Abstract
Mood processes are theorized to play a role in the initiation and progression of smoking behavior. Available work using real-time assessments in samples of young smokers, including several reports from the Social and Emotional Contexts of Adolescent Smoking Patterns (SECASP) study, has indicated that smoking events acutely improve mood and that escalating smoking frequency may stabilize mood. However, prior analyses have not specifically evaluated within-person change in nicotine dependence, which is conceptually distinguishable from frequent smoking and may be associated with unique mood consequences. The current investigation addressed this question using data from 329 adolescent SECASP participants (9th or 10th grade at recruitment) who contributed mood reports via ecological momentary assessment in up to four 1-week bursts over the course of 24 months. Mixed-effects location-scale analyses revealed that within-person increases in scores on the Nicotine Dependence Syndrome Scale were associated with elevations in negative mood level and increased variability of both positive and negative moods. These effects remained when within-person changes in smoking frequency were covaried and were not fully attributable to a subgroup of youth who rapidly escalated their smoking frequency over time. The findings indicate that adolescents tend to show increasing levels of positive mood states, decreasing levels of negative mood, and diminishing mood variability between ages 16 to 18, but progression of nicotine dependence may counteract some of these developmental gains. Emergence of withdrawal symptoms is a likely explanation for the adverse mood effects associated with dependence progression.
Keywords: adolescence, nicotine dependence, mood, ecological momentary assessment, measurement burst design
Smokers routinely cite mood management among their top motives for cigarette use (e.g., Fidler & West, 2009; Piasecki, Richardson & Smith, 2007) and theorists have frequently looked to mood-related processes to help account for the acquisition and progression of smoking behavior (Baker, Brandon, & Chassin, 2004; Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Eissenberg, 2004; Glautier, 2004; H. Leventhal & Cleary, 1980; Solomon & Corbit, 1973; Tomkins, 1966). This basic notion that mood processes play a role in smoking has generated a voluminous and complex literature. In an authoritative review, Kassel, Stroud, and Paronis (2003) pointed out that existing theory actually contains a number of variations on the central theme, and that these generate an array of testable between- and within-subject hypotheses concerning mood-smoking relations. Further, they highlighted the importance of considering the stage of smoking as a potential moderator because the motivational underpinnings of smoking may shift or evolve over the smoking career. Thus, improving our understanding of the mood-smoking relation requires careful experimental and field studies probing focused, developmentally specific questions (Kassel, et al., 2003).
Ecological momentary assessment (EMA; Shiffman, Stone & Hufford, 2008) methodology is well suited to characterizing associations between mood features and youth smoking behaviors. In EMA research, participants are asked to repeatedly report on their immediate experiences as they go about their daily activities in their natural environments. This strategy yields ecologically valid, representative samples of participants' affective experiences that are free from many of the errors and heuristic biases that can influence global, retrospective self-reports (Piasecki, Hufford, Solhan, & Trull, 2007; Shiffman, 2009). Because EMA typically results in a large number of repeated observations from each participant, this strategy also permits the variability in mood states to be quantified (Hedeker, Mermelstein, Berbaum, & Campbell, 2009). This is important because mood level and mood instability may have separable relations with smoking behavior (Weinstein, Mermelstein, Shiffman, & Flay, 2008; Weinstein & Mermelstein, 2013a). Much of the available EMA evidence addressing links between mood states and adolescent smoking comes from the Social and Emotional Contexts of Adolescent Smoking Project (SECASP), a large-scale observational study of Chicago-area adolescents selected to represent varying levels of smoking involvement at recruitment, when participants were in 9th or 10th grade. A subsample of the SECASP cohort participated in a multi-wave EMA sub-study involving four 1-week periods of EMA recording spanning 24 months.
Some existing EMA investigations have used cross-sectional designs (i.e., data from a single period of EMA recording), examining mood processes with reference to contemporaneous, between-person differences in self-reported typical smoking rate. Analyses of EMA data from current smokers collected at the baseline observation wave in SECASP indicated that smoking events were associated with immediate elevations in positive mood and decreases in negative mood, but these effects were smaller among youth who smoke more frequently (Hedeker, et al., 2009). Another analysis of EMA data at the baseline wave in SECASP found that, compared to nonsmokers, young smokers reported lower mean levels of positive mood, higher mean levels of negative mood, and more variable negative moods (Pugach, Hedeker, Richmond, Sokolovsky, & Mermelstein, 2014). Again, these effects were diminished among heavier users, such that the most frequent smokers' mood levels and variability were equivalent to those of nonsmokers (Pugach, et al., 2014).
A few investigations have examined data from discrete periods of EMA, collected apart in time, to examine how transitions in adolescent smoking behaviors are related to changes in mood processes. Weinstein, Mermelstein, Shiffman & Flay (2008) examined EMA data collected during three 1-week periods over the course of one year in a sample of 8th and 10th graders. Mood variability was indexed at each wave by computing the within-person standard deviation of all available EMA negative mood ratings. Retrospective calendar-based reports of smoking behaviors were used to identify discrete trajectories of smoking frequency over the yearlong study period and to classify participants into trajectory groups. Youth who belonged to trajectory groups characterized by escalating smoking frequency showed higher negative mood variability at baseline compared to other groups. These `escalators' also showed decreases in negative mood variability over time. In contrast, negative mood variability remained stable among youth who either remained infrequent smokers or who smoked frequently over the entire study period. These findings suggest that negative mood variability, presumably an indicator of mood dysregulation, is a risk for smoking escalation, and that negative reinforcement in the form of mood stabilizing effects may contribute to the maintenance of frequent smoking.
Weinstein & Mermelstein (2013a, 2013b) applied a similar approach to EMA mood data collected at baseline and at 15 months in SECASP. Youth were classified into 7 trajectory groups based upon various thresholds of self-reported smoking frequency (e.g., monthly, weekly, daily) at the two time points. A group of `rapid escalators' progressed from infrequent use at baseline to daily or near-daily use 15 months later. Analyses of aggregated EMA negative mood reports comparing these rapid escalators to youth with other smoking trajectories revealed a complex pattern of findings that differed by gender. Among girls, negative mood variability at baseline was associated with rapid escalation. Planned contrasts between rapid escalators and girls who consistently smoked over time revealed a group × time interaction; negative mood variability decreased significantly over time among the rapid escalators, but remained stable in the regular smokers. Among boys, higher mean levels of negative mood at baseline were associated with escalating smoking frequency. Boys who rapidly escalated smoking showed larger decreases in mean levels of negative mood over time compared to boys who either smoked consistently or experimented with smoking during the observation period.
Taken together, the existing EMA evidence seems to support a dose-dependent self-medication or stress/coping account of early smoking. That is, adverse mood seems to confer risk for smoking experimentation, but the mood-smoking association dissipates as smoking becomes more frequent, perhaps reflecting more complete pharmacologic management of negative affect. However, the immediate mood-enhancing consequences of smoking also diminish with more frequent smoking, suggesting tolerance to these effects may develop with repeated use.
Existing studies have largely examined the correlates of smoking frequency rather than the pathological state of nicotine dependence. These two constructs are correlated, but not identical -- tobacco use is a prerequisite for nicotine dependence, but individual differences in vulnerability to dependence are important as well (Belsky, et al., 2013; Maes, et al., 2004; Pomerleau, Collins, Shiffman, & Pomerleau, 1993). Accordingly, one can identify light smokers with symptoms of dependence and regular smokers who do not experience dependence symptoms (Dierker, Donny, Tiffany, Colby, Perrine & Clayton, 2007; Donny & Dierker, 2007; Zhan, Dierker, Rose, Selya, & Mermelstein, 2012; Rubenstein, Rait, Sen, & Shiffman, 2014; Shiffman, Tindle, Li, Scholl, Dunbar, & Mitchell-Miland, 2012). Nicotine dependence requires separate scrutiny because it is theorized to entail compensatory neuroadaptations to chronic nicotine exposure that contribute to the emergence of withdrawal symptoms between cigarettes (Baker, et al., 2012; Benowitz, 2010; Kassel, Evatt, Greenstein, Wardle, Yates, & Veilleux, 2007; Parrott, 1999), and may lead to a dysregulation of brain reward and stress systems (Epping-Jordan, Watkins, Koob, & Markou; Koob & LeMoal, 2008).
The current investigation sought to identify mood features that are uniquely related to the within-person progression of nicotine dependence. We analyzed EMA reports from SECASP participants collected during four 7-day bursts occurring at baseline, 6-, 15-, and 24-months. Participants also completed questionnaires assessing nicotine dependence and past-month smoking frequency at each study wave. A mixed-effects location scale analysis permitting simultaneous investigation of mood level and variability (Hedeker, Mermelstein, & Demirtas, 2008) was used to test the mood correlates of within-person changes in nicotine dependence. Based on theories suggesting drug dependence has adverse effects on mood (e.g., Koob & LeMoal, 2008; Parrot, 1999), we hypothesized that the progression of nicotine dependence would be associated with lower positive mood, higher negative mood, and elevated mood instability. We also investigated whether effects of within-person changes in nicotine dependence remained after accounting for between- and within-person differences in smoking frequency or membership in a group of `rapid escalators' who substantially increased their smoking frequency over the course of the study.
Method
Participants
Participants were part of the SECASP project, a longitudinal, observational study of adolescent smoking behaviors. Portions of the data from this study have been used in prior reports examining mood-smoking associations (Hedeker, et al., 2008; Hedeker, et al., 2009; Hedeker & Mermelstein, 2012; Pugach, et al., 2014; Weinstein & Mermelstein, 2013a; Weinstein & Mermelstein, 2013b), but none of these has specifically tested effect of within-person changes in nicotine dependence. All 9th and 10th graders in 16 Chicago-area high schools (N = 12,970) were screened for smoking behavior, and responses were used to form a longitudinal cohort (N = 1,263) involving participants with varying levels of smoking experience.
The current investigation used data from youth who participated in an EMA sub-study (N = 461). From these, we retained for analysis 329 individuals (71%) who (a) reported smoking in the past 30 days at one or more waves, and (b) completed EMA recordings and the nicotine dependence scale for at least two waves. These selection criteria were imposed to ensure that the analyzed participants could contribute information regarding within-person changes in dependence, smoking rate, and mood. All participants completed EMA recording at baseline, and more than 80% of the sample contributed EMA data at subsequent waves (6 months: n = 302, 92%; 15 months: n = 269, 82%; 24 months: n = 289, 88%). Of the 329 participants included in the analyses, 102 (31%) reported having smoked in the past 30 days at all 4 waves, 78 (24%) smoked at 3 waves, 67 (20%) smoked at 2 waves, and the remainder (82, 25%) reported past-month smoking at a single wave. The sample contained 189 females (57%). The racial composition was as follows: White (n = 245, 75%), African American (n = 51, 16%), Native American (n = 6, 2%), Asian (n = 5, 1.5%), Native Hawaiian/Pacific Islander (n = 5, 2%), and more than one category (n = 17, 16%). Hispanic ethnicity was reported by 64 participants (20%). At baseline, participants' mean age was 15.7 years (SD = .59; range = 14.4 to 17.2).
Procedure
Participants in the EMA sub-study were invited to participate in diary monitoring at baseline and at 6, 15, and 24 months post-baseline. At each wave, participants received non-EMA questionnaires approximately one week prior to their first day of carrying the EMA device and handed in the questionnaire at the first day of EMA training for each wave. Participants were paid $20 for questionnaire completion at the baseline, 6-, and 15-month waves, and $40 at the 24-month wave. Participants also were paid for completion of each EMA wave, escalating from $50 at baseline to $70 at 24 months.
During periods of EMA monitoring, participants carried palmtop computers programmed to serve as electronic diaries for 7 days. The diaries audibly prompted participants to make an entry at random moments up to five times per day. Compliance with the prompts averaged between 68% complete at baseline to better than 85% at subsequent waves. Additionally, participants were instructed to initiate a diary recording whenever they (a) smoked a cigarette, (b) had the opportunity to smoke but elected not to do so, and (c) wanted to smoke but could not do so at that time. The main analyses included mood ratings from 41,931 diary reports. Of these, 12,033 (28.7%) reports were recorded at the baseline phase, 10,251 (24.4%) at the 6-month wave, 9,250 (22.1%) at 15 months, and 10,397 (24.8%) at the 24-month assessment. Most records (35,658, 85%) were from prompted assessments, with 3,930 (9%) from smoking events, 1,033 (3%) from moments the adolescent chose not to smoke, and 1,310 (2%) were from moments when the adolescent could not smoke.
Measures
Diary-Assessed Mood
In each diary assessment, participants were asked to describe how they felt “just before the signal” (random prompts) or “right now” (user-initiated entries) using 19 adjectives rated on a 1–10 Likert scale. On the basis of a preliminary factor analysis of baseline random prompt data, then replicated in other record types, composite scales were computed indexing positive mood (average score on happy, relaxed, cheerful, confident, and accepted by others) and negative mood (average score on frustrated, angry, stressed, irritable, and sad). Coefficient alpha for these scales were consistently good across waves of EMA assessment (positive mood α = .86 to .87; negative mood α = .94 to .95).
Nicotine Dependence
At each wave of assessment, a gating question asked whether they had ever smoked at least one puff of a cigarette. Those who answered “yes” were administered a 10-item version of the Nicotine Dependence Syndrome Scale (NDSS; Shiffman, Waters, & Hickcox, 2004) optimized for young smokers (Sterling, Mermelstein, Turner, Diviak, Flay, & Shiffman, 2009). The items retained for the modified NDSS primarily represent the Drive and Tolerance scales from the original instrument. Participants responded to each of the items on a 4-point Likert scale ranging from 1 (not at all true) to 4 (very true). Two items included an additional response option, scored 0, allowing the respondent to indicate that the premise of the question did not apply (e.g., respondent could not report on improved function after morning smoking because s/he did not smoke in the morning). Responses across the 10 items were averaged, yielding a total score that could range from 0.8 to 4.0. In the analyzed subsample, internal consistency was excellent for the NDSS (αs = .93 to .95 across waves).
Smoking Frequency
At each wave of assessment, participants were asked to report the number of days smoked in the past 30 days using a 9-level response metric, scored as follows for the current analyses: 0 days (scored 0), 1 day (1), 2 to 3 days (2), 4 to 5 days (3), 6 to 7 days (4), 8 to 10 days (5), 11 to 20 days (6), 21 to 29 days (7), and all 30 days (8).
Following Weinstein & Mermelstein (2013a, 2013b), we also used responses to this question at baseline and 24 months to identify a group of rapid escalators whose smoking frequency increased sharply over the study period. To be classified as a rapid escalator, a participant had to report smoking 0–5 of the past 30 days at baseline and 11–30 of the past 30 days at 24 months. Forty-three participants (13.1%) met these criteria.
Data Analysis
A preliminary set of multilevel regression analyses were estimated to evaluate the degree of within-person variation in nicotine dependence during the 24-month observation period (approximately ages 16–18) and characterized overall trends in NDSS scores over time
A mixed-effects location scale analysis was used to model mood ratings (Hedeker, et al., 2008). This is an extension of the basic multilevel regression analysis to include a log-linear submodel for the error variance, permitting tests of the influence of covariates on both the mean and variance structures. The location-scale mixed model allows the error variance to be modeled in terms of covariates using a log link function, which is identical to the link function used in Poisson regression to ensure a positive count outcome from the estimated model. Similar to what is done in Poisson regression, we calculated variance ratios (VRs) by exponentiating the estimated coefficients (note that in Poisson regression, the exponentiated coefficients represent count ratios) to represent the covariate effects on the WS variance. These represent the ratio of within-subject variance per unit change of the covariate. A VR greater (lesser) than one represent increased (decreased) variability with increased levels of the covariate.
Due to the nesting of observations within waves and multiple waves within subjects, three-level models (Level 1 = moments, Level 2 = assessment wave, Level 3 = person) were estimated using PROC NLMIXED in SAS (see Hedeker & Mermelstein, 2012; sample syntax is available from the 2nd author on request). The corresponding three variances of the model were therefore between-subjects (BS), within-subjects between-waves (WS-BW) and within-subjects-within-waves (WS-WW). The models included random effects for the mean at the person and wave levels, and a random subject effect for the WS-WW variance. The two subject-level random effects (mean and WS-WW variance) were allowed to be correlated.
Submodels were estimated for mood level and WS-WW mood variance. We used two nicotine dependence variables in each submodel: one indexing between-person differences (the person-mean on the NDSS across all waves of assessment) and one isolating the effect of intra-individual change (deviations of the NDSS score at each wave from the individual's overall mean across waves). The within-person NDSS deviation score was the focal predictor in each submodel. The following covariates were included in all submodels: gender (male coded 1, female coded 0), grade level at baseline (10th grade coded 1, 9th grade coded 0), and time (baseline = 0, 6 months = .25, 15 months = .63, 24 months = 1).
Existing EMA work has explored how mood experiences are associated with smoking frequency. The NDSS contains items tapping smoking heaviness. Not surprisingly, past-month reports of smoking frequency were strongly correlated with nicotine dependence scores at each wave of assessment (rs = .75 to .80, ps < .001). To evaluate whether NDSS scores contribute incremental information relative to simple measures of recent smoking behavior, we estimated a second set of models that included between- and within-person effects for past-month smoking frequency as additional covariates.
Conceivably, including diary data from user-initiated reports tied to smoking events and occasions where smoking was resisted or was desired but not possible might bias the analyses1. To investigate whether this affected the obtained results, each set of models was re-estimated after limiting the data to mood reports provided in random prompts only.
Prior studies suggested that rapid escalation of smoking frequency during adolescence may be associated with improved mood (Weinstein, et al., 2008; Weinstein & Mermelstein, 2013a, 2013b). Notably, prior SECASP analyses found that rapid escalators' nicotine dependence scores increased as their smoking became more frequent (Weinstein & Mermelstein, 2013a; 2013b), suggesting any mood effects of within-person changes in dependence might be driven by this subgroup of individuals. To investigate this, we conducted supplementary location-scale analyses of positive and negative moods comparing rapid escalators to the remaining adolescents. Submodels were again estimated for mood level and WS-WW mood variance, incorporating gender, grade at baseline, and time as covariates. In these models, we included a dichotomous indicator of rapid escalator status (1 = yes, 0 = no) and a Rapid Escalator x Time interaction term. We then expanded these models to include between- and within-person NDSS differences to test whether within-person changes in dependence contributed incremental information after accounting for rapid escalator effects.
Results
Preliminary Analyses
The major goal of the analyses was to characterize mood effects related to within-person variation in nicotine dependence scores. Meaningful tests of this question require that there be evidence for appreciable within-person changes in nicotine dependence in the analytic sample. We evaluated this using a two-level (Level 1 = wave, Level 2 = participant), random intercept, unconditional means mixed model (i.e., a model with no predictors) with NDSS score as the dependent measure. This model permitted computation of the intraclass correlation coefficient (ICC), which was .68. This indicated that the majority of the total variance in NDSS scores (68%) was attributable to between-person differences, but approximately one third of the total variance (32%) was due to intra-individual differences. Expanding this model to include a linear time trend revealed a significant effect (b = 0.18, p < .001), indicating that dependence scores tended to increase slightly over the course of the study. Including a random slope for time indicated there was significant heterogeneity in the within-person trajectories of NDSS scores over time (variance = 0.29, SE = 0.05, Wald z = 6.10, p < .001).
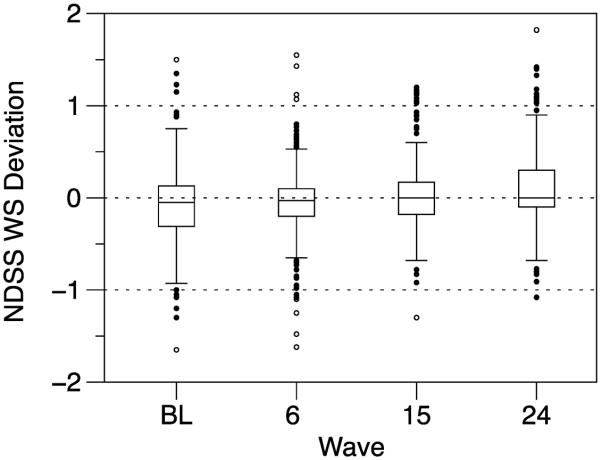
Figure 1 depicts boxplots showing the distributions of individual's deviations from his or her person-mean at each wave. These plots reveal that individuals' dependence scores changed substantially in both directions over time. Consistent with the overall trend toward increasing dependence scores over time, the distributions of within-subject deviations shifted subtly such that median deviations tended to be slightly lower at early vs. later waves. However, there was substantial heterogeneity of within-person deviations at every wave.
Figure 1.
Distributions of within-person deviations on the NDSS at each study wave. WS = within-subject; BL = baseline. For each plot, the box depicts the interquartile range and the median, the whiskers extend to the 2.5 and 97.5 percentiles, and the circles show the locations of outliers in the top and bottom 2.5% of the distribution.
Positive Mood
Results from the three-level mixed location-scale models predicting positive mood states from NDSS scores and covariates are given in the Table 1. The base model using all records indicated that mean levels of positive mood increased over time (b = .24, p < .001), but NDSS scores were not related to positive mood level. The corresponding WS-WW variance submodel showed that mood variability decreased significantly across time. The VR of 0.81 (p < .001) indicates that within-subject within-wave positive mood variance decreased 19% over the study period. Both between-person (VR = 1.09, p < .05) and within-person (VR = 1.10, p < .001) differences in NDSS scores were associated with greater WS-WW positive mood variance (9% and 10% change per 1-point increase on the NDSS, respectively).
Table 1.
Results from mixed-effects location-scale analyses predicting positive mood from NDSS scores and covariates.
| Base Model |
Smoking Frequency Covaried |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model/Predictor | Mean Level |
WS-WW Variance |
Mean Level |
WS-WW Variance |
||||||||
| b | SE | P | VR | SE | P | b | SE | P | VR | SE | P | |
| All Records | ||||||||||||
| Intercept | 6.90 | 0.17 | < .001 | 1.92 | 0.17 | < .001 | 7.10 | 0.18 | < .001 | 1.77 | 0.17 | < .001 |
| Gender | 0.01 | 0.12 | .908 | 0.88 | 0.06 | .054 | −0.01 | 0.12 | .955 | 0.89 | 0.06 | .074 |
| 10th Grade | 0.004 | 0.12 | .971 | 0.97 | 0.06 | .589 | −0.04 | 0.12 | .745 | 0.98 | 0.01 | .739 |
| Time | 0.24 | 0.05 | < .001 | 0.81 | 0.02 | < .001 | 0.23 | 0.05 | < .001 | 0.81 | 0.02 | < .001 |
| BS NDSS | −0.13 | 0.08 | .128 | 1.09 | 0.05 | .039 | −0.47 | 0.15 | .003 | 1.27 | 0.10 | .003 |
| WS NDSS | −0.05 | 0.05 | .370 | 1.10 | 0.02 | < .001 | −0.07 | 0.06 | .260 | 1.08 | 0.02 | < .001 |
| BS Smoking | -- | -- | -- | -- | -- | -- | 0.12 | 0.04 | .009 | 0.95 | 0.02 | .031 |
| WS Smoking | -- | -- | -- | -- | -- | -- | 0.01 | 0.02 | .457 | 1.01 | 0.01 | .269 |
| Random Prompts | ||||||||||||
| Intercept | 6.92 | 0.17 | < .001 | 1.86 | 0.16 | < .001 | 7.12 | 0.19 | < .001 | 1.71 | 0.16 | < .001 |
| Gender | 0.01 | 0.12 | .940 | 0.88 | 0.06 | .044 | −0.01 | 0.12 | .929 | 0.88 | 0.06 | .058 |
| 10th Grade | −0.002 | 0.12 | .981 | 0.97 | 0.06 | .671 | −0.05 | 0.12 | .703 | 0.99 | 0.06 | .831 |
| Time | 0.21 | 0.05 | < .001 | 0.84 | 0.02 | < .001 | 0.21 | 0.05 | < .001 | 0.83 | 0.02 | < .001 |
| BS NDSS | −0.13 | 0.08 | .127 | 1.08 | 0.05 | .079 | −0.46 | 0.16 | .004 | 1.25 | 0.10 | .006 |
| WS NDSS | −0.05 | 0.05 | .317 | 1.08 | 0.02 | < .001 | −0.06 | 0.06 | .361 | 1.05 | 0.03 | .047 |
| BS Smoking | -- | -- | -- | -- | -- | -- | 0.11 | 0.04 | .012 | 0.95 | 0.02 | .035 |
| WS Smoking | -- | -- | -- | -- | -- | -- | 0.003 | 0.15 | .832 | 1.01 | 0.01 | .062 |
Note: BS = between-subjects, WS = within-subjects WS-WW = within-subjects, within-wave, VR = variance ratio. WS-WW variance parameters were estimated using a log-linear model and then exponentiated so that they represent variance ratios (i.e., the ratio of WS-WW variance per one-unit change on the covariate).
In the model accounting for smoking frequency, results were similar with the exception that higher NDSS person-means were associated with lower mean levels of positive mood (b = −.47, p < .01) with smoking covaried. Higher person-means for smoking frequency were associated with elevated mean levels of positive mood (b = .12, p < .01) and decreased positive mood variability (VR = 0.95, p < .05).
The pattern of results was little changed by restricting the analyses to prompted assessments; the only differences were observed in the base WS-WW submodel, such that between-person differences in NDSS scores were no longer significantly associated with positive mood variability (VR = 1.08, p = .08) and the gender difference crossed the nominal significance threshold (VR = 0.88, p = .044).
Negative Mood
Table 2 summarizes findings from models predicting negative mood states from NDSS scores. In the base model using all records, mean levels of negative affect were lower in boys (b = −.34, p < .05), decreased over time (b = −.27, p < .001), and increased with within-person elevations in NDSS score (b = .17, p < .05). The corresponding WS-WW variance submodel indicated that boys showed lower mood variability compared to girls (VR = 0.82, p < .01), negative mood variability decreased over time (VR = 0.78, p < .001), and both higher NDSS person-means (VR = 1.14, p < .01) and within-person growth in NDSS scores (VR = 1.09, p < .001) were associated with elevated negative mood variability.
Table 2.
Results from mixed-effects location-scale analyses predicting negative mood from NDSS scores and covariates.
| Base Model |
Smoking Frequency Covaried |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model/Predictor | Mean Level |
WS-WW Variance |
Mean Level |
WS-WW Variance |
||||||||
| b | SE | P | VR | SE | P | b | SE | P | VR | SE | P | |
| All Records | ||||||||||||
| Intercept | 3.56 | 0.20 | < .001 | 2.42 | 0.23 | < .001 | 3.23 | 0.22 | < .001 | 2.16 | 0.23 | < .001 |
| Gender | −0.34 | 0.15 | .021 | 0.82 | 0.06 | .004 | −0.30 | 0.15 | .044 | 0.83 | 0.06 | .010 |
| 10th Grade | −0.02 | 0.15 | .873 | 0.94 | 0.07 | .411 | 0.04 | 0.15 | .771 | 0.96 | 0.07 | .560 |
| Time | −0.27 | 0.07 | < .001 | 0.78 | 0.02 | < .001 | −0.26 | 0.07 | < .001 | 0.79 | 0.02 | < .001 |
| BS NDSS | 0.15 | 0.10 | .149 | 1.14 | 0.05 | .004 | 0.68 | 0.18 | < .001 | 1.37 | 0.12 | < .001 |
| WS NDSS | 0.17 | 0.06 | .011 | 1.09 | 0.02 | < .001 | 0.24 | 0.08 | .003 | 1.12 | 0.03 | < .001 |
| BS Smoking | -- | -- | -- | -- | -- | -- | −0.18 | 0.05 | < .001 | 0.94 | 0.02 | .017 |
| WS Smoking | -- | -- | -- | -- | -- | -- | −0.03 | 0.02 | .141 | 0.99 | 0.01 | .175 |
| Random Prompts | ||||||||||||
| Intercept | 3.53 | .020 | < .001 | 2.41 | 0.24 | < .001 | 3.20 | 0.22 | < .001 | 2.06 | 0.23 | < .001 |
| Gender | −0.34 | 0.15 | .024 | 0.79 | 0.06 | .001 | −0.29 | 0.15 | .049 | 0.80 | 0.06 | .004 |
| 10th Grade | −0.03 | 0.15 | .852 | 0.93 | 0.07 | .310 | 0.04 | 0.15 | .789 | 0.95 | 0.07 | .507 |
| Time | −0.24 | 0.07 | < .001 | 0.82 | 0.02 | < .001 | −0.22 | 0.07 | .001 | 0.82 | 0.02 | < .001 |
| BS NDSS | 0.15 | 0.10 | .134 | 1.12 | 0.06 | .024 | 0.38 | 0.19 | < .001 | 1.44 | 0.13 | < .001 |
| WS NDSS | 0.18 | 0.07 | .007 | 1.08 | 0.02 | < .001 | 0.24 | 0.08 | .003 | 1.09 | 0.03 | < .001 |
| BS Smoking | -- | -- | -- | -- | -- | -- | −0.18 | 0.05 | < .001 | 0.92 | 0.01 | .002 |
| WS Smoking | -- | -- | -- | -- | -- | -- | −0.02 | 0.02 | .257 | 1.00 | 0.001 | .626 |
Note: BS = between-subjects, WS = within-subjects WS-WW = within-subjects, within-wave, VR = variance ratio. WS-WW variance parameters were estimated using a log-linear model and then exponentiated so that they represent variance ratios (i.e., the ratio of WS-WW variance per one-unit change on the covariate).
In the model including smoking frequency as a covariate, all effects from the base model remained significant, and higher NDSS person-means were additionally associated with higher mean levels of negative mood (b = .68, p < .001). Higher overall mean smoking frequency was associated with lower mean levels (b = −.18, p < .001) and variability (VR = 0.94, p < .05) in negative mood. The pattern of findings was identical in models limited to mood reports from prompted assessments.
Rapid Escalators
Mean NDSS scores increased over time in the subsample of rapid escalators (baseline: M = 1.21 SD= 0.49, 6 months: M = 1.49, SD = .67, 15 months: M = 1.87, SD = 0.94, 24 months: M = 2.25, SD = 0.81), but were more consistent over time for the non-escalators (Ms = 1.51 to 1.56). A 2-level mixed model (Level 1 = wave, Level 2 = participant) with NDSS scores as the dependent measure revealed a significant Rapid Escalator x Time interaction, b = 0.87, p < .001. Stratified analyses confirmed a significant, positive relation between NDSS score and time in rapid escalators, b = 1.04, p <.001, and suggested there was no systematic trend among non-escalators, b = 0.05, p = .12.
Tables 3 and 4 summarize results from location-scale analyses testing mood effects associated with rapid escalator status. No effects involving rapid escalator status were found in submodels predicting mean levels of positive or negative moods. However, significant Rapid Escalator x Time interactions were found in every WS-WW submodel (positive mood interaction VRs = 1.22–1.37, negative mood interaction VRs = 1.15–1.38). These interaction effects consistently indicated that the rate of decline in mood variability was lower among rapid escalators compared to the remaining participants. After accounting for rapid escalator status, within-person changes in NDSS scores were still associated with higher mean levels of negative mood and increased WS-WW mood variability (although the effects for mood variability were reduced to marginal significance in analyses limited to random prompts).
Table 3.
Results from mixed-effects location-scale analyses testing effects of Rapid Escalator status and time on positive mood level and variability, with and without covarying nicotine dependence.
| Escalator Effects |
Escalator and NDSS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model/Predictor | Mean Level |
WS-WW Variance |
Mean Level |
WS-WW Variance |
||||||||
| b | SE | p | VR | SE | p | b | SE | p | VR | SE | p | |
| All Records | ||||||||||||
| Intercept | 6.72 | 0.11 | < .001 | 2.19 | 0.12 | < .001 | 6.91 | 0.17 | < .001 | 1.93 | 0.17 | < .001 |
| Gender | 0.02 | 0.12 | .860 | 0.89 | 0.06 | .056 | 0.03 | 0.12 | .836 | 0.88 | 0.06 | .038 |
| 10th Grade | 0.02 | 0.12 | .875 | 0.96 | 0.06 | .549 | 0.01 | 0.12 | .961 | 0.97 | 0.06 | .594 |
| Time | 0.20 | 0.06 | < .001 | 0.80 | 0.02 | < .001 | 0.23 | 0.05 | < .001 | 0.79 | 0.02 | < .001 |
| Escalator | −0.23 | 0.19 | .240 | 1.05 | 0.10 | .640 | −0.21 | 0.19 | .279 | 1.06 | 0.10 | .537 |
| Escalator x Time | 0.08 | 0.16 | .575 | 1.28 | 0.07 | < .001 | 0.10 | 0.16 | .549 | 1.22 | 0.07 | .001 |
| BS NDSS | -- | -- | -- | -- | -- | -- | −0.12 | 0.08 | .144 | 1.09 | 0.05 | .053 |
| WS NDSS | -- | -- | -- | -- | -- | -- | −0.05 | 0.05 | .303 | 1.07 | 0.02 | < .001 |
| Random Prompts | ||||||||||||
| Intercept | 6.74 | 0.11 | < .001 | 2.09 | 0.12 | < .001 | 6.93 | 0.17 | < .001 | 1.87 | 0.17 | < .001 |
| Gender | 0.02 | 0.12 | .879 | 0.88 | 0.06 | .048 | 0.02 | 0.12 | .862 | 0.87 | 0.06 | .031 |
| 10th Grade | 0.01 | 0.12 | .918 | 0.97 | 0.06 | .657 | −0.01 | 0.12 | .990 | 0.97 | 0.06 | .688 |
| Time | 0.18 | 0.06 | .002 | 0.82 | 0.02 | < .001 | 0.20 | 0.06 | < .001 | 0.81 | 0.02 | < .001 |
| Escalator | −0.22 | 0.20 | .259 | 1.01 | 0.10 | .927 | −0.20 | 0.20 | .305 | 1.02 | 0.10 | .881 |
| Escalator x Time | 0.03 | 0.16 | .849 | 1.37 | 0.08 | < .001 | 0.04 | 0.16 | .827 | 1.33 | 0.09 | < .001 |
| BS NDSS | -- | -- | -- | -- | -- | -- | −0.12 | 0.08 | .146 | 1.07 | 0.05 | .105 |
| WS NDSS | -- | -- | -- | -- | -- | -- | −0.05 | 0.05 | .313 | 1.04 | 0.02 | .064 |
Note: BS = between-subjects, WS = within-subjects WS-WW = within-subjects, within-wave, VR = variance ratio. WS-WW variance parameters were estimated using a log-linear model and then exponentiated so that they represent variance ratios (i.e., the ratio of WS-WW variance per one-unit change on the covariate).
Table 4.
Results from mixed-effects location-scale analyses testing effects of Rapid Escalator status and time on negative mood level and variability, with and without covarying nicotine dependence.
| Escalator Effects |
Escalator and NDSS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model/Predictor | Mean Level |
WS-WW Variance |
Mean Level |
WS-WW Variance |
||||||||
| b | SE | p | VR | SE | p | b | SE | p | VR | SE | p | |
| All Records | ||||||||||||
| Intercept | 3.72 | 0.13 | < .001 | 2.94 | 0.18 | < .001 | 3.54 | 0.20 | < .001 | 2.43 | 0.23 | < .001 |
| Gender | −0.35 | 0.15 | .017 | 0.83 | 0.06 | .008 | −0.37 | 0.15 | .013 | 0.81 | 0.06 | .003 |
| 10th Grade | −0.02 | 0.15 | .900 | 0.95 | 0.07 | .468 | −0.03 | 0.15 | .855 | 0.95 | 0.07 | .412 |
| Time | −0.24 | 0.07 | < .001 | 0.77 | 0.02 | < .001 | −0.26 | 0.07 | < .001 | 0.77 | 0.02 | < .001 |
| Escalator | 0.34 | 0.23 | .149 | 0.98 | 0.10 | .853 | 0.42 | 0.08 | .076 | 1.00 | 0.11 | .964 |
| Escalator x Time | 0.08 | 0.20 | .692 | 1.24 | 0.07 | < .001 | −0.13 | 0.21 | .548 | 1.15 | 0.07 | .021 |
| BS NDSS | -- | -- | -- | -- | -- | -- | 0.13 | 0.10 | .183 | 1.14 | 0.05 | .005 |
| WS NDSS | -- | -- | -- | -- | -- | -- | 0.18 | 0.07 | .009 | 1.07 | 0.02 | < .001 |
| Random Prompts | ||||||||||||
| Intercept | 3.70 | 0.13 | < .001 | 2.85 | 0.19 | < .001 | 3.50 | 0.20 | < .001 | 2.43 | 0.24 | < .001 |
| Gender | −0.35 | 0.15 | .019 | 0.80 | 0.06 | .002 | −0.36 | 0.15 | .015 | 0.78 | 0.06 | .001 |
| 10th Grade | −0.02 | 0.15 | .886 | 0.94 | 0.07 | .365 | −0.03 | 0.15 | .835 | 0.93 | 0.07 | .320 |
| Time | −0.22 | 0.07 | .003 | 0.80 | 0.02 | < .001 | −0.23 | 0.07 | .002 | 0.80 | 0.02 | < .001 |
| Escalator | 0.32 | 0.24 | .170 | 0.93 | 0.11 | .544 | 0.41 | 0.24 | .083 | 0.95 | 0.11 | .654 |
| Escalator x Time | 0.15 | 0.20 | .439 | 1.38 | 0.09 | < .001 | −0.06 | 0.21 | .765 | 1.30 | 0.09 | < .001 |
| BS NDSS | -- | -- | -- | -- | -- | -- | 0.14 | 0.10 | .168 | 1.12 | 0.06 | .028 |
| WS NDSS | -- | -- | -- | -- | -- | -- | 0.19 | 0.07 | .007 | 1.04 | 0.02 | .054 |
Note: BS = between-subjects, WS = within-subjects WS-WW = within-subjects, within-wave, VR = variance ratio. WS-WW variance parameters were estimated using a log-linear model and then exponentiated so that they represent variance ratios (i.e., the ratio of WS-WW variance per one-unit change on the covariate).
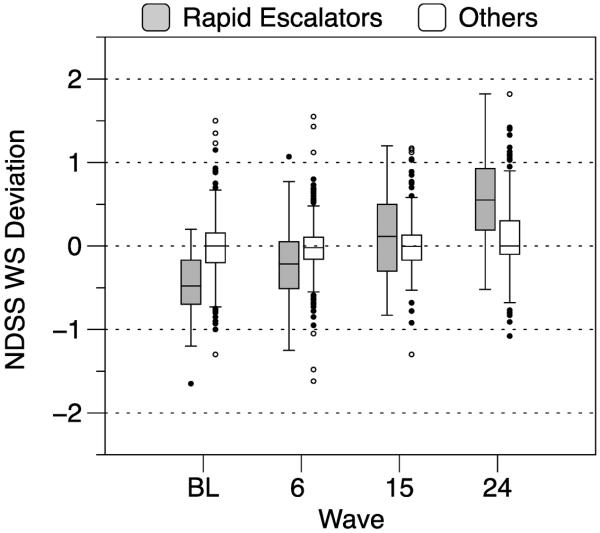
Figure 2 depicts boxplots showing the distributions of within-person NDSS deviations as a function of rapid escalator status and time. This illustrates that, as a group, the rapid escalators showed progression in nicotine dependence, but group status did not fully resolve all of the within-person changes in dependence scores. For instance, at each wave there was considerable heterogeneity among the escalators with respect to the degree to which they deviated from their person-mean NDSS score. Additionally, there was a good deal of overlap of within-person NDSS deviations between escalators and non-escalators at each time point.
Figure 2.
Distributions of within-person deviations on the NDSS at each study wave by Rapid Escalator status. WS = within-subject; BL = baseline. For each plot, the box depicts the interquartile range and the median, the whiskers extend to the 2.5 and 97.5 percentiles, and the circles show the locations of outliers in the top and bottom 2.5% of the distribution.
Discussion
The current study used data from repeated ecological assessments of adolescents' momentary moods to isolate and describe the affective correlates of the progression of nicotine dependence. Findings indicated that mean levels of negative mood and variability of both positive and negative moods increased as an individual's level of nicotine dependence increased.
Prior EMA analyses focused on variations in smoking frequency appear compatible with self-medication or stress/coping accounts of youthful smoking (Hedeker, et al., 2009; Pugach, et al, 2014; Weinstein & Mermelstein, 2013a; Weinstein & Mermelstein, 2013b; Weinstein, et al., 2008). Consistent with these earlier reports, the current analyses revealed that adolescents who smoked more frequently reported higher mean levels of positive mood, lower levels of negative mood, and less mood variability. Smoking frequency and NDSS scores were substantially correlated, but within-person progression of nicotine dependence was associated with adverse mood effects even after taking smoking frequency into account. This illustrates the importance of scrutinizing changes in dependence in addition to changes in frequency of cigarette use in order to develop a fuller understanding of the causes and consequences of adolescent tobacco use.
Using the criteria described by Weinstein and Mermelstein (2013a; 2013b), we identified a small group of rapid escalators in the analytic sample (~13%) who increased their smoking frequency substantially between baseline and 24 months. Mean nicotine dependence scores increased significantly over time in this group, whereas no systematic trend in dependence scores emerged in the remainder of the sample. Location-scale analyses indicated that rapid escalators' WS-WW positive and negative mood variances declined less steeply compared to the other participants. Thus, these findings are consistent with the picture emerging from the primary analyses, namely that progression of nicotine dependence is associated with a blunted decline in mood variability. Within-person increases in NDSS scores were associated with higher mean levels of negative mood and increased mood variability even after accounting for the rapid escalator effects. Descriptive analyses (Figure 2) revealed that this was possible because rapid escalator status did not fully resolve all of the within-person fluctuations in dependence severity. Again, the pattern of findings points to the conclusion that smoking frequency and dependence are strongly correlated but conceptually and empirically separable constructs. Presumably nicotine dependence symptoms arise from a combination of nicotine exposure and individual differences in vulnerability to the disorder. If so, classifying participants according to levels or rates of change in tobacco use alone will serve as an imprecise proxy for dependence progression because this does not account for variation in dependence risk or resilience.
In contrast to the current findings, prior longitudinal EMA studies, including reports from the SECASP study, found that rapid escalators tended to show larger decreases in mood variability compared to some other groups with distinct smoking patterns (Weinstein, et al., 2008; Weinstein & Mermelstein, 2013a; 2013b). Several differences in analytic approach may help to explain this discrepancy. The prior studies classified participants into numerous smoking trajectory groups and tested focused, sex-specific contrasts between selected pairs (e.g. rapid escalators vs. consistent smokers; rapid escalators vs. nonescalators), whereas the current analysis simply compared rapid escalators to the remainder of the analytic sample. The earlier analyses included large numbers of never smokers; these individuals were omitted from the current analyses because they would not be informative with respect to variations in nicotine dependence. The current study used a multilevel location-scale model to simultaneously investigate mood level and variability, whereas the earlier studies tested summary statistics (within-person means and standard deviations) aggregated at each wave. The prior SECASP analyses used data from two waves (baseline and 15 months; Weinstein & Mermelstein, 2013a; 2013b). In contrast, we modeled data from 4 waves and identified rapid escalators with respect to this longer time frame.
The NDSS contains items that tap multiple symptoms of dependence, including withdrawal symptomatology (“After not smoking for a while, I need to smoke to relieve feelings of restlessness and irritability”), craving, (“Whenever I go without a smoke for a few hours, I experience craving”), difficulty refraining from smoking (“If there were no cigarettes in the house and there was a big rainstorm, I would still go out of the house and find a cigarette”) and tolerance (“Compared to when I first started smoking, I need to smoke a lot more now in order to be satisfied.”). The high internal consistency of the NDSS suggests that these features form a coherent syndrome. Of these features, withdrawal symptomatology represents the most natural explanation for the elevated negative mood and increased mood variability associated with dependence progression (Goedeker & Tiffany, 2008; Parrott, 1999). Negative affect and anhedonia are prominent features of tobacco withdrawal (Cook, Piper, Leventhal, Schlam, Fiore, & Baker, 2015; Hendricks, Ditre, Drobes, & Brandon, 2006; A.M. Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010). As commonly envisioned by theoretical accounts (e.g., Baker, Brandon, et al., 2004; Baker, Piper, et al., 2004; Parrott, 1999; Koob & LeMoal, 2008; H. Leventhal & Cleary, 1980; Solomon & Corbit, 1973) early smoking trials may result in mood improvements that motivate continued experimentation or escalation of smoking frequency. However, after sufficient exposure (at least in susceptible individuals), the acquisition of dependence may represent an inflection point where these initial hedonic benefits begin to fade and mood dysregulation starts to emerge. Focusing analyses of mood data on within-person changes in dependence per se may more clearly demonstrate these adverse effects.
The findings highlight the value of using repeated bursts of EMA to fully characterize how young smokers' mood experiences are altered by the progression of nicotine dependence. Because mood levels, variability, and capacity for mood regulation change substantially during adolescence (Larson, Moneta, Richards, & Wilson, 2002; Maciejewski, van Lier, Branje, Meeus, & Koot, 2015; Silvers, McRae, Gabrieli, Gross, Remy, & Ochsner, 2012), mood measures are “moving targets” during the period in which most smokers begin to use cigarettes. Indeed, the current analyses consistently identified effects of time, with levels of positive affect increasing, and levels of negative mood and degree of mood variability decreasing over the two-year assessment period. This underscores the importance of prospective designs with repeated, intensive mood assessments in this age range. Nicotine dependence was related to mood variability. EMA is uniquely well-suited to characterizing mood variation (Hedeker, et al., 2009; Trull et al., 2008).
Youths' expectancies that smoking will alleviate negative mood states predict later smoking behavior (Guller, Zapolski, & Smith, 2015). The current findings might encourage development and dissemination of health communications for youth to counter affective enhancement expectancies by emphasizing the risk of adverse mood consequences from protracted smoking and nicotine dependence (Parrott & Murphy, 2012). It is notable that we observed both within-person decreases and increases in NDSS scores over time. Thus, the findings for within-person NDSS can be restated as indicating that mood improves as nicotine dependence dissipates. Health messaging emphasizing mood-related benefits associated with reducing dependence symptoms might be useful for promoting youth smoking cessation.
A number of limitations should be acknowledged. The current work focused on mood states in light of prior research and theory, but nicotine dependence is a multifaceted construct (e.g., Piper, et al., 2004; Shiffman, et al., 2004) and a comprehensive characterization of the sequelae of dependence progression would require attention to other cognitive and behavioral processes. Because there is uncertainty about the best way to assess nicotine dependence in adolescents (Tiffany, Conklin, Shiffman, & Clayton, 2004), it would be valuable to extend the work to examine the consistency of the findings using alternative dependence measures. The analyses focused on reports of the momentary mood states, not psychiatric symptoms or diagnoses, and it should not assumed that the effects we observed are indicative of mood or anxiety disorders co-occurring with youthful nicotine dependence. We interpret dependence-related increases in mood variability as maladaptive or adverse effects, but moods are influenced by many factors and mood variation is not necessarily bad. However, it is notable that the normative trend in this sample was for mood variation to decrease over time by 19–22% (Tables 1 and 2). Decreases in mood variation may be a sign of emotional maturity (Maciejewski, et al., 2015), and nicotine dependence may offset some of the expected age-related gains. The current analyses (base models, Tables 1 and 2) indicate that a one-point increase on the NDSS was associated with an 8–10% increase in WS-WW mood variance, canceling out approximately half the time effect.
Strengths include the availability of four bursts of EMA mood assessment collected from a sample of adolescents at risk for smoking, a two-year observation period during which there were substantial within-person changes in nicotine dependence, and the application of a mixed-effects location-scale regression analysis permitting scrutiny of both mood level and variability. This combination of techniques represents an innovative strategy for characterizing how behaviors and subjective experiences change in conjunction with the levels of nicotine dependence. Future research is needed to elucidate the specific mechanisms accounting for dependence-related mood effects and to investigate other correlates and consequences of dependence progression.
Acknowledgments
This research was supported by the National Cancer Institute of the National Institutes of Health under award number 5P01CA098262. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Multilevel regression analyses predicting moods from record type indicated that, relative to random prompts, smoking records were associated with lower negative mood (b = −0.25, p < .001) and higher positive mood (b = 0.29, p < .001) and reports of not being able to smoke were associated with elevated negative mood (b = 0.94, p < .001) and reduced positive mood (b = −0.97, p < .001). A multinomial generalized linear mixed model predicting record type (with prompted assessments as the reference category) indicated that within-person increases in dependence were associated with increased odds of logging smoking events (OR = 2.32, 95% CI = 2.00– 2.70, p < .001), increased odds of being prevented from smoking (OR = 1.38, 95% CI = 1.14– 1.66, p = .001), and decreased odds of reporting having resisted smoking given the opportunity (OR = 0.67, 95% CI = 0.53– 0.84, p = .001).
References
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, Schlam TR, Cook JW, Smith SS, Loh W, Bolt D. Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualization of dependence. Journal of Abnormal Psychology. 2012;121:909–921. doi: 10.1037/a0027889. doi: 10.1037/a0027889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrintgton H, Caspi A. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence. JAMA Psychiatry. 2013;70:534–542. doi: 10.1001/jamapsychiatry.2013.736. doi: 10.1001/jamapsychiatry.2013.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. New England Journal of Medicine. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. Journal of Abnormal Psychology. 2015;124:215–225. doi: 10.1037/abn0000016. doi: 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker LC, Donny E, Tiffany S, Colby SM, Perrine N, Clayton RR. The association between cigarette smoking and DSM-IV nicotine dependence among first year college students. Drug and Alcohol Dependence. 2007;86:106–114. doi: 10.1016/j.drugalcdep.2006.05.025. doi: 10.1016/j.druglcdep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy smokers. Drug and Alcohol Dependence. 2007;89:93–96. doi: 10.1016/j.drugalcdep.2006.11.019. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99(Suppl. 1):5–99. doi: 10.1111/j.1360-0443.2004.00735.x. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. doi: doi:10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fidler JA, West R. Self-perceived smoking motives and their correlates in a general population sample. Nicotine & Tobacco Research. 2009;11:1182–1188. doi: 10.1093/ntr/ntp120. doi: 10.1093/ntr/ntp120. [DOI] [PubMed] [Google Scholar]
- Glautier S. Measures and models of nicotine dependence: Positive reinforcement. Addiction. 2004;99(Suppl. 1):30–50. doi: 10.1111/j.1360-0443.2004.00736.x. doi: 10.1111/j.1360-0443.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Goedeker KC, Tiffany ST. On the nature of nicotine addiction: A taxometric analysis. Journal of Abnormal Psychology. 2008;117:896–909. doi: 10.1037/a0013296. doi: 10.1037/a0013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller L, Zapolski TCB, Smith GT. Longitudinal test of a reciprocal model of smoking expectancies and smoking experience in youth. Psychology of Addictive Behaviors. 2015;29:201–210. doi: 10.1037/adb0000002. doi: 10.1037/adb0000002. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein R. Mood changes associated with smoking in adolescents: An application of a mixed-effects location scale model for longitudinal ecological momentary assessment (EMA) data. In: Hancock GR, Harring J, editors. Advances in longitudinal methods in the social and behavioral sciences. Information Age Publishing; Charlotte, NC: 2012. pp. 59–79. [Google Scholar]
- Hedeker D, Mermelstein RJ, Berbaum ML, Campbell RT. Modeling mood variation associated with smoking: An application of a heterogeneous mixed-effects model for analysis of ecological momentary assessment. Addiction. 2009;104:297–307. doi: 10.1111/j.1360-0443.2008.02435.x. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Demirtas H. An application of a mixed-effects location scale model for analysis of Ecological Momentary Assessment (EMA) data. Biometrics. 2008;64:627–634. doi: 10.1111/j.1541-0420.2007.00924.x. doi: 10.1111/j.1541-0420.2007.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Evatt DP, Greenstein JE, Wardle MC, Yates MC, Veilleux JC. The acute effects of nicotine on positive and negative affect in adolescent smokers. Journal of Abnormal Psychology. 2007;116:543–533. doi: 10.1037/0021-843X.116.3.543. doi: 10.1037/0021-843X.116.3.543. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Development. 2002;73:1151–1165. doi: 10.1111/1467-8624.00464. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors. 2010;35:1120–1130. doi: 10.1016/j.addbeh.2010.08.007. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal H, Cleary PD. The smoking problem: A review of the research and theory in behavioral risk modification. Psychological Bulletin. 1980;88:370–405. doi: 10.1037/0033-2909.88.2.370. doi: 10.1037//0033-2909.88.2.370. [DOI] [PubMed] [Google Scholar]
- Maciejewski DF, van Lier PAC, Branje SJT, Meeus WJJ, Koot HM. A 5-year longitudinal study on mood variability across adolescence using daily diaries. Child Development. 2015;86:1908–1921. doi: 10.1111/cdev.12420. doi: 10.1111/cdev.12420. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use, and nicotine dependence. Psychological Medicine. 2004;34:1–11. doi: 10.1017/s0033291704002405. doi: 10.1017/S0033291704002405. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? American Psychologist. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. doi: 10.1037//0003-066X.54.10.817. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Murphy RS. Explaining the stress-inducing effects of nicotine to cigarette smokers. Human Psychopharmacology: Clinical and Experimental. 2012;27:150–155. doi: 10.1002/hup.1247. doi: 10.1002/hup.1247. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Hufford MR, Solhan M, Trull TJ. Assessing clients in their natural environments with electronic diaries: Rationale, benefits, limitations, and barriers. Psychological Assessment. 2007;19:25–43. doi: 10.1037/1040-3590.19.1.25. doi: 10.1037/1040-3590.19.1.25. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Richardson AE, Smith SM. Self-monitored motives for smoking among college students. Psychology of Addictive Behaviors. 2007;21:328–337. doi: 10.1037/0893-164X.21.3.328. doi: 10.1037/0893-164X.21.3.328. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS. Why some people smoke and others do not: New perspectives. Journal of Consulting and Clinical Psychology. 1993;61:723–731. doi: 10.1037//0022-006x.61.5.723. doi:10.1037/0022-006X.61.5.723. [DOI] [PubMed] [Google Scholar]
- Pugach O, Hedeker D, Richmond MJ, Sokolovsky A, Mermelstein R. Modeling mood variation and covariation among adolescent smokers: Application of a bivariate location-scale mixed effects model. Nicotine & Tobacco Research. 2014;16(Suppl. 2):S151–S158. doi: 10.1093/ntr/ntt143. doi: 10.1093/ntr/ntt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein ML, Rait MA, Sen S, Shiffman S. Characteristics of adolescent intermittent and daily smokers. Addictive Behaviors. 2014;39:1337–1341. doi: 10.1016/j.addbeh.2014.04.021. doi: 10.1016/j.addbeh.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–497. doi: 10.1037/a0017074. doi:10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Experimental and Clinical Psychopharmacology. 2012;20:264–277. doi: 10.1037/a0027546. doi: 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–1247. doi: 10.1037/a0028297. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: II. Cigarette addiction. Journal of Abnormal Psychology. 1973;81:158–171. doi: 10.1037/h0034534. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Sterling KL, Mermelstein R, Turner L, Diviak K, Flay B, Shiffman S. Examining the psychometric properties and predictive validity of a youth-specific version of the Nicotine Dependence Syndrome Scale (NDSS) among teens with varying levels of smoking. Addictive Behaviors. 2009;34:616–619. doi: 10.1016/j.addbeh.2009.03.016. doi: 10.1016/j.addbeh.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA, Shiffman S, Clayton RR. What can dependence theories tell us about assessing the emergence of tobacco dependence? Addiction. 2004;99(Suppl 1):78–86. doi: 10.1111/j.1360-0443.2004.00734.x. doi: 10.1111/j.1360-0443.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- Tomkins SS. Psychological model for smoking behavior. American Journal of Public Health. 1966;56(Suppl. 12):17–20. doi: 10.2105/ajph.56.12_suppl.17. doi: 10.2105/AJPH.56.12_Suppl.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Trageeser SL, Jahng S, Wood PK, Piasecki TM, Watson D. Affective instability: Measuring a core feature of borderline personality disorder with ecological momentary assessment. Journal of Abnormal Psychology. 2008;117:647–661. doi: 10.1037/a0012532. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein RJ. Dynamic associations of negative mood and smoking across the development of smoking in adolescence. Journal of Clinical Child & Adolescent Psychology. 2013a;42:629–642. doi: 10.1080/15374416.2013.794698. doi: 10.1080/15374416.2013.794698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein RJ. Influences of mood variability, negative moods, and depression on adolescent cigarette smoking. Psychology of Addictive Behaviors. 2013b;27:1068–1078. doi: 10.1037/a0031488. doi: doi: 10.1037/a0031488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein R, Shiffman S, Flay B. Mood variability and cigarette smoking escalation among adolescents. Psychology of Addictive Behaviors. 2008;22:504–513. doi: 10.1037/0893-164X.22.4.504. doi: 10.1037/0893-164X.22.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W, Dierker LC, Rose JS, Selya A, Mermelstein RJ. The natural course of nicotine dependence symptoms among adolescent smokers. Nicotine & Tobacco Research. 2012;14:1445–1452. doi: 10.1093/ntr/nts031. doi: 10.1093/ntr/nts031. [DOI] [PMC free article] [PubMed] [Google Scholar]