ATP-linked respiration, proton leak respiration, maximal respiration, reserve capacity, and non-mitochondrial respiration.24 Basal respiration served as a baseline for mitochondrial respiration and was calculated by subtracting the basal OCR from the non-mitochondrial OCR. Oligomycin, an ATP synthase inhibitor was injected to evaluate ATP-linked and proton leak respiration. ATP-linked OCR was determined by subtracting basal OCR from the amount of respiration left after oligomycin injection. Proton leak was calculated by subtracting non-mitochondrial OCR from the amount of respiration left after oligomycin injection. FCCP, a mitochondrial uncoupler, was injected next to evaluate maximal respiration and reserve capacity. Maximal respiration was determined by subtracting maximal OCR from non-mitochondrial OCR. Reserve capacity was calculated by subtracting the maximal OCR from the basal OCR. Lastly, antimycin A, a complex III inhibitor was injected to evaluate oxygen consumption not linked to mitochondria and is referred to as non-mitochondrial OCR. All mitochondrial OCR data was normalized to cell count. The bioenergetic health index (BHI), an integrated value comprised of the individual parameters, was determined using the following equation: (ATP–Linked x Reserve Capacity)/(Proton Leak x Non–mitochondrial respiration).15
Figure 1.
Mitochondrial function in monocytes from calcium oxalate (CaOx) stone formers and healthy subjects (HS). (A) Illustration of the mitochondrial assay used to measure oxygen consumption rate (OCR) over time using the mitochondrial stress test. (B) OCR profiles of monocytes from healthy subjects (black circle) and CaOx stone formers (white circle) using the mitochondrial stress test. Indices of mitochondrial function—(C) maximal respiration and (D) reserve capacity were calculated from all study participants based on their respective bioenergetic profile. (E) The BHI was calculated using the equation: (ATP-linked OCR x Reserve Capacity)/(Proton Leak x Non-mitochondrial OCR). The indices are represented in a box plot with lower 25th percentile, median, upper 75th percentile, and whiskers drawn at 1.5 × interquartile range. Results are n=18 healthy subjects and n=11 CaOx stone formers. *p<0.05 compared to healthy subject monocytes.
Enzyme Linked Immunosorbent Assays
IL-6 is a pro-inflammatory cytokine that has been shown to be upregulated in the urine of kidney stone formers and is considered a marker of inflammation.22,25 Plasma IL-6 was measured using a Human IL-6 Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The absorbance was measured at 490 nm using a Synergy HT microplate reader (BioTek Instruments, Winooksi, VT).
Statistics
Statistical analyses were performed using GraphPad Prism (La Jolla, CA, USA) or JMP version 12.1.0 (SAS, Cary, NC, USA) software for multivariate analysis. All data are presented as mean ± SE. The difference between groups was determined using a two-tailed student’s t test. A P<.05 was considered statistically significant.
RESULTS
Demographic and Clinical Data
The mean ± SE age for the two study populations was 44.5 ± 3.0 years for healthy subjects and 42.3 ± 4.8 years for CaOx stone formers and was not significantly different (p=0.6905). Of the 18 healthy subjects, 60% were women. Of the 12 patients, 50% were women, 40% had a family history of kidney stone disease, and 70% were recurrent stone formers. The BMI was not different between the two populations (28.3 ± 1.5 healthy subjects and 29.3 ± 1.9 CaOx stone formers kg/m2). Two patients had a history of hypertension and 2 had prior episodes of gout. Calcium, sodium, potassium, uric acid, and creatinine serum values, and white blood cell counts were all within normal clinical ranges for CaOx stone formers (data not shown).
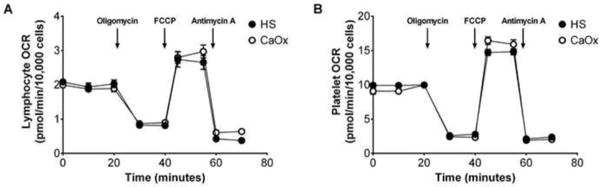
The overall OCR profile was lower in the monocytes of CaOx stone formers compared to healthy subjects (Figure 1B). Both maximal respiration (p=0.0011, Figure 1C) and reserve capacity (p<0.0001, Figure 1D) were significantly lower in CaOx stone former monocytes compared to healthy subjects. The remaining parameters in monocytes (Basal, ATP-linked, proton leak, and non-mitochondrial respiration) were not different between the two cohorts. The BHI in monocytes was significantly lower in the CaOx stone formers compared to healthy subjects (p=0.0041) (Figure 1E). In addition, the ECAR, a marker of glycolysis, was not different between the two groups (data not shown). Mitochondrial functional was similar in the lymphocytes and platelets of both cohorts (Figure 2A and 2B). In addition, there were was no apparent difference in mitochondrial function between first time and recurrent stone formers.
Figure 2.
Mitochondrial function in lymphocytes and platelets from calcium oxalate (CaOx) stone formers and healthy subjects (HS). OCR profiles of A) lymphocytes and (B) platelets isolated from healthy subjects (black circle) and CaOx stone formers (white circle) using the mitochondrial stress test. Results are presented as mean ± SE; n=5–6 replicates per group; n=14–17 healthy subjects and n=10–11 CaOx stone formers.
Plasma IL-6 concentrations were three-fold higher in CaOx stone formers (n=8) compared to healthy subjects (n=10) (healthy subjects 0.66 ± 0.21 vs CaOx stone formers 1.81 ± 0.49 pg/mL, p=0.0324) (Figure 3). There was no difference in IL-6 levels between first time and recurrent stone formers (data not shown). There was no correlation between serum IL-6 and mitochondrial function.
Figure 3.
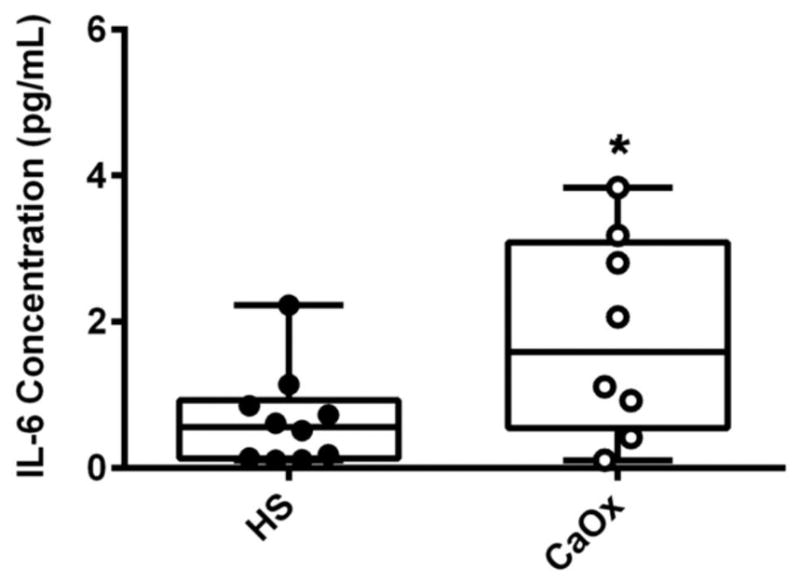
Plasma Interleukin-6 levels in calcium oxalate (CaOx) stone formers (SF) and healthy subjects (HS). Plasma from HS (black circle) and CaOx SF (white circle) were analyzed for the expression of IL-6 using ELISA. Results are represented in a box plot with lower 25th percentile, median, upper 75th percentile, and whiskers drawn at 1.5 × interquartile range; n = 10 healthy subjects and n=8 CaOx SF and presented as mean ± SE; *p<0.05 compared to HS monocytes.
COMMENT
It has been previously determined that mitochondrial function is disrupted in a number of pathologies linked to inflammation and oxidative stress such as atherosclerosis, CKD, and acute kidney injury.26–28 In the present pilot study, we sought to assess whether mitochondrial function is suppressed in monocytes, lymphocytes, and platelets from CaOx stone formers compared to healthy subjects. To our knowledge, this is the first study to evaluate cellular bioenergetics in immune cells from CaOx stone formers. Cellular bioenergetics is an overall assessment of cellular metabolism that consists of pathways such as oxidative phosphorylation, glycolysis, and fatty acid oxidation. To study mitochondrial metabolism in immune cells from CaOx stone formers and healthy subjects, we utilized the mitochondrial stress test. Our data provide evidence for a significant decrease in monocyte mitochondrial function in CaOx stone formers. In particular, both maximal respiration and reserve capacity were significantly lower in monocytes from CaOx stone formers compared to healthy subjects. We also determined that the BHI is significantly depressed in CaOx stone former monocytes. In contrast, both lymphocyte and platelet mitochondrial function from CaOx stone formers was similar to those from healthy subjects.
One rationale for why monocytes from CaOx stone formers are affected rather than lymphocytes is that monocytes are a part of the innate immunity and are one of the first responders to sites of inflammation; whereas, lymphocytes are involved in adaptive immunity. Monocytes also have different mitochondrial programs compared to lymphocytes due to their physiological functions. Monocytes can differentiate into two classes of macrophages (M1 and M2) to regulate inflammation using glycolysis or oxidative phosphorylation, respectively.10 Importantly, both subtypes can convert to the other form during inflammation and this conversion could feasibly be modulated by the extent of crystal exposure. The exact reason why lymphocytes and platelets are not affected warrants further investigation.
Our results (increased plasma IL-6) and those of others demonstrate that patients with CaOx stones have increased markers of inflammation18,21,22,25. While there was no correlation between IL-6 and mitochondrial function in this study, the number of subjects was relatively small and other markers of inflammation were not assessed. However, these results may be different with a larger cohort of patients. The decline observed in monocyte mitochondrial function in CaOx stone formers could be due to decreased mitochondrial mass, compromised electron transport chain integrity, or decreased substrate supply. It is possible that inflammation may still be a potential candidate or other factors such as calcium oxalate crystals may perturb mitochondrial function and will be investigated further. It was reported that calcium oxalate crystal exposure to monocytes alters mitochondrial proteins involved in metabolism.29 Additional studies are currently underway to identify potential mechanisms responsible for decreasing mitochondrial function in monocytes from patients with CaOx kidney stones.
We recognize that this study has certain limitations. The number of subjects was relatively small, their degree of stone activity was mixed, and a longitudinal profile of mitochondrial function was not undertaken. Determining whether these findings are present in a larger cohort of patients should be of value. In addition, monocyte mitochondrial function in the blood may not be representative of what’s occurring within tissue. A further limitation is the lack of understanding of the role immune cells play in stone formation as they have not been identified in papillary tip biopsies taken from CaOx stone formers.30 Several future studies are planned to elucidate the mechanisms of monocyte mitochondrial dysfunction in CaOx stone formers. These include renal epithelial crystal exposure, impact of monocyte and macrophage sub-types, and relationships with IL-6 and monocyte recruitment stimulated by proteins such as MCP-1. In addition, a search for such mitochondrial dysfunction in other types of kidney stone formers is planned.
CONCLUSION
The findings from this study suggest that mitochondrial cellular processes in monocytes may be linked to CaOx stone formation. Further work is needed to determine the significance of monocytes/macrophages in kidney stone disease.
Acknowledgments
Research Support: Research reported in this publication was supported by NIH grants: DK079337, UL1TR001417, DK054468 (RH), and DK054468-S1 (TM), and funds from the UAB Faculty Development Grant Program, Office of the Provost (TM).
Footnotes
Conflict of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scales CD, Smith AC, Hanley JM, Saigal CS Project UDiA. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H, Zisman AL, Coe FL, Worcester EM. Kidney stones: an update on current pharmacological management and future directions. Expert Opin Pharmacother. 2013;14:435–47. doi: 10.1517/14656566.2013.775250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoag J, Halpern J, Goldfarb DS, Eisner BH. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol. 2014;192:1440–5. doi: 10.1016/j.juro.2014.05.117. [DOI] [PubMed] [Google Scholar]
- 4.Holmes RP, Assimos DG. The impact of dietary oxalate on kidney stone formation. Urol Res. 2004;32:311–6. doi: 10.1007/s00240-004-0437-3. [DOI] [PubMed] [Google Scholar]
- 5.Goodman HO, Brommage R, Assimos DG, Holmes RP. Genes in idiopathic calcium oxalate stone disease. World J Urol. 1997;15:186–94. doi: 10.1007/BF02201856. [DOI] [PubMed] [Google Scholar]
- 6.Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3:256–76. doi: 10.3978/j.issn.2223-4683.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada A, Yasui T, Fujii Y, et al. Renal macrophage migration and crystal phagocytosis via inflammatory-related gene expression during kidney stone formation and elimination in mice: Detection by association analysis of stone-related gene expression and microstructural observation. J Bone Miner Res. 2010;25:2701–11. doi: 10.1002/jbmr.158. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi K, Okada A, Kitamura H, et al. Colony-stimulating factor-1 signaling suppresses renal crystal formation. J Am Soc Nephrol. 2014;25:1680–97. doi: 10.1681/ASN.2013060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusmartsev S, Dominguez-Gutierrez PR, Canales BK, Bird VG, Vieweg J, Khan SR. Calcium Oxalate Stone Fragment and Crystal Phagocytosis by Human Macrophages. J Urol. 2016;195:1–9. doi: 10.1016/j.juro.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravi S, Mitchell T, Kramer PA, Chacko B, Darley-Usmar VM. Mitochondria in monocytes and macrophages-implications for translational and basic research. Int J Biochem Cell Biol. 2014;53:202–7. doi: 10.1016/j.biocel.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chacko BK, Kramer PA, Ravi S, et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Laboratory investigation; a journal of technical methods and pathology. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. Journal of visualized experiments : JoVE. 2014;85:1–9. doi: 10.3791/51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chacko BK, Kramer PA, Ravi S, et al. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci (Lond) 2014;127:367–73. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M, Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Critical care medicine. 2007;35:2702–8. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 17.Cordero MD, De Miguel M, Moreno Fernandez AM, et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease. Arthritis research & therapy. 2010;12:R17. doi: 10.1186/ar2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boonla C, Hunapathed C, Bovornpadungkitti S, et al. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int. 2008;101:1170–7. doi: 10.1111/j.1464-410X.2008.07461.x. [DOI] [PubMed] [Google Scholar]
- 19.Joshi S, Wang W, Peck AB, Khan SR. Activation of the NLRP3 inflammasome in association with calcium oxalate crystal induced reactive oxygen species in kidneys. J Urol. 2015;193:1684–91. doi: 10.1016/j.juro.2014.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada A, Yasui T, Hamamoto S, et al. Genome-wide analysis of genes related to kidney stone formation and elimination in the calcium oxalate nephrolithiasis model mouse: detection of stone-preventive factors and involvement of macrophage activity. J Bone Miner Res. 2009;24:908–24. doi: 10.1359/jbmr.081245. [DOI] [PubMed] [Google Scholar]
- 21.Shoag J, Eisner BH. Relationship between C-reactive protein and kidney stone prevalence. J Urol. 2014;191:372–5. doi: 10.1016/j.juro.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Suen JL, Liu CC, Lin YS, Tsai YF, Juo SH, Chou YH. Urinary chemokines/cytokines are elevated in patients with urolithiasis. Urol Res. 2010;38:81–7. doi: 10.1007/s00240-010-0260-y. [DOI] [PubMed] [Google Scholar]
- 23.Ghazali A, Fuentes V, Desaint C, et al. Low bone mineral density and peripheral blood monocyte activation profile in calcium stone formers with idiopathic hypercalciuria. J Clin Endocrinol Metab. 1997;82:32–8. doi: 10.1210/jcem.82.1.3649. [DOI] [PubMed] [Google Scholar]
- 24.Hill BG, Benavides GA, Lancaster JR, Jr, et al. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem. 2012;393:1485–512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee E, Santiago L, Park E, Lad P, Bellman GC. Urinary IL-6 is elevated in patients with urolithiasis. J Urol. 1998;160:2284–8. doi: 10.1097/00005392-199812010-00101. [DOI] [PubMed] [Google Scholar]
- 26.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 27.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–95. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Cao Q, Wang Y, Harris DC. Pathogenic and protective role of macrophages in kidney disease. Am J Physiol Renal Physiol. 2013;305:F3–11. doi: 10.1152/ajprenal.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singhto N, Sintiprungrat K, Sinchaikul S, Chen ST, Thongboonkerd V. Proteome changes in human monocytes upon interaction with calcium oxalate monohydrate crystals. J Proteome Res. 2010;9:3980–8. doi: 10.1021/pr100174a. [DOI] [PubMed] [Google Scholar]
- 30.Miller NL, Evan AP, Lingeman JE. Pathogenesis of renal calculi. Urol Clin North Am. 2007;34:295–313. doi: 10.1016/j.ucl.2007.05.007. [DOI] [PubMed] [Google Scholar]