Abstract
Introduction
The frequency and causes of hypertrophic olivary degeneration (HOD) are unknown. We compared the clinical and radiological characteristics of unilateral HOD and bilateral HOD.
Methods
We performed a search of a radiologic report database for patients who were radiologically diagnosed as having HOD. This database includes the patients examined at the Mayo Clinic in Florida and Arizona. We used the search terms “hypertrophic olivary degeneration,” “HOD,” and “olivary” in the reports recorded from 1995 to 2015. Pertinent medical records and magnetic resonance imaging (MRI) scans of the brain for those with HOD were reviewed retrospectively.
Results
We identified 142 MRI studies on 95 cases who had radiologically proven HOD, 39 cases had unilateral HOD and 56 with bilateral HOD. In symptomatic cases, the most common symptom was ataxia. Palatal tremor was observed in almost half of all HOD cases. While cerebrovascular diseases were the most frequent etiology in both types of HOD (n=24, 62% in unilateral; n=17, 30% in bilateral), more than half of bilateral HOD cases had an unknown etiology (52%, n=29), whereas only 13% (n=5) of the unilateral cases had an unknown etiology (χ2 test, P<0.001). The lesions of unilateral HOD had a tendency to improve radiologically over time, whereas those associated with bilateral HOD were likely to worsen (χ2 test, P<0.05).
Conclusions
Our study showed that bilateral HOD is more common than unilateral HOD. Half of bilateral HOD cases had no obvious cause and some worsened over time. This may implicate a possible primary neurodegenerative process.
Keywords: Hypertrophic olivary degeneration, inferior olivary nucleus, MRI, palatal tremor, progressive ataxia and palatal tremor, etiology
1. Introduction
Hypertrophic olivary degeneration (HOD), also known as inferior olivary hypertrophy, is a rare condition affecting the inferior olivary nucleus (ION) in the medulla [1]. Transsynaptic degeneration caused by the loss of afferent signals into ION is considered to cause the characteristic hypertrophic changes that are unique to HOD [1]. Pathologically, vacuolation in the ION and enlargement of the cell bodies represent the hypertrophic appearance of HOD [1]. These changes are well demonstrated by magnetic resonance imaging (MRI), which typically shows that the affected side of the ION is enlarged and has increased signaling on T2-weighted images (T2WI) [2, 3]. HOD usually develops following a lesion involving the dentato-rubro-olivary pathway within the triangle of Guillain and Mollaret. Afferent fibers derived from the dentate nucleus of cerebellum ascend through the dentatorubral tract in the superior cerebellar peduncle (SCP) and reach the contralateral red nucleus in midbrain across midline. The afferent fibers from the red nucleus descend to the ipsilateral ION through the central tegmental tract (CTT) [4]. Any lesions involved in any components of this pathway from dentate nucleus to ION can cause HOD [3]. Olivodentate fibers within the inferior cerebellar peduncle, the remaining element of the triangle, do not participate in the formation of HOD because they are deafferent fibers leading from the ION to the contralateral dentate nucleus [3, 4]. HOD is classified as unilateral or bilateral. Bilateral HOD is considered rare because both sides of the afferent fibers into ION from dentate nucleus are damaged [5]. Several gene mutations in addition to structural lesions contribute to bilateral HOD [6–11].
The epidemiology and etiology of HOD are not fully understood, and the differences between the unilateral and bilateral forms of HOD require further elucidation. We performed a clinico-radiological analysis on 95 radiologically proven HOD cases and focused on the characteristic differences between unilateral and bilateral HOD.
2. Methods
2.1. Searching patients and extracting data
We searched the radiologic report database of Mayo Clinic Florida (MCF) and Arizona (MCA) for patients who had radiologically proven HOD using the terms “hypertrophic olivary degeneration,” “HOD,” and “olivary.” We limited the search to only MRI scans. All MRI scans were recorded from April 1, 1995 to April 20, 2015 at either MCF or MCA. These study materials were entirely separate from those used in previous studies by the Mayo Clinic Rochester group [12, 13]. The definition of HOD used in this study included the presence of a hyperintense signal in the ION on T2WI or on fluid-attenuated inversion recovery images (FLAIR) [3]. Patients who did not have any evidence of HOD were excluded. Pertinent medical records were reviewed. The data was then tabulated. This study was approved by the Mayo Clinic Institutional Review Board Committee.
2.2. Statistical Analysis
We used student t test for comparison of continuous data. For comparison of categorical data, we performed χ2 test with Yates’ correction or Fisher’s exact test if appropriate. Residual analysis for χ2 test was applied when necessary. Significance was assigned at P<0.05.
3. Results
3.1. Demographic profiles
We found 172 MRI studies on 121 patients in our initial search of the Mayo Clinic radiologic database. Of these, we identified 142 MRI reports on 95 cases who had radiologically-proven HOD (Table 1); thirty-nine cases had unilateral HOD (41%; men, n=15; women, n=24), and fifty-six cases had bilateral HOD (59%; men, n=39; women, n=17). Age at onset in which HOD was initially detected by MRI was not significantly different between unilateral HOD and bilateral HOD cases (60±17 years [range 30–89] and 63±13 years [range 31–82], respectively (P=0.31). Bilateral HOD was observed more frequently in men (P<0.01).
Table 1.
Demographic and clinical findings of hypertrophic olivary degeneration in our cohort
| Unilateral HOD | Bilateral HOD | p Value | |||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | ||
| Total | 39 | 100 | 56 | 100 | 0.08 |
| Age at onseta (y) | 60 ± 17 (30–89)b | 63 ± 13 (31–82)b | 0.31 | ||
| Men:Women | 15:24 | 38:62 | 39:17 | 70:30 | 0.003 |
|
| |||||
| Symptomatic Clinical findingsc | 9 | 23 | 22 | 39 | 0.10 |
| Ataxia | 8 | 89 | 18 | 82 | 1.00 |
| Palatal tremor | 4 | 44 | 11 | 50 | 1.00 |
| Dysarthria | 5 | 56 | 11 | 50 | 1.00 |
| Tremor | 2 | 22 | 11 | 50 | 0.24 |
| Pyramidal signs | 6 | 67 | 9 | 41 | 0.25 |
| Nystagmus | 5 | 56 | 8 | 36 | 0.43 |
| Ophthalmoplegia | 7 | 78 | 6 | 27 | 0.02 |
| Dysphagia | 1 | 11 | 5 | 23 | 0.64 |
| Ocular myoclonus | 0 | 0 | 3 | 14 | 0.54 |
| Myoclonus | 0 | 0 | 1 | 5 | 1.00 |
HOD: hypertrophic olivary degeneration, n: number
Initially detected by MRI,
mean ± SD (range),
presenting each ratio in the number of symptomatic cases
3.2. Clinical findings
The clinical findings observed in the patients with HOD are shown in Table 1. The number of symptomatic cases were 9 (23%) out of 39 in unilateral HOD and 22 (39%) out of 56 in bilateral HOD. Frequently observed features in the symptomatic cases were as follows (unilateral; bilateral): ataxia (n=8, 89%; n=18, 82%), palatal tremor (n=4, 44%; n=11, 50%), dysarthria (n=5, 56%; n=11, 50%), tremor (n=2, 22%; n=11, 50%), pyramidal signs (n=6, 67%; n=9, 41%), nystagmus (n=5, 56%; n=8, 36%), ophthalmoplegia (n=7, 78%; n=6, 27%) and dysphagia (n=1, 11%; n=5, 23%). Ocular myoclonus (n=3, 14%) and myoclonus (n=1, 5%) were observed only in bilateral HOD patients. There were no significant differences in symptoms between unilateral and bilateral HOD, but ophthalmoplegia was observed more frequently in unilateral HOD (P<0.05). Ear clicking was seen in one case with bilateral HOD that had palatal tremor.
3.3. Etiologies of HOD
Causes of HOD are listed in Table 2. Cerebrovascular diseases including hemorrhage, infarction, and vascular malformations were the most frequent etiologies in both types of HOD, 62% (n=24) in unilateral and 30% (n=17) in bilateral HOD. Other causes included tumor, prior surgery of the posterior fossa, metronidazole intoxication, multiple sclerosis (MS), encephalitis, progressive multifocal leukoencephalopathy (PML), and Dandy-Walker variant. The inciting lesion involved the dentato-rubro-olivary pathway in 34 of the 39 unilateral HOD cases (87%). All of these cases had a clear correlation between the location impacted by HOD and the location of the causative lesion. In one case, a focal lesion in medulla associated with MS was suspected to represent HOD. Cases who had bilateral HOD more frequently had an unknown etiology (52% [n=29] bilateral HOD vs. 13% [n=5] unilateral HOD [P<0.001]).
Table 2.
Causes of hypertrophic olivary degeneration in our cohort
| Unilateral HOD | Bilateral HOD | |||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
| Cerebrovascular diseases | 24 | 62 | 17 | 30 |
| Hemorrhage | 14a | 36 | 5a | 9 |
| Infarction | 8 | 21 | 12 | 21 |
| Arteriovenous malformation | 1 | 3 | 0 | 0 |
| Capillary venous malformation | 1 | 3 | 0 | 0 |
| Tumor | 5b | 13 | 3b | 5 |
| Prior surgery of the posterior fossa | 3 | 8 | 1 | 2 |
| Metronidazole | 1 | 3 | 2 | 4 |
| Multiple sclerosis | 1 | 3 | 1 | 2 |
| Encephalitis | 0 | 0 | 1 | 2 |
| Progressive multifocal leukoencephalopathy | 0 | 0 | 1 | 2 |
| Dandy-Walker variant | 0 | 0 | 1 | 2 |
| Unknownc | 5 | 13 | 29 | 52 |
HOD: hypertrophic olivary degeneration, n: number
Including hemorrhages associated with cavernous malformations: 5 cases in unilateral HOD and 4 cases in bilateral HOD.
Three cases had glioma and two cases had metastatic tumors in unilateral HOD. All three cases who had bilateral HOD had glioma.
P<0.001 by χ2 test.
3.4. Temporal changes of MRI findings in follow-up cases
There were 20 cases of unilateral HOD and 29 cases of bilateral HOD with at least two separate MRI records with various follow-up intervals (Table 3). We assessed temporal radiological changes of the HOD lesions between the first and the last MRI scans of these follow up cases regardless of whether the patient was symptomatic. They were classified into three categories; “worsened,” “improved,” and “unchanged.” We considered the cases to have “worsened” if their lesions had stronger signaling and/or were getting larger, but if the reverse was true then the cases fell into the “improved” category. The median follow-up duration was 35 months (range 4 – 140 months) for the unilateral HOD cases and 25 months (range 1 – 130 months) for the bilateral cases. While unilateral HOD lesions had a tendency to improve with time (P<0.05), bilateral HOD lesions were likely to worsen (P<0.01). Seven of the 13 bilateral HOD cases that worsened over time also had an unknown etiology. Only four of these seven cases were symptomatic. Three of the four cases were stable, but one case had a progressive clinical course throughout the follow-up period.
Table 3.
Temporal changes in follow up cases who had hypertrophic olivary degeneration
| Unilateral HOD | Bilateral HOD | p Value | |||
|---|---|---|---|---|---|
|
| |||||
| n | Durationa (m) | n | Durationa (m) | ||
| Total | 20 | 35 (4–140) | 29 | 25 (1–130) | 0.26 |
| Worsened | 2 | 51 (10–92) | 13 | 23 (3–130) | |
| Improved | 8 | 45 (10–126) | 3 | 3 (1–29) | <0.05b |
| Unchanged | 10 | 19 (4–140) | 13 | 32 (1–127) | |
HOD: hypertrophic olivary degeneration, n: number, m: minutes
Median (range)
P<0.05 by χ2 test. We revealed that MRI abnormalities in affected ION of unilateral HOD had a tendency to improve (P<0.05), and that of bilateral HOD was likely to worsen (P<0.01) by residual analysis.
3.5. Case presentations
Case 1
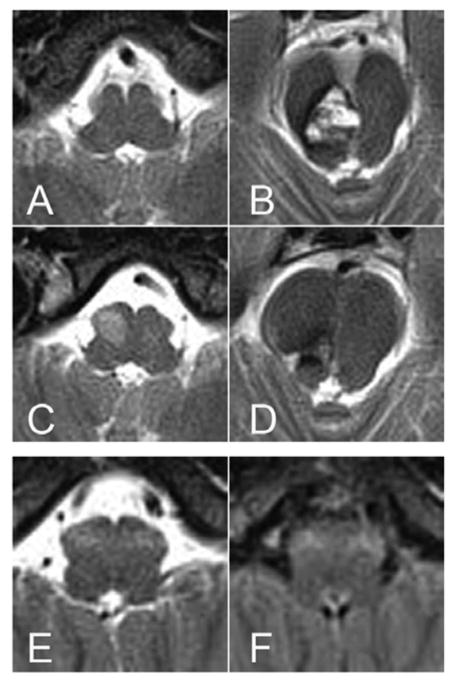
A 28-year-old woman had an episode of numbness on the left side of her face. She had a second episode of numbness a year later in which the numbness worsened and spread through her left arm and leg. She also had some difficulty with balance. At the age of 29 years, she had a neurological examination that identified horizontal diplopia to extreme right lateral gaze and subtle hypesthesia in her left face and arm. Her gait was found to be steady but her tandem gait was performed with slight difficulty. Her brain MRI revealed a cavernous malformation extending from the right lower mesencephalon to the upper pons, and there was some evidence of bleeding. At that time, the ventral medulla appeared normal (Figure 1A, B). She underwent surgery for resection of the brainstem cavernous malformation. Two months after surgery, she developed ophthalmoplegia, nystagmus, and significant dysmetria on her left side and imbalance. A follow-up MRI scan four months after the surgery showed newly hyperintense lesion with enlargement of the right ION (Figure 1C, D). This woman was diagnosed as having unilateral HOD that developed after resection of the cavernous malformation.
Figure 1. MR images of example cases who had unilateral and bilateral hypertrophic olivary degeneration (HOD).
(A–D): unilateral HOD. (E, F): bilateral HOD. On a day before surgery, there was no abnormal signals in the medulla (A), but a large cavernous malformation with evolving blood products was evident in right pontomesencephalic junction (B). Four months after resection of the cavernous malformation, a newly developed hyperintense lesion was observed in right ION (C), while there were only postoperative changes in pontomesencephalic junction (D). At age of 54 years, symmetrical hyperintense lesions were detected in bilateral ION (E, F). (A–E, T2-weighted images; F, fluid-attenuated inversion recovery image)
Case 2
A 52-year-old, right-handed woman noticed tremor in her right hand. Subsequently, head shaking and voice tremors appeared. She visited our clinic at the age of 53 years. Her previous medical history was unremarkable, and she was not on any medications. There was no family history of tremor. Upon examination, she had a mild tremor that was mainly postural in both of her hands, but it was more prevalent on her right side. She also had evidence of irregular head shaking, voice tremors, and occasional body jerking. Saccadic dysmetria, square wave jerks, nystagmus, dysarthria, palatal tremor, and wobbly and unsteady gait were also present. She had difficulty with visual focus due to her oscillopsia. She had bilateral eyelid twitching when she closed her eyes (Supplemental Video 1). Her brain MRI scan showed hyperintense T2WI/FLAIR signal and mild enlargement of the bilateral ION without gadolinium enhancement (Figure 1E, F). There were no detectable lesions within the dentato-rubro-olivary pathway. The results of laboratory tests for paraneoplastic neurologic syndromes, Wilson’s disease, thyroid dysfunction, and vitamin deficiency were all negative. GAD65 antibody, which could be associated with cerebellar ataxia, was also negative. Although MRI abnormalities were stable at her 16-month follow-up exam, her symptoms were slowly progressive. We diagnosed her as having progressive ataxia and palatal tremor (PAPT) with bilateral HOD [14].
4. Discussion
This study is a retrospective clinico-radiologic analysis of cases with radiologically-proven HOD. We found more cases of bilateral HOD than unilateral HOD (56% vs. 44%) in our radiologic database. Similar to our results, a recent retrospective study reported that 76% of their cohort had bilateral HOD [12]. These results suggest that bilateral HOD is more common than previously expected [3]. We also found that bilateral HOD was more frequently seen in men. A reason for this difference is unknown; however, a similar observation has been previously described [13]. The frequency of symptomatic HOD was 23% in unilateral and 39% in bilateral HOD, indicating that HOD was often asymptomatic and could be found incidentally on MRI scans. The factors that lead to development of the clinical signs associated with HOD remain unclear. The development of these features, their severity and extend may depend on the amount of residual functional neurons in the ION and their afferent fibers; or it may be related to the severity of initial insult. Classical presentations of HOD include palatal tremor, cerebellar ataxia (slurred speech, dysmetria and ataxic gait), ocular symptoms (nystagmus, ophthalmoplegia and ocular myoclonus), and other involuntary movements, such as tremor and myoclonus [15–17]. In our cohort, ataxia was the most frequent symptom and was associated with both types of HOD.
Palatal tremor was seen in almost half of the cases of unilateral and bilateral HOD. Palatal tremor has both essential and symptomatic forms, and the latter is mostly associated with HOD [18]. Among the 15 cases with symptomatic palatal tremor, only one case (6.7%) had ear clicking sounds in our study. Ear clicks are known to be common in essential palatal tremor, but they are rarely observed in cases of symptomatic palatal tremor; a frequency was reported as 90% and 8%, respectively [18]. Our observation is compatible with this well. PAPT is known as a subgroup of symptomatic palatal tremor and is associated with bilateral HOD [14, 19]. HOD in PAPT does not usually accompany any insults to the dentato-rubro-olivary pathway. The etiology of PAPT is unknown; however, a degenerative process is presumed [14, 20]. In addition to palatal tremor, patients with PAPT present with progressive cerebellar ataxia and ocular movement abnormalities combined with mild cerebellar atrophy on MRI imaging. In this study, we identified six patients with bilateral HOD who could be clinically diagnosed as having PAPT (Case 2 is one example). All were sporadic, but one was suspected to have a family history of PAPT. All of these patients had a slowly-progressive course, indicating a neurodegenerative disease. On the other hand, there has been a single case report of PAPT that was associated with gluten sensitivity [21]. In this case, palatal tremor and MRI abnormalities in the ION were improved by a gluten-free diet, suggesting that an inflammatory process instead of degeneration may play a role. Pathological analysis could help us understand the underlying pathological process in PAPT; however, no autopsy cases have been reported so far.
Symptoms associated with the inciting lesions in the dentato-rubro-olivary pathway could not be fully excluded because they could overlap with symptoms related to HOD, such as ataxia and some ocular manifestations. This limitation could partially explain the high frequency of ataxia both types of HOD. As most of the preceding lesions associated with unilateral HOD involved in the brainstem, ophthalmoplegia might be more frequently observed in unilateral HOD cases. However, variable preceding lesions involving the dentato-rubro-olivary pathway could be a cause of HOD; for example, hemorrhage, infarction, tumor, trauma, prior surgery of a posterior fossa, inflammation, and demyelination [18]. In addition, abscesses [22], Wilson’s disease [23], gluten sensitivity [21], metronidazole intoxication [24] and PML [25] have been reported as rare causative conditions presenting HOD. In this study, cerebrovascular diseases including hemorrhage, infarction, and vascular malformations were the most common causes of both types of HOD.
Interestingly, three HOD cases were induced by metronidazole intoxication; one had unilateral HOD, and the other two had bilateral HOD. The olivary lesions in these cases were associated with lesions in dentate nucleus or CTT. In contrast to a previous report of HOD not improving after withdrawing metronidazole [24], the lesions in one of our cases almost completely resolved within a month after discontinuance of metronidazole.
We also identified rare cases of bilateral HOD caused by PML and Dandy-Walker variant. The case with PML had contiguous lesions throughout the dentate nucleus, SCP, and tegmentum of the brainstem bilaterally. These lesions were likely the cause of bilateral HOD in this case. In the case of the Dandy-Walker variant, bilateral ION lesions seemed to occur after marked thinning of the bilateral SCP related to marked enlargement of the fourth ventricle.
The majority of unilateral HOD had identifiable causes and had correlation between the affected side of the ION and the location of the causative lesion, whereas more than half of the cases who had bilateral HOD had no obvious cause. Similarly, Gu et al. showed that a majority of HOD patients without any structural lesions in the dentato-rubro-olivary pathway had bilateral HOD [13]. These results suggest that the underlying etiology may be different between unilateral HOD and some cases of bilateral HOD. In general, unilateral HOD occurs secondary to a lesion involving the dentato-rubro-olivary pathway. On the other hand, bilateral HOD can develop as either a primary or secondary event. Nearly half of all bilateral HOD cases who had an unknown etiology worsened radiologically over time in this study. These observations imply that some of bilateral HOD cases may also have a neurodegenerative process. Indeed, some pediatric cases who had ION involvement were found to have neurodegenerative diseases [26].
To date, several gene mutations have been identified in patients presenting with bilateral HOD. POLG encodes a mitochondrial DNA polymerase and was identified firstly as a causative gene of progressive external ophthalmoplegias [27]. It is currently known that POLG mutations cause more heterogeneous clinical phenotypes, including bilateral HOD [6]. SURF1 is a causative gene of Leigh syndrome, which is a severe neurodegenerative disorder associated with cytochrome c oxidase deficiency usually affecting infants [28]. Bilateral HOD is a characteristic finding of patients with SURF1 mutations [7, 8]. A nonsense mutation of TTC19 leads to mitochondrial respiratory chain complex III deficiency and neurodegeneration. Some patients with TTC19 mutations show bilateral HOD on MRI scans [9]. A male patient with an adult-onset mitochondrial disease associated with an AIFM1 mutation has recently been reported [10]. His brain MRI scan exhibited hyperintense lesions in the bilateral ION on proton density-weighted images. Given that all of these genes are associated with mitochondria, mitochondrial dysfunction may underlie the pathogenesis of cases of bilateral HOD without any obvious etiologies. Mitochondrial disorders were also suspected in some patients with PAPT because hearing loss was observed in some of them [14]. Interestingly, two of the six patients with PAPT had hearing loss in our cohort. Bilateral HOD has also been observed in patients with spinocerebellar ataxia (SCA) type 20 [11]. If the patient with bilateral HOD has family history or physiological and neurological signs indicating mitochondrial disease or SCA, genetic analysis would be helpful for providing a correct diagnosis.
HOD is pathologically characterized by enlargement and vacuolation of neurons, astrocytic hyperplasia and proliferation with gliosis, and demyelination [1, 29]. These pathologies are considered transsynaptic degeneration secondary to loss of afferent input to the ION [1]. Pathologies in bilateral HOD with unknown etiology, including PAPT, might be different. Indeed, HOD observed in progressive supranuclear palsy showed severe neurofibrillary degeneration of the ION, indicating that direct neurodegeneration could occur rather than transsynaptic degeneration [30]. Thus, it should be noted that radiologically-proven HOD could include heterogeneous pathological conditions. A prospective study combined with pathological analysis is needed to verify our observation and to unveil the pathological basis of the radiological changes in the ION.
Supplementary Material
The patient had a fine horizontal pendular nystagmus in primary and all directions of gaze. There was significant ocular dysmetria of saccadic eye movements. Rhythmic twitching in her eyelids appeared bilaterally when she closed her eyes. Palatal tremor with irregular rate was present. She had postural tremor with a kinetic component in both hands that was more prevalent on the right side. She was able to walk independently but wobbled slightly. She was exceedingly cautious while walking with a tandem gait.
HIGHLIGHTS.
Bilateral HOD is more common than unilateral HOD.
Half of the bilateral HOD cases we studied had no obvious cause.
Some of these bilateral HOD cases worsened radiologically over time.
Bilateral HOD may be caused by a primary neurodegenerative process.
Acknowledgments
We would like to thank Ms. Kelly Viola, ELS for her assistance with the technical preparation of this manuscript.
Abbreviations
- HOD
hypertrophic olivary degeneration
- MRI
magnetic resonance imaging
- ION
inferior olivary nucleus
- T2WI
T2-weighted images
- SCP
superior cerebellar peduncle
- CTT
central tegmental tract
- PAPT
progressive ataxia and palatal tremor
- FLAIR
fluid-attenuated inversion recovery images
- PML
progressive multifocal leukoencephalopathy
- SCA
spinocerebellar ataxia
Footnotes
Author Contributions:
Takuya Konno: drafting the manuscript, acquisition, analysis, and interpretation of the data, statistical analysis, and final approval of the manuscript. Daniel F. Broderick: drafting/reviewing the manuscript, acquisition and interpretation of the data, and final approval of the manuscript. Pawel Tacik: reviewing the manuscript, acquisition of the data, and final approval of the manuscript. John N. Caviness: drafting/reviewing the manuscript, acquisition of the data, and final approval of the manuscript. Zbigniew K. Wszolek: drafting/reviewing the manuscript, design and conceptualization of the study, interpretation of the data, and final approval of the manuscript.
Study Funding: No targeted funding reported.
Disclosures: T. Konno received research support from the Uehara Memorial Foundation postdoctoral fellowship and is partially supported by a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. D. Broderick reports no disclosures relevant to the manuscript. P. Tacik received support from the Max Kade Foundation, an Allergan Medical Educational Grant, and a Jaye F. and Betty F. Dyer Foundation Fellowship in progressive supranuclear palsy research. J. Caviness is funded by the Michael J. Fox Foundation for Parkinson’s Disease Research. Z. Wszolek is supported by the NIH P50 NS072187, Mayo Clinic Center for Regenerative Medicine, Mayo Clinic Center for Individualized Medicine, Mayo Clinic Neuroscience Focused Research Team, and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. Z. Wszolek is also the co-editor-in-chief of Parkinsonism and Related Disorders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gautier JC, Blackwood W. Enlargement of the inferior olivary nucleus in association with lesions of the central tegmental tract or dentate nucleus. Brain. 1961;84:341–361. doi: 10.1093/brain/84.3.341. [DOI] [PubMed] [Google Scholar]
- 2.Yokota T, Hirashima F, Furukawa T, Tsukagoshi H, Yoshikawa H. MRI findings of inferior olives in palatal myoclonus. J Neurol. 1989;236:115–116. doi: 10.1007/BF00314408. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Versnick E, Tuite P, Cyr JS, Kucharczyk W, Montanera W, Willinsky R, Mikulis D. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol. 2000;21:1073–1077. [PMC free article] [PubMed] [Google Scholar]
- 4.Lapresle J, Hamida MB. The dentato-olivary pathway. Somatotopic relationship between the dentate nucleus and the contralateral inferior olive. Arch Neurol. 1970;22:135–143. doi: 10.1001/archneur.1970.00480200041004. [DOI] [PubMed] [Google Scholar]
- 5.Gerace C, Fele MR, Luna R, Piazza G. Neurological picture. Bilateral hypertrophic olivary degeneration. J Neurol Neurosurg Psychiatry. 2006;77:73. doi: 10.1136/jnnp.2005.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzoulis C, Engelsen BA, Telstad W, Aasly J, Zeviani M, Winterthun S, Ferrari G, Aarseth JH, Bindoff LA. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain. 2006;129:1685–1692. doi: 10.1093/brain/awl097. [DOI] [PubMed] [Google Scholar]
- 7.Sonam K, Khan NA, Bindu PS, Taly AB, Gayathri N, Bharath MM, Govindaraju C, Arvinda HR, Nagappa M, Sinha S, Thangaraj K. Clinical and magnetic resonance imaging findings in patients with Leigh syndrome and SURF1 mutations. Brain Dev. 2014;36:807–812. doi: 10.1016/j.braindev.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Bindu PS, Taly AB, Sonam K, Govindaraju C, Arvinda HR, Gayathri N, Bharath MM, Ranjith D, Nagappa M, Sinha S, Khan NA, Thangaraj K. Bilateral hypertrophic olivary nucleus degeneration on magnetic resonance imaging in children with Leigh and Leigh-like syndrome. Br J Radiol. 2014;87:20130478. doi: 10.1259/bjr.20130478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghezzi D, Arzuffi P, Zordan M, Da Re C, Lamperti C, Benna C, D’Adamo P, Diodato D, Costa R, Mariotti C, Uziel G, Smiderle C, Zeviani M. Mutations in TTC19 cause mitochondrial complex III deficiency and neurological impairment in humans and flies. Nat Genet. 2011;43:259–263. doi: 10.1038/ng.761. [DOI] [PubMed] [Google Scholar]
- 10.Ardissone A, Piscosquito G, Legati A, Langella T, Lamantea E, Garavaglia B, Salsano E, Farina L, Moroni I, Pareyson D, Ghezzi D. A slowly progressive mitochondrial encephalomyopathy widens the spectrum of AIFM1 disorders. Neurology. 2015;84:2193–2195. doi: 10.1212/WNL.0000000000001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight MA, Gardner RJ, Bahlo M, Matsuura T, Dixon JA, Forrest SM, Storey E. Dominantly inherited ataxia and dysphonia with dentate calcification: spinocerebellar ataxia type 20. Brain. 2004;127:1172–1181. doi: 10.1093/brain/awh139. [DOI] [PubMed] [Google Scholar]
- 12.Carr CM, Hunt CH, Kaufmann TJ, Kotsenas AL, Krecke KN, Wood CP. Frequency of bilateral hypertrophic olivary degeneration in a large retrospective cohort. J Neuroimaging. 2015;25:289–295. doi: 10.1111/jon.12118. [DOI] [PubMed] [Google Scholar]
- 13.Gu CN, Carr CM, Kaufmann TJ, Kotsenas AL, Hunt CH, Wood CP. MRI Findings in Nonlesional Hypertrophic Olivary Degeneration. J Neuroimaging. 2015;25:813–817. doi: 10.1111/jon.12267. [DOI] [PubMed] [Google Scholar]
- 14.Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE. Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain. 2004;127:1252–1268. doi: 10.1093/brain/awh137. [DOI] [PubMed] [Google Scholar]
- 15.Deuschl G, Toro C, Valls-Solé J, Zeffiro T, Zee DS, Hallett M. Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain. 1994;117:775–788. doi: 10.1093/brain/117.4.775. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd GM, Tauböll E, Bakke SJ, Nyberg-Hansen R. Midbrain tremor and hypertrophic olivary degeneration after pontine hemorrhage. Mov Disord. 1997;12:432–437. doi: 10.1002/mds.870120327. [DOI] [PubMed] [Google Scholar]
- 17.Cachia D, Izzy S, Smith T, Ionete C. A rare presentation of hypertrophic olivary degeneration secondary to primary central nervous system lymphoma. JAMA Neurol. 2013;70:1192–1193. doi: 10.1001/2013.jamaneurol.218. [DOI] [PubMed] [Google Scholar]
- 18.Deuschl G, Mischke G, Schenck E, Schulte-Mönting J, Lücking CH. Symptomatic and essential rhythmic palatal myoclonus. Brain. 1990;113:1645–1672. doi: 10.1093/brain/113.6.1645. [DOI] [PubMed] [Google Scholar]
- 19.Sperling MR, Herrmann C., Jr Syndrome of palatal myoclonus and progressive ataxia: two cases with magnetic resonance imaging. Neurology. 1985;35:1212–1214. doi: 10.1212/wnl.35.8.1212. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni PK, Muthane UB, Taly AB, Jayakumar PN, Shetty R, Swamy HS. Palatal tremor, progressive multiple cranial nerve palsies, and cerebellar ataxia: a case report and review of literature of palatal tremors in neurodegenerative disease. Mov Disord. 1999;14:689–693. doi: 10.1002/1531-8257(199907)14:4<689::aid-mds1022>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Kheder A, Currie S, Romanowski C, Hadjivassiliou M. Progressive ataxia with palatal tremor due to gluten sensitivity. Mov Disord. 2012;27:62–63. doi: 10.1002/mds.23987. [DOI] [PubMed] [Google Scholar]
- 22.Chhetri SK, Dayanandan R, Bindman DC, Mathur S, Mills RJ. Symptomatic palatal tremor following multiple listerial brainstem abscesses. Park Relat Disord. 2014;20:253–255. doi: 10.1016/j.parkreldis.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Otto J, Guenther P, Hoffmann KT. Bilateral hypertrophic olivary degeneration in Wilson disease. Korean J Radiol. 2013;14:316–320. doi: 10.3348/kjr.2013.14.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seok JI, Yi H, Song YM, Lee WY. Metronidazole-induced encephalopathy and inferior olivary hypertrophy: lesion analysis with diffusion-weighted imaging and apparent diffusion coefficient maps. Arch Neurol. 2003;60:1796–1800. doi: 10.1001/archneur.60.12.1796. [DOI] [PubMed] [Google Scholar]
- 25.Mathew RM, Murnane M. MRI in PML: bilateral medullary lesions. Neurology. 2004;63:2380. doi: 10.1212/01.wnl.0000141860.97900.8a. [DOI] [PubMed] [Google Scholar]
- 26.Mirabelli-Badenier M, Morana G, Bruno C, Di Rocco M, Striano P, De Grandis E, Veneselli E, Rossi A, Biancheri R. Inferior olivary nucleus involvement in pediatric neurodegenerative disorders: does it play a role in neuroimaging pattern-recognition approach? Neuropediatrics. 2015;46:104–109. doi: 10.1055/s-0035-1544185. [DOI] [PubMed] [Google Scholar]
- 27.Van Goethem G, Dermaut B, Löfgren A, Martin JJ, Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet. 2001;28:211–212. doi: 10.1038/90034. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Z, Yao J, Johns T, Fu K, De Bie I, Macmillan C, Cuthbert AP, Newbold RF, Wang J, Chevrette M, Brown GK, Brown RM, Shoubridge EA. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat Genet. 1998;20:337–343. doi: 10.1038/3804. [DOI] [PubMed] [Google Scholar]
- 29.Horoupian DS, Wiśniewski H. Neurofilamentous hyperplasia in inferior olivary hypertrophy. J Neuropathol Exp Neurol. 1971;30:571–582. doi: 10.1097/00005072-197110000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Katsuse O, Dickson DW. Inferior olivary hypertrophy is uncommon in progressive supranuclear palsy. Acta Neuropathol. 2004;108:143–146. doi: 10.1007/s00401-004-0878-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The patient had a fine horizontal pendular nystagmus in primary and all directions of gaze. There was significant ocular dysmetria of saccadic eye movements. Rhythmic twitching in her eyelids appeared bilaterally when she closed her eyes. Palatal tremor with irregular rate was present. She had postural tremor with a kinetic component in both hands that was more prevalent on the right side. She was able to walk independently but wobbled slightly. She was exceedingly cautious while walking with a tandem gait.