Abstract
Introduction
While regular exercise has been shown to alleviate the motor symptoms of Parkinson’s disease (PD), it remains unclear whether a physically active lifestyle may prevent PD.
Methods
To examine physical activities across the lifespan and risk of PD, we relied on data from a population-based case-control study that enrolled 357 incident PD cases and 341 controls. We assessed physical activity levels via self-report of (1) overall physical activity (PA) over 4 age periods; (2) competitive sports; and (3) occupational histories.
Results
PD risks were lower comparing the overall PA highest quartile (moderate to vigorous activities ≥180 metabolic equivalent task-hours/week (MET-h/wk)) with the lowest quartile (<47.8 MET-h/wk) in age-period 18–24 years (adjusted odds ratio (OR) 0.64, 95% confidence interval (CI) 0.40–1.02), and 45–64 years (OR 0.50, 95%CI 0.31–0.83) but not in age-period 25–44. Individuals who consistently engaged in overall PA at high levels (before age 65 years) had a 51% lower PD risk than those with low levels. Also, having participated in competitive sports prior to age 25 was inversely associated with PD (OR 0.53, 95% CI 0.31–0.91 for high level versus never). There was no association for measures of occupational physical activity though.
Conclusion
The long prodromal stage of PD makes it difficult to conclude whether insidious disease leads to a reduction of physical activity years before motor symptom onset and PD diagnosis. However, sports activities and high levels of overall PA in youth appear protective unless they are markers for biologic or genetic factors that lower PD risk.
Keywords: Parkinson’s disease, Physical activity, Exercise, Occupations
1. Introduction
Parkinson’s disease (PD) is characterized by progressive motor and non-motor impairments leading to disability, a considerable decline in health related quality of life, and a large financial and caretaker burden in aging societies. Many clinical trials have shown beneficial effects of exercise therapies in people with PD as summarized in meta-analyses; for example, two recent meta-analyses found that aerobic exercise or physical therapy improved motor scores based on the Unified Parkinson’s disease rating scale (UPDRS), as well as balance and gait compared with no intervention [1, 2]. Even though there is some evidence from animal experiments and PD intervention studies that intensive physical exercise induces neuroplasticity in the nigrostriatal dopaminergic system [3–6], less is known about the role lifetime physical activity may play for PD risk.
Some cohort studies (Supplementary Table 1) have suggested that those physically inactive or reporting prolonged daily TV viewing are more likely to develop PD [7–12]. However, many of these cohort studies relied on self-report of physical activity levels or recorded activity patterns at baseline only [11, 12], accrued small numbers of incident PD cases or followed populations with a life-style different from the general population [10, 11]. In addition, most previous studies focused on leisure time activities while only two considered work related activities [8, 10]. Activities at work and during leisure time likely entail very different physical demands, in terms of intensity, frequency and duration, and are known to affect all-cause mortality and cardiovascular disease differently [13, 14]. Our aim here is to examine whether the type and/or the timing of physical activity throughout adulthood affects risk of PD.
2. Methods
Data collection and procedures described in this study have been approved by the University of California, Los Angeles (UCLA), Institutional Review Board for human subjects, and written informed consent has been obtained from all participants.
2.1. Study Population
We conducted a population-based case-control study in largely agricultural counties in Central California. Details of the study have been provided elsewhere [15]. Briefly, we enrolled recently diagnosed PD patients (within 3 years of diagnosis) between 1998–2007, residing in Fresno, Kern, or Tulare counties, who at recruitment had lived in California for 5 years or more. Potential cases and controls were contacted by mail, by telephone, or both with eligibility criteria including: (1) being at least 35 years of age; (2) not too ill to participate; (3) currently living in one of the three designated counties; (4) having lived in California for five years or more; and (5) having PD for cases.
Of the 1,167 PD patients who responded to invitations, we excluded 604 who had their initial PD diagnosis 3 years prior to contact or did not fulfill the above inclusion criteria. Of the 563 eligible cases, 90 were too ill to be examined, moved or died prior to exam. The remaining eligible cases were examined by movement disorder specialists from UCLA to confirm PD diagnoses while 94 did not meet published criteria for idiopathic PD [16], an additional 13 were reclassified as not having idiopathic PD during our follow-up study [17], and 6 withdrew between examination and interview. Of the remaining 360 cases, 357 provided complete information on physical activity.
Population controls 65 years or older were first identified from Medicare lists (in 2001). Later, due to implementation of the Health Insurance Portability and Accountability Act (HIPPA), we recruited around 70% of controls of all ages from residential parcel tax assessor records in the tri-county area. Of the 1,212 potential controls, 457 were ineligible, and 409 declined participation due to illness or moving; a total of 341 individuals provided complete information on physical activity.
2.2. Assessment of Physical Activity
Trained interviewers blinded to case/control status conducted structured telephone interviews to obtain demographic and physical activity information, including self-report of (1) overall physical activity level across four age periods; (2) history of participation in competitive sports; and (3) occupational histories to create a job exposure matrix (JEM) and estimate occupational physical activity.
2.2.1. Overall Physical activity
Participants were asked to report average number of days per week and average number of hours per day during which they performed mild, moderate, or vigorous physical activity at work and leisure time, during 4 periods of adulthood: 18–24, 25–44, 45–64, and ≥ 65 years. Definitions and examples for intensity of activities were provided during interview. To account for both the effects of duration and intensity, we assigned metabolic-equivalent (MET) values to the activity intensities (vigorous activities as 8 and moderate activities as 4) [18] and created a cumulative physical activity measure — MET-hour per week (MET-h/wk) at each age period. Previous studies suggested that only moderate to vigorous activities were associated with PD risk and no effect was observed for mild activities [7, 8]; therefore we set “mild” activities to a MET value of zero to maximize the specificity of the physical activity measures. We also calculated the sum of the MET-hour per week for every year of adulthood before index date (PD onset in cases, interview date in controls) and divided by the total number of adult years to derive the average lifetime activity score.
2.2.2. Competitive sports history
If a participant reported ever having engaged in competitive sports, we collected information about type of sports, and ages at which they started and stopped. For every type of sport, a MET value was assigned according to published standard equivalents [19]. We then multiplied the MET value with the reported years for each sport, and summed those to derive cumulative sports measures (MET-year).
2.2.3. Occupational history
Participants were asked to report job titles, tasks, companies, industries, and duration (years) and frequency (hours per week) for all jobs in which they had worked for 6-months or more throughout their lifetime. We created a JEM to estimate occupational physical activity by coding information about jobs and industries based on the Integrated Public Use Microdata Series (IPUMS-USA) 2000 Occupation Code System [20], and assigned MET value to each job code [21]. We first multiplied the MET value with the reported years in each job, and summed those to derive cumulative occupational physical activity measures, and then calculated an average lifetime score by dividing the cumulative score by the total number of working years.
2.3. Statistical analyses
Logistic regression analysis were performed using SAS software version 9.3 (SAS Institute, Inc., Cary, North Carolina), with adjustment for age (continuous), gender, race (white, non-white), education (<12 years, 12 years, >12 years), smoking status (never, past, or current smoker), having a 1st degree family member with PD (yes, no), residential pesticide exposures (ever or never exposed) [22] and pesticide exposure estimates previously derived from a JEM (never, low, median or high exposure) [23]. We reported odds ratio (OR), 95% confidence intervals (95%CI), and p-values for trend based on the median of each exposure category.
We categorized the physical activity scores into quartiles based on the distribution of average lifetime MET scores in controls: <47.8, 47.8–93.0, 93.0–180.0, ≥180.0 MET-h/wk. Participants who never performed moderate and/or vigorous physical activities and those who fell in the 1st quartile of the MET distribution were considered less active and formed our reference group. Furthermore, we examined whether changes in overall physical activity over lifetime until age 64 were associated with PD risk. For each age period, participants were categorized as having high or low activity based on the overall median (93.0 MET-h/wk). We compared those who reported a consistently high activity, and those who reported either low-high or high-low trajectories to those who reported consistently low activity throughout life. For occupational physical activity measures, we categorized cumulative (MET-year) and average (MET) scores into quartiles based on the control distribution in each age period, and for cumulative sport activity scores, we considered those who never participated in any strenuous sport as reference and examined age periods specific tertiles (MET-year). We also included all three measures in the same model to mutually adjust for the different types of activity.
In sensitivity analyses we stratified by gender or excluded subjects with a PD diagnosis prior to age 60. Examining occupational physical activity, we also excluded participants with high occupational pesticide exposures, because we previously found pesticide exposure to contribute to PD risk in this population [23]. To assess the potential influence of preclinical changes in physical activity due to insidious disease onset, we used exposure lagging and discounted activities within 10-years or 20-years prior to the index date for cumulative measures.
3. Results
Study participants were in average 68 years of age at eligibility screen and predominantly white (Table 1). Cases were more frequently male, less highly educated, never smokers, and residentially and occupationally more heavily exposed to pesticides. The mean score on the Hoehn and Yahr scale was 2.10 (range 1–5) at baseline (mean PD duration: 1.71±1.18 years).
Table 1.
Sociodemographic characteristics of the study population in Central Valley of California, 2001–2007.
| PD Cases
|
Controls
|
|||
|---|---|---|---|---|
| N=357 | % | N=341 | % | |
| Mean age (years) [SD] | 68.29 | ±10.22 | 68.20 | ±11.42 |
| Gender | ||||
| Male | 205 | 57 | 176 | 52 |
| Female | 152 | 43 | 165 | 48 |
| Race | ||||
| White | 287 | 80 | 279 | 82 |
| Non-white | 70 | 20 | 62 | 18 |
| Education | ||||
| < 12 years | 66 | 18 | 38 | 11 |
| 12 years | 96 | 27 | 64 | 19 |
| > 12 years | 195 | 55 | 239 | 70 |
| Cigarette smoking status | ||||
| Never | 187 | 52 | 146 | 43 |
| Former | 150 | 42 | 161 | 47 |
| Current | 20 | 6 | 34 | 10 |
| PD Family History | ||||
| Yes | 52 | 15 | 37 | 11 |
| No | 305 | 85 | 281 | 82 |
| Residential pesticide exposure | ||||
| Dithiocarbamates | ||||
| Never | 198 | 55 | 228 | 67 |
| Ever | 159 | 46 | 113 | 33 |
| Organochlorines | ||||
| Never | 148 | 41 | 159 | 47 |
| Ever | 209 | 59 | 182 | 53 |
| Organophosphorus | ||||
| Never | 60 | 17 | 83 | 24 |
| Ever | 297 | 83 | 258 | 76 |
| Paraquat | ||||
| Never | 128 | 36 | 139 | 41 |
| Ever | 229 | 64 | 202 | 59 |
| Occupational Pesticide exposure | ||||
| None | 227 | 64 | 242 | 71 |
| Low | 23 | 6 | 25 | 7 |
| Medium | 56 | 16 | 47 | 14 |
| High | 51 | 14 | 27 | 8 |
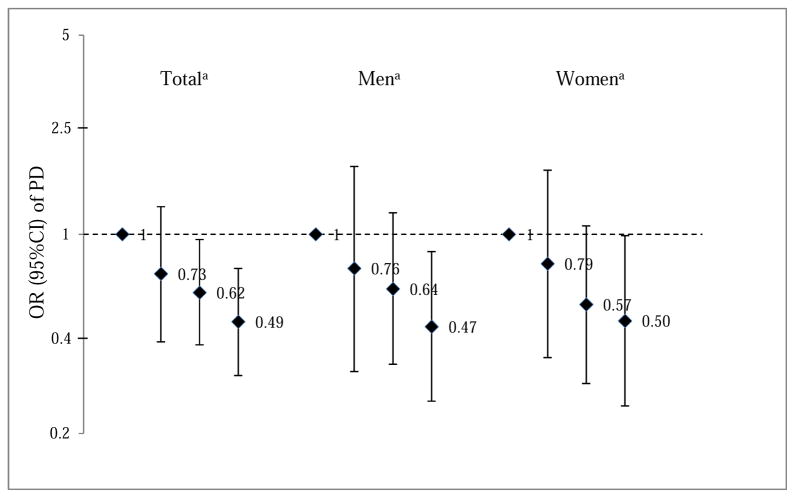
Higher levels of self-reported lifetime physical activity were inversely associated with PD risks (Table 2). Specifically, we found a 44% (OR 0.56, 95% CI=0.34–0.92) lower risk of PD among those with moderate to vigorous physical activity of at least 180 MET-hours per week on average in adulthood compared with the less active reference group (<47.8 MET-h/wk). Similar results were obtained for all age periods except for activity during 25–44 years of age. Examining the influence of changes in physical activity from youth to later adulthood, those who remained highly active throughout their lifetime were at the lowest risk of PD (OR=0.49, 95%CI 0.32–0.76), followed by those with a high-low or a low-high trajectory, compared with those who were consistently less active (Fig. 1).
Table 2.
Multivariable-adjusted odds ratios (aOR) with 95% confidence intervals (CI) and p-for-trend for Parkinson’s disease (PD) risk by four age periods of self-reported overall moderate to vigorous physical activitya.
| Overall physical activity [MET-hour/week]b | PD/Control | aOR | 95%CI | P-for-trendc |
|---|---|---|---|---|
| Lifetime Average | ||||
| <47.8 | 107/84 | 1 (Reference) | ||
| 47.8–93.0 | 75/79 | 0.68 | 0.43–1.07 | |
| 93.0–180.0 | 73/79 | 0.68 | 0.43–1.07 | |
| ≥180.0 | 81/78 | 0.56 | 0.34–0.92 | 0.05 |
| 18–24 years | ||||
| <47.8 | 86/68 | 1 (Reference) | ||
| 47.8–93.0 | 82/69 | 1.00 | 0.62–1.62 | |
| 93.0–180.0 | 63/79 | 0.64 | 0.39–1.05 | |
| ≥180.0 | 105/104 | 0.64 | 0.40–1.02 | 0.03 |
| 25–44 years | ||||
| <47.8 | 111/93 | 1 (Reference) | ||
| 47.8–93.0 | 67/73 | 0.78 | 0.49–1.22 | |
| 93.0–180.0 | 53/62 | 0.73 | 0.45–1.19 | |
| ≥180.0 | 105/92 | 0.82 | 0.53–1.28 | 0.33 |
| 45–64 years | ||||
| <47.8 | 136/112 | 1 (Reference) | ||
| 47.8–93.0 | 65/67 | 0.82 | 0.52–1.29 | |
| 93.0–180.0 | 63/58 | 0.97 | 0.60–1.56 | |
| ≥180.0 | 65/72 | 0.50 | 0.31–0.83 | 0.01 |
| ≥65 years | ||||
| <47.8 | 145/112 | 1 (Reference) | ||
| 47.8–93.0 | 51/48 | 0.89 | 0.54–1.46 | |
| 93.0–180.0 | 24/35 | 0.50 | 0.27–0.92 | |
| ≥180.0 | 18/21 | 0.65 | 0.31–1.37 | 0.08 |
Logistic regression models adjusted for age, gender, race, education, smoking, family history of PD, and residential and occupational pesticide exposures.
Overall Physical Activity (MET-h/wk) = 8*vigorous activity hour/week + 4*moderate activity hour/week; quartiles according to the average lifetime physical activity distribution in controls.
Linear trend was tested using the midpoint of each exposure category as a continuous variable in the regression model.
Fig 1.
Multivariable-adjusted odds ratios (OR) with 95% confidence intervals (CI) of Parkinson’s disease (PD) according to changes of overall moderate to vigorous physical activities before age 65. The analysis adjusted for age, gender, race, education, smoking, family history of PD, and residential and occupational pesticide exposures.
- Low-Low trajectory (reference group): low activity at 18–24, 25–44 and 45–64 age periods.
- Low-High trajectory: low activity at 18–24, and high activity at either 25–44 or 45–64 or both age periods.
- High-Low trajectory: high activity at 18–24, and low activity at either 25–44 or 45–64 or both age periods.
- High-High trajectory: high activity at 18–24, 25–44 and 45–64 age periods.
Having participated in competitive sports was inversely associated with PD risk (Table 3, supplementary Table 2). Compared with individuals who never participated in competitive sports, for those who reported a high sports activity level prior to 25 years of age, we estimated a 47% lower risk of PD (OR 0.53, 95%CI 0.31–0.91; p for trend = 0.04). Cumulative or average scores for occupational physical activity were not associated with PD risk (Table 3).
Table 3.
Multivariable-adjusted odds ratios (aOR) with 95% confidence intervals (CI) and p-for-trend for Parkinson’s disease (PD) risk by competitive sports and occupational activitya.
| Cumulative physical activity [MET-year]b | PD/Control | aOR | 95%CI | P-for-trendc |
|---|---|---|---|---|
| Competitive Sports | ||||
| Lifetime | ||||
| Never | 184/160 | 1 (Reference) | ||
| <42 | 69/61 | 0.99 | 0.63–1.56 | |
| 42–105 | 58/59 | 0.94 | 0.58–1.52 | |
| ≥105 | 40/58 | 0.62 | 0.37–1.04 | 0.06 |
| 18–24 years | ||||
| Never | 192/168 | 1 | ||
| <40 | 63/59 | 0.97 | 0.61–1.52 | |
| 40–91 | 66/56 | 1.06 | 0.67–1.70 | |
| >91 | 30/55 | 0.53 | 0.31–0.91 | 0.04 |
| Occupational Activity | ||||
| Lifetime | ||||
| <56.7 | 89/85 | 1 (Reference) | ||
| 56.7–89.3 | 69/86 | 0.76 | 0.47–1.22 | |
| 89.3–130.8 | 86/84 | 0.85 | 0.51–1.43 | |
| ≥130.8 | 109/85 | 0.78 | 0.44–1.39 | 0.49 |
| 18–24 years | ||||
| <12.0 | 122/113 | 1 (Reference) | ||
| 12.0–22.5 | 70/76 | 0.92 | 0.60–1.43 | |
| 22.0–37.6 | 66/74 | 0.76 | 0.47–1.21 | |
| ≥37.6 | 94/76 | 0.97 | 0.59–1.59 | 0.86 |
| 25–44 years | ||||
| <30.7 | 90/102 | 1 (Reference) | ||
| 30.7–50.0 | 94/83 | 1.46 | 0.92–2.33 | |
| 50.0–65.8 | 62/75 | 0.87 | 0.52–1.46 | |
| ≥65.8 | 106/79 | 1.21 | 0.70–2.08 | 0.72 |
| 45–64 years | ||||
| <22.5 | 121/126 | 1 (Reference) | ||
| 22.5–36.5 | 71/68 | 0.97 | 0.61–1.55 | |
| 36.5–52.5 | 69/73 | 0.86 | 0.53–1.39 | |
| ≥52.5 | 91/72 | 0.86 | 0.52–1.42 | 0.51 |
Logistic regression models were adjusted for age, gender, race, education, smoking, family history of PD, and residential and occupational pesticide exposures.
Competitive Sports = sum of sport MET value*year of participation (MET-year); tertiles – age periods specific .
Occupational Activity = sum of job MET value*year of participation (MET-year); quartiles – age periods specific.
Linear trend was tested using the midpoint of each exposure category as a continuous variable in the regression model.
In sensitivity analyses, no apparent gender specific effect was found for overall physical activity or competitive sports measures, and higher levels of occupational physical activity did not reduce PD risk overall or in males only (supplementary Table 3). Results from lagged analyses that excluded physical activity 10-years or 20-years prior to PD diagnosis (supplementary Table 4) for cumulative measures were similar to unlagged estimates. Excluding participants with high occupational pesticide exposure (supplementary Table 5) or excluding PD cases diagnosed prior to age 60 also did not substantially change all physical activity estimates (results not shown).
4. Discussion
In this population-based case-control study, we examined three different measures of physical activity and found that higher lifetime moderate to vigorous activity, especially consistently high level of such activities throughout adulthood, reduced the risk of developing PD. Controls who did not developed PD had more often engaged in strenuous competitive sports in their youth. However, occupational physical activity did not influence PD risk.
Our findings agree with a meta-analysis of five prospective studies reporting that being physically active reduces PD risk with a 34% lower risk estimated for the highest versus the lowest activity levels [10]. Some of these studies, however, only examined leisure time physical activity at baseline (between age 50–60) [7, 9, 24], and did not account for other types of physical activity (e.g. occupational, household and commuting activities). A Swedish study [10], reported an inverse association with PD risk for the sum of household, commuting and leisure time exercise and total physical activity, but no associations for occupational activity or leisure time exercise when analyzed separately. Similarly, our overall physical activity measure included activities at work and leisure time, and higher activities according to this overall measure were inversely associated with PD risk. Our occupational measure covered all lifetime job-related activities and considered intensity and duration while the Swedish study only asked about job intensity in the past year [10]. Both studies do not support a protective role for occupational activities. Interestingly, the lack of beneficial effects for job-related physical activity in cardiovascular disease and all-cause mortality [13, 14] has recently been coined the ‘health paradox’ of physical activity [25].
We collected physical activity data according to age periods, and found inverse association with PD in all except the 25–44 year period. Careers and child rearing demands may restrict leisure time or sports activities in this period such that occupational activity may be the main activity to overall physical activity. The only other paper [8] that examined physical activity during several age periods reported only on leisure time activity and reported inverse associations with PD during 35–39 years and 50–60 years of age but not in young adulthood (ages 15–29). Yet, our evaluation of activity trajectories throughout adulthood is consistent with this study’s findings suggesting that individuals who remain highly active throughout life are at lowest risk of PD [8].
Similar to previous study, we cannot dismiss the possibility of reverse causation because decreasing physical activity before disease onset might be a prodromal PD symptom. However, our estimates did not substantially change after excluding physical activity reported during 10- or 20-years before diagnosis. We also found that those who were consistently active throughout life or active in at least one of the age periods were at lower risk of PD, compared with those maintaining low physical activity levels throughout life. This suggests that staying or becoming active in adulthood may protect against PD. Further, the protective associations found for high physical activity and sports activities in youth would not be affected by reverse causation unless an active lifestyle depends on genetic factors that also lower PD risk.
Physical activity may reduce the risk of PD by increasing cerebrovascular circulation and improving the production of neurotransmitters, including neurotrophic substances [3, 4]. In rodent models of PD, animals forced to exercise before or after toxin treatments had more remaining dopaminergic neurons and terminals as well as less motor deficits than immobilized animals [26, 27]. Forced exercise was found to stimulate neurotrophic factors and downregulation of the dopamine transporter, which may contribute to neuroplasticity and reduce vulnerability to neurotoxicants [3, 4]. In early stages of PD, 8 weeks of high-intensity treadmill training (MET ≥3 or 60–80% age-adjusted maximal heart rate) has been shown to induce cortico-motor excitability and an increase in dopamine D2 receptor binding potential in the dorsal striatum, along with motor function improvement [5, 6]. Alternatively, high intensity exercise may induce elevations in plasma urate [28], an anti-oxidant, and high urate levels have been associated with lower PD risk and slower disease progression [29].
Compared with other studies, we measured physical activity levels more comprehensively using multiple approaches. First, we accounted for the two most important sources of physical activity – occupation and leisure time sports activity – by age periods. This allowed us to evaluate the effect of changes in physical activity levels, reflecting a life course perspective and possibly identify periods of importance for interventions. Second, to over-come some of the recall problems in our case-control design, we asked participants to report their lifetime history of competitive sports and job-related activities. Based on the latter, we created an exposure matrix for which raters assigned physical activity levels to job titles and tasks while blinded to case status. To our knowledge, we are the first PD study that used a JEM to examine occupational physical activity. Though the possibility exists that cases spent more effort to recall details of their job histories, we found that the reported number of occupations and work years was similar for cases and controls [23].
Our study with nearly 360 PD cases enrolled a substantially larger number of PD affected participants than most previous studies [10, 11, 24, 30]. Moreover, ours is the only epidemiologic population-based study in which movement disorder specialists examined 71% of patients multiple times over almost a decade to confirm a diagnosis of idiopathic PD, minimizing disease misclassification potential. PD ascertainment in prospective cohort studies is generally based on self-reported PD diagnoses and medical records review that relies heavily on a patients’ access to quality health care [7, 8, 24, 30]. PD diagnoses strongly depends on clinical evaluations, thus diagnostic and reporting errors may be differential for those who are more health conscious and thus more physically active.
The present study has several limitations. Our JEM approach assumes that everyone with the same job title/tasks had a similar level of physical activity and thus was given the same MET value. This inevitably introduces non-differential misclassification errors because of likely within-job variability. Moreover, female participants may simply report housewife/homemaker, and this category is hard to judge in terms of physical activity. In our farming population, ‘housework’ may also be physically intensive for women who engaged in farming or gardening but do not consider this a job – these activities, however, would have been captured by our overall physical activity measure. Furthermore, our study was conducted in largely agricultural counties in Central California, which may limit the generalizability of our findings. Given the high correlation between active manual labor on farms and occupational pesticide exposure, controlling for pesticide exposure was important. Also, sensitivity analyses excluding participants with high occupational pesticide exposure did not change our results for occupational physical activity. An advantage of our rural study population is that our study included a wide range of occupational PA including physically demanding farm labor that should have increased our ability to detect associations. Although non-differential exposure misclassification remains a concern and may have biased our results towards the null, our findings indicate that the beneficial effects of overall physical activity and leisure time sports activity are not observed for occupational physical activity in this agricultural population.
In conclusion, we found that lifetime overall physical activity, and participation in competitive sports during young ages were negatively associated with PD risk, but found no beneficial role for occupational physical activity. Our results provide further support for a previous meta-analysis of 5 studies that concluded higher leisure-time physical activity levels are associated with lower PD risk [10].
Supplementary Material
HIGHLIGHTS.
Engaging in moderate to vigorous activities in young and later adulthood is associated with lower risk of PD.
Individuals physically active in youth who remain active throughout life are at the lowest risk of developing PD.
Participation in competitive sports in youth but not lifetime occupational physical activity lowers PD risk.
Acknowledgments
Funding: Research reported in this publication was supported by the NIEHS of the National Institutes of Health under award number R01ES10544; pilot funding for the study was received from the SCEHSC# 5P30 ES07048 and The American Parkinson Disease Association.
We thanks Dr. Bronstein and Dr. Bordelon, all study participants, and the Parkinson’s disease support groups in the California central valley for their support and contributions.
Footnotes
Conflict of interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shu H-F, Yang T, Yu S-X, Huang H-D, Jiang L-L, Gu J-W, et al. Aerobic exercise for Parkinson's disease: a systematic review and meta-analysis of randomized controlled trials. PloS one. 2014;9:e100503. doi: 10.1371/journal.pone.0100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ. 2012;345:e5004. doi: 10.1136/bmj.e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch M, Farley B. Exercise and neuroplasticity in persons living with Parkinson’s disease. European journal of physical and rehabilitation medicine. 2009;45:215–29. [PubMed] [Google Scholar]
- 4.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288–94. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher BE, Li Q, Nacca A, Salem GJ, Song J, Yip J, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport. 2013;24:509–14. doi: 10.1097/WNR.0b013e328361dc13. [DOI] [PubMed] [Google Scholar]
- 6.Fisher BE, Wu AD, Salem GJ, Song J, Lin C-HJ, Yip J, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Archives of physical medicine and rehabilitation. 2008;89:1221–9. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thacker EL, Chen H, Patel AV, McCullough ML, Calle EE, Thun MJ, et al. Recreational physical activity and risk of Parkinson's disease. Movement disorders. 2008;23:69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–8. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Zhang S, Schwarzschild M, Hernan M, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–9. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Lagerros YT, Bellocco R, Adami H-O, Fang F, Pedersen NL, et al. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain. 2015;138:269–75. doi: 10.1093/brain/awu323. [DOI] [PubMed] [Google Scholar]
- 11.Sääksjärvi K, Knekt P, Männistö S, Lyytinen J, Jääskeläinen T, Kanerva N, et al. Reduced risk of Parkinson’s disease associated with lower body mass index and heavy leisure-time physical activity. European journal of epidemiology. 2014;29:285–92. doi: 10.1007/s10654-014-9887-2. [DOI] [PubMed] [Google Scholar]
- 12.Keadle SK, Moore SC, Sampson JN, Xiao Q, Albanes D, Matthews CE. Causes of death associated with prolonged TV viewing: NIH-AARP Diet and Health Study. American journal of preventive medicine. 2015;49:811–21. doi: 10.1016/j.amepre.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen LB, Schnohr P, Schroll M, Hein HO. All-cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Archives of internal medicine. 2000;160:1621–8. doi: 10.1001/archinte.160.11.1621. [DOI] [PubMed] [Google Scholar]
- 14.Krause N. Physical activity and cardiovascular mortality-disentangling the roles of work, fitness, and leisure. Scandinavian journal of work, environment & health. 2010:349–55. doi: 10.5271/sjweh.3077. [DOI] [PubMed] [Google Scholar]
- 15.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. American journal of epidemiology. 2009;169:919–26. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 2001;57:S34–S8. [PubMed] [Google Scholar]
- 17.Ritz B, Rhodes SL, Bordelon Y, Bronstein J. alpha-Synuclein genetic variants predict faster motor symptom progression in idiopathic Parkinson disease. PloS one. 2012;7:e36199. doi: 10.1371/journal.pone.0036199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Committee IR. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)–short and long forms. 2005;17:2008. Retrieved September. [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and science in sports and exercise. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed June 2010];Public Use Microdata Series (IPUMS-USA) Occupation Code System. 2000 http://usa.ipums.org/usa/volii/00occup.shtml.
- 21.Tudor-Locke C, Ainsworth BE, Washington TL, Troiano R. Assigning metabolic equivalent values to the 2002 census occupational classification system. Journal of Physical Activity and Health. 2011;8:581–6. doi: 10.1123/jpah.8.4.581. [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. European journal of epidemiology. 2011;26:547–55. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew Z, Wang A, Bronstein J, Ritz B. Job Exposure Matrix (JEM)-Derived Estimates of Lifetime Occupational Pesticide Exposure and the Risk of Parkinson's Disease. Archives of environmental & occupational health. 2014;69:241–51. doi: 10.1080/19338244.2013.778808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logroscino G, Sesso HD, Paffenbarger R, Lee I-M. Physical activity and risk of Parkinson’s disease: a prospective cohort study. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77:1318–22. doi: 10.1136/jnnp.2006.097170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtermann A, Hansen J, Burr H, Søgaard K, Sjøgaard G. The health paradox of occupational and leisure-time physical activity. British Journal of Sports Medicine. 2012;46:291–5. doi: 10.1136/bjsm.2010.079582. [DOI] [PubMed] [Google Scholar]
- 26.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. Journal of neurochemistry. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 27.Tillerson J, Caudle W, Reveron M, Miller G. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 28.Green HJ, Fraser I. Differential effects of exercise intensity on serum uric acid concentration. Medicine and science in sports and exercise. 1988;20:55–9. doi: 10.1249/00005768-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson's disease risk, diagnosis and prognosis. Biomarkers in medicine. 2010;4:701–12. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson’s disease in Greece. European journal of epidemiology. 2013;28:67–77. doi: 10.1007/s10654-012-9760-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.