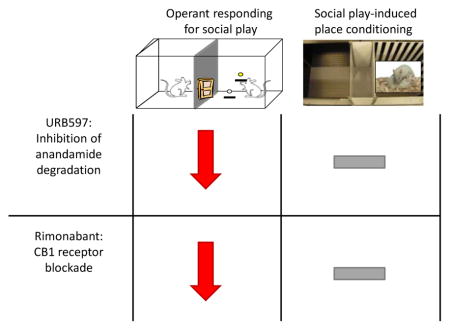
Abstract
Social play behaviour is a vigorous form of social interaction, abundant during the juvenile and adolescent phases of life in many mammalian species, including humans. Social play is highly rewarding and it is important for social and cognitive development. Being a rewarding activity, social play can be dissociated in its pleasurable and motivational components. We have previously shown that endocannabinoids modulate the expression of social play behaviour in rats. In the present study, we investigated whether endocannabinoids modulate the motivational and pleasurable properties of social play behaviour, using operant and place conditioning paradigms, respectively. Treatment with the anandamide hydrolysis inhibitor URB597 did not affect operant responding or social play-induced conditioned place preference (CPP) when administered at a dose (0.1 mg/kg) known to increase the expression of social play behaviour, while it modestly reduced operant responding at a higher dose (0.2 mg/kg). The cannabinoid-1 (CB1) receptor antagonist rimonabant reduced operant responding when administered at a dose (1 mg/kg) known to decrease the expression of social play behaviour, although this effect may be secondary to concurrent drug-induced stereotypic behaviours (i.e., grooming and scratching). These data demonstrate that enhancing endocannabinoid levels does not differentially affect the motivational and pleasurable aspects of social play behaviour, whereas CB1 receptor blockade reduces the motivational aspects of social play behaviour, possibly due to response competition. Thus, endocannabinoids likely drive the expression of social play behaviour as a whole, without differentially affecting its motivational or pleasurable properties.
Keywords: Social play behaviour, endocannabinoids, operant conditioning, place conditioning, URB597, rimonabant
Graphical abstract
1. Introduction
Social play behaviour is a highly vigorous form of social interaction, in which components of other social behaviours can be discerned, but in an adapted and/or out-of-context manner (Panksepp et al., 1984; Pellis and Pellis, 2009; Vanderschuren et al., 1997). Social play is abundantly expressed throughout the juvenile and adolescent periods of life in the majority of mammalian species (Panksepp et al., 1984; Pellis and Pellis, 2009; Spear, 2000). Engaging in social play behaviour is thought to be important for social and cognitive development (Baarendse et al., 2013; Potegal and Einon, 1989; Van den Berg et al., 1999) as it equips animals and humans with a rich and flexible behavioural repertoire (Špinka et al., 2001; Vanderschuren and Trezza, 2014).
Social play behaviour is highly rewarding (Trezza et al., 2011a; Vanderschuren, 2010). Indeed, its expression is modulated through neural systems implicated in other rewards such as food, sex, and drugs of abuse (Vanderschuren et al., 1997; Trezza et al., 2010; Siviy and Panksepp, 2011). It is generally thought that reward processes consist of different components: pleasure (‘hedonics’), incentive motivation, and learning processes, that are mediated via different neural systems (Berridge et al., 2009). For example, opioids and endocannabinoids are thought to primarily influence the pleasurable properties of a reward, whereas dopamine is considered to be mainly involved in its motivational aspects (Berridge et al., 2009; Barbano and Cador et al., 2007; Fattore et al., 2007; Friemel et al., 2014; Kelley, 2004; Mahler et al., 2007; Salamone and Correa, 2012; Solinas et al., 2008).
Previous studies have shown that the expression of social play behaviour is modulated by endocannabinoid neurotransmission. Indirect cannabinoid agonists, i.e. drugs that prolong endocannabinoid signaling, such as URB597 (which inhibits FAAH, the enzyme that degrades the endocannabinoid anandamide) or VDM11 (which blocks endocannabinoid reuptake) enhanced social play in rats (Manduca et al., 2014; Trezza et al., 2012; Trezza and Vanderschuren, 2009; Trezza and Vanderschuren 2008a,b). Interestingly, the effects of endocannabinoids on social play were found to depend on opioid receptor stimulation, and vice versa (Trezza and Vanderschuren, 2008a).
In the present study, we aimed to dissociate the role of endocannabinoids in the motivational and pleasurable properties of social play behaviour. To measure the motivational aspects of social play behaviour, we used an operant conditioning task that we recently developed, in which rats respond for access to a playful partner under a progressive ratio schedule of reinforcement (Achterberg et al., 2016). Place conditioning was used to assess the pleasurable aspects of social play (Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Douglas et al., 2004; Thiel et al., 2008; Trezza et al., 2009b). Since treatment with URB597 was previously found not to affect responding for food and drug rewards in rats and non-human primates (Adamcyk et al., 2009; Forget et al., 2009; Justinova et al., 2008; Kangas et al., 2016; Oleson et al., 2012; Scherma et al., 2008; Solinas et al., 2005), we hypothesized that enhancing anandamide levels with URB597 would increase the pleasurable aspects of social play without affecting responding for social play. Conversely, since genetic and pharmacological blockade of CB1 cannabinoid receptors decreases the motivation to respond for food (Hernandez and Cheer, 2012; Rasmussen and Huskinson, 2008; Solinas and Goldberg, 2005; Ward and Dykstra, 2005) and drugs of abuse (Economidou et al., 2006; Maccioni et al., 2010), and affect food- (Chaperon et al., 1998; Méndez-Diaz et al., 2012) and drug-induced (Forget et al., 2004; Hu et al., 2014; Singh et al., 2004; Thanos et al., 2005; Yu et al., 2011) conditioned place preference, we hypothesized that treatment with the CB1 cannabinoid receptor antagonist rimonabant would decrease both the motivational and pleasurable aspects of social play.
2. Materials and methods
2.1 Animals
Male Wistar rats (Charles River, Sulzfeld, Germany) arrived in our animal facility at 21 days of age and were housed in groups of four in 40 × 26 × 20 cm (l × w × h) Macrolon cages under controlled conditions (ambient temperature 20–21°C, 60–65% relative humidity, and 12/12 h light cycle with lights on at 7.00 a.m.). Food and water were available ad libitum. All animals used were experimentally naïve. All experiments were approved by the Animal Ethics Committee of Utrecht University and were conducted in accordance with Dutch laws (Wet op de Dierproeven, 1996) and European regulations (Guideline 86/609/EEC).
2.2 Drugs
URB597 (Tocris Cookson, Avonmouth, UK) and rimonabant (National Institute of Mental Health’s Chemical Synthesis and Drug Supply Program, Bethesda, MD, USA) were dissolved in 5% Tween-80/5% polyethylene glycol/saline. URB597 and rimonabant were administered intra-peritoneally (i.p.), 2 h and 30 min before testing, respectively. Drug doses and pre-treatment intervals were based on previous studies (Trezza and Vanderschuren, 2008a,b; 2009).
2.3 Operant conditioning paradigm
2.3.1 Apparatus
Behavioural testing was conducted in an operant conditioning chamber (Med Associates, Georgia, VT, USA) divided into two equally sized compartments (25 × 30 × 25 cm, l x w x h). The compartments were separated by a Plexiglas wall with 42 small holes (Ø 0.5 cm) and an automated metal door in the middle. Both compartments had a metal grid floor and a Plexiglas lid which contained a house-light (2 W). One compartment (the ‘lever pressing compartment’) was equipped with two 4.8 cm-wide retractable levers, located on opposite sides of the compartment. Above each lever was a cue light (2.5 W). One lever was designated as the active lever and the other as the inactive lever; allocation of the left or right lever as active was counterbalanced between animals. Experimental events and data recording were controlled using Med PC software (Med Associates, Georgia, VT, USA).
2.3.2 Experimental procedure
Operant conditioning was performed as previously described (Achterberg et al., 2016). All experiments were performed under red light conditions, since the performance of social play behaviour is inhibited by bright light conditions (Vanderschuren et al., 1995). Animals were randomly paired with a test partner from another home cage. Animals in a test pair did not differ by more than 10 grams in body weight at the start of the experiment. A test pair consisted of one experimental animal and its stimulus partner. At 24 days of age, test pairs were habituated to the test cage for 10 min. After the habituation session, animals were isolated for 24 h/day for 5 consecutive days/week, except in the experiment in which both animals received URB597. In this experiment, animals were also isolated for 2 h/day prior to testing after being socially housed for at least 24 h. Next, the animals received two shaping sessions on two consecutive days. During these shaping sessions, the cue light was presented, the lever retracted and the door opened when the experimental animal approached the active lever. Rats were allowed to interact for two minutes after which the door closed and each rat was placed back into its starting compartment by the experimenter. This procedure was repeated 7 times in each shaping session. In addition, if an animal did not perform any active lever presses during acquisition sessions, it received an additional shaping session later that day or on the next day.
On the fourth day, the lever pressing sessions (20 min) commenced under a fixed ratio (FR)-1 schedule of reinforcement. Under this FR-1 schedule of reinforcement, each active lever press resulted in presentation of the cue light, retraction of both levers, and opening of the door, after which animals were allowed to freely interact for 2 min. After acquisition of the task under the FR-1 schedule (i.e., when an animal obtained at least six out of eight possible rewards on two consecutive days), a progressive ratio (PR) schedule of reinforcement was introduced. When rats met the response requirement, the cue light was illuminated, both levers retracted and the door opened for 1 min, during which the animals could freely interact. Animals received one session per day, for 5 consecutive days/week. During the other 2 days/week animals were socially housed with their original cage-mates. After responding had stabilized, defined as obtaining at least six rewards on three consecutive days with a variation of no more than two rewards, drug treatment started according to a Latin Square design. Inactive lever presses were recorded, but had no programmed consequences.
2.3 Place conditioning paradigm
2.3.1 Apparatus
The place conditioning setup (TSE System, Bad Homburg, Germany) comprised eight boxes, each consisting of three compartments with removable Plexiglas lids. The two conditioning compartments were equally sized (30 cm × 25 cm × 30 cm; l x w x h) and separated by the third, neutral, compartment (10 cm × 25 cm × 30 cm; l x w x h). The two conditioning compartments had different visual and tactile cues: one had black-and-white striped walls and a floor with wide metal mesh, and the other had black walls and a floor with fine metal mesh. The compartment with black walls had a white light (2 W) mounted on the Plexiglas lid, to achieve a comparable light intensity in both conditioning compartments. The middle compartment had white walls, a smooth floor, and a white light (2 W) on the lid. The position of the animal in the apparatus was monitored by an array of photo-beam sensors located 2.5 cm above the floor. The time spent in each compartment (in msec) was recorded by a computer. All experiments were performed in a dimly lit room, since testing under bright light conditions reduces the performance of social play behaviour (Vanderschuren et al., 1995).
2.3.2 Experimental procedure
Place conditioning was performed as previously described (Achterberg et al., 2012; Trezza et al., 2009b; Trezza et al., 2011b). At 26 days of age (experimental day 1), each rat was placed in the middle compartment of the apparatus and pre-conditioning side preference was determined by allowing the rats to move freely in the three compartments for 15 min. On the basis of their preference scores, rats were assigned to a compartment in which they would be allowed social interaction during conditioning. A counterbalanced place conditioning design was used (Tzschentke, 2007; Veeneman et al., 2011). Thus, on the basis of their baseline preference scores (i.e., time spent in each of the two conditioning compartments), the rats were assigned to a compartment in which they would be allowed social interaction during conditioning, so that the baseline preference in each test group for the (to be) social-paired and (to be) non-social paired compartments approximated 50%. Thus, based on their pre-conditioning performance, some of the rats were conditioned in their preferred compartment, while some were conditioned in their non-preferred compartment. After the pre-conditioning test, the rats were individually housed to increase their motivation for social interaction and to facilitate the development of social play-induced CPP (Achterberg et al., 2012; Niesink and Van Ree, 1989; Trezza et al., 2009b; Vanderschuren et al., 2008). Place conditioning began on day 2. On days 2, 4, 6, and 8, the rats were placed for 30 min in one compartment with an initially unfamiliar partner (social session) in the morning and were placed alone in the other compartment (non-social session) in the afternoon. On day 3, 5, 7, and 9 the order of the sessions was reversed. Social and non-social sessions were separated by at least three hours. Drugs were administered 30 min (rimonabant) or 2 h (URB597) before the start of each social session. On day 10, the rats were placed in the middle compartment and were allowed to explore the entire apparatus for 15 min. The time spent in each compartment during this test was recorded to determine place preference.
2.4 Statistical analysis
Data were analysed using SPSS software 23.0 for Windows and expressed as mean + SEM. Data were analysed using a repeated measures ANOVA with drug dose as within-subjects factor followed by a paired Student’s t-test when appropriate. Operant responding was analysed with lever, treatment and, where appropriate, isolation time as a within-subjects factor. The breakpoints under the PR schedule of reinforcement are derived from an escalating curve, which violates the homogeneity of variance. Therefore, breakpoints were analysed using the non-parametric Friedman test, followed by a post-hoc Wilcoxon signed ranks test when appropriate. The duration of rimonabant-induced scratching behaviour was calculated as a percentage of time.
Place conditioning data were expressed as mean time spent in the social paired and non-social paired compartment + SEM. Place conditioning data were analysed using a two-way ANOVA, with compartment and treatment as factors, followed by paired Student’s t-test when appropriate.
3. Results
3.1 Effects of the FAAH inhibitor URB597 on responding for social play: influence of dose, isolation period and treatment of the stimulus animal
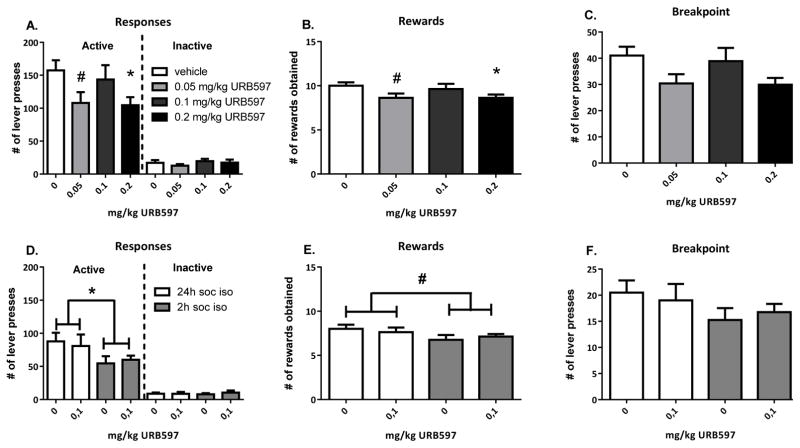
When experimental rats were treated with the FAAH inhibitor URB597 and had the opportunity to interact with an vehicle-treated stimulus partner upon lever pressing, URB597 (URB: 0.05–0.1–0.2 mg/kg) altered operant responding for social play (Ftreatment(3,21)=3.47, p=0.03; Flever*treatment(3,21)=4.42, p=0.02, n=8). Administration of URB597 tended to reduce responding on the active lever at the lowest dose (t(7)veh-0.05=2.26, p=0.06) and significantly decreased it at the highest dose (t(7)veh-0.2=3.17, p=0.02), but did not affect inactive lever presses (Flever(1,7)=86.95, p<0.001) (Fig. 1A). In addition, URB597 tended to reduce the number of rewards obtained at the lowest dose and significantly decreased it at the highest dose (Ftreatment(3,21)=3.62, p=0.03; t(7)veh-0.05=2.31, p=0.05; t(7)veh-0.2=3.27, p=0.01) (Fig. 1B). There was a tendency for URB597 treatment to reduce the breakpoint (χ2=7.34, df=3, p=0.06) (Fig. 1C).
Figure 1. Effect of the FAAH inhibitor URB597 on responding for social play behaviour: influence of dose, isolation period and treatment of the stimulus animal.
Experimental rats treated with the FAAH inhibitor URB597 (0.05–0.1–0.2 mg/kg, n=8) and given the opportunity to interact with an untreated stimulus partner upon lever pressing following a 24 h isolation period showed reduced active responses (A) and rewards obtained (B) at the highest dose tested, with no changes in inactive responses (A) and a tendency towards a reduced breakpoint (C). In a separate experiment (n=8), rats treated with URB597 (0.1 mg/kg) were given the opportunity to interact with a URB597-treated stimulus partner upon lever pressing following a 2 h isolation period. No effect of treatment with URB597 was observed, while reducing the isolation time (soc iso) from 24 hours to 2 hours before testing decreased the number of active responses and tended to reduce the rewards obtained, without affecting inactive responses or the breakpoint (D,E,F). Data are represented as mean+SEM. #0.08>p>0.05 (trend), *p<0.05, relative to vehicle (0 mg/kg URB597/24h social isolation) treatment.
URB597 was previously found to enhance social play behaviour, but only when both animals in a test pair were treated, and following a short-time isolation period before testing (Trezza and Vanderschuren, 2008b). Therefore, we conducted an experiment in which we treated both the experimental and stimulus rats with 0.1 mg/kg URB597 or vehicle, and isolated them for 2 h prior to testing in the operant paradigm. In this experiment, a significant effect of the isolation period before testing was observed, with no treatment effects (Fisolation(1,7)=5.90, p=0.05; Fisolation*lever(1,7)=6.02, p=0.04; Fisolation*treatment(1,7)=0.34, p=0.58; n=8). Animals discriminated between the levers (Flever(1,7)=57.73, p<0.001) but URB597 did not affect the number of lever presses (Ftreatment(1,7)=0.003, p=0.96; Flever*treatment(1,7)=0.08, p=0.79; Fisolation*lever*treatment(1,7)=0.25, p=0.63) (Fig. 2D). The number of rewards tended to decrease with a shorter isolation time (Fisolation(1,7)=4.83, p=0.06) (Fig. 2E) but this was unaffected by treatment (Ftreatment(1,7)=0.001, p=0.99; Fisolation*treatment(1,7)=0.80, p=0.40). The breakpoint was not altered as a result of isolation time or treatment (χ2=4.39, df=3, p=0.22) (Fig. 2F).
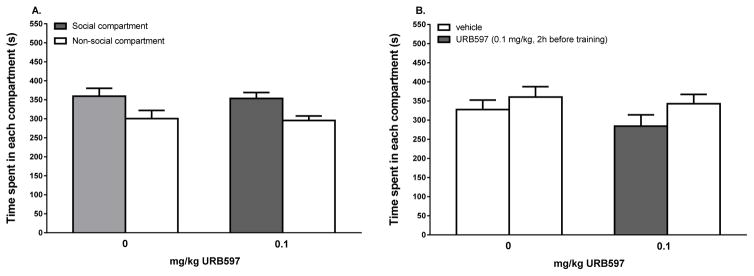
Figure 2. Treatment with URB597 (0.1 mg/kg) did not alter social play-induced CPP (A) and did not induce CPP by itself (B).
(A) Data represent the mean time (sec + SEM) spent in the social compartment (grey bars) and the non-social compartment (white bars) during a 15 min session. Both vehicle-treated animals (n = 16) and URB597-treated animals (0.1 mg/kg, n = 17) showed a significant preference for the social-play associated compartment. (B) Data represent the mean time (sec + SEM) spent in the compartment where animals received vehicle (white bars) and the compartment where animals received URB597 (grey bar) during a 15 min session. URB597 did not induce conditioned place preference or aversion.
3.2 Effects of the FAAH inhibitor URB597 on social play-induced place conditioning
URB597 did not affect the acquisition of social play-induced CPP (Ftreatment(1,62)=0.09, p=0.76; Fcompartment*treatment(1,62)=0.001, p=0.98). The animals spent more time in the social compartment compared to the non-social compartment (Fcompartment(1,62)=11.00, p=0.002; n(vehicle)=16, n(URB)=17) (Fig. 3A). URB597 did not induce CPP by itself (Fcompartment(1,28)=0.82, p=0.37; Ftreatment(1,28)=1.26, p=0.27; Ftreatment*compartment(1,28)=1.18, p=0.29; n=8) (Fig. 3B).
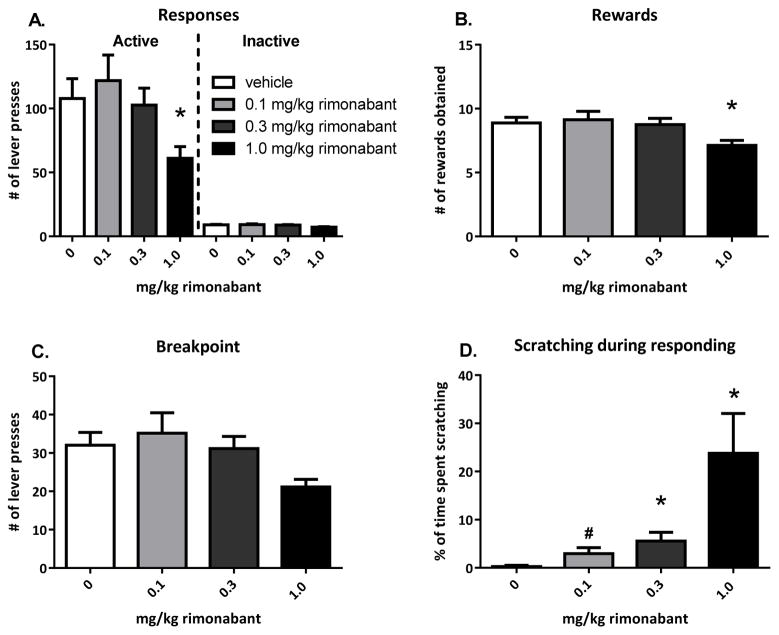
Figure 3. Effects of the CB1 receptor antagonist rimonabant on responding for social play behaviour.
Treatment with rimonabant (0.1–0.3–1.0 mg/kg, n=8) reduced active responses (A) and rewards obtained (B) at the highest dose tested, did not affect inactive responses (A) and tended to reduce the breakpoint (C). The percentage of time spent scratching was increased by the two highest doses of rimonabant (D). Data is represented as mean+SEM. #0.08>p>0.05, *p<0.05, relative to vehicle (0 mg/kg rimonabant) treatment.
3.3 Effects of the CB1 cannabinoid receptor antagonist rimonabant on operant responding for social play
Treatment with the CB1 receptor antagonist rimonabant (0.1–0.3–1.0 mg/kg), reduced responding for social play (Ftreatment(3,21)=3.59, p=0.03, n=8). Animals discriminated between the active and inactive lever (Flever(1,7)=136.81, p<0.001; Flever*treatment(3,21)=2.72, p=0.07). Treatment with the highest dose of rimonabant (1 mg/kg), decreased the number of active responses (t(7)veh-1.0=2.57, p=0.04) (Fig. 3A). In addition, the number of rewards obtained was significantly reduced at the highest dose (Ftreatment(3,21)=4.16, p=0.02; t(7)veh-1.0=3.13, p=0.02) (Fig. 3B), and a trend toward a reduced breakpoint was observed (χ2=6.46, df=3, p=0.09) (Fig. 3C). Since rimonabant is known to induce pruritic effects (Cook et al., 1998; Rubino et al., 2000; Tallett et al., 2007; Vickers et al., 2003), the time animals spent scratching was also scored. The time spent scratching during responding was significantly affected by rimonabant treatment (Ftreatment(3,21)=6.67, p=0.03) (Fig. 3D). Scratching was increased significantly by 0.3 and 1.0 mg/kg rimonabant (t(7)veh-0.1=−2.03, p=0.08; t(7)veh-0.3=−2.77, p=0.03; t(7)veh-1.0=−2.80, p=0.03).
3.4 Effects of rimonabant on social play-induced place conditioning
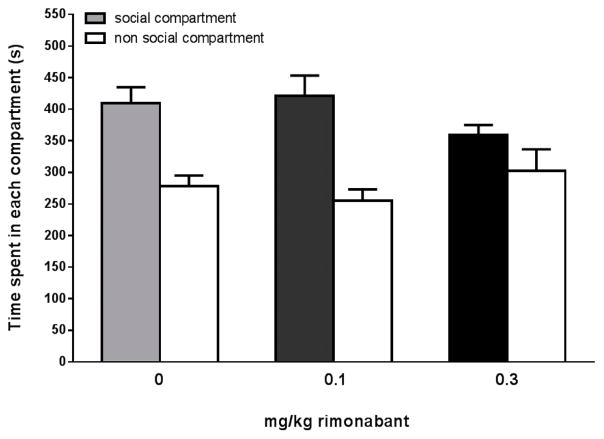
Rimonabant treatment (0.1–0.3 mg/kg) did not affect acquisition of social play-induced CPP. Animals showed a significant preference for the play associated compartment (Fcompartment(1,58)=43.72, p<0.001) but there was no effect of treatment (Fdose(2,58)=0.15, p=0.86; Fcompartment*dose(2,58)=2.48, p=0.09) (Fig. 4).
Figure 4. Effects of the CB1 receptor antagonist rimonabant on social play-induced place conditioning.
Data represent the mean time (sec + SEM) spent in the social compartment (grey/black bars) and the non-social compartment (white bars) during a 15 min session. Vehicle-treated animals (2 ml/kg, n = 12) and rimonabant-treated animals (0.1 mg/kg, n=12, 0.3 mg/kg, n=12) showed a significant preference for the social-play associated compartment..
4. Discussion
We have previously identified an important role for endocannabinoids in social play behaviour. Social play increases levels of the endocannabinoid anandamide in brain areas involved in reward and motivation (Trezza et al., 2012) and treatment with drugs that increase endocannabinoid signalling by blocking endocannabinoid deactivation enhances social play (Manduca et al., 2014; Trezza and Vanderschuren, 2009; Trezza and Vanderschuren 2008a,b; Trezza et al., 2012). Conversely, systemic and intracranial blockade of CB1 cannabinoid receptors decreases social play (Trezza and Vanderschuren, 2009; Trezza et al., 2012).
The aim of this study was to investigate whether endocannabinoids differentially affect the motivational and pleasurable aspects of social play behaviour. We hypothesized that enhancing anandamide levels with URB597 would increase the pleasurable aspects of social play without affecting responding for social play (Adamcyk et al., 2009; Forget et al., 2009; Justinova et al., 2008; Kangas et al., 2016; Oleson et al., 2012; Scherma et al., 2008; Solinas et al., 2005), whereas treatment with the CB1 cannabinoid receptor antagonist rimonabant would decrease both the motivational (Economidou et al., 2006; Hernandez and Cheer, 2012; Maccioni et al., 2010; Rasmussen and Huskinson, 2008; Solinas and Goldberg, 2005; Ward and Dykstra, 2005) and pleasurable aspects (Chaperon et al., 1998; Forget et al., 2004; Hu et al., 2014; Méndez-Diaz et al., 2012; Singh et al., 2004; Thanos et al., 2005; Yu et al., 2011) of social play.
To test this hypothesis, we investigated the consequences of pharmacological enhancement or reduction of endocannabinoid activity in two setups designed to assess the motivational or pleasurable properties of social play, i.e., responding for social play under a progressive ratio schedule of reinforcement (Achterberg et al., 2016) and social play-induced conditioned place preference (Trezza et al., 2009b), respectively.
4.1 Effects of URB597 and rimonabant on responding for social play behaviour
At a dose known to increase the expression of social play behaviour (i.e. 0.1 mg/kg; Manduca et al., 2014; Trezza and Vanderschuren, 2008a,b), treatment with URB597 did not affect responding for social play, neither in animals isolated for 24 h nor for 2 h before testing. URB597 modestly reduced responding for social play at a higher dose (0.2 mg/kg) in animals isolated for 24 h prior to testing. These findings are in line with previous studies showing that treatment with URB597 does not affect responding for food (Adamcyk et al., 2009; Kangas et al., 2016; Oleson et al., 2012), nicotine (Forget et al., 2009; Scherma et al., 2008), heroin (Solinas et al., 2005), anandamide, THC and cocaine (Adamcyk et al., 2009; Justinova et al., 2008) in rats and non-human primates. Similarly, inhibiting the reuptake of anandamide with AM404 had no effect on responding for food in rats (Gamaleddin et al., 2013). Together, these data show that increasing anandamide tone through inhibition of its degradation or reuptake does not profoundly alter the incentive motivational properties of rewards.
Blocking CB1 cannabinoid receptors with rimonabant reduced operant responding for social play. In previous studies, treatment with rimonabant was found to reduce operant responding for food (Evenden and Ko, 2007; Meye et al., 2013; Solinas and Goldberg, 2005; Ward et al., 2008), chocolate-drinks (Maccioni et al. 2008) but not intracranial self-stimulation (Arnold et al., 2001). Rimonabant treatment also reduced the intake of food (Verty et al., 2004a,b,c), chocolate-drink, and ethanol (Arnone et al., 1997), suggesting that a decrease in the pleasurable properties of rewards contributes to the reduction in operant responding induced by rimonabant. However, the reduction in operant responding induced by rimonabant may also be secondary to frequent episodes of scratching induced by the drug (Cook et al., 1998; Rubino et al., 2000; Tallett et al., 2007; Vickers et al., 2003). Indeed, during lever pressing for social play, the animals treated with the highest dose of rimonabant (1.0 mg/kg) spent about 25% of their time scratching, which likely interfered with operant responding. Thus, the reduction in operant responding could also be the result of behavioural competition, which has been proposed to underlie certain other behavioural effects of rimonabant as well (e.g. Tallett et al., 2007). In sum, decreases in responding for rewards, including social play behaviour, after treatment with rimonabant may result from effects on incentive motivation, pleasure, behavioural competition, or a combination of these. Future studies with intracranial administration may clarify the consequences of central blockade of CB1-receptors on operant responding for rewards without the interference of competing stereotypic behaviours.
In contrast to the lack of consistent effects on incentive motivation by indirectly enhancing endocannabinoid levels, targeting CB1-receptors with direct agonists has been found to affect the motivational aspects of both food and drug rewards (for reviews see Panilio et al., 2013; Parsons and Hurd, 2015). The discrepancies between effects of direct vs indirect cannabinoid agonists illustrate the peculiar dynamics of endocannabinoid signaling, whereby these neuromodulators are released ‘on demand’ after changes in synaptic activity (Freund et al., 2003). Thus, endocannabinoid degradation inhibitors or reuptake blockers only increase ongoing endocannabinoid signaling, whereas direct receptor agonists will increase cannabinoid activity throughout the central nervous system. As a result, administration of direct and indirect cannabinoid agonists can evoke differential effects on behaviour (e.g. Trezza and Vanderschuren, 2008a). With regard to operant responding for rewards, we think that there is modest, if any, endocannabinoid activity in brain regions that support incentive motivational processes, so that interfering with endocannabinoid degradation will hardly affect this behaviour. In contrast, stimulating cannabinoid neurotransmission in other brain regions may evoke mental states that do result in changes in responding for rewards.
4.2 Effects of URB597 and rimonabant on social play-induced place conditioning
Enhancing endocannabinoid levels by URB597 treatment during place conditioning did not alter the expression of social play-induced CPP, although at this dose, URB597 has been found to enhance social play behaviour (Manduca et al., 2014; Trezza and Vanderschuren, 2008a,b). A likely explanation for the lack of effect social play behaviour-induced CPP is that in the place conditioning paradigm, the animals are isolated for 24 hours, which leads to near-maximal levels of social play behaviour (Niesink and Van Ree, 1989; Vanderschuren et al., 2008). Possibly, this is associated with maximal levels of endocannabinoid signalling, so that treatment with URB597 can not influence this behaviour further. Consistent with previous work (Gobbi et al., 2005; Scherma et al., 2008a), URB597 did not induce CPP by itself, although URB597 has been found to prevent the development of nicotine-induced CPP in adult rats (Scherma et al., 2008b).
Blocking CB1 receptors with rimonabant did not significantly affect the acquisition of social play-induced CPP. However, visual inspection of Fig. 4 shows that the social play-induced CPP was somewhat reduced after treatment with 0.3 mg/kg rimonabant. Indeed, Trezza and Vanderschuren (2009) showed that rimonabant, at doses of 0.3 mg/kg and higher reduced social play behaviour. Thus, rimonabant may have a modest effect on the pleasurable properties of social play, although we cannot exclude that the pruritic effects of rimonabant interfered with behaviour in this case as well. Previous studies have shown that food- and drug-induced CPP was inhibited after CB1 receptor blockade (Chaperon et al., 1998; Forget et al., 2004; Hu et al., 2014; Méndez-Diaz et al., 2012; Singh et al., 2004; Thanos et al., 2005; Yu et al., 2011). The present data indicate that CB1 receptor antagonist treatment only modestly affected the pleasurable properties of social play behaviour.
Contrary to our hypothesis, enhancing endocannabinoid tone as well as blocking CB1 receptors did not lead to robust changes in the pleasurable aspects of social play behaviour as measured in our place conditioning set-up. This indicates that endocannabinoids have no primary role in the pleasurable properties of social play behaviour, but future studies with suboptimal conditioning paradigms and/or central administration of cannabinoid drugs may shed light on the role of endocannabinoid signalling in social play reward.
5. Conclusion
These data provide new insights into the role of endocannabinoids in the motivational and pleasurable aspects of social play behaviour. Endocannabinoids modulate the expression of social play behaviour (Trezza et al., 2012; Trezza and Vanderschuren 2009, 2008a,b). The present results extend these findings by showing that they do not differentially affect the motivational and pleasurable properties of social play. Thus, rather than by specifically interfering with one of its emotional components, endocannabinoid signaling may modulate the expression of social play behaviour by altering the coherence of its behavioural components or prolonging the playful interaction.
Supplementary Material
Acknowledgments
6. Role of funding source
Supported by National Institute on Drug Abuse Grant R01DA022628 (LJMJV), Netherlands Organization for Scientific Research (NWO) Veni grant 91611052 (VT) and Marie Curie Career Reintegration Grant PCIG09-GA-2011-293589 (VT). The funding organizations had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the paper for publication.
Footnotes
Chemical compounds: URB597 (Pubmed Chem CID: 1383884), Rimonabant (Pubmed Chem CID: 104849)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Achterberg EJM, Trezza V, Vanderschuren LJMJ. Beta-adrenoreceptor stimulation mediates reconsolidation of social reward-related memories. PLoS One. 2012;7:e39639. doi: 10.1371/journal.pone.0039639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achterberg EJM, van Kerkhof LWM, Servadio M, van Swieten MM, Houwing DJ, Aalderink M, Driel NV, Trezza V, Vanderschuren LJMJ. Contrasting roles of dopamine and noradrenaline in the motivational properties of social play behaviour in rats. Neuropsychopharmacology. 2016;41:858–868. doi: 10.1038/npp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behaviour in rats. J Physiol Pharmacol. 2009;60:119–125. [PubMed] [Google Scholar]
- 4.Arnold JC, Hunt GE, McGregor IS. Effects of the cannabinoid receptor agonist CP 55,940 and the cannabinoid receptor antagonist SR 141716 on intracranial self-stimulation in Lewis rats. Life Sci. 2001;70:97–108. doi: 10.1016/s0024-3205(01)01366-2. [DOI] [PubMed] [Google Scholar]
- 5.Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 6.Baarendse PJJ, Counotte DS, O’Donnell P, Vanderschuren LJMJ. Social experience during adolescence is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 2013;38:1485–1494. doi: 10.1038/npp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- 8.Barbano MF, Le Saux M, Cador M. Involvement of dopamine and opioids in the motivation to eat: Influence of palatability, homeostatic state, and behavioural paradigms. Psychopharmacology. 2009;203:475–487. doi: 10.1007/s00213-008-1390-6. [DOI] [PubMed] [Google Scholar]
- 9.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 11.Chaperon F, Soubrié P, Puech AJ, Thiébot M-H. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology. 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- 12.Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–1156. [PubMed] [Google Scholar]
- 13.Crowder WF, Hutto CW., Jr Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav. 1992;41:817–824. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- 14.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 15.Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 16.Evenden J, Ko T. The effects of anorexic drugs on free-fed rats responding under a second-order FI15-min (FR10:S) schedule for high incentive foods. Behav Pharmacol. 2007;18:61–69. doi: 10.1097/FBP.0b013e32801456c6. [DOI] [PubMed] [Google Scholar]
- 17.Fattore L, Fadda P, Fratta W. Endocannabinoid regulation of relapse mechanisms. Pharmacol Res. 2007;56:418–427. doi: 10.1016/j.phrs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration--comparison with CB(1) receptor blockade. Psychopharmacology. 2009;205:613–24. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- 19.Friemel CM, Zimmer A, Schneider M. The CB1 receptor as an important mediator of hedonic reward processing. Neuropsychopharmacology. 2014;39:2387–2396. doi: 10.1038/npp.2014.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 21.Gamaleddin I, Guranda M, Scherma M, Fratta W, Makriyannis A, Vadivel SK, Goldberg SR, Le Foll B. AM404 attenuates reinstatement of nicotine seeking induced by nicotine-associated cues and nicotine priming but does not affect nicotine- and food-taking. J Psychopharmacol. 2013;27:564–571. doi: 10.1177/0269881113477710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez G, Cheer JF. Effect of CB1 receptor blockade on food-reinforced responding and associated nucleus accumbens neuronal activity in rats. J Neurosci. 2012;32:11467–11477. doi: 10.1523/JNEUROSCI.1833-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, Bergman J. Comparisons of Δ9-tetrahydrocannabinol and anandamide on a battery of cognition-related behaviour in nonhuman primates. J Pharmacol Exp Ther. 2016;357:125–133. doi: 10.1124/jpet.115.228189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behaviour and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Maccioni P, Colombo G, Carai MA. Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets. 2010;9:55–59. doi: 10.2174/187152710790966623. [DOI] [PubMed] [Google Scholar]
- 28.Maccioni P, Pes D, Carai MAM, Gessa GL, Colombo G. Suppression by the cannabinoid CB1 receptor antagonist, rimonabant, of the reinforcing and motivational properties of a chocolate-flavoured beverage in rats. Behav Pharmacol. 2008;19:197–209. doi: 10.1097/FBP.0b013e3282fe8888. [DOI] [PubMed] [Google Scholar]
- 29.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 30.Manduca A, Servadio M, Campolongo P, Palmery M, Trabace L, Vanderschuren LJMJ, Cuomo V, Trezza V. Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behaviour in rats. Eur Neuropsychopharmacol. 2014;24:1337–1348. doi: 10.1016/j.euroneuro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Méndez-Díaz M, Rueda-Orozco PE, Ruiz-Contreras AE, Prospéro-García O. The endocannabinoid system modulates the valence of the emotion associated to food ingestion. Addict Biol. 2012;17:725–735. doi: 10.1111/j.1369-1600.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meye FJ, Trezza V, Vanderschuren LJMJ, Ramakers GMJ, Adan RAH. Neutral antagonism at the cannabinoid 1 receptor: a safer treatment for obesity. Mol Psychiatry. 2013;18:1294–1301. doi: 10.1038/mp.2012.145. [DOI] [PubMed] [Google Scholar]
- 33.Niesink RJM, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–418. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 34.Oleson E, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, Lichtman AH, Cheer JF. Endocannabinoids shape accumbal encoding of cue-motivated behaviour via CB1 receptor activation in the ventral tegmentum. Neuron. 2012;73:360–373. doi: 10.1016/j.neuron.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panksepp J, Siviy SM, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 36.Panksepp J, Beatty WW. Social deprivation and play in rats. Behav Neural Biol. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- 37.Panlilio LV, Justinova Z, Goldberg SR. Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacol Ther. 2013;138:84–102. doi: 10.1016/j.pharmthera.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellis SM, Pellis VC. The playful brain. OneWorld Publications; Oxford: 2009. [Google Scholar]
- 40.Potegal M, Einon D. Aggressive behaviours in adult rats deprived of playfighting experience as juveniles. Dev Psychobiol. 1989;22:159–172. doi: 10.1002/dev.420220206. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen EB, Huskinson SL. Effects of rimonabant on behavior maintained by progressive ratio schedules of sucrose reinforcement in obese Zucker (fa/fa) rats. Behav Pharmacol. 2008;19:735–742. doi: 10.1097/FBP.0b013e3283123cc2. [DOI] [PubMed] [Google Scholar]
- 42.Rubino T, Vigano D, Zagato E, Sala M, Parolaro D. In vivo characterization of the specific cannabinoid receptor antagonist, SR141716A: behavioural and cellular responses after acute and chronic treatments. Synapse. 2000;35:8–14. doi: 10.1002/(SICI)1098-2396(200001)35:1<8::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 43.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008a;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, Justinová Z, Mikics E, Haller J, Medalie J, Stroik J, Barnes C, Yasar S, Tanda G, Piomelli D, Fratta W, Goldberg SR. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioural and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008b;327:482–490. doi: 10.1124/jpet.108.142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh ME, Verty ANA, McGregor IS, Mallet PEA. cannabinoid receptor antagonist attenuates conditioned place preference but not behavioural sensitization to morphine. Brain Res. 2004;1026:244–253. doi: 10.1016/j.brainres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 48.Siviy SM, Panksepp J. In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci Biobehav Rev. 2011;35:1821–1830. doi: 10.1016/j.neubiorev.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- 50.Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- 52.Spear LP. The adolescent brain and age-related behavioural manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 53.Špinka M, Newberry RC, Bekoff M. Mammalian play: training for the unexpected. Q Rev Biol. 2001;76:141–168. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- 54.Tallett AJ, Blundell JE, Rodgers RJ. Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacol. 2007;195:27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- 55.Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci. 2011a;1:444–458. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ. Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci. 2011b;31:6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trezza V, Damsteegt R, Manduca A, Petrosino S, Van Kerkhof LWM, Pasterkamp RJ, Zhou Y, Campolongo P, Cuomo V, Di Marzo V, Vanderschuren LJMJ. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J Neurosci. 2012;32:14899–14908. doi: 10.1523/JNEUROSCI.0114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trezza V, Damsteegt R, Vanderschuren LJMJ. Conditioned place preference induced by social play behaviour: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009b;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trezza V, Vanderschuren LJMJ. Bidirectional cannabinoid modulation of social behaviour in adolescent rats. Psychopharmacology. 2008a;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- 62.Trezza V, Vanderschuren LJMJ. Divergent effects of anandamide transporter inhibitors with different target selectivity on social play behaviour in adolescent rats. J Pharmacol Exp Ther. 2009;328:343–350. doi: 10.1124/jpet.108.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trezza V, Vanderschuren LJMJ. Cannabinoid and opioid modulation of social play behaviour in adolescent rats: differential behavioural mechanisms. Eur Neuropsychopharmacol. 2008b;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 65.Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- 66.Vanderschuren LJMJ, Trezza V. What the laboratory rat has taught us about social play behaviour: Role in behavioural development and neural mechanisms. Curr Topics Behav Neurosci. 2014;16:189–212. doi: 10.1007/7854_2013_268. [DOI] [PubMed] [Google Scholar]
- 67.Vanderschuren LJMJ. How the brain makes play fun. Am J Play. 2010;2:315–337. [Google Scholar]
- 68.Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Influence of environmental factors on social play behavior of juvenile rats. Physiol Behav. 1995;58:119–123. doi: 10.1016/0031-9384(94)00385-i. [DOI] [PubMed] [Google Scholar]
- 69.Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behaviour in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 70.Vanderschuren LJMJ, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, Schoffelmeer ANM. Methylphenidate disrupts social play behaviour in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veeneman MMJ, Boleij H, Broekhoven MH, Snoeren EMS, Guitart MM, Cousijn J, Spooren W, Vanderschuren LJMJ. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology. 2011;214:863–876. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verty ANA, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004a;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 73.Verty ANA, McFarlane JR, McGregor IS, Mallet PE. Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake. Endocrinology. 2004b;145:3224–3231. doi: 10.1210/en.2004-0059. [DOI] [PubMed] [Google Scholar]
- 74.Verty ANA, McFarlane JR, McGregor IS, Mallet PE. Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology. 2004c;47:593–603. doi: 10.1016/j.neuropharm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology. 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- 76.Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Ward SJ, Lefever TW, Jackson C, Tallarida RJ, Walker EA. Effects of a cannabinoid1 receptor antagonist and serotonin 2c receptor agonist alone and in combination on motivation for palatable food: A dose-addition analysis study in mice. J Pharmacol Exp Ther. 2008;325:567–576. doi: 10.1124/jpet.107.131771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.