Abstract
Background
Exposure-based therapy parallels extinction learning of conditioned fear. Prior research points to the ventromedial prefrontal cortex as a potential site for the consolidation of extinction learning and subsequent retention of extinction memory.
Objective/hypothesis
The present study aimed to evaluate whether the application of non-invasive transcranial direct current stimulation (tDCS) during extinction learning enhances late extinction and early recall in human participants.
Methods
Forty-four healthy volunteers completed a 2-day Pavlovian fear conditioning, extinction, and recall paradigm while skin conductance activity was continuously measured. Twenty-six participants received 2 mA anodal tDCS over EEG coordinate AF3 during extinction of a first conditioned stimulus. The remaining 18 participants received similar tDCS during extinction of a second conditioned stimulus. Sham stimulation was applied for the balance of extinction trials in both groups. Normalized skin conductance changes were analyzed using linear mixed models to evaluate effects of tDCS over late extinction and early recall trials.
Results
We observed a significant interaction between timing of tDCS during extinction blocks and changes in skin conductance reactivity over late extinction trials. These data indicate that tDCS was associated with accelerated late extinction learning of a second conditioned stimulus after tDCS was combined with extinction learning of a previous conditioned stimulus. No significant effects of tDCS timing were observed on early extinction recall.
Conclusions
Results could be explained by an anxiolytic aftereffect of tDCS and extend previous studies on tDCS-induced modulation of fear and threat related learning processes. These findings support further exploration of the clinical use of tDCS.
Keywords: Neuromodulation, Anxiety, Emotion, Cognition, Learning, Memory
Introduction
Extinction of conditioned fear has been used as a model to explain the therapeutic benefits of exposure-based therapy for anxiety and stress disorders [1,2]. Successful extinction of conditioned fear has been associated with “top-down” modulation by the ventromedial prefrontal cortex (vmPFC) of fear responses originating in the amygdala [3–9]. In addition, other factors besides the amygdala–vmPFC connectivity contribute to extinction success, e.g. time of extinction after fear conditioning [10] and reinforcement rate during conditioning [11]. Nonetheless, facilitating activation of vmPFC during extinction learning may be one mechanism to improve fear extinction as well as the retention of extinction memories.
The idea of increasing neural activity in vmPFC to impact fear expression and extinction has been previously tested in rats. Specifically, invasive electrical stimulation of the rat infralimbic subregion of the rat vmPFC during presentation of conditioned stimulus reduced fear expression, thereby simulating extinction in non-extinguished rats [12]. Similar electrical stimulation during extinction learning reduced conditioned fear expression during extinction and extinction recall [13]. Recent review papers outline the rationale for evaluating non-invasive neuromodulation techniques during extinction-based processes to assess their clinical potential [14–16].
Transcranial direct current stimulation (tDCS) is one such non-invasive technique that alters cortical excitability via subthreshold modulation of neuronal resting membrane potentials using a weak constant electrical current [17]. Anodal or ‘excitatory’ stimulation is thought to increase the likelihood of action potentials in underlying cortex, whereas cathodal or ‘inhibitory’ stimulation may decrease the likelihood of action potentials. There is a rapidly growing body of research showing that prefrontal tDCS in the range of 1–2 mA affects various cognitive functions such as learning, memory and emotional processing [18].
So far, two studies suggest that tDCS can modify fear memories in line with the direction of stimulation. Asthana et al. [19] showed that inhibitory cathodal tDCS over the left dorsolateral pre-frontal cortex after fear conditioning resulted in reduced fear expression to the conditioned stimulus during fear extinction 24 hours later. In another study, Mungee et al. [20] observed that excitatory anodal tDCS vs. sham over the right dorsolateral prefrontal cortex after providing a reminder of the conditioned fear stimulus resulted in increased fear expression. These results indicate that pre-frontal tDCS could impact fear memory processes.
To our knowledge there are no studies to date that examine the effect of tDCS during extinction learning of conditioned fear in order to improve fear extinction learning and subsequent extinction recall. In this study we evaluated the hypothesis that, compared to sham stimulation, 2 mA anodal tDCS over EEG coordinate AF3 during extinction learning would enhance fear extinction learning as well as extinction recall. In particular, we predicted that active tDCS would result in a greater reduction in skin conductance values, an index of conditioned fear response, compared to sham stimulation across late extinction trials as well as across early recall trials in healthy volunteers. This focus on late extinction and early recall is based on an extensive amount of research in which extinction success has been quantified as a reduction in skin conductance during late extinction trials and early recall trials [7,9,21–23].
We employed a within-subjects design in which all participants received both active tDCS as well as sham stimulation during the extinction of a conditioned stimulus (CS+). This allowed us to evaluate whether tDCS during the extinction of one CS+, but not CS+ paired with sham, would affect extinction learning and subsequent extinction recall within participants. This design allowed timing of tDCS to occur during extinction of an initial CS+ or a second, subsequent CS+. Although exploratory, this may provide insight into a temporal order effect of tDCS in relation to extinction learning. The selection of excitatory, anodal tDCS and area AF3 as the target location was based on previously discussed literature on the association between increased vmPFC activity and successful extinction learning [3–9,12,13].
Materials and methods
Participants
Fifty-two participants aged 18–50 years were recruited from the Providence metro area by online advertisements and were included in the study. Eight participants were removed from all data analyses: two participants did not tolerate the unconditioned stimulus; one participant did not tolerate skin sensation associated with tDCS; equipment failure during fear conditioning prevented data collection for four participants; one participant screened out after the psychiatric interview. This resulted in a group of 44 participants, 21 females and 23 males for further analyses. All 44 participants denied using psychoactive or other potentially confounding medication, or smoking/use of nicotine replacement options.
Participants were randomly assigned to two study groups: 26 received active tDCS immediately at the onset of the first extinction learning block and sham during the second extinction block. The remaining 18 participants received sham stimulation during the first extinction block and active tDCS during the second extinction block. Table 1 describes the demographic characteristics of the participant sample. Exclusion criteria included current psychiatric disorders or past anxiety or psychotic disorder as assessed by the Mini International Neuropsychiatric Interview (MINI) [24] and contra-indications for tDCS. Study procedures were performed in accordance with Declaration of Helsinki and the local IRB (Butler Hospital and Brown University) approved the study. Informed consent was obtained prior to the onset of any study procedures.
Table 1.
Demographics of participants included for analyses.
| All participants (N = 44) | tDCS during 1st extinction block | tDCS during 2nd extinction block | |
|---|---|---|---|
| Age (in years) | 27.34 (SD 8.18; range 18–50) | 27.77 (SD 8.45) | 26.72 (SD 7.97) |
| Sex (F:M ration) | 21 F:23 M | 10 F:16 M | 11 F:7 M |
| Handedness (# R; L; A) | 33; 4; 7 | 18; 2; 6 | 15; 2; 1 |
| Ethnicity (#) | 31 White/Caucasian; 6 African-American; 4 Hispanic; 2 Asian; 1 biracial. | 19 White/Caucasian; 3 African-American; 2 Hispanic; 1 Asian; 1 biracial. | 12 White/Caucasian; 3 African-American; 2 Hispanic; 1 Asian; 0 biracial. |
| Educational level (in years) | 14.75 (2.32) | 14.42 (SD 2.50) | 15.22 (SD 2.02) |
Fear conditioning paradigm and procedures
The experimental protocol was adapted from Milad et al. [7,9] and administered over two days. On Day 1, participants underwent three different phases: habituation, conditioning, and extinction. On Day 2, approximately 24 hours after conditioning and extinction, participants were tested on extinction recall.
During both days participants were asked to passively view photographs that would appear on a computer monitor. A set of electrodes was placed over the middle phalanges of the index and middle fingers of the dominant hand and was used to deliver the unpleasant unconditioned stimulus (US), i.e. a non-harmful electrical shock. Before initiation of the habituation phase, the intensity of the electric shock was set individually by each participant, and determined to be ‘highly annoying but not painful.’ The shock was generated by a Coulbourn Transcutaneous Aversive Finger Stimulator [25]. The mean shock level selected by participants was 2.32 mA out of 4 mA max (SD: 0.97; range: 0.8–4 mA). There was no significant difference of tDCS Timing Group on average shock level, t(42) = 0.74, p = 0.47. A second set of electrodes was placed on the non-dominant hand to measure skin conductance throughout testing sessions (see details on skin conductance recording and analyses below).
Day 1 – habituation phase
Participants were instructed that the purpose of this phase was to show them all of the possible pictures that they would see in the experiment, and that no shock would be delivered in this phase. Two future conditioned stimuli (CS+: red and blue light) and one unconditioned stimulus (CS−: yellow light) were presented in a counterbalanced manner once within the fear acquisition context (CX+: picture of an office) and once within the fear extinction context (CX−: picture of a room with bookcase), resulting in a total of 6 trials. The selection of the CS and CS− light colors and the CX+ and CX− rooms was predetermined and remained constant between participants. For each trial during the experiment, the CX was presented for 9 sec: 3 sec alone, followed by 6 sec in combination with the CS+ or CS−. The average inter-trial interval was 15 sec (12–18 sec).
Day 1 – conditioning phase
Participants were instructed that they ‘may or may not be shocked’ during this phase and the following phases of the experiment. Both CS+ (red and blue light) were always depicted within the CX+ and paired with the US (i.e., finger shock) at a 60% reinforcement rate. The CS− (yellow light) was also depicted within the CX+ but was never paired with the US. Each participant was administered 16 CS+ (8 red lights; 8 blue lights) and 8 CS− trials using a block design in which first one of the two CS+ was intermixed with the CS−, and after which the other CS+ was intermixed with the CS−. Out of the 16 CS+ total, 10 CS+ were followed by the delivery of the US. The US occurred during the last 500 msec of the CS+ presentation. This phase was followed by a 1-minute break.
Day 1 – extinction training phase
Participants were reminded that they ‘may or may not be shocked’ during this phase. All CS+ and CS− were presented in the absence of the US and solely in the CX−. Extinction was divided into two blocks separated by a 5 minute rest period. Twenty-six participants were randomized to receive active tDCS during the first extinction block and sham stimulation during the second extinction block. The remaining eighteen participants received sham stimulation during the first extinction block and sham stimulation during the second extinction block (see tDCS specific below). This allowed a within-subjects design in which each participant received anodal tDCS during the extinction of one CS+, so-called CS++DC (plus Direct Current) trials, and sham stimulation during the extinction of the second CS+, so-called CS+-DC (minus Direct Current) trials.
Six CS+ and three CS− trials were presented within both extinction blocks, for a total of 18 trials across both blocks. Median split determined the last three CS+ trials as late extinction trials within each extinction block. This suboptimal number of extinction trials was chosen in order to examine the hypothesized influence of tDCS on late extinction trials while preventing a ceiling effect of extinction learning.
Day 2 – extinction recall phase
Participants were told that the experiment would be similar to the previous day, and that they ‘may or may not be shocked.’ The recall phase followed a similar procedure as the conditioning phase, i.e. 16 CS+ (8 red and 8 blue lights) and 8 CS− using a block design. During recall, all CS were shown within the CX− similar to extinction procedures. Participants did not receive any shocks during the recall phase. Early recall trials were calculated by median split defining the first 4 CS+ as early recall trials out of the 8 recall trials for both CS++DC and CS+-DC.
Skin conductance recording
A Coulbourn Modular Instruments System [25] was used to record skin conductance levels via a Coulbourn Isolated Skin Conductance Coupler using a constant 0.5 V through 8 mm (sensor diameter) electrodes. Electrodes were filled with isotonic paste and placed on the palm of the participant’s non-dominant hand. The skin conductance electrodes were separated by approximately 8 mm, as determined by the width of the adhesive collar. Before starting habituation 2 minutes of baseline skin conductance were recorded, followed by two external stimuli, a sigh and handclap, in order to ensure a correct attachment and conductance of the electrodes.
Transcranial direct current stimulation (tDCS)
tDCS was delivered using a 9 V battery-driven Soterix Medical 1 × 1 device [25]. We used a 1 (anode) × 1 (cathode) unilateral anodal electrode montage [26]. Both electrodes measured 2.9 × 2.4 cm and were placed in a 3 × 5 cm (15 cm2) sponge pocket saturated with normal saline resulting in a 1.33 A/m2 current density. Sponge pockets with inserted electrodes were fixed to the participant’s skull using a rubber headband.
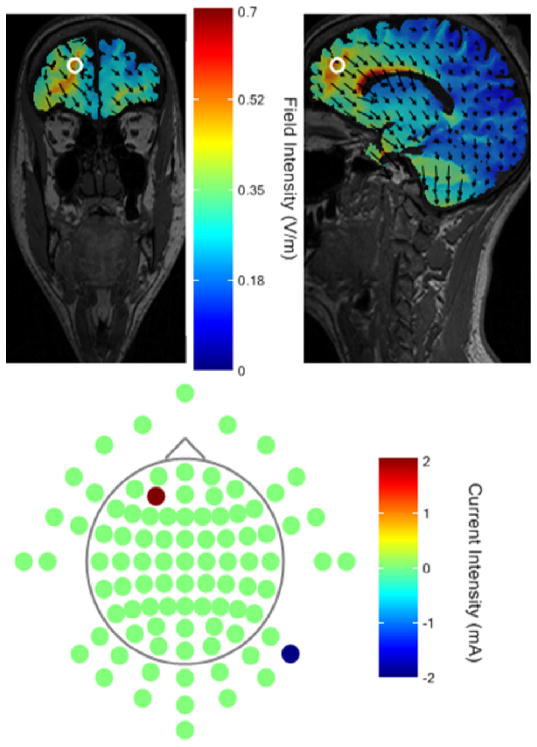
The anodal electrode was placed over 10–20 EEG position AF3 and the cathodal electrode was placed over the contralateral mastoid process. This electrode placement was chosen to avoid prefrontal cathodal modulation and prior finite element modeling indicated current distributions to reach the vmPFC (personal communication Soterix Medical). Fig. 1 shows additional post-hoc computer modeling of current distributions using HD-Explore neurotargeting software by Soterix Medical [27, 28]. We selected a 2 mA stimulation intensity because tDCS montages in which electrodes are placed further away from one another to potentially reach deeper neural structures [28] may need to ‘correct’ for stimulation intensity by increasing from 1 mA to 2 mA to obtain comparable tDCS effects [29].
Figure 1.
Modeling of transcranial direct current distribution based on a 1 anode electrode over the EEG coordinate AF3 and a 1 cathode electrode over the contra-lateral mastoid process using HD-Explore neurotargeting software by Soterix Medical.
Although tDCS electrodes were placed prior to fear conditioning onset, tDCS was applied for a total of 10 minutes using a current of 2 mA either before and during the first extinction block, or immediately after the first extinction block continuing during the second extinction block (see Fig. 2). During the part of the task where stimulation was off, participants received sham that consisted of ramping up the device to 1 mA over a period of 30 seconds and then back down to zero in 30 seconds. Participants were pseudo-randomly assigned to receive active tDCS during the first or second extinction block in order to examine the effects of tDCS within subjects. We however realize that participants who received tDCS during the first extinction block might still experience tDCS aftereffects when they received sham during the second extinction block.
Figure 2.
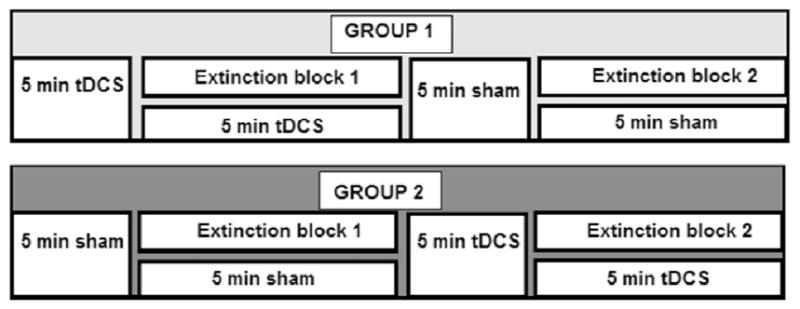
Time of tDCS and sham application in relation to the two extinction blocks for the two different groups.
To prevent side effects such as a skin burn, the skin under the stimulation sites was inspected and participants were instructed to notify the experimenter of any discomfort during testing so stimulation could be discontinued if discomfort occurred. To further ensure tDCS tolerability to study procedures, all participants initially received brief stimulation (1 mA for 30 seconds, with a ramp up/down of 30 seconds each). The Soterix Medical tDCS device provides an indication of electrode contact quality using a 20-segment light bar display. When converted to numbers this would resemble a 0–20 point scale. Average contact quality obtained during this study was 17.93 (SD: 1.14) and no study procedures were started unless contact quality reached a level of 15 or above. There was no significant difference of tDCS Timing Group (tDCS during first extinction block vs. tDCS during second extinction block) on tDCS contact quality, t(42) = − 1.15, p = 0.26.
Skin conductance analyses
Skin conductance for each trial was calculated by subtracting the mean skin conductance level during the 2 sec before CS onset (during which the context alone was being presented) from the highest skin conductance level during the 6-sec CS duration. Thus, skin conductance to each CS reflected changes beyond any change produced by the presentation of context. This method of analysis has been extensively used and published in human psychophysiological research supporting its validity [7–9,30]. Skin conductance data were normalized using a square root transformation resulting in a skewness and kurtosis of 0.83 for both.
Normalized data were analyzed using the linear mixed effects model function in SPSS v. 21 [31], which allows adjustment for correlations due to repeated observations within each participant over trials following McLaughlin et al. [32]. Instead of averaging across CS+ and CS− trials, linear mixed effects models permit examining the potential effect of tDCS on learning over trials during late extinction and early recall to test our main hypotheses. Separate models were performed for each of the experimental sections: habituation, conditioning, extinction and recall. The dependent variable was the normalized skin conductance value. The following variables were added as predictors (factors): tDCS Timing Group (two levels: ‘active tDCS during first extinction block’ and ‘active tDCS during second extinction block’); Trial Order (number of levels reflecting the number of trials within each experiment phase); Stimulus Type (max. three levels: (future) CS++DC, (future) CS+-DC and CS−). The variable Subject was entered as a correlated random effects variable. A two-sided alpha level of 0.05 was applied to determine significance in all analyses.
Results
Demographics
Table 1 depicts demographic variables for the entire participant sample as well as broken down by tDCS Timing Group. There were no significant differences between participants who received tDCS during the first extinction block and participants who received tDCS during the second extinction block on age (t(42) = 0.41, p = 0.68), gender distribution (Chi-Square = 2.19, df = 1, p = 0.14), handedness (Chi-square = 2.47, df = 2, p = 0.29), ethnicity (Chi-Square = 1.16, df = 4, p = 0.88), or education level (t(42) = − 1.13, p = 0.27).
tDCS
Although we did not systematically assess tDCS side effects, one participant out of three participants excluded from the analyses did not tolerate tDCS accompanied skin sensations. All other participants reported an itchy or sometimes prickly sensation that diminished over time associated with tDCS.
Habituation – day 1
One participant was excluded from habituation data analyses due to equipment data storage failure during habituation only. A linear mixed effects model showed no significant difference in skin conductance values for Stimulus Type (F(2,213) = 0.69, p = 0.50) with a value of 0.17 for the first CS+, 0.19 for the second CS+, and 0.13 for CS−. Also, no significant tDCS Timing Group difference was observed (F(1,41) = 1.27, p = 0.27), i.e. skin conductance values for participants who were randomized to receive tDCS during the first extinction block were comparable to participants who were randomized to receive tDCS during the second extinction block. However, the magnitude of skin conductance reactivity decreased significantly over the six habituation trials (F(5,210) = 7.55, p < 0.0001).
Conditioning – day 1
Three participants were excluded from further analyses (conditioning, extinction and recall) due to evidence of unclear conditioning as defined by not meeting CS+ > CS− during conditioning and skin conductance deflections below 0.05 3S on less than two CS+ conditioning trials [32]. We performed an initial linear mixed model analysis to confirm the presence of conditioning (CS+ conditioning > CS+ habituation) in both tDCS Timing Groups. Analyzing CS+ only, results revealed a significant main effect of phase of paradigm: habituation vs. conditioning (F(1,774.9) = 9.91, p = 0.003) demonstrating larger skin conductance reactivity to CS+ during conditioning (0.32) versus CS+ during habituation (0.18). There was no significant main effect of tDCS Timing Group (F(1,47.8) = 0.52, p = 0.48) and no significant interaction between tDCS Timing Group and phase of paradigm (F(1,774.9) = 0.06, p = 0.81). This confirms that skin conductance to the CS+ increased during conditioning as compared to habituation and that this effect was similar for both tDCS Timing Groups.
In a second linear mixed effects model we further examined adequate conditioning to both CS+ during this phase (CS+ > CS−). For this analysis we excluded the first trial to limit an effect due to an orienting response. Result showed that skin conductance values for the three CS differed significantly (F(2,900) = 38.84, p < 0.0001), which was due to a significantly higher skin conductance to either CS+ (CS+ 1: 0.27; CS+ 2: 0.30; p = 0.49). These data confirm the successful and equal conditioning to both CS+. Moreover, there were no significant tDCS Timing Group differences (F(1,39) = 0.39, p = 0.53), indicating no evidence of differences in levels of fear conditioning between the two groups. The magnitude of skin conductance reactivity decreased over trials (F(20,800) = 11.71, p < 0.0001) (see Fig. 3A).
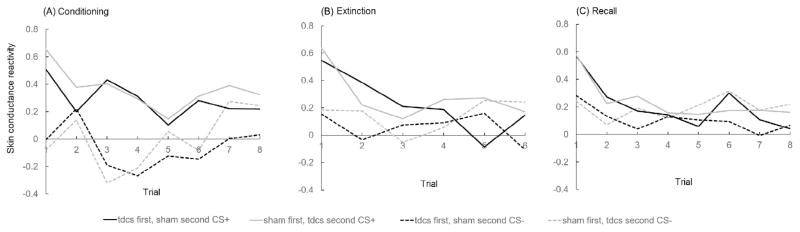
Figure 3.
Normalized skin conductance values for CS+ and CS− trials over time for conditioning (A), extinction (B), and recall (C) separated by tDCS Timing Group.
Extinction – day 1
An initial linear mixed model was performed to examine effects of Stimulus Type (CS−; CS++DC; CS+-DC); tDCS Timing Groups; Trial Order; and the interaction between tDCS Timing Group and Trial Order. The addition of this interaction was specifically done to test our hypothesis that changes in skin conductance magnitude over extinction trials may differ for tDCS Timing Groups. Results showed a significant main effect of Stimulus Type (F(2,695) = 8.78, p = 0.0002) and skin conductance was higher for both CS+-DC (0.21) and CS++DC (0.31) versus CS− (0.098) across all extinction trials. We further observed a significant main effect of Trial Order (F(17,663) = 3.97, p = < 0.0001) and a non-significant main effect of tDCS Timing Group (F(1,39) = 0.56, p = 0.46), suggesting that magnitude of skin conductance reactivity decreased over trials and that skin conductance across all trials was similar for both tDCS groups. However, we also observed a borderline significant Trial Order*tDCS Timing Group interaction (F(17,663) = 1.58, p = 0.06), suggesting that reduction in skin conductance reactivity over all extinction trials differed marginally between tDCS Groups. This borderline significance is not surprising; we explicitly hypothesized that the effects of tDCS should be most apparent during late extinction when (at least some) extinction learning had taken place.
Given our explicit hypothesis of tDCS on late CS+ extinction, we repeated the above-mentioned linear mixed model selecting CS+ (+DC and − DC) only to examine the effect of tDCS as learning continued over late extinction trials including the variables tDCS Timing Group, Trial Order, and the interaction tDCS Timing Group*Trial Order as predictors. Results showed non-significant main effects of tDCS Timing Group (F(1,39) = 1.95, p = 0.17) and Trial Order (F(5,195) = 0.82, p = 0.54), but a significant interaction between tDCS Timing Group and Trial Order (F(5,195) = 2.47, p = 0.034). This suggests that the reduction in skin conductance magnitude over CS+ (+DC/−DC) late extinction trials differed significantly between tDCS groups, and thus shows a temporal order effect of tDCS application as can also be seen in Fig. 3B (late extinction are trials 4–6). Post-hoc general linear model analyses to disentangle this finding showed that skin conductance continued to marginally decrease across all late extinction trials in participants who received active tDCS during the first extinction block (F(5,110) = 1.93, p = 0.09). There was no change in skin conductance magnitude across all late extinction trials for participants who received active tDCS during the second extinction block (F(5,85) = 1.42, p = 0.23). Furthermore, we observed a borderline significant difference between tDCS Timing Group during second block CS+ late extinction only (F(1,39) = 3.37, p = 0.07), with lower skin conductance values for participants who received tDCS during the first extinction block (0.06) versus participants who received tDCS during the second extinction block (0.33).
In order to determine whether the observed significant tDCS*Trial Order interaction for late extinction trials was specific for late extinction as we would expect, we repeated the above linear mixed effects model selecting early extinction CS+ trials only (early extinction trials were thus defined by taking the first three trials out of six of each tDCS extinction block). Results showed a significant main effect of Trial Order (F(5,195) = 4.78, p < 0.001), suggesting that skin conductance values decreased over trials. No significant main effect of tDCS Timing Group (F(1,39) = 0.21, p = 0.65) and no significant tDCS Timing Group*Trial Order interaction was observed (F(5,195) = 0.88, p = 0.50), demonstrating no effect of tDCS on learning across early extinction trials.
Recall – day 2
Five participants did not return to complete Day 2 and data collection of one participant was unsuccessful due to equipment failure. We examined whether (a decline in) skin conductance reactivity across the first four early recall trials of both CS+ and CS− differed for the two tDCS Timing Groups using a linear mixed model including the variables tDCS Timing Group, Trial order, and the tDCS Timing Group*Trial Order interaction as predictors. Results showed a non-significant main effect of tDCS Timing Group (F(1,33) = 0.05, p = 0.82), but significant main effects of Stimulus Type (F(2,383) = 5.20, p = 0.006) with higher values for both CS+ (0.26 and 0.34) versus CS− (0.15) and Trial Order (F(11,363) = 7.01, p < 0.0001). The interaction between tDCS Timing Group and Trial Order was non-significant (F(11,363) = 0.52, p = 0.89). These data suggest a return of fear response during early recall (CS+ > CS−) with magnitude of this response diminishing over recall trials, but no effect of tDCS on recall (see Fig. 3C).
Discussion
Our results on late extinction showed that participants who received active tDCS during the first extinction block (and sham during the second extinction block) continued to display significantly lower skin conductance reactivity during late extinction of the second extinction block in comparison to participants who received sham during the first extinction block (and tDCS during the second extinction block). Despite a tDCS modulation on second block late extinction learning, there were no effects of (timing of) tDCS on extinction recall as evidenced by a lack of significant tDCS timing group differences on magnitude of skin conductance over early recall trials. This was surprising as vmPFC activation during extinction learning has been shown to predict success of extinction recall [5–7].
Our observation of a continued reduction in skin conductance during late extinction of the second extinction block for those who received active tDCS during the first extinction block could be due the presence of tDCS aftereffects. These tDCS aftereffects could have had a brief anxiolytic effect. Prior research has shown that tDCS aftereffects can be expected to last for at least as long as the duration of stimulation and potentially longer [33], although it is likely that any tDCS aftereffects had dissipated 24 hours after tDCS offset during the assessment of extinction recall. Thus, participants who received active tDCS during the first extinction block and sham during the second extinction block most likely continued to be affected by tDCS aftereffects during the second extinction block. If tDCS aftereffects resulted in a brief anxiolytic effect, we would not necessarily expect tDCS to impact recall the next day, which indeed we did not observe. In fact, Milad et al. [13] observed possibly similar anxiolytic effects of electrical stimulation prior to stimuli presentation in rats despite a rather different stimulation paradigm.
In contrast, participants who received sham during the first extinction block and active tDCS during the second extinction block did not appear to benefit from active tDCS during late extinction. However, a close examination of the results may suggest that participants receiving active tDCS during second block extinction learning show higher skin conductance magnitude during late extinction in the second block. It can be argued that the skin sensation associated with active tDCS could have resulted in increased levels of psychophysiological arousal in participants who received tDCS during the second extinction block. This is however not supported by the absence of significant skin conductance differences during early extinction trials or elevated skin conductance magnitude during the first extinction block for participants who received active tDCS at that time. If the physical sensation of active tDCS elicits higher levels of skin conductance, one would expect this effect to be most apparent at tDCS onset when people are not yet habituated to the sensation. Moreover, our experimental design included five minutes of active tDCS (or sham) before the onset of extinction learning to allow habituation to the physical sensations associated with tDCS.
A more likely explanation is that tDCS for participants receiving active stimulation during the second extinction block may have impaired extinction learning. This is based on a lack of decline in skin conductance magnitude late extinction trials in both extinction blocks for this group. Magnitude of skin conductance did show a marginal continued reduction during late extinction over both extinction blocks in participants who received active tDCS during the first extinction block. This suggests that augmentation of late extinction due to tDCS aftereffects might somewhat better explain the tDCS group differences than tDCS-induced extinction impairments, although both appear to be present.
Although the exact physiological basis of tDCS is not yet fully understood, indirect data show that anodal tDCS and its aftereffects depend on synaptic modulation involving intracortical neurons resembling LTP-like plasticity affecting neural networks [34–36]. A growing body of human tDCS studies suggests that the aftereffects of anodal tDCS appear to be driven by N-methyl-D-aspartate (NMDA) receptor modulation [37–39]. Such synaptic change, or plasticity, is the signal to drive learning and is relevant to extinction [40,41]. Studies aimed to evaluate the mechanism of action of tDCS-augmented fear extinction will be crucial. For instance, previous studies have shown that neuronal modulation after tDCS might be non-linear and in fact has opposite effects [42]. Reversed tDCS aftereffects were particularly pronounced after 2 mA cathodal tDCS and resulted in excitability instead of inhibition [43], although reversed aftereffects have also been observed after 1 mA anodal tDCS [44]. We explicitly tried avoiding prefrontal (after)effects of cathodal stimulation by placing the cathodal electrode over the right mastoid process.
Our observation that tDCS appears to affect extinction learning is particularly interesting in light of previous research demonstrating tDCS to affect fear memory. More specifically, Asthana et al. [19] showed that the application of 1 mA inhibitory cathodal tDCS over the left dorsolateral prefrontal cortex (dlPFC) after fear conditioning resulted in lower skin conductance responses to the conditioned stimulus during fear extinction 24 hours later. Moreover, Mungee et al. [20] demonstrated that the application of 1 mA excitatory anodal tDCS over the right dlPFC after participants were reminded of a previously conditioned fear stimulus resulted in higher skin conductance responses to conditioned stimuli as compared to participants who had received sham stimulation. Further support for the ability of tDCS to influence threat processing comes from two recent studies. Ironside et al. [45] demonstrated that 20 minutes of 2 mA anodal left dlPFC - cathodal right dlPFC tDCS reduced threat sensitivity as measured with a short duration (100 ms) dot-probe task to fearful faces. A second study by Clarke et al. [46] reported that 1 mA anodal left dlPFC tDCS for about 17 minutes enhanced attentional threat bias consistent with the direction of attention (toward or away). Taken together with our findings, tDCS appears to be able to modulate various fear and threat related learning processes.
There are several important differences to note between our study and the studies cited above [19,20,45,46]. First, prior studies aimed to stimulate the dlPFC, whereas we aimed to stimulate the vmPFC because of its role in extinction learning. In order to best target the vmPFC we applied the anode over EEG coordinate AF3 and the cathode over the contralateral mastoid process. tDCS does not typically penetrate deeply into the brain and directly stimulating the vmPFC can be challenging using a conventional 1 × 1 unilateral electrode tDCS montage. The selection of the contralateral mastoid as reference point, instead of the contralateral supraorbital region, dlPFC [47] or a bipolar balanced montage [45] was done to reach the less superficially deeper brain structures such as the vmPFC and avoid cathodal effects on the prefrontal cortex. However, this may have resulted in a less focal current distribution and reduced intensity of tDCS aftereffects [29], although we applied a 2 mA intensity to counteract such reduction in intensity. Furthermore, we cannot rule out possible tDCS modulation of other brain regions, including the dlPFC. The dlPFC has been associated with the cognitive strategy of explicit fear regulation, whereas the vmPFC has been linked to extinction learning [48,49].
Neuroimaging data show that the dlPFC has reciprocal connections to the mPFC [50,51] and activity in the dlPFC can modulate activity in the mPFC [52,53]. Nonetheless, this inherent disadvantage might in fact be beneficial as it increases the likelihood that (multiple) nodes of targeted circuitry may be affected for potential neuropsychiatric treatment [54]. For instance, transcranial magnetic stimulation over the left dlPFC appears to influence subgenual anterior cingulate for the treatment of depression [55].
Other important differences between prior studies on tDCS-induced fear modulation [19,20] and ours are timing of tDCS in relation to the task as well as experimental conditioning procedures. Asthana et al. [19] and Mungee et al. [20] focused on modulating the consolidation or reconsolidation of the conditioned fear memory respectively, instead of modulating novel learning associated with extinction. Moreover, we partially used a so-called ‘online’ tDCS protocol, in which stimulation is combined with a particular learning procedure such as extinction similar to Clarke et al. [46]. This is relevant given the modulatory nature of tDCS [17] and careful manipulation of what the brain does during stimulation might be just as vital as the stimulation itself. ‘Online’ tDCS might therefore produce different results as compared to ‘offine’ tDCS protocols [19,20,45].
Some limitations and deviations from previous extinction protocols should be mentioned. The extinction phase of our protocol purposely included only six trials per CS+ and was thus designed in a suboptimal manner as compared to the more typical average of 10–15 extinction trials [6,9,56]. The rationale for suboptimal extinction was to allow tDCS to have an augmentation effect on extinction learning while preventing ceiling effects due to over-learning. It is possible this extinction procedure was not robust enough to see any tDCS-related effects on extinction recall and we observed a return of fear across recall trials based on larger skin conductance magnitude for extinguished CS+ versus CS−. Future research might want to examine whether tDCS or its aftereffects in combination with full extinction learning will affect extinction recall the next day. In addition, the use of a within-subjects design in which extinction of one CS+ was paired with active tDCS and extinction of the other CS+ paired with sham may have further limited effects of tDCS on extinction and subsequent recall. This design allowed comparing active tDCS vs. sham during the first extinction block and tDCS aftereffects vs. active tDCS during a second extinction block. A comparison between tDCS aftereffects vs. sham on extinction learning was however not possible, which in hindsight of our results would have been interesting.
Nonetheless, our findings support further exploring the potential clinical value of tDCS for anxiety and stress disorders, such as obsessive–compulsive disorder and posttraumatic stress disorder, following tDCS research for depression [57]. Although more research is needed on stimulation timing, effects of multiple stimulation sessions, and the mechanisms of action, the potential of tDCS to affect a clinically relevant cognitive process such as fear extinction is promising. Especially given the relative inexpensiveness, minimal associated risk, high portability and limited disruptive sensation during stimulation, tDCS lends itself well to be combined with cognitive–behavioral treatments including exposure-based therapies.
Conclusion
Our results show that the application of tDCS applied during extinction learning of an initial CS could potentially have anxiolytic aftereffects on late extinction learning of a future, second CS. This result is cautiously promising for clinical tDCS applications to facilitate treatment for anxiety and traumatic stress related disorders, such as PTSD. Future studies should examine (the timing of) tDCS-augmentation during extinction as well as its effects on more therapeutically relevant stimuli in clinical populations.
Acknowledgments
We would like to thank Jason Kirschner and Palmira Angelova for assistance with recruitment and data collection. This work was supported by a NARSAD Young Investigator Grant (17821) to partially support salary to MvtW. This funding source had no involvement in study design, collection, analysis and interpretation of data, writing and submission of the manuscript for publication. This material is the result of work supported with resources and the use of facilities at the Providence VA Medical Center, Providence, RI, USA.
References
- 1.Pitman RK, Orr SP. Test of the conditioning model of neurosis: differential aversive conditioning of angry and neutral facial expressions in anxiety disorder patients. J Abnorm Psychol. 1986;95:208–13. doi: 10.1037//0021-843x.95.3.208. [DOI] [PubMed] [Google Scholar]
- 2.Eysenck HJ. The conditioning model of neurosis. Behav Brain Sci. 1979;2:155–99. [Google Scholar]
- 3.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–64. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebrón K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–8. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- 6.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–11. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–23. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant DA, Hake HW, Hornseth JP. Acquisition and extinction of a verbal conditioned response with differing percentages of reinforcement. J Exp Psychol. 1951;42:1–5. doi: 10.1037/h0054051. [DOI] [PubMed] [Google Scholar]
- 12.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 13.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–94. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 14.Marin MF, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depress Anxiety. 2014;31:269–78. doi: 10.1002/da.22252. [DOI] [PubMed] [Google Scholar]
- 15.Marin MF, Milad MR. Neuromodulation approaches for the treatment of post-traumatic stress disorder: stimulating the brain following exposure-based therapy. Curr Behav Neurosci Rep. 2015;2:67–71. [Google Scholar]
- 16.Milad MR, Rosenbaum BL, Simon NM. Neuroscience of fear extinction: implications for assessment and treatment of fear-based and anxiety related disorders. Behav Res Ther. 2014;62:17–23. doi: 10.1016/j.brat.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Tremblay S, Lepage JF, Latulipe-Loiselle A, Fregni F, Pascual-Leone A, Théoret H. The uncertain outcome of prefrontal tDCS. Brain Stimul. 2014;7:773–83. doi: 10.1016/j.brs.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asthana M, Nueckel K, Mühlberger A, Neueder D, Polak T, Domschke K, et al. Effects of transcranial direct current stimulation on consolidation of fear memory. Front Psychiatry. 2013;4:107. doi: 10.3389/fpsyt.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mungee A, Kazzer P, Feeser M, Nitsche MA, Schiller D, Bajbouj M. Transcranial direct current stimulation of the prefrontal cortex: a means to modulate fear memories. Neuroreport. 2014;25:480–4. doi: 10.1097/WNR.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 21.Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–64. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 22.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 25.Coulbourn Inc, Allentown, PA.
- 26.Nasseri P, Nitsche MA, Ekhtiari H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum Neurosci. 2015;9:54. doi: 10.3389/fnhum.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soterix Medical Inc., New York, NY, USA.
- 28.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8:046011. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- 29.Moliadze V, Antal A, Paulus W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin Neurophysiol. 2010;121:2165–71. doi: 10.1016/j.clinph.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–8. [PubMed] [Google Scholar]
- 31.IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; Released 2013. [Google Scholar]
- 32.McLaughlin NC, Strong D, Abrantes A, Garnaat S, Cerny A, O’Connell C, et al. Extinction retention and fear renewal in a lifetime obsessive–compulsive disorder sample. Behav Brain Res. 2015;280:72–7. doi: 10.1016/j.bbr.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 34.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 35.Merzagora AC, Foffani G, Panyavin I, Mordillo-Mateos L, Aguilar J, Onaral B, et al. Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage. 2010;49:2304–10. doi: 10.1016/j.neuroimage.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 36.Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, et al. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci. 2013;33:11425–31. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 38.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex. 2004;14:1240–5. doi: 10.1093/cercor/bhh085. [DOI] [PubMed] [Google Scholar]
- 40.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–63. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–80. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Bestmann S, de Berker AO, Bonaiuto J. Understanding the behavioural consequences of noninvasive brain stimulation. Trends Cogn Sci. 2015;19:13–20. doi: 10.1016/j.tics.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amadi U, Allman C, Johansen-Berg H, Stagg CJ. The homeostatic interaction between anodal transcranial direct current stimulation and motor learning in humans is related to GABA A activity. Brain Stimul. 2015;8:898–905. doi: 10.1016/j.brs.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ironside M, O’Shea J, Cowen PJ, Harmer CJ. Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.012. < http://dx.doi.org/10.1016/j.biopsych.2015.06.012>. [DOI] [PubMed]
- 46.Clarke PJ, Browning M, Hammond G, Notebaert L, MacLeod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation. Biol Psychiatry. 2014;76:946–52. doi: 10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Chib VS, Yun K, Takahashi H, Shimojo S. Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Transl Psychiatry. 2013;3:e268. doi: 10.1038/tp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–46. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–75. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 51.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 52.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 53.Goel V, Dolan RJ. Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. Neuroimage. 2003;20:2314–21. doi: 10.1016/j.neuroimage.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Kuo MF, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage. 2014;85:948–60. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- 55.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–52. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 57.Brunoni AR, Valiengo L, Baccaro A, Zanao TA, de Oliveira JF, Goulart A, et al. The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–91. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]