Abstract
In the last few years, a rapidly growing number of autoantibodies targeting neuronal cell-surface antigens have been identified in patients presenting with neurological symptoms. Targeted antigens include ionotropic receptors such as N-methyl-D-aspartate receptor or the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, metabotropic receptors such as mGluR1 and mGluR5, and other synaptic proteins, some of them belonging to the voltage-gated potassium channel complex. Importantly, the cell-surface location of these antigens makes them vulnerable to direct antibody-mediated modulation. Some of these autoantibodies, generally targeting ionotropic channels or their partner proteins, define clinical syndromes resembling models of pharmacological or genetic disruption of the corresponding antigen, suggesting a direct pathogenic role of the associated autoantibodies. Moreover, the associated neurological symptoms are usually immunotherapy-responsive, further arguing for a pathogenic effect of the antibodies. Some studies have shown that some patients’ antibodies may have structural and functional in vitro effects on the targeted antigens. Definite proof of the pathogenicity of these autoantibodies has been obtained for just a few through passive transfer experiments in animal models. In this review we present existing and converging evidence suggesting a pathogenic role of some autoantibodies directed against neuronal cell-surface antigens observed in patients with central nervous system disorders. We describe the main clinical symptoms characterizing the patients and discuss conflicting arguments regarding the pathogenicity of these antibodies.
Keywords: antibodies, cerebellitis, encephalitis, NMDAR, synaptopathies
Introduction
In the last few years, a rapidly growing number of patients with non-infectious, autoimmune encephalitis associated with autoantibodies targeting the central nervous system (CNS) have been described. The first anti-neuronal autoantibodies – anti-Hu (Szabo et al., 1991), anti-Yo, anti-CV2/CRMP5 (Honnorat et al., 1996) and anti-Ri (Pittock et al., 2003) – were identified about 25 years ago in paraneoplastic neurological syndromes (PNSs). These anti-neuronal autoantibodies recognize intracellular antigens and therefore do not appear to be directly pathogenic, but may be used as diagnostic biomarkers of cancer (Honnorat, 2006; Raspotnig et al., 2011). Cytotoxic neuronal damage and neuronal death are responsible for symptoms found in these PNSs and immunization with the paraneoplastic antigen does not induce disease in rodents, further supporting a non-pathogenic role of the respective autoantibodies (Sillevis Smitt et al., 1995). Rather, the antibodies may reflect a T-cell-mediated immune response against neurons (Bien et al., 2012). On the other hand, an increasing number of autoantibodies targeting neuronal cell-surface antigens have been identified recently in patients presenting with neurological symptoms resembling PNSs. Targets include ionotropic receptors including N-methyl-D-aspartate receptor (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), γ-aminobutyric acid (GABA)AR and glycine receptor (GlyR); metabotropic receptors such as mGluR1, mGluR5 and GABABR; and proteins belonging to the voltage-gated potassium channel (VGKC) complex, namely Lgi1 and Caspr2. Several key features differentiate patients with cell-surface autoantibodies from patients with autoantibodies recognizing intracellular targets: (i) associated neurological symptoms are usually immunotherapy-responsive and reversible, suggesting a direct pathogenic effect of antibodies without cytotoxically induced neuronal loss; and (ii) tumour association is far less consistent. Encephalitis associated with antibodies directed against cell-surface antigens such as NMDAR or VGKC complex are much more common than PNSs with intracellular targets and were identified to account for ~8% of encephalitis cases in a population-based prospective study in England (Granerod et al., 2010). The location of cell-surface antigens allows their recognition by autoantibodies on the intact cell and evidence suggests that these antibodies may play a direct pathogenic role in the neurological and psychiatric symptoms of patients with encephalitis. The purpose of this review is to present existing and converging evidence suggesting a pathogenic role of some autoantibodies directed against neuronal cell-surface antigens observed in patients with encephalitis. We describe the main clinical symptoms characterizing the patients and discuss conflicting arguments regarding the pathogenicity of these antibodies.
Encephalitis associated with antibodies directed against NMDAR
Definition and clinical presentation
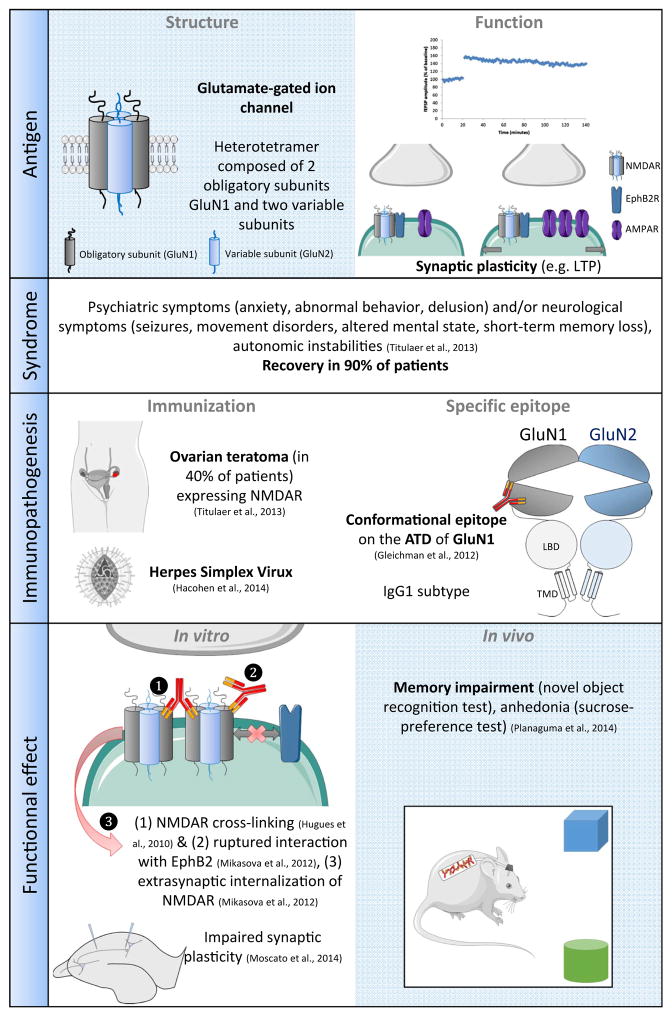
Encephalitis associated with anti-NMDAR autoantibodies is a clinico-biological entity defined by a clinical presentation of encephalitis, and the presence of IgG antibodies in the cerebrospinal fluid (CSF) that are directed against the GluN1 subunit of the NMDAR (Dalmau et al., 2008). Detection of anti-NMDAR antibodies relies on two tests: a specific immunohistochemical staining pattern on rat brain section with patients’ IgG; and patients’ IgG binding to NMDAR-expressing HEK cells in a cell-based assay (Dalmau et al., 2008; Gresa-Arribas et al., 2014; Viaccoz et al., 2014). Encephalitis with anti-NMDAR antibodies was initially described in 2007 in 12 women with associated ovarian teratoma (Dalmau et al., 2007). The clinical presentation was more comprehensively described 1 year later in 100 autoantibody-positive encephalitis patients (Dalmau et al., 2008). To date, several hundred cases have been published (Titulaer et al., 2013), indicating a relatively frequent disorder. Accordingly, NMDAR encephalitis was identified as the most common cause of non-infectious encephalitis in patients under 30 years old (Gable et al., 2012). Among all autoimmune encephalitides, NMDAR encephalitis is the most frequent (Thomas et al., 2014). The clinical disorder, predominantly affecting young women, shows a progression of similar symptoms (Titulaer et al., 2013), usually starting with a prodromal syndrome including headache, low-grade fever or non-specific viral-like illness followed by the development of psychiatric symptoms (anxiety, agitation, delusional thoughts, hallucinations, and personality or behavioural changes) and/or neurological symptoms (seizures, movement disorders, catatonia, alteration of mental state, speech impairment and rapid development of short-term memory loss) within the next days or weeks. During the course of the disease, patients often develop decreased responsiveness, abnormal movements (the most characteristic being oro-facial dyskinesia) and autonomic instabilities (Dalmau et al., 2008, 2011). Only a few adult male patients have been described and tend to present with seizures as first symptoms whereas female patients usually present with psychiatric symptoms (Viaccoz et al., 2014). Psychiatric symptoms and abnormal movements are more prominent in children (Florance et al., 2009). A good recovery is generally observed in about 80% of patients but is typically slow, occurring in stages following the reverse order from symptom appearance (Dalmau et al., 2011; Titulaer et al., 2013; Gresa-Arribas et al., 2014). In the largest published series, more than 75% of patients recovered fully or retained only mild deficits (Dalmau et al., 2008). An underlying neoplasm is found in about 40% of patients, primarily in females aged between 12 and 45 years (Dalmau et al., 2011; Titulaer et al., 2013). More than 90% of all tumours associated with anti-NMDAR encephalitis are ovarian teratoma, while the frequency of an underlying tumour is less than 5% in men and children (Titulaer et al., 2013). All immunopathological studies of teratomas associated with NMDAR encephalitis revealed the presence of GluN1-expressing nervous tissue (Dalmau et al., 2008; Tüzün et al., 2009). In more than 60% of cases, no tumour is found and mechanisms leading to the breaking of immunological tolerance remain unclear. In a few cases, anti-NMDAR antibodies have been observed a few weeks after a typical herpes simplex encephalitis, suggesting that viral encephalitis may sometimes trigger anti-NMDAR auto-immunity (Prüss et al., 2012; Armangue et al., 2013; Hacohen et al., 2014) (Fig. 1).
Fig. 1.
Example of encephalitis associated with anti-NMDAR autoantibodies.
Antigen
NMDAR belong to the family of ionotropic glutamate receptors (iGluRs). They are involved in excitatory neurotransmission and are the pivots of synaptic plasticity (e.g. long-term potentiation and long-term depression) underlying cognitive functions such as learning and memory. Functional NMDAR are heterotetramers consisting of two obligatory GluN1 subunits and GluN2 or GluN3 subunits. Antibodies of patients with anti-NMDAR encephalitis target the obligatory subunit GluN1 (Dalmau et al., 2008; Gleichman et al., 2012). Each NMDAR subunit comprises two extracellular domains, the amino-terminal domain (ATD) and the ligand-binding domain, one transmembrane domain and a large intracellular domain (Paoletti et al., 2013). Remarkably, this subunit structure is conserved for all members of the iGluR family (Traynelis et al., 2010). Antibodies of patients with anti-NMDAR encephalitis target a conformational epitope located in the ATD of the GluN1 subunit, in a small region located near the hinge of a clamshell-like structure (Gleichman et al., 2012). Within this region, the amino acid N368 was identified as crucial for patients’ anti-NMDAR antibody binding (Gleichman et al., 2012).
Antibody pathogenicity
Strong clinical arguments support the hypothesis of an antibody-mediated pathogenicity in anti-NMDAR encephalitis. First, pharmacological antagonists of NMDAR (e.g. ketamine) may induce specific clinical symptoms similar to those presented by patients with anti-NMDAR encephalitis. Moreover, the majority of patients respond well to plasma exchanges and intravenous immunoglobulins, often with full recovery (Titulaer et al., 2013). Furthermore immunotherapy targeting an antibody producing CD20+ B-cells (e.g. Rituximab) improves patients’ recovery (Titulaer et al., 2013). Substantial evidence points toward an intrathecal synthesis of autoantibodies in anti-NMDAR encephalitis. Indeed, in patients with preserved blood–brain barrier integrity, antibody titres are higher in the CSF than in the serum (Dalmau et al., 2007, 2008). Accordingly, several immunopathological studies reported deposits of IgG, B-cells and plasma cells cuffing in the brain perivascular spaces (Dalmau et al., 2007, 2008; Tüzün et al., 2009; Hughes et al., 2010; Camdessanché et al., 2011). Although anti-NMDAR antibodies are of the subclasses IgG1 and IgG3 that are capable of complement activation, no deposits of complement were found (Tüzün et al., 2009; Hughes et al., 2010). Taken together, these data support the hypothesis of an antibody-mediated pathogenicity, without major involvement of T-cells or complement-mediated cytotoxicity.
Incubation of neuronal cultures with patients’ NMDAR autoantibodies caused a selective and reversible decrease in NMDAR surface density and synaptic localization without affecting other synaptic components, neuronal morphology or causing neuronal loss (Hughes et al., 2010; Moscato et al., 2014). The effect size correlated with patients’ autoantibody titres. The mechanism of this decrease is selective antibody-mediated capping and internalization of surface NMDAR (Hughes et al., 2010). NMDAR antibody binding does not appear to distinguish between excitatory and inhibitory neurons (Moscato et al., 2014). Interestingly, patients’ antibody-mediated decrease of NMDAR clusters relies upon the presence of the Fc domain of anti-NMDAR IgG that allows cross-linking of NMDAR (Hughes et al., 2010; Moscato et al., 2014) and suggests an antibody-mediated alteration of NMDAR surface diffusion. In accordance with those results, a subsequent study confirmed the effect of patients’ NMDAR antibodies on NMDAR surface diffusion by disruption of NMDAR and EphrinB2 receptor (EPHB2R) interactions (Mikasova et al., 2012) leading to NMDAR internalization. Activation of EPHB2R by its ligand EphrinB2 prevents patients’ NMDAR antibodies’ effects on their target (Mikasova et al., 2012). Data from several electrophysiological recordings performed in in vitro and ex vivo studies point toward a functional effect of patients’ NMDAR antibodies. Whole cell-patch clamp recordings showed that application of patients’ CSF decreased the NMDAR-mediated component of excitatory currents (Hughes et al., 2010). A decrease of the NMDAR-mediated current seems to be caused only by internalization of NMDAR and not by an antagonistic effect (Moscato et al., 2014). Acute application of patients’ CSF also decreased neuronal network activity in neuronal cultures (Jantzen et al., 2013). Finally, two studies reported an alteration of NMDAR-mediated plasticity by patients’ NMDAR antibodies as they prevented induction of chemical long-term potentiation (LTP) (Mikasova et al., 2012) and electrophysiologically induced LTP in neuronal cell cultures (Zhang et al., 2012).
Independent studies reported a reduction of NMDAR clusters after intrathecal administration of NMDAR antibodies (Hughes et al., 2010; Mikasova et al., 2012; Planaguma et al., 2014). Interestingly, this effect was partly reversed by co-injection of EphrinB2 (Mikasova et al., 2012) confirming the above described in vitro studies. Microdialysis after hippocampal injection of patients’ NMDAR antibodies in rodents revealed an increase in extracellular glutamate concentration (Manto et al., 2010). Furthermore, injection of the motor prefrontal cortex with patients’ NMDAR antibodies increased motor stimulation after high-frequency stimulation (Manto et al., 2011a). Both studies support the hypothesis of induction of hyperactivity of glutamatergic pathways by patients’ NMDAR antibodies. Recently, an in vivo study (Planaguma et al., 2014) demonstrated that continuous intrathecal infusion of patients’ CSF induced behavioural changes in mice (memory impairment, depressive-like and anhedonic behaviour; all symptoms also observed in patients). These effects were reversible upon cessation of patients’ CSF infusion. No effect of patients’ CSF infusion was observed on anxiety, aggressiveness and locomotor activity (Planaguma et al., 2014).
To summarize, anti-NMDAR encephalitis is the best-described autoimmune encephalitis and appears to be a disorder much more common than initially expected. A direct pathogenic effect of NMDAR antibodies is suggested by altered behaviour in mice partly mimicking anti-NMDAR encephalitis symptoms after infusion with patients’ CSF (Planaguma et al., 2014), clinical data, patients’ outcome, and other in vitro and in vivo studies. The possible mechanisms of NMDAR antibody action on NMDAR have started to be elucidated in vitro (Dalmau et al., 2008; Hughes et al., 2010; Gleichman et al., 2012; Mikasova et al., 2012), arguing for an antigenic modulation. Yet studies on the long-term effect of NMDAR antibodies on neuronal networks and pathways underlying synaptic plasticity are still lacking. Although clinical data such as treatment response and histopathological studies are not in favour of the involvement of cellular-mediated cytotoxicity, further experimental research is still needed to confirm this impression.
Encephalitis associated with antibodies directed against AMPAR
Definition and clinical presentation
Limbic encephalitis associated with antibodies directed against AMPAR was identified in 2009 in 10 of 109 patients with limbic encephalitis (Lai et al., 2009). Subsequently, three studies describing a few cases were reported (Graus et al., 2010; Hoftberger et al., 2015; Joubert & Honnorat, 2015). The majority of patients were women (60%) with a median age around 60 years (Lai et al., 2009; Graus et al., 2010; Hoftberger et al., 2015; Joubert & Honnorat, 2015). Clinical features are more variable than in anti-NMDAR encephalitis. The most common presentation reported is classical limbic encephalitis dominated by short-term memory loss, confusion and abnormal behaviour (Lai et al., 2009; Hoftberger et al., 2015). Diffuse encephalitis with limbic dysfunction associated with various other symptoms (e.g. seizures, psychiatric manifestations, ataxia, abnormal movements, aphasia and neuropathy) has also been described (Spatola et al., 2014; Hoftberger et al., 2015). A few patients presented with fulminant encephalitis (Wei et al., 2013; Joubert & Honnorat, 2015), and a few cases of atypical presentation with prominent psychiatric symptoms resembling acute psychosis have also been reported (Graus et al., 2010; Hoftberger et al., 2015). Additional associated antibodies recognizing cell-surface (GABABR, NMDAR, LGI1, VGCC), intracellular (SOX1, Hu) or synaptic (amphiphysin, GAD) targets have been identified in about 30% of these patients (Lai et al., 2009; Hoftberger et al., 2015; Joubert et al., 2015). Overlapping immune responses may complicate clinical presentations and explain some aspects of the clinical heterogeneity observed in patients with anti-AMPAR encephalitis (Lai et al., 2009; Hoftberger et al., 2015). Associated tumours (thymus, lung, breast) were observed in 65% of patients with anti-AMPAR encephalitis (Lai et al., 2009; Hoftberger et al., 2015) and these tumours expressed AMPAR (Lai et al., 2009). This finding supports the hypothesis of a break in immune tolerance induced by ectopic expression of AMPAR by the underlying neoplasm. However, anti-AMPAR encephalitis can occur without an identified tumour (Lai et al., 2009). In these cases, the immune trigger remains unknown. Patients usually respond to immunotherapy although relapses may occur (Lai et al., 2009). Aggressive immunotherapy significantly reduces risks of relapses (Hoftberger et al., 2015). Regarding clinical presentation and severity, the outcome is heterogeneous and the presence of an underlying tumour seems to be associated with a poor outcome (Hoftberger et al., 2015).
Antigen
Like NMDAR, AMPAR belong to the family of iGluRs. AMPAR mediate fast excitatory transmission in the brain. They are required for basal synaptic transmission and are involved in the mechanisms of synaptic plasticity (Santos et al., 2009). AMPAR are heterotetramers composed of four subunits (GluA1 to GluA4) (Traynelis et al., 2010). Patients’ antibodies are IgG of uncharacterized subtype targeting GluA1 and/or GluA2 subunits of AMPAR, with a higher incidence of GluA2 antibodies (Lai et al., 2009; Hoftberger et al., 2015). Like NMDAR subunits, each AMPAR subunit is composed of two extracellular domains, the ATD and the ligand-binding domain, a transmembrane domain and an intracellular domain. The bottom lobe of the ATD is the main receptor epitope recognized by AMPAR antibodies (Gleichman et al., 2014). This region matches the location of the epitope on the NMDAR in patients with anti-NMDAR encephalitis, suggesting that the bottom lobe of the iGluRs might be particularly antigenic (Gleichman et al., 2014). The possible detection of AMPAR antibodies by western blot suggests a non-conformational epitope (Gleichman et al., 2014).
Antibody pathogenicity
As in patients with anti-NMDAR encephalitis, clinical data tend to support the hypothesis of AMPAR-antibody-mediated pathogenicity. Clinical improvement after immune therapy occurred concurrent with a decline in CSF antibody titre, supporting a pathogenic role of the antibodies (Wei et al., 2013). Some evidence indicates that patients’ AMPAR antibody synthesis is intrathecal; antibody titres were higher in the CSF than in the serum (Lai et al., 2009) and some patients have antibodies only detected in CSF (Hoftberger et al., 2015). Moreover, serum AMPAR antibodies differ in their epitope specificity from CSF antibodies (Gleichman et al., 2014). Concerning the pathological effects of antibodies on affected brain structures, diffuse cortical atrophy was observed in a few patients with anti-AMPAR encephalitis (Joubert & Honnorat, 2015), suggesting a possible neuronal loss in these patients. However, neuropathological data to support this hypothesis are lacking.
Patients’ CSF specifically decreases the number of AMPAR and alter their synaptic localization in cultured neurons. This effect is reversible upon removal of antibodies (Lai et al., 2009). In vitro, patients’ AMPAR antibodies do not seem to alter other components of glutamatergic synapses, or excitatory synapse density or cell viability (Lai et al., 2009; Peng et al., 2015). Cross-linking assay using patients’ F(ab) fragment antibodies or full patient antibodies have not been published and the mechanisms leading to this AMPAR internalization have not yet been demonstrated. Functionally, patients’ AMPAR antibodies specifically alter AMPAR-mediated synaptic transmission, as demonstrated by the reduction of amplitude and frequency of excitatory current recordings in cultured neurons treated with patients’ CSF (Gleichman et al., 2014; Peng et al., 2015). Interestingly, patients’ AMPAR antibodies also reduced inhibitory currents and increased neuronal excitability in cultured neurons, suggesting a compensatory decrease of inhibitory synaptic strength leading to a homeostatic increase in intrinsic neuronal excitability to counteract the effects of patients’ AMPAR antibodies (Peng et al., 2015). This imbalance between excitatory and inhibitory transmission has been suggested to account for seizures observed in some patients suffering from AMPAR encephalitis (Peng et al., 2015). No published in vivo experimental data are available regarding a possible role of patients’ AMPAR antibodies.
To summarize, contrary to anti-NMDAR encephalitis patients, the clinical presentations of anti-AMPAR encephalitis are more variable. This heterogeneity could reflect a wider diversity of pathological mechanisms in anti-AMPAR encephalitis that remains to be elucidated. While in vitro data strongly suggest a possible direct pathological effect of patients’ AMPAR antibodies, the proof of their pathogenicity remains lacking and other immune factors such as T-cells or some cytokines could be also involved in the pathogenesis.
Encephalitis associated with antibodies directed against the GABAAR
Clinical presentation
GABAAR antibodies have been recently described in patients with encephalitis and refractory seizures or status epilepticus (Ohkawa et al., 2014; Petit-Pedrol et al., 2014; Pettingill et al., 2015). Two subgroups of patients can be differentiated based on immunological and clinical criteria. The first group presents with encephalitis and refractory seizures and high titres of serum and CSF GABAAR antibodies. The second group presents with diverse clinical features (encephalitis with seizures, stiff-person syndrome and opsoclonus–myoclonus) and low titres of GABAAR antibodies present only in the serum (Pettingill et al., 2015). No strong tumour association has been reported in this disorder. These initial reports suggest a treatment-responsive disorder but the novelty of this discovery needs certitude regarding treatment response and patient outcome.
Antigen
GABAARs belong to the family of heteropentameric ligand-gated ion channels, which also includes the glycine receptor. Various subunit combinations generate structurally and functionally distinct GABAAR subtypes with different pharmacology and channel gating properties (Luscher et al., 2011). Synaptic GABAARs are composed of three α subunits (α1–3), two β subunits (β2 or β3) and a single γ2 subunit and mediate most fast inhibitory neurotransmissions in the adult brain (Jacob et al., 2008). Mutations in GABAAR subunits associate with genetic epilepsy syndrome in humans (Macdonald et al., 2010). In patients with encephalitis and high GABAAR anti-body serum titres, the antibodies are mainly of the IgG1 or rarely IgG3 subtype with subunit specificity (α1 or β3 or γ2) (Ohkawa et al., 2014; Petit-Pedrol et al., 2014; Pettingill et al., 2015). No further epitope mapping has been reported. In patients with low GABAAR antibody serum titre, the antibodies were mainly of the IgM subtype without subunit specificity (Pettingill et al., 2015).
Antibody pathogenicity
Patients with anti-GABAAR encephalitis tend to improve when treated with immunosuppressors and at least in one case clinical improvement was correlated with reduction of GABAAR antibody titre in the serum (Pettingill et al., 2015). Studies linking clinical evolution to CSF antibody titres are still lacking. Patients’ CSF and sera reduce the synaptic density of GABAAR when applied to neuronal culture with no effect on other synaptic components (Ohkawa et al., 2014; Petit-Pedrol et al., 2014; Pettingill et al., 2015). This effect is not mediated by complement activation (Ohkawa et al., 2014; Pettingill et al., 2015). A functional effect of GABAAR anti-bodies on inhibitory synaptic currents in vitro has been demonstrated by whole-cell patch clamp (Ohkawa et al., 2014). However, effects of patients’ antibodies on extrasynaptic GABAAR are still unclear, with some contradictory data (Ohkawa et al., 2014; Petit-Pedrol et al., 2014) and there are as yet no in vivo studies published on the effect of GABAAR antibodies.
To summarize, the clinical manifestation of encephalitis with anti-GABAARs seem to be dependent on the CSF autoantibody titre. In vitro data suggest a potential structural and functional effect of the patients’ CSF antibodies on their target antigen by a mechanism of antigenic modulation, similar to that demonstrated for other antibodies directed against the ionotropic channel receptor, but this effect must be confirmed by other studies.
Encephalitis associated with antibodies directed against Lgi1
Clinical presentation
Anti-Lgi1 antibodies were initially described in a cohort of patients with ‘VGKC-Ab’-associated encephalitis (Shillito et al., 1995; Lai et al., 2010). Indeed, autoantibodies called ‘anti-VGKC antibodies’ were first described in patients with neuromyotonia, and later in patients with autoimmune limbic encephalitis and Morvan’s syndrome (Lee et al., 1998; Buckley et al., 2001). Further studies revealed that the target of these autoantibodies was not the potassium channel itself but other proteins interacting with the channel, namely contactin-associated protein-like 2 (Caspr2) and leucin-rich, glioma inactivated 1 (Lgi1) (Irani et al., 2010; Lai et al., 2010; Lancaster et al., 2011a).
Lgi1 antibodies are predominantly encountered in patients with a clinical picture of autoimmune encephalitis (Lai et al., 2010; Klein et al., 2013; Ohkawa et al., 2013). Age of onset is usually in the sixth decade but can vary from 20 to 80 years (Lai et al., 2010; Shin et al., 2013; Malter et al., 2014). Both limbic (anterograde amnesia, behavioural/psychiatric disturbances, seizures) and extra-limbic symptoms (motor, cerebellar, extrapyramidal involvement) can be observed. Epilepsy is found in 80% of the patients and may represent the initial symptom (Lai et al., 2010; Klein et al., 2013; Shin et al., 2013; Malter et al., 2014). Insomnia, paradoxical sleep disorders, severe bradyarrythmias and hyponatraemia are other typical features (Lai et al., 2010; Klein et al., 2013; Kim et al., 2014; Malter et al., 2014). Importantly, abnormal movements called facio-brachial dystonic seizures are closely associated with anti-Lgi1 encephalitis (Irani et al., 2011, 2013). Interestingly, facio-brachial seizures respond only poorly to anti-epileptic drugs, but are highly responsive to immunotherapy (Irani et al., 2011, 2013). Anti-Lgi1 encephalitis seems to be associated with a poor cognitive outcome and frequent evolution to hippocampal atrophy (Malter et al., 2014), but no prospective studies are available. Relapses occur in 10% of patients (Lai et al., 2010; Kim et al., 2014; Malter et al., 2014). Aggressive and prolonged immunotherapy seems important to relieve symptoms and prevent relapses (Irani et al., 2013; Kim et al., 2014). The prevalence of cancers varies among different studies but does not appear to exceed 20% of patients (Irani et al., 2010; Klein et al., 2013; Shin et al., 2013; Malter et al., 2014).
Antigen
Lgi1 is a secreted synaptic protein expressed in neural tissues. It interacts with the synaptic receptors ADAM22 and ADAM23 to create a trans-synaptic complex bridging post-synaptic glutamatergic AMPAR and pre-synaptic Kv1.1 (Fukata et al., 2010). Lgi1 was proposed to act as a regulating factor for neuronal excitability at synapses. Indeed, Lgi1 mutations in humans are related to a hereditary epileptic syndrome (Ottman et al., 1995) and Lgi1-knockout mice develop a phenotype of lethal epilepsy (Fukata et al., 2010). Anti-Lgi1 antibodies are of the IgG4 type, as demonstrated by subclass-specific staining (Irani et al., 2012). The results of epitope mapping performed using patients’ serum antibodies suggest that different parts of the protein are recognized by these antibodies (Ohkawa et al., 2013).
Antibody pathogenicity
The clinical symptoms of anti-Lgi1 encephalitis suggest CNS hyper-excitability, similar to what is reported in Lgi1 genetic deletion models (human hereditary epileptic syndrome and mutant mice), suggesting that patients’ antibodies may alter Lgi1 functions leading to neuronal hyperexcitability. In vitro, Lgi1 antibodies were shown to impair Lgi1 binding to ADAM22 and to decrease surface expression of post-synaptic AMPAR in a reversible and dose-dependent manner (Ohkawa et al., 2013). Although anti-Lgi1 antibodies are mainly of the IgG4 subtype (Irani et al., 2012) and thus unlikely to fix complement, deposition of complement on neuronal membranes has been demonstrated in the brain of patients with anti-Lgi1 encephalitis, suggesting the involvement of complement-dependent cytotoxicity (Bien et al., 2012). Moreover, Lgi1 antibodies fixed complement on the surfaces of Lgi1-transfected cells (Irani et al., 2012). These observations might explain the more frequent occurrence of hippocampal atrophy associated with Lgi1 antibodies compared to other anti-cell surface antigen antibodies associated with autoimmune encephalitis, such as anti-NMDAR or anti-Caspr2 antibodies. No in vivo experimental data arguing for Lgi1 antibody pathogenicity are available.
Encephalitis associated with antibodies directed against Caspr2
Clinical-resentation
Anti-Caspr2 antibodies (Caspr2 Abs) were initially described in eight ‘VGKC Ab’ patients with encephalitis and/or peripheral nervous system symptoms (Shillito et al., 1995; Irani et al., 2010). Caspr2 Abs correlate with the presence of peripheral symptoms, mostly neuromyotonia and Morvan’s syndrome (Shillito et al., 1995; Irani et al., 2010; Lancaster et al., 2011a; Klein et al., 2013). By definition, CNS involvement is excluded in patients with pure neuromyotonia. Morvan’s syndrome, by contrast, is a rare autoimmune disorder presenting neuromyotonia features together with marked dysautonomic symptoms (profuse sweating, tachycardia, genito-urinary dysfunction), complete disruption of sleep organization and encephalopathy (visual hallucinations, delusion and impaired vigilance) (Irani et al., 2012). Caspr2 Abs are found in most of Morvan’s syndromes, either alone or with moderately elevated anti-Lgi1 Abs (Irani et al., 2012; Ohkawa et al., 2013). Nevertheless, Caspr2 Abs can also be found in patients with pure limbic encephalitis, whose specific clinical pattern and prognosis remain to be precisely determined (Lancaster et al., 2011a; Malter et al., 2014). Differences in epitope specificities and location of the production of autoantibodies have been hypothesized to account for such a variety of clinical presentations. An associated malignant thymoma is observed in more than 50% of Caspr2 Ab-positive neuromyotonia/Morvan’s syndrome patients (Irani et al., 2012), while the prevalence of tumours is much lower in patients with pure limbic encephalitis (Lai et al., 2010; Klein et al., 2013; zShin et al., 2013; Malter et al., 2014). Patients with Caspr2 Ab-associated syndrome seem to have low titre of multiple autoantibodies such as Lgi1 Abs, anti-DCC or anti-DPP10 antibodies (Ohkawa et al., 2013). The pathogenic role of these antibodies has not yet been evaluated.
Antigen
Caspr2 is a membrane cell adhesion molecule that clusters voltage-gated potassium channels (Kv1.1/1.2) at the juxtaparanodes of myelinated axons and may regulate axonal excitability (Poliak, 2003; Labasque & Faivre-Sarrailh, 2010). Experimental findings suggest that Caspr2 may also be present in the CNS at pre-synaptic sites where it may interact with the pre-synaptic Kv1 channels (Zweier et al., 2009). Caspr2 was also suggested to play a role in synapse formation and dendritic arborization (Labasque & Faivre-Sarrailh, 2010). Polymorphisms of the CASPR2 gene (CNTNAP2) have notably been described in the autistic spectrum disorders (Anderson et al., 2012).
Antibody pathogenicity
A recent in vitro study, using Caspr2 Abs from patients affected by pure limbic encephalitis, determined that Caspr2 Abs from patients’ CSF mainly recognize the N-terminal Discoïdin and LamininG1 modules of Caspr2 and strongly target inhibitory interneurons (Pinatel et al., 2015). Functional assays indicated that limbic encephalitis with Caspr2 Abs may induce alteration of Gephyrin clusters at inhibitory synaptic contacts (Pinatel et al., 2015). This work provides new insight into the potential pathogenic effect of Caspr2 Abs in central hyperexcitability that may be related to perturbation of inhibitory interneuron activity. However, in vivo studies are still lacking to assess the potential pathogenic effects of Caspr2 Abs according to the different associated clinical presentations.
To conclude, despite the overlap of clinical syndromes, neurological symptoms specific to Lgi1 or Caspr2 Abs begin to emerge, but further work is needed to understand the exact role of the respective autoantibodies (Table 1).
Table 1.
Main autoantibodies suspected to play a direct role in neurological symptoms
| Antigen | Nature of the antigen | Number of described cases | Clinical syndrome | Tumour association | Described mechanisms |
|---|---|---|---|---|---|
| NMDAR | Ionotropic Glutamate Receptor | > 500 (Titulaer et al., 2013) | Psychiatric symptoms (anxiety, abnormal behaviour, delusion) and/or neurological symptoms (seizures, movement disorders, altered mental state, short-term memory loss), autonomic instabilities (Titulaer et al., 2013). | Age-dependent, up to 40% (ovarian teratoma) in young women (Titulaer et al., 2013) | Antibodies disrupt NMDAR function by cross-linking and internalization of receptors (Hughes et al., 2010; Mikasova et al., 2012). In vitro: reduced surface expression (Hughes et al., 2010), disrupted interaction with EphB2R (Mikasova et al., 2012). In vivo: hyperactivity of glutamatergic pathway (Manto et al., 2010), reduced number of NMDAR clusters (Hughes et al., 2010), impaired memory and depression-like behaviour (Planaguma et al., 2014). |
| AMPAR | Ionotropic Glutamate Receptor | ≈58 (Lai et al., 2009; Bataller et al., 2010; Graus et al., 2010; Wei et al., 2013; Gleichman et al., 2014; Spatola et al., 2014; Hoftberger et al., 2015; Joubert et al., 2015) | Four distinct clinical presentations: classical limbic encephalitis, diffuse encephalitis, fulminant encephalitis, psychiatric presentation (Hoftberger et al., 2015; Joubert et al., 2015) | 34% (thymus, breast, lung) | In vitro: antibody-induced internalization of AMPAR (Lai et al., 2009; Peng et al., 2015), reduced excitatory transmission (Gleichman et al., 2014). |
| mGluR1 | Metabotropic Glutamate Receptor | 5 (Sillevis Smitt et al., 2000; Marignier et al., 2010; Lancaster et al., 2011b; Iorio et al., 2013) | Cerebellitis with cerebellar ataxia, balance and gait disturbances, dysarthria and nystagmus | 2 cases with Hodgkin’s lymphoma (Sillevis Smitt et al., 2000), 1 case with prostate adenocarcinoma (Iorio et al., 2013) | In vitro: blockage of mGluR1 activation (Sillevis Smitt et al., 2000), reduced cerebellar LTD (Coesmans et al., 2003). In vivo: severe but transient ataxia induced by patients’ IgG infusion in mice (Sillevis Smitt et al., 2000), impaired compensatory eye movement (Coesmans et al., 2003). |
| mGluR5 | Metabotropic Glutamate Receptor | 4 (Lancaster et al., 2011b; Mat et al., 2013; Pruss et al., 2014) | Limbic encephalitis | 3 cases with Hodgkin’s lymphoma (Lancaster et al., 2011b; Mat et al., 2013) | No experimental study available. |
| GABAAR | Ligand-gated ion Channel | ≈35 (Ohkawa et al., 2014; Petit-Pedrol et al., 2014; Pettingill et al., 2015) | Encephalitis with refractory seizures | 2 cases with thymoma (Ohkawa et al., 2014), 1 case with a neuroepithelial tumour (Pettingill et al., 2015) | In vitro: reduction of GABAAR synaptic density (Ohkawa et al., 2014; Petit-Pedrol et al., 2014; Pettingill et al., 2015), reduced inhibitory currents (Ohkawa et al., 2014). |
| GABABR | Metabotropic Receptor | ≈67 (Lancaster et al., 2010; Boronat et al., 2011; Höftberger et al., 2013a,b; Jeffery et al., 2013; Kim et al., 2014) | Limbic encephalitis with prominent seizures | 50% (small-cell lung cancer) (Höftberger et al., 2013a,b) | In vitro: blockage of GABABR activation by baclofen without decrease of surface GABABRs (Jain et al., 2015) |
| GlyR | Chloride pentameric channel | ≈77 (Hutchinson et al., 2008; Clerinx et al., 2011; Mas et al., 2011; Turner et al., 2011; Iizuka et al., 2012; Damásio et al., 2013; Kyskan et al., 2013; McKeon et al., 2013; Carvajal-González et al., 2014; Kenda et al., 2015; Bourke et al., 2014; Carvajal-González et al., 2014) | Stiff-person syndrome & progressive encephalomyelitis with rigidity and myoclonus | ≈20% (thymoma, lymphoma, malignant melanoma, breast cancer) | In vitro: internalization of GlyR by GlyR in recombinant cellular model (Wuerfel et al., 2014). |
| LGI1 | Secreted Protein | > 150 (Irani et al., 2010, 2011; Lai et al., 2010; Ohkawa et al., 2013; Shin et al., 2013) | Encephalitis with limbic (amnesia, behavioural alterations, seizures) and extra-limbic symptoms | No consistent tumour association | In vitro: patients’ LGI1 antibodies impair LGI1 binding to partner proteins and decrease synaptic AMPAR expression (Ohkawa et al., 2013) |
| Caspr2 | Adhesion Protein | 95 (Irani et al., 2010, 2012; Lancaster et al., 2011a; Klein et al., 2013) | Limbic encephalitis, Morvan’s syndrome | 50% (thymoma) (Irani et al., 2012) | In vitro: Caspr2 antibodies from patients with limbic encephalitis alter gephyrin clusters (Pinatel et al., 2015). |
| DPPX | Membrane glycoprotein | 28 | Pronounced gastrointestinal symptoms, agitation, hallucinations, confusion, myoclonus, tremor and seizures, sleep-disturbance, PERM-like syndrome | No tumour associations reported | In vitro: patient’s IgG reduce expression of DPPX and Kv4.2 in cultured neurons (Piepgras et al., 2015). |
| Amphiphysin | Synaptic vesicle protein | > 35 (Antoine et al., 1999; Murinson & Guarnaccia, 2008; Moon et al., 2014) | Stiff-person syndrome | ≈90% (breast cancer) | In vivo: transfer of IgG from patients to rat causes muscles stiffness and spasms (Sommer et al., 2005). |
| GAD | GABA synthesis enzyme | > 100 (Saiz et al., 2008; Ariño et al., 2015; Fouka et al., 2015) | Limbic encephalitis, cerebellitis | Usually none. Associated with neuroendocrine tumours and lung cancer (Ariño et al., 2014) in 2 case series (Saiz et al., 2008; Ariño et al., 2014). | In vitro: autoantibodies uptake in heterologous cell systems expressing GAD-65 (Hampe et al., 2013). In vivo: altered behaviour induced by GAD antibodies transfer to rodents (Manto et al., 2007, 2015; Hampe et al., 2013). |
| VGCC | Calcium channel | > 40 (Mason et al., 1997; Lorenzoni et al., 2008; Bürk et al., 2010; Ogawa et al., 2011) | Cerebellar ataxia | Small cell lung carcinoma | In vivo: passive transfer of patients’ antibodies to mice induces acute ataxia (Martín-García et al., 2013). |
| Tr/DNER | Neuron-specific signalling protein | > 40 (Graus et al., 1997; de Graaff et al., 2012; Probst et al., 2015) | Cerebellar ataxia | ≈90% (Hodgkin’s lymphoma) | No experimental study available. |
Encephalitis associated with antibodies directed against GABABR
Clinical presentation
Antibodies against GABABR were described in an initial series of 15 patients (Lancaster et al., 2010) and subsequently followed by several case series (Boronat et al., 2011; Höftberger et al., 2013b; Jeffery et al., 2013; Kim et al., 2014). Patients with GABABR antibodies usually present a pure limbic encephalitis with prominent severe seizures or status epilepticus (Lancaster et al., 2010; Höftberger et al., 2013b). Other rare clinical presentations such as opsoclonus–myoclonus or cerebellar ataxia have also been described (Lancaster et al., 2010; Höftberger et al., 2013b; Jarius et al., 2013; Defelipe-Mimbrera et al., 2014). About 50% of patients with GABABR antibodies present an underlying small-cell lung carcinoma (Höftberger et al., 2013b). In this paraneoplastic subgroup, the median age of patients is higher (around 60 years) with a shorter overall survival (Höftberger et al., 2013b). Almost all patients achieve partial to complete neurological recovery with immunotherapy and appropriate oncological treatment (Lancaster et al., 2010; Höftberger et al., 2013b; Jeffery et al., 2013; Kim et al., 2014). As observed with anti-NMDAR encephalitis, recovery might be improved using more aggressive immunotherapy targeting B-cells and antibody production (e.g. rituximab) (Kim et al., 2014). Interestingly, as observed in patients with GABAAR antibodies, a subset of patients with low titres of GABABR antibodies in the serum or the CSF and with heterogeneous clinical syndrome has also been described (Lancaster et al., 2010; Jeffery et al., 2013). These patients present with additional antibodies (CV2/CRMP5, ANNA-1 or ANNA-3) revealing a larger antineuronal immunization and their prognosis is less favourable in spite of treatment (Jeffery et al., 2013).
Antigen
The GABABRs are transmembrane metabotropic G-protein coupled receptors that trigger cAMP cascades and thereby regulate specific ion channel properties. GABABRs have been found to play a key role in regulating membrane excitability and synaptic transmission in the brain. Indeed, they can stimulate the opening of K+ and Ca2+ channels and elicit both presynaptic and slow postsynaptic inhibition (Benarroch, 2012; Gassmann & Bettler, 2012). GABABR is an obligatory heterodimer formed by 2 subunits (GABAB1 and GABAB2) (Bettler, 2004; Gassmann & Bettler, 2012). Patients’ antibodies target the GABAB1 subunit and are mainly of the IgG1 subtype, but IgG2 and IgG3 also occur (Lancaster et al., 2010). Failure of GABABR detection by patient antibody in immunoblots suggests that the antibodies are directed against a conformational epitope (Höftberger et al., 2013b). Recent deletion mapping experiments suggest that patients’ anti-GABABR antibodies bind to alternatively spliced Sushi domains present in the presynaptically localized GABAB1A isoform (Jain et al., 2015).
Antibody pathogenicity
Aside from the good neurological outcome under immunosuppressive treatment, no in vivo experimental data are available on the direct neuronal pathogenicity of GABABR antibodies. However, the above in vitro experiments by Jain et al. (2015) showed that patients’ anti-GABABR antibodies block baclofen-mediated activation of the GABABR without decreasing GABABR surface expression. These data suggest that anti-GABABR antibodies interfere with GABA-mediated inhibition, thereby contributing to seizures.
Encephalomyelitis associated with antibodies directed against the glycine receptor
Clinical presentation
Glycine receptor (GlyR) antibodies were first described in association with progressive encephalomyelitis rigidity and myoclonus (Hutchinson et al., 2008). Stiff-person syndrome (SPS), defined as axial rigidity with or without hyperreflexia and/or autonomic disturbances (Ciccotto et al., 2013), progressive encephalomyelitis with rigidity and myoclonus (PERM or SPS with brainstem involvement) are the most common clinical presentations of patients with GlyR antibodies (McKeon et al., 2013; Carvajal-González et al., 2014). However, a strong phenotypic association is lacking, because GlyR antibodies could also be associated with demyelinating phenotypes including optic neuritis (Woodhall et al., 2013; Martinez-Hernandez et al., 2015). SPS and PERM symptoms are acute or subacute in 70% of cases (Carvajal-González et al., 2014). In the largest described cohort of patients, median age at symptom onset was 50 years (range: 1–75 years) with equal male/female presence (Carvajal-González et al., 2014). Contrary to patients with GlyR antibody-negative SPS/PERM, antibody-positive patients improved markedly with symptomatic treatment and immunotherapy (McKeon et al., 2013; Carvajal-González et al., 2014) although relapses and relapsing/remitting courses are frequent (Carvajal-González et al., 2014). Associated tumours (mainly thymoma and lymphoma, but also malignant melanoma and metastatic breast cancer) have been reported in a few patients (Clerinx et al., 2011; McKeon et al., 2013; Bourke et al., 2014; Carvajal-González et al., 2014). A clear causality link is lacking and the majority of cases are not paraneoplastic.
Antigen
GlyRs are chloride pentameric channels composed of a variable arrangement of α and β subunits (Legendre, 2001; Betz & Laube, 2006). GlyRs are distributed mainly in the spine and brainstem and have a prominent role in the inhibitory modulation of motor, visual, auditory and autonomic networks (Lynch, 2004; Dutertre et al., 2012). GlyR antibodies are predominantly of the IgG1 subclass, intrathecally synthesized and directed against an extracellular epitope on the GlyRα subunit, probably common to the three isoforms of the subunit (Carvajal-González et al., 2014).
Antibody pathogenicity
GlyR antibody titres in serum and CSF may correlate with the clinical course (Hutchinson et al., 2008; Iizuka et al., 2012). In one case, rituximab treatment (anti-CD20 antibody) led to a marked clinical improvement, suggesting a B-cell-mediated pathology. Interestingly, an increase in CSF GABA levels and a decrease in glutamate level suggestive of a compensatory mechanism have been reported in one patient (Carvajal-González et al., 2014). Recently, the ability of patients’ GlyR antibodies to induce GlyR internalization in HEK-transfected cells was reported (Carvajal-González et al., 2014), but no in vivo experimental data arguing for GlyR antibody pathogenicity are available.
Other autoimmune encephalitis associated with pathogenic antibodies
Encephalitis associated with antibodies directed against mGluR5
Four patients with limbic encephalitis and antibodies against mGluR5 were recently described (Lancaster et al., 2011b; Mat et al., 2013; Pruss et al., 2014). All presented with pure limbic encephalitis. This syndrome was previously described and named Ophelia syndrome by Carr (1982). The first described patients with mGluR5 antibodies were three patients with Hodgkin’s lymphoma (Lancaster et al., 2011b; Mat et al., 2013), but no tumour was observed in a recently published case (Pruss et al., 2014). Patients appear to respond well to immunosuppressive treatment (Lancaster et al., 2011b; Mat et al., 2013; Pruss et al., 2014) and this recovery correlates with serum antibody titre reduction (Pruss et al., 2014), indirectly arguing for a link between mGluR5 antibodies and pathogenicity. However, no in vitro or in vivo experimental data on the effect of mGluR5 antibodies have been reported.
Encephalitis associated with antibodies directed against DPPX
Antibodies against dipeptidyl-peptidase-like protein X (DPPX) (DPP6), an accessory subunit of Kv4.2 potassium channels, were detected in four patients with hallucinations and confusion associated with symptoms reflecting CNS hyperexcitability (e.g. myoclonus, tremor, hyperekplexia and seizures) (Boronat et al., 2013). Subsequently, three additional patients with anti-DPPX antibodies were reported with a syndrome resembling PERM (Balint et al., 2014). Until today, 21 patients with this disorder have been described (Tobin et al., 2014; Piepgras et al., 2015). Interestingly, half of the reported encephalitic patients with anti-DPPX had prominent gastrointestinal symptoms, mainly severe diarrhoea, but also constipation (Boronat et al., 2013; Balint et al., 2014; Tobin et al., 2014). DPPX is a membrane glycoprotein that critically determines the conductance of neuronal Kv4.2 channels and thus regulates membrane excitability (Kim et al., 2008; Kaulin et al., 2009). DPPX expression is spread in the hippocampus, striatum and cerebellum, and remarkably in the myenteric plexus, which may explain the characteristic gastrointestinal symptoms of anti-DPPX encephalitis. DPPX-knockout mice have hyperexcitability of CNS neurons (Boronat et al., 2013; Balint et al., 2014). Recently, it was observed that patients’ purified IgG reduced expression of DPPX and Kv4.2 channels on hippocampal neurons and demonstrated a significantly increased activity of enteric neurons after application of anti-DPPX sera to myenteric plexus preparations (Piepgras et al., 2015). These in vitro data suggest that downregulation of DPPX and Kv4.2 by a patient’s anti-DPPX antibodies results in CNS and enteric neuron hyperexcitability, which may underlie the neuropsychiatric and gastrointestinal manifestations of anti-DPPX encephalitis. Therefore, these first in vitro data support a pathogenic role of anti-DPPX antibodies in this newly identified autoimmune encephalitis.
CNS syndromes associated with antibodies directed against amphiphysin
Amphiphysin antibodies were first described in patients with paraneoplastic SPS (Camilli et al., 1993), although they can also be found in other neurological manifestations such as limbic encephalitis, dysautonomia and cerebellar dysfunction (Pittock et al., 2005; Moon et al., 2014). Classically, patients with SPS and anti-amphiphysin antibodies are female with a median age at symptom onset around 60 years and associated breast cancer (Murinson & Guarnaccia, 2008). These patients improve with immunotherapy and tumour removal (Murinson & Guarnaccia, 2008). An underlying tumour (lung cancer, ovarian cancer, gastric cancer) can also be found in some cases of non-SPS associated with amphiphysin antibodies and some clinical improvement can be achieved through immunotherapy (Moon et al., 2014). Amphiphysin is an intracellular synaptic vesicle protein (Lichte et al., 1992) involved in clathrin-mediated endocytosis. Alteration of GABAergic neurotransmission induced by patients’ anti-amphiphysin antibodies has been reported (Geis et al., 2010). Moreover, transfer of amphiphysin antibodies from patients to rodents causes stiffness and muscle spasms (Sommer et al., 2005). Taken together, these data suggest that amphiphysin antibodies might be pathogenic although the mechanisms of antibody action on their intracellular target remain unknown.
Encephalitis and cerebellitis associated with antibodies directed against glutamate decarboxylase (GAD)
Clinical presentation
GAD autoantibodies associated with neurological syndromes were initially described by Solimena et al. (1988). Several neurological syndromes have been described in association with high titres of GAD autoantibodies, including SPS, cerebellar ataxia, epilepsy and limbic encephalitis (Saiz et al., 2008; Ali et al., 2011; Alexopoulos & Dalakas, 2013; Fouka et al., 2015; Gresa-Arribas et al., 2015). While GAD autoantibodies are generally not associated with cancer, they can occur as a paraneoplastic event (Saiz et al., 2008; Ariño et al., 2015). Patients with limbic encephalitis associated with GAD autoantibodies usually do not respond well to treatment (Malter et al., 2010). Patients with cerebellar ataxia are mainly women with a median age around 55 years (Ariño et al., 2014). Clinical presentation is insidious or subacute (Honnorat et al., 2001; Ariño et al., 2014) with the most common presenting symptom being gait ataxia, followed by limb ataxia, dysarthria and nystagmus (Ariño et al., 2014). In contrast to limbic encephalitis patients, good outcome (modified Rankin scale < 3) with immunotherapy has been reported in 50% of patients with cerebellar ataxia (Ariño et al., 2014). Finally, patients with SPS associated with GAD antibodies appear to have the best probability of clinical improvement compared to patients who developed another GAD-associated neurological syndrome (Ariño et al., 2015).
Antigen
GAD is the rate-limiting enzyme implicated in the synthesis of GABA, which is the major inhibitory neurotransmitter in the CNS (Buddhala et al., 2009). Two isoforms of GAD (65 and 67 kDa) are expressed predominantly in the brain. GAD-65 can be found concentrated in presynaptic terminals and appears to be the dominant target of patients’ autoantibodies that seem to recognize mainly the decarboxylase catalytic domain of GAD-65 (Gresa-Arribas et al., 2015). Although GAD-65 is an intracellular enzyme, it has been suggested that autoantibodies may interact with their target during the synaptic release event (Gresa-Arribas et al., 2015). GAD-65 antibody titres are significantly higher in patients with cerebellar ataxia than in patients with SPS associated with GAD antibodies (Gresa-Arribas et al., 2015). Notably, GAD autoantibodies present in patients with SPS and cerebellar ataxia recognize an epitope distinct from that recognized by GAD autoantibodies present in patients with type 1 diabetes mellitus or limbic encephalitis (Manto et al., 2015), arguing for GAD-65 antibodies recognizing disease-specific epitopes.
Antibody pathogenicity
In vitro and in vivo experiments with purified IgGs of patients with GAD-65 autoantibodies and SPS or cerebellar ataxia further supported the hypothesis that GAD-65 autoantibodies target and inhibit GAD-65 function in these diseases (Manto et al., 2007, 2011b; Holmøy & Geis, 2011; Hampe et al., 2013; Hansen et al., 2013). Moreover, the administration of a monoclonal GAD-65 antibody directed against the epitope recognized by SPS patients disrupted in vitro the association of GAD with GABAergic synaptic vesicles and depressed the inhibitory synaptic transmission in cerebellar slices (Manto et al., 2015). In vivo, this monoclonal antibody induced decreased exploratory behaviour and impaired locomotor function in rats (Manto et al., 2007, 2015). These findings support a specific pathological role of GAD-65 autoantibodies in the pathogenesis of SPS and cerebellar ataxia. In contrast, no effect of GAD-65 antibodies from a patient with limbic encephalitis was observed in vitro on GABAergic neurotransmission (Stemmler et al., 2015).
To summarize, numerous evidence suggests a direct role of GAD-65 antibodies in some associated neurological syndromes, but the antibodies may not always play a direct role. Differences in epitope specificities according the neurological syndromes may explain at least in part the spectrum of anti-GAD-associated neurological disorders (Mitoma et al., 2016). Strong experimental evidence exists in favour of a pathogenic effect of GAD-65 antibodies in SPS and cerebellar ataxia.
Cerebellitis associated with antibodies directed against mGluR1
Clinical presentation
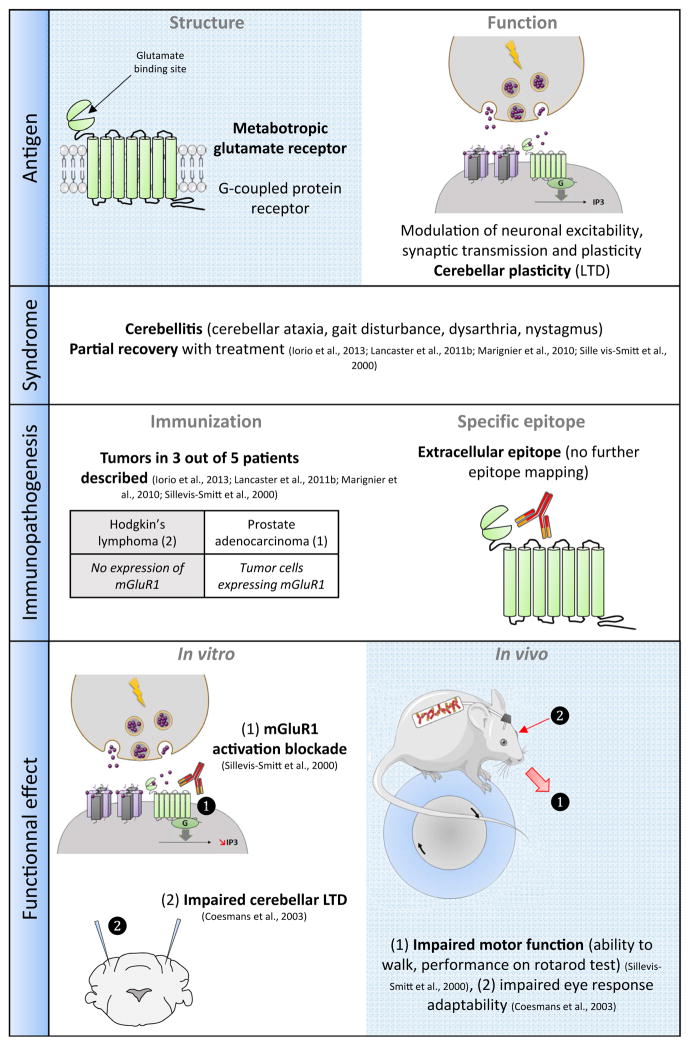
Since their first description in 2000, mGluR1 autoantibodies have been reported in five patients with cerebellitis. All patients experienced severe cerebellar ataxia with balance and gait disturbances, dysarthria and nystagmus. In two cases, mGluR1 cerebellitis was associated with a Hodgkin’s lymphoma (Sillevis Smitt et al., 2000) and in one case with a prostate adenocarcinoma (Iorio et al., 2013). In the two remaining published cases, no underlying tumours were found in spite of an extensive examination (Marignier et al., 2010; Lancaster et al., 2011b), raising the hypothesis of a ‘primary’ autoimmune-mediated disorder for which mechanisms leading to cross-reactivity remain unknown. Treatment allows only limited recovery (Sillevis Smitt et al., 2000; Marignier et al., 2010; Lancaster et al., 2011b; Iorio et al., 2013) (Fig. 2).
Fig. 2.
Example of cerebellitis associated with anti-mGluR1 autoantibodies.
Antigen
mGluR1 is a metabotropic glutamate receptor localized postsynaptically in somatodendritic domains and strongly expressed in hippocampus and cerebellum (Martin et al., 1992). In Purkinje cells, mGluR1 is critically involved in long-term depression (LTD) and motor coordination (Ichise et al., 2000). Interestingly, mGluR1-knockout mice exhibit cerebellar symptoms and impaired cerebellar LTD (Aiba et al., 1994). A role for mGluR1 in eye response movement adaption has also been reported (Shutoh et al., 2002). The mGluR1 epitope is located extracellularly as indicated by the detection of patients’ antibodies in cell-based assay experiments, but no further epitope mapping has yet been published. Although there is a strong sequence homology between mGluR1 and other members of the mGluR family (for instance mGluR5), antibodies do not cross-react (Lancaster et al., 2011b). The IgG subtypes of patient antibodies have not been reported.
Antibody pathogenicity
The rarity of the disease does not allow a conclusion to be made regarding a pathogenic role of the associated autoantibody. Interestingly, antibodies to Homer-3, which co-localizes with and organizes mGluR1 at synapses in the dendritic spines of the Purkinje cells, have been reported in two exceptional patients who presented with clinical symptoms similar to patients with mGluR1-ab antibodies (Zuliani et al., 2007; Höftberger et al., 2013a). In one case of anti-mGluR1 cerebellitis, early immunosuppressive treatment led in one patient to a reduction of mGluR1 antibody titres in serum and CSF and recovery (Sillevis Smitt et al., 2000) but the other described patients developed cerebellar atrophy and responded only partially to treatment (Coesmans et al., 2003; Marignier et al., 2010; Lancaster et al., 2011b; Iorio et al., 2013). In the only reported postmortem study of brain tissue, Purkinje cell loss was observed, suggesting involvement of cytotoxic mechanisms although no cytotoxic CD8+ T-lymphocytes were observed (Coesmans et al., 2003). Patients’ antibodies are able to block mGluR1 activation in mGluR1-expressing CHO cells (Sillevis Smitt et al., 2000). Accordingly, slice recordings and whole-cells patch clamp recordings indicate that patients’ mGluR1 antibodies reduce Purkinje neuron excitability and spontaneous firing rate (Coesmans et al., 2003). Extracellular bath application onto cultured embryonic Purkinje cells but not intracellular microinjection reduces LTD, further confirming that patients’ mGluR1 antibodies are directed against the extracellular part of the receptor and that they can block mGluR1 activation and impair synaptic plasticity (Coesmans et al., 2003). Passive transfer of patients’ mGluR1 antibodies into mouse brain provoked transient ataxia and this behavioural effect was abolished by patients’ CSF pre-absorption on CHO cells expressing mGluR1α, confirming that this effect was directly caused by mGluR1 IgG (Sillevis Smitt et al., 2000). Moreover, antibody infusion into the flocculus of mice acutely and reversibly decreases compensatory eye movements in a way similar to the impairment of saccadic eye movements observed in patients (Coesmans et al., 2003). These experimental models provided the ultimate proof of the pathogenicity of patients’ mGluR1 antibodies.
To summarize, cerebellitis associated with mGluR1 is an exceptional disorder for which the pathogenicity of the patients’ antibodies has been clearly demonstrated. The metabotropic nature of the target of this antibody-mediated neurological disease is unique in this group of autoimmune encephalitides that generally share ion channel dysfunction as a common pathogenesis. Elucidation of the cellular consequences of this singular antibody-mediated metabotropic receptor dysfunction needs further investigation.
Cerebellitis associated with antibodies directed against the VGCC
Antibodies targeting the P/Q-type of the voltage-gated calcium channels (VGCC-Abs) are associated with Lambert-Eaton myasthenic syndrome, cerebellar ataxia or both (Lennon et al., 1995). Cerebellar ataxia associated with VGCC-Abs is essentially paraneoplastic, associated with small cell lung carcinoma expressing functional VGCC (Meriney et al., 1996). The accepted hypothesis for paraneoplastic cases proposes that the immune adapted reaction against the tumour triggers the autoimmune response. VGCC-Ab-associated cerebellar ataxia is usually subacute with symmetrical gait and limb ataxia, dysarthria and nystagmus. However, anti-VGCC cerebellar ataxia may sometimes develop progressively, mimicking idiopathic sporadic late-onset ataxia (Bürk et al., 2010). Patients respond poorly to immunotherapy. This poor response is imputed to the early and diffuse loss of Purkinje cells observed in these patients (Fukuda et al., 2003). In contrast, some case reports suggest that patients with the rare non-paraneoplastic VGCC-Ab-associated cerebellar ataxia may respond well to immunotherapy (Rigamonti et al., 2014). Numerous observations support the hypothesis of a direct pathogenic role of VGCC-Abs in the development of cerebellar ataxia: P/Q-type VGCCs, which are involved in neurotransmitter release in the synaptic cleft (Simms & Zamponi, 2014), are prominent in the cerebellum (Hillman et al., 1991), and mutations of P/Q VGCCs cause ataxia in mice (Hillman et al., 1991; Fletcher & Lennon, 2003). Post-mortem brain examination revealed a diffuse loss of Purkinje cells along with Bergmann’s gliosis, with only minor or absent lymphocytic perivascular infiltration (Fukuda et al., 2003), arguing against a major role of cellular immunity. Furthermore, P/Q type VGCC density is decreased by 70–80% in the cerebellum of VGCC-Ab cerebellar ataxia patients compared to control subjects (Fukuda et al., 2003). Indirect experimental arguments were provided by a study using a polyclonal antibody generated against a major epitope in the P/Q-type VGCCs that inhibited VGCC function in neuronal and recombinant VGCCs, altered cerebellar synaptic transmission, and conferred the phenotype of cerebellar ataxia (Liao et al., 2008). More importantly, passive immunization of mice with antibodies from VGCC-Ab cerebellar ataxia patients induced acute ataxia in mice, demonstrating the pathogenic potential of VGCC-Abs (Martín-García et al., 2013). Questions remain concerning the variability of the clinical manifestations associated with VGCC-Abs between patients. Differences in the production site of VGCC-Abs and in antibody epitope specificities may provide an explanation for this variability.
Cerebellitis associated with antibodies directed against Tr/DNER
Anti-Tr/Delta/notch-like epidermal growth factor-related receptor (DNER) antibodies are associated with paraneoplasic cerebellar degeneration and Hodgkin’s lymphoma (Graus et al., 1997; de Graaff et al., 2012; Greene et al., 2014). More than 40 patients have been reported with symptoms typically including progressive nystagmus, limb ataxia, dysarthria and gait ataxia (Trotter et al., 1976; Graus et al., 1997; Bernal et al., 2003; de Graaff et al., 2012; Greene et al., 2014; Probst et al., 2015). Even with successful Hodgkin disease treatment, ataxia correlated with cerebellar atrophy is irreversible in many patients (de Graaff et al., 2012). DNER, the actual target antigen of anti-Tr antibodies, is a CNS-specific Notch ligand transmembrane protein expressed on Purkinje cells (de Graaff et al., 2012). During development, DNER is involved in the crucial interaction between Purkinje cells and Bergmann’s glia (Eiraku et al., 2005). Anti-Tr/DNER antibodies are mainly of the IgG1 and IgG3 subclass (Bernal et al., 2003) and intrathecally produced (Graus et al., 1997). Their main epitopes have been mapped to an N-glycosylated extracellular region of DNER (de Graaff et al., 2012). While it is plausible that DNER antibodies act by disrupting DNER-Notch signalling, this remains to be confirmed. Thus, proof of the pathogenicity of anti-DNER antibodies is still lacking and the exact pathogenic mechanisms of this disorder remain to be demonstrated.
Conclusions
The rapidly expanding types of autoimmune encephalitis have stimulated fruitful clinical and fundamental investigations, aiming to specify the role of the autoantibodies associated with these disorders (Table 1). Interestingly, some of these autoantibodies, in general targeting ionotropic channels or their partner proteins, define clinical syndromes that resemble the models of pharmacological or genetic disruption of the corresponding antigen, arguing for a pathogenic role of the associated autoantibodies. Different studies have shown that patients’ antibodies may have structural and functional in vitro effects on the targeted antigens. However, definitive proof of the pathogenicity of these autoantibodies has been obtained for only a few antibodies by passive transfer experiments in murine models reproducing the human disease symptoms. Examples for autoantibodies with a direct effect are mGluR1R, NMDAR, amphiphysin, VGCC and GAD-65 antibodies. However, although strong evidence points toward a dysfunction of the targeted antigen’s signalling and the synaptic apparatus that regulates it, the molecular cascades and cellular dynamics by which these autoantibodies induce pathological processes remain often poorly understood. In diseases for which no direct pathogenic role for associated autoantibodies has been demonstrated, patients often show great variability of the clinical presentations, which may possibly reflect a more complex pathogenicity. In these cases, the autoantibodies may serve as disease biomarkers and for characterization of the involvement of the immune system in the disease. However, they may present only one factor among other immune agents. Further clinico-biological correlation and experimental investigations will be necessary to understand the mechanisms of the underlying immune reaction.
Finally, these autoimmune encephalitides offer unique models of brain–immune interactions in which the targeted antigens have critical roles in synaptic transmission and plasticity. Therefore, studies of the role of each associated autoantibody should open new avenues to understand the molecular mechanisms of some neurological and psychiatric disorders. These rare diseases with specific antibodies may provide novel experimental tools to decipher the role of the targeted synaptic proteins and their involvement in neurological and psychiatric dysfunctions observed in patients.
Acknowledgments
The authors received granted support from the Fédération pour la Recherche sur le Cerveau (FRC), the Agence Nationale pour la Recherche (ANR-14-CE15-0001) and the LECMA-Vaincre Alzheimer (pilot grant 12751). Images from Servier Medical Art (http://www.servier.fr/smart/banque-dimages-powerpoint), which are covered by the Creative Commons Attribution 3.0 France, were used in the figures.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- ATD
amino-terminal domain
- Caspr2
contactin-associated protein-like 2
- CNS
central nervous system
- CRMP5
collapsin response mediator protein 5
- CSF
cerebrospinal fluid
- DNER
Delta/notch-like epidermal growth factor-related receptor
- DPPX
dipeptidyl-peptidase-like protein X
- EPHB2R
ephrin B2 receptor
- GABA
γ-aminobutyric acid
- GAD
glutamate decarboxylase
- GlyR
glycine receptor
- iGluR
ionotropic glutamate receptor
- Lgi1
leucine-rich glioma inactivated 1
- LTD
long-term depression
- LTP
long-term potentiation
- mEPSC
miniature excitatory post-synaptic current
- mGluR
metabotropic glutamate receptor
- mIPSC
miniature inhibitory post-synaptic current
- NMDA
N-methyl-D-aspartate
- PERM
progressive encephalomyelitis with rigidity and myoclonus
- PNS
paraneoplastic neurological syndrome
- SPS
stiff-person syndrome
- VGCC
voltage-gated calcium channel
- VGKC
voltage-gated potassium channel
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Rosenmund C, Stevens C, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Alexopoulos H, Dalakas MC. Immunology of stiff person syndrome and other GAD-associated neurological disorders. Exp Rev Clin Immunol. 2013;9:1043–1053. doi: 10.1586/1744666X.2013.845527. [DOI] [PubMed] [Google Scholar]
- Ali F, Rowley M, Jayakrishnan B, Teuber S, Gershwin ME, Mackay IR. Stiff-person syndrome (SPS) and anti-GAD-related CNS degenerations: protean additions to the autoimmune central neuropathies. J Autoimmun. 2011;37:79–87. doi: 10.1016/j.jaut.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Anderson GR, Galfin T, Xu W, Aoto J, Malenka RC, Sudhof TC. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc Natl Acad Sci. 2012;109:18120–18125. doi: 10.1073/pnas.1216398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine JC, Absi L, Honnorat J, Boulesteix JM, De Broucker T, Vial C, Butler M, De Camilli P, Michel D. Antiamphiphysin antibodies are associated with various paraneoplastic neurological syndromes and tumors. Arch Neurol. 1999;56:172–177. doi: 10.1001/archneur.56.2.172. [DOI] [PubMed] [Google Scholar]
- Ariño H, Gresa-Arribas N, Blanco Y, Martínez-Hernández E, Sabater L, Petit-Pedrol M, Rouco I, Bataller L, Dalmau J, Saiz A, Graus F. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. 2014;71:1009–1016. doi: 10.1001/jamaneurol.2014.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariño H, Höftberger R, Gresa-Arribas N, Martínez-Hernández E, Armangue T, Kruer MC, Arpa J, Domingo J, Bojan R, Bataller L, Saiz A, Dalmau J, Graus F. Paraneoplastic neurological syndromes and glutamic acid decarboxylase antibodies. JAMA Neurol. 2015;72:1–8. doi: 10.1001/jamaneurol.2015.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armangue T, Titulaer MJ, Málaga I, Bataller L, Gabilondo I, Graus F, Dalmau J. Pediatric anti-N-methyl-D-aspartate receptor encephalitis – clinical analysis and novel findings in a series of 20 patients. J Pediatr. 2013;162:850–856. doi: 10.1016/j.jpeds.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint B, Jarius S, Nagel S, Haberkorn U, Probst C, Blocker IM, Bahtz R, Komorowski L, Stöcker W, Kastrup A, Kuthe M, Meinck HM. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology. 2014;82:1521–1528. doi: 10.1212/WNL.0000000000000372. [DOI] [PubMed] [Google Scholar]
- Bataller L, Galiano R, Garcia-Escrig M, Martinez B, Sevilla T, Blasco R, Vilchz JJ, Dalmau J. Reversible paraneoplastic limbic encephalitis associated with antibodies to the ampa receptor. Neurology. 2010;74:265–267. doi: 10.1212/WNL.0b013e3181cb3e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. GABAB receptors: structure, functions, and clinical implications. Neurology. 2012;78:578–584. doi: 10.1212/WNL.0b013e318247cd03. [DOI] [PubMed] [Google Scholar]
- Bernal F, Shams’ili S, Rojas I, Sanchez-Valle R, Saiz A, Dalmau J, Honnorat J, Sillevis-Smitt P, Graus F. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60:230–234. doi: 10.1212/01.wnl.0000041495.87539.98. [DOI] [PubMed] [Google Scholar]
- Bettler B. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F, Jellinger KA, Reuss DE, Ribalta T, Schlegel J, Sutton I, Lassman H, Bauer J. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–1638. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABAB receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76:795–800. doi: 10.1212/WNL.0b013e31820e7b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat A, Gelfand JM, Gresa-Arribas N, Jeong HY, Walsh M, Roberts K, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R, Graus F, Rudy B, Dalmau J. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol. 2013;73:120–128. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke D, Roxburgh R, Vincent A, Cleland J, Jeffery O, Dugan N, Abernethy D, King A, Anderson N. Hypoventilation in glycine-receptor antibody related progressive encephalomyelitis, rigidity and myoclonus. J Clin Neurosci. 2014;21:876–878. doi: 10.1016/j.jocn.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Buckley CJ, Oger J, Clover L, Tüzün E, Carpenter K, Jackson M, Vincent A. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50:73–78. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- Buddhala C, Hsu CC, Wu JY. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem Int. 2009;55:9–12. doi: 10.1016/j.neuint.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Bürk K, Wick M, Roth G, Decker P, Voltz R. Antineuronal antibodies in sporadic late-onset cerebellar ataxia. J Neurol. 2010;257:59–62. doi: 10.1007/s00415-009-5262-8. [DOI] [PubMed] [Google Scholar]
- Camdessanché JP, Streichenberger N, Cavillon G, Rogemond V, Jousserand G, Honnorat J, Convers P, Antoine JC. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur J Neurol. 2011;18:929–931. doi: 10.1111/j.1468-1331.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Thomas A, Cofiell R, Folli F, Lichte B, Piccolo G, Meinck HM, Autoni M, Fasseta G, Bottazon G, Bates D, Cartlidge N, Solimena M, Kilimann MW. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of stiff man syndrome with breast cancer. J Exp Med. 1993;178:2219–2223. doi: 10.1084/jem.178.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr I. The Ophelia syndrome: memory loss in Hodgkin’s disease. Lancet. 1982;1:844–845. doi: 10.1016/s0140-6736(82)91887-6. [DOI] [PubMed] [Google Scholar]
- Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, Lang B, Pettingill P, Carr A, Sheerin UM, Press R, Lunn MP, Lim M, Maddison P, Meinck HM, Vandenberghe W, Vincent A. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137:2178–2192. doi: 10.1093/brain/awu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccotto G, Blaya M, Kelley RE. Stiff person syndrome. Neurol Clin. 2013;31:319–328. doi: 10.1016/j.ncl.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Clerinx K, Breban T, Schrooten M, Leite MI, Vincent A, Verschakelen J, Tousseyn T, Vandenberghe W. Progressive encephalomyelitis with rigidity and myoclonus: resolution after thymectomy. Neurology. 2011;76:303–304. doi: 10.1212/WNL.0b013e318207b008. [DOI] [PubMed] [Google Scholar]
- Coesmans M, Sillevis Smitt P, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, Van Alphen AM, Luo C, Van Der Geet JN, Kros JM, Gaillard CA, Frens MA, De Zeeuw CI. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol. 2003;53:325–336. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfled MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon RJ. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damásio J, Leite MI, Coutinho E, Waters P, Woodhall M, Santos MA, Carrilho I, Vincent A. Progressive encephalomyelitis with rigidity and myoclonus. JAMA Neurol. 2013;70:498. doi: 10.1001/jamaneurol.2013.1872. [DOI] [PubMed] [Google Scholar]
- Defelipe-Mimbrera A, Masjuan J, Corral Í, Maria L, Graus F, Garcíabarragán N. Opsoclonus – myoclonus syndrome and limbic encephalitis associated with GABAB receptor antibodies in CSF. J Neuroimmunol. 2014;272:91–93. doi: 10.1016/j.jneuroim.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Becker CM, Betz H. Inhibitory glycine receptors: an update. J Biol Chem. 2012;287:40216–40223. doi: 10.1074/jbc.R112.408229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Tohgo A, Ono K, Kaneko M, Fujishima K, Hirano T, Kengaku M. DNER acts as a neuron-specific notch ligand during Bergmann glial development. Nat Neurosci. 2005;8:873–880. doi: 10.1038/nn1492. [DOI] [PubMed] [Google Scholar]
- Fletcher CF, Lennon VA. Do calcium channel autoantibodies cause cerebellar ataxia with Lambert-Eaton syndrome? Ann Neurol. 2003;53:5–7. doi: 10.1002/ana.10497. [DOI] [PubMed] [Google Scholar]
- Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, Campen CJ, Moss H, Peter N, Gleichman AJ, Glaser CA, Lynch DR, Rosenfeld MR, Dalmau J. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouka P, Alexopoulos H, Akrivou S, Trohatou O, Politis PK, Dalakas MC. GAD65 epitope mapping and search for novel autoantibodies in GAD-associated neurological disorders. J Neuroimmunol. 2015;281:73–77. doi: 10.1016/j.jneuroim.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Lovero KL, Iwanaga T, Watanabe A, Yokoi N, Tabuchi K, Shigemoto R, Nicoll RA, Fukata M. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci. 2010;107:3799–3804. doi: 10.1073/pnas.0914537107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Motomura M, Nakao Y, Shiraishi H, Yoshimura T, Iwanaga K, Tsujihata M, Eguchi K. Reduction of P/Q-type calcium channels in the postmortem cerebellum of paraneoplastic cerebellar degeneration with Lambert-Eaton myasthenic syndrome. Ann Neurol. 2003;53:21–28. doi: 10.1002/ana.10392. [DOI] [PubMed] [Google Scholar]
- Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the california encephalitis project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Bettler B. Regulation of neuronal GABAB receptor functions by subunit composition. Nat Rev Neurosci. 2012;13:380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- Geis C, Weishaupt A, Hallermann S, Grunewald B, Wessig C, Wultsch T, Reif A, Byts N, Beck M, Jablonka S, Boettger MK, Üçeyler N, Fouquet W, Gerlach M, Meinck HM, Sirén AL, Sigrist SJ, Toyka KV, Heckmann M, Sommer C. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain. 2010;133:3166–3180. doi: 10.1093/brain/awq253. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci. 2012;32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichman AJ, Panzer JA, Baumann BH, Dalmau J, Lynch DR. Antigenic and mechanistic characterization of anti-AMPA receptor encephalitis. Ann Clin Transl Neurol. 2014;1:180–189. doi: 10.1002/acn3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaff E, Hulsenboom E, Van Den Berg R, Demmers J, Lugtenburg PJ, Hoogenraad CC, Sillevis-Smitt P. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2012;71:815–824. doi: 10.1002/ana.23550. [DOI] [PubMed] [Google Scholar]
- Granerod J, Ambrose HE, Davies NWS, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- Graus F, Dalmau J, Valldeoriola F, Ferrer I, Rene R, Marin C, Vecht CJ, Arbizu T, Targa C, Moll JW. Immunological characterization of a neuronal antibody (anti-Tr) associated with paraneoplastic cerebellar degeneration and Hodgkin’s disease. J Neuroimmunol. 1997;74:55–61. doi: 10.1016/s0165-5728(96)00205-6. [DOI] [PubMed] [Google Scholar]
- Graus F, Boronat A, Xifro X, Boix M, Svigelj V, Garcia A, Palomino A, Sabater L, Alberch J, Saiz A. The expanding clinical profile of anti-AMPA receptor encephalitis. Neurology. 2010;74:857–859. doi: 10.1212/WNL.0b013e3181d3e404. [DOI] [PubMed] [Google Scholar]
- Greene M, Lai Y, Baella N, Dalmau J, Lancaster E. Antibodies to Delta/notch-like epidermal growth factor-related receptor in patients with anti-Tr, paraneoplastic cerebellar degeneration, and Hodgkin lymphoma. JAMA Neurol. 2014;71:1003–1008. doi: 10.1001/jamaneurol.2014.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R, Rosenfeld MR, Lynch D, Graus F, Dalmau J. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresa-Arribas N, Ariño H, Martínez-Hernández E, Petit-Pedrol M, Sabater L, Saiz A, Dalmau J, Graus F. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS One. 2015;10:e0121364. doi: 10.1371/journal.pone.0121364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, Menson E, Lin JP, Tardieu M, Vincent A, Lim MJ. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord. 2014;29:90–96. doi: 10.1002/mds.25626. [DOI] [PubMed] [Google Scholar]
- Hampe CS, Petrosini L, De Bartolo P, Caporali P, Cutuli D, Laricchiuta D, Foti F, Radtke JR, Vidova V, Honnorat J, Manto M. Monoclonal antibodies to 65 kDa glutamate decarboxylase induce epitope specific effects on motor and cognitive functions in rats. Orphanet J Rare Dis. 2013;8:82. doi: 10.1186/1750-1172-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N, Grünewald B, Weishaupt A, Colaço MN, Toyka KV, Sommer C, Geis C. Human stiff person syndrome IgG-containing high-titre anti-GAD65 autoantibodies induce motor dysfunction in rats. Exp Neurol. 2013;239:202–209. doi: 10.1016/j.expneurol.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, Llinas RR. Localization of P-type calcium channels in the central-nervous-system. Proc Natl Acad Sci USA. 1991;88:7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftberger R, van Sonderen A, Leypoldt F, Houghton D, Geschwind M, Gelfand J, Paredes M, Sabater L, Saiz A, Titulaer MJ, Graus F, Dalmau J. Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology. 2015;84:2403–2412. doi: 10.1212/WNL.0000000000001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höftberger R, Sabater L, Ortega A, Dalmau J, Graus F. Patient with Homer-3 antibodies and cerebellittis. JAMA Neurol. 2013a;70:506–509. doi: 10.1001/jamaneurol.2013.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, de Felipe A, Saiz A, Dalmau J, Graus F. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013b;81:1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmøy T, Geis C. The immunological basis for treatment of stiff person syndrome. J Neuroimmunol. 2011;231:55–60. doi: 10.1016/j.jneuroim.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Honnorat J. Onconeural antibodies are essential to diagnose paraneoplastic neurological syndromes. Acta Neurol Scand. 2006;183:64–68. doi: 10.1111/j.1600-0404.2006.00620.x. [DOI] [PubMed] [Google Scholar]
- Honnorat J, Antoine JC, Derrington E, Aguera M, Belin MF. Antibodies to a subpopulation of glial cells and a 66 kDa developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neurosur Ps. 1996;61:270–278. doi: 10.1136/jnnp.61.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnorat J, Saiz A, Giometto B, Vincent A, Brieva L, de Andres C, Maestre J, Fabien N, Vighetto A, Casamitjana R, Thivolet C, Tavolato B, Antoine J, Trouillas P, Graus F. Cerebellar ataxia with anti-glutamic acid decarboxylase antibodies. Arch Neurol. 2001;58:225. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balice-Gordon RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M, Waters P, McHugh J, Gorman G, O’Riordan S, Connolly S, Hager H, Yu P, Becker CM, Vincent A. Progressive encephalomyelitis, rigidity and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71:1291–1292. doi: 10.1212/01.wnl.0000327606.50322.f0. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Iizuka T, Leite MI, Lang B, Waters P, Urano Y, Miyakawa S, Hamada J, Sakai F, Mochizuki H, Vincent A. Glycine receptor antibodies are detected in progressive encephalomyelitis with rigidity and myoclonus (PERM) but not in saccadic oscillations. J Neurol. 2012;259:1566–1573. doi: 10.1007/s00415-011-6377-2. [DOI] [PubMed] [Google Scholar]
- Iorio R, Damato V, Mirabella M, Vita MG, Hulsenboom E, Plantone D, Bizzaro A, Del Grande A, Sillevis Smitt P. Cerebellar degeneration associated with mGluR1 autoantibodies as a paraneoplastic manifestation of prostate adenocarcinoma. J Neuroimmunol. 2013;263:155–158. doi: 10.1016/j.jneuroim.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, Schott JM, Armstrong RJ, Zagami SA, Bleasel A, Somerville ER, Smith SM, Vincent A. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, Zuliani L, Watanabe O, Lang B, Buckley C, Vincent A. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72:241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- Irani SR, Stagg CJ, Schott JM, Rosenthal CR, Schneider SA, Pettingill P, Pettingill R, Waters P, Thomas A, Voets NL, Cardoso MJ, Cash DM, Manning EN, Lang B, Smith SJ, Vincent A, Johnson MR. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. 2013;136:3151–3162. doi: 10.1093/brain/awt212. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Lancaster E, Dalmau J, Balice-Gordon RJ. Autoantibodies in the CSF of Anti-GABAB receptor encephalitis patients block activation of GABAB receptors in vitro. Ann Neurol Special Issue: 2015 Annual Meetings. 2015;78(S19):S77. [Google Scholar]
- Jantzen SU, Ferrea S, Wach C, Quasthoff K, Illes S, Scherfeld D, Hartung HP, Seitz RJ, Dihné M. In vitro neuronal network activity in NMDA receptor encephalitis. BMC Neurosci. 2013;14:17. doi: 10.1186/1471-2202-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Steinmeyer F, Knobel A, Streitberger K, Hotter B, Horn S, Heuer H, Schreiber SJ, Wilhelm T, Trefzer U, Wildemann B, Ruprecht K. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol. 2013;256:94–96. doi: 10.1016/j.jneuroim.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology. 2013;81:882–887. doi: 10.1212/WNL.0b013e3182a35271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert B, Honnorat J. Autoimmune channelopathies in paraneoplastic neurological syndromes. Biochim Biophys Acta. 2015;1848:2665–2676. doi: 10.1016/j.bbamem.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Joubert B, Kerschen P, Zekeridou A, Desestret V, Rogemond V, Chaffois MO, Ducray F, Larrue V, Daubail B, Idbaih A, Psimaras D, Antoine JC, Delattre JY, Honnorat J. Clinical spectrum of encephalitis associated with antibodies against the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor. JAMA Neurol. 2015;72:1163–1169. doi: 10.1001/jamaneurol.2015.1715. [DOI] [PubMed] [Google Scholar]
- Kaulin YA, De Santiago-Castillo JA, Rocha CA, Nadal MS, Rudy B, Covarrubias M. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels. J Neurosci. 2009;29:3242–3251. doi: 10.1523/JNEUROSCI.4767-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenda J, Švigelj V, Rodi Z, Koritnik B, Graus F, Kojović M. Glycine receptor antibodies and progressive encephalomyelitis with rigidity and myoclonus with predominant motor neuron degeneration – expanding the clinical spectrum. J Neurol Sci. 2015;353:177–178. doi: 10.1016/j.jns.2015.03.049. [DOI] [PubMed] [Google Scholar]
- Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, Rudy B, Hoffman DA. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100:1835–1847. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Lee ST, Shin JW, Moon J, Lim JA, Byun JI, Shin YW, Lee KJ, Jung KH, Kim YS, Park KI, Chu K, Lee SK. Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol. 2014;270:45–50. doi: 10.1016/j.jneuroim.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Klein CJ, Lennon VA, Aston PA, McKeon A, O’Toole O, Quek A, Pittock SJ. Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA Neurol. 2013;70:229. doi: 10.1001/jamaneurol.2013.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyskan R, Chapman K, Mattman A, Sin D. Antiglycine receptor antibody and encephalomyelitis with rigidity and myoclonus (PERM) related to small cell lung cancer. BMJ Case Rep. 2013;2013:1–3. doi: 10.1136/bcr-2013-010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasque M, Faivre-Sarrailh C. GPI-anchored proteins at the node of Ranvier. FEBS Lett. 2010;584:1787–1792. doi: 10.1016/j.febslet.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, Matá S, Kremens D, Vitaliani R, Geschwind MD, Bataller L, Kalb RG, Davis R, Graus F, Lynch DR, Balice-Gordon RJ, Dalmau J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Huijbers MGM, Lancaster E, Graus F, Bataller L, Balice-Gordon RJ, Cowell JK, Dalmau J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Lai M, Peng X, Hughes EG, Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura A, Ohta K, Iizuka T, Guzman M, Graus F, Moss SJ, Balice-Gordon R, Dalmau J. Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Huijbers MGM, Bar V, Boronat A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker RW, Graus F, Bataller L, Illa I, Markx S, Strauss KA, Peles E, Scherer SS, Dalmau J. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 2011a;69:303–311. doi: 10.1002/ana.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Martinez-Hernandez E, Titulaer MJ, Boulos M, Weaver S, Antoine JC, Liebers E, Kornblum C, Bien CG, Honnorat J, Wong S, Xu J, Contractor A, Balice-Gordon R, Dalmau J. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011b;77:1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, Maselli RA, Ellis WG, Agius MA. Morvan’s fibrillary chorea: a paraneoplastic manifestation of thymoma. J Neurol Neurosur Ps. 1998;65:857–862. doi: 10.1136/jnnp.65.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Griesmann GE, O’Suilleabhain PE, Windebank AJ, Woppmann A, Miljanich GP, Lambert EH. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med. 1995;332:1467–1475. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Safa P, Chen YR, Sobel RA, Boyden ES, Tsien RW. Anti-Ca2+ channel antibody attenuates Ca2+ current and mimics cerebellar ataxia in vivo. Proc Natl Acad Sci USA. 2008;105:2705–2710. doi: 10.1073/pnas.0710771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichte B, Veh RW, Meyer HE, Kilimann MW. Amphiphysin, a novel protein associated with synaptic vesicles. EMBO J. 1992;11:2521–2530. doi: 10.1002/j.1460-2075.1992.tb05317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzoni PJ, Scola RH, Lang B, Kay CSK, Teive HAG, Kowacs PA, Werneck LC. Cerebellar ataxia in non-paraneoplastic Lambert-Eaton myasthenic syndrome. J Neurol Sci. 2008;270:194–196. doi: 10.1016/j.jns.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. 2010;588(Pt 11):1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67:470–478. doi: 10.1002/ana.21917. [DOI] [PubMed] [Google Scholar]
- Malter MP, Frisch C, Schoene-Bake JC, Helmstaedter C, Wandinger KPP, Stoecker W, Urbach H, Surges R, Elger CE, Vincent AV, Bien CG. Outcome of limbic encephalitis with VGKC-complex antibodies: relation to antigenic specificity. J Neurol. 2014;261:1695–1705. doi: 10.1007/s00415-014-7408-6. [DOI] [PubMed] [Google Scholar]
- Manto MU, Laute MA, Aguera M, Rogemond V, Pandolfo M, Honnorat J. Effects of anti–glutamic acid decarboxylase antibodies associated with neurological diseases. Ann Neurol. 2007;61:544–551. doi: 10.1002/ana.21123. [DOI] [PubMed] [Google Scholar]
- Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet J Rare Dis. 2010;5:31. doi: 10.1186/1750-1172-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. Afferent facilitation of corticomotor responses is increased by IgGs of patients with NMDA-receptor antibodies. J Neurol. 2011a;258:27–33. doi: 10.1007/s00415-010-5674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto MU, Hampe CS, Rogemond V, Honnorat J. Respective implications of glutamate decarboxylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet J Rare Dis. 2011b;6:3. doi: 10.1186/1750-1172-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M, Honnorat J, Hampe CS, Guerra-Narbona R, López-Ramos JC, Delgado-García JM, Saitow F, Suzuki H, Yanagawa Y, Mizusawa H, Mitoma H. Disease-specific monoclonal antibodies targeting glutamate decarboxylase impair GABAergic neurotransmission and affect motor learning and behavioral functions. Front Behav Neurosci. 2015;9:1–14. doi: 10.3389/fnbeh.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marignier R, Chenevier F, Rogemond V, Sillevis Smitt P, Renoux C, Cavillon G, Androdias G, Vukusic S, Graus F, Honnorat J, Confavreux C. Metabotropic glutamate receptor type 1 autoantibody–associated cerebellitis. Arch Neurol. 2010;67:627–630. doi: 10.1001/archneurol.2010.51. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez E, Sepulveda M, Rostásy K, Höftberger R, Graus F, Harvey RJ, Saiz A, Dalmau J. Antibodies to aquaporin 4, myelin-oligodendrocyte glycoprotein, and the glycine receptor α1 subunit in patients with isolated optic neuritis. JAMA Neurol. 2015;72:187–193. doi: 10.1001/jamaneurol.2014.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-García E, Mannara F, Gutiérrez-Cuesta J, Sabater L, Dalmau J, Maldonado R, Graus F. Intrathecal injection of P/Q type voltage-gated calcium channel antibodies from paraneoplastic cerebellar degeneration cause ataxia in mice. J Neuroimmunol. 2013;261:53–59. doi: 10.1016/j.jneuroim.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Mas N, Saiz A, Leite MI, Waters P, Baron M, Castano D, Sabater L, Vincent A, Graus F. Anti glycine-receptor encephalomyelitis with rigidity. J Neurol Neurosur Ps. 2011;82:1399–1401. doi: 10.1136/jnnp.2010.229104. [DOI] [PubMed] [Google Scholar]
- Mason WP, Graus F, Lang B, Honnorat J, Delattre JY, Valldeoriola F, Antoine JC, Rosenblum MK, Rosenfeld MR, Newsom-Davis J, Posner JB, Dalmau J. Small-cell lung cancer, paraneoplastic cerebellar degeneration and the Lambert-Eaton myasthenic syndrome. Brain. 1997;120:1279–1300. doi: 10.1093/brain/120.8.1279. [DOI] [PubMed] [Google Scholar]
- Mat A, Adler H, Merwick A, Chadwick G, Gullo G, Dalmau J, Tubridy N. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80:1349–1350. doi: 10.1212/WNL.0b013e31828ab325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon A, Martinez-Hernandez E, Lancaster E, Matsumoto JY, Harvey RJ, McEvoy KM, Pittock SJ, Lennon VA, Dalmau J. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol. 2013;70:44. doi: 10.1001/jamaneurol.2013.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriney SD, Hulsizer SC, Lennon VA, Grinnel AD. Lambert-Eaton myasthenic syndrome immunoglobulins react with multiple types of calcium channels in small-cell lung carcinoma. Ann Neurol. 1996;40:739–749. doi: 10.1002/ana.410400510. [DOI] [PubMed] [Google Scholar]
- Mikasova L, De Rossi P, Bouchet D, Georges F, Rogemond V, Didelot A, Meissirel C, Honnorat J, Groc L. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, Honnorat J, Joubert B, Kakei S, Lee J, Manto M, Matsunaga A, Mizusawa H, Nanri K, Shanmugarajah P, Yoneda M, Yuki N. Consensus paper: neuroimmune mechanisms of cerebellar ataxias. Cerebellum. 2016;15:213–232. doi: 10.1007/s12311-015-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Lee ST, Shin JW, Byun JI, Lim JA, Shin YW, Kim TJ, Lee KJ, Park KI, Jung KH, Jung KY, Lee SK, Chu K. Non-stiff anti-amphiphysin syndrome: clinical manifestations and outcome after immunotherapy. J Neuroimmunol. 2014;274:209–214. doi: 10.1016/j.jneuroim.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;76:108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murinson BB, Guarnaccia JB. Stiff-person syndrome with amphiphysin antibodies: distinctive features of a rare disease. Neurology. 2008;71:1955–1958. doi: 10.1212/01.wnl.0000327342.58936.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E, Sakakibara R, Kawashima K, Yoshida T, Kishi M, Tateno F, Kataoka M, Kawashima T, Yamamoto M. VGCC antibody-positive paraneoplastic cerebellar degeneration presenting with positioning vertigo. Neurol Sci. 2011;32:1209–1212. doi: 10.1007/s10072-011-0648-7. [DOI] [PubMed] [Google Scholar]
- Ohkawa T, Fukata Y, Yamasaki M, Miyazaki T, Yokoi N, Takashima H, Watanabe M, Watanabe O, Fukata M. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci. 2013;33:18161–18174. doi: 10.1523/JNEUROSCI.3506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa T, Satake S, Yokoi N, Miyazaki Y, Ohshita T, Sobue G, Takashima H, Watanabe O, Fukata Y, Fukata M. Identification and characterization of GABAA receptor autoantibodies in autoimmune encephalitis. J Neurosci. 2014;34:8151–8163. doi: 10.1523/JNEUROSCI.4415-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman R, Risch N, Hauser WA, Pedley TA, Lee JH, Barker-Cummings C, Lustenberger A, Nagle KJ, Lee KS, Scheuer ML, Wilhelmsen KC. Localization of a gene for partial epilepsy to chromosome 10q. Nat Genet. 1995;10:56–60. doi: 10.1038/ng0595-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Peng X, Hughes EG, Moscato EH, Parsons TD, Dalmau J, Balice-Gordon RJ. Cellular plasticity induced by anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor encephalitis antibodies. Ann Neurol. 2015;77:381–398. doi: 10.1002/ana.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Pedrol M, Armangué T, Peng X, Bataller L, Cellucci T, Davis RL, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcalà C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettingill P, Kramer HB, Coebergh JA, Pettingill R, Maxwell S, Nibber A, Malaspine A, Jacob A, Irani SR, Buckley C, Lang B, Waters P, Vincent A. Antibodies to GABAA receptor α1 and γ2 subunits: clinical and serologic characterization. Neurology. 2015;84:1233–1241. doi: 10.1212/WNL.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepgras J, Holtje M, Michel K, Li Q, Otto C, Drenckhahn C, Probst C, Schemann M, Jarius S, Stöcker W, Balint B, Meinck HM, Buchert R, Dalmau J, Ahnert-Hilger G, Ruprecht K. Anti-DPPX encephalitis: pathogenic effects of antibodies on gut and brain neurons. Neurology. 2015;85:890–897. doi: 10.1212/WNL.0000000000001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinatel D, Hivert B, Boucraut J, Saint-Martin M, Rogemond V, Zoupi L, Karagogeos D, Honnorat J, Faivre-Sarrailh C. Inhibitory axons are targeted in hippocampal cell culture by anti-Caspr2 autoantibodies associated with limbic encephalitis. Front Cell Neurosci. 2015;9:265. doi: 10.3389/fncel.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53:580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, Kim KK, Kilimann MW, Lennon VA. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58:96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- Planaguma J, Leypoldt F, Mannara F, Gutierrez-Cuesta J, Martin-Garcia E, Aguilar E, Titulaer MJ, Petit-Pedrol M, Jain A, Balice-Gordon R, Lakadamyali M, Graus F, Maldonado R, Dalmau J. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2014;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C, Komorowski L, de Graaff E, van Coevorden-Hameete M, Rogemond V, Honnorat J, Sabeter L, Graus F, Jarius S, Voltz R, Wildemann B, Franciotta D, Blöcker IM, Schlumberger W, Stöcker W, Sillevis Smitt PA. Standardized test for anti-Tr/DNER in patients with paraneoplastic cerebellar degeneration. Neurol Neuroimmunol Neuroinflamm. 2015;2:e68. doi: 10.1212/NXI.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Rothkirch M, Kopp U, Hamer HM, Hagge M, Sterzer P, Saschenbrecker S, Stöcker W, Harms L, Endres M. Limbic encephalitis with mGluR5 antibodies and immunotherapy-responsive prosopagnosia. Neurology. 2014;83:1384–1386. doi: 10.1212/WNL.0000000000000865. [DOI] [PubMed] [Google Scholar]
- Prüss H, Finke C, Höltje M, Hofmann J, Klingbeil C, Probst C, Borowski K, Ahnert-Hilger G, Harms L, Schwab JM, Ploner CJ, Komorowski L, Stoecker W, Dalmau J, Wandinger KP. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72:902–911. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspotnig M, Vedeler CA, Storstein A. Onconeural antibodies in patients with neurological symptoms: detection and clinical significance. Acta Neurol Scand Suppl. 2011;124:83–88. doi: 10.1111/j.1600-0404.2011.01549.x. [DOI] [PubMed] [Google Scholar]
- Rigamonti A, Lauria G, Stanzani L, Mantero V, Andreetta F, Salmaggi A. Non-paraneoplastic voltage-gated calcium channels antibody-mediated cerebellar ataxia responsive to IVIG treatment. J Neurol Sci. 2014;336:169–170. doi: 10.1016/j.jns.2013.10.031. [DOI] [PubMed] [Google Scholar]
- Saiz A, Blanco Y, Sabater L, González F, Bataller LL, Casamitjana R, Ramió-Torrentà L, Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009;158:105–125. doi: 10.1016/j.neuroscience.2008.02.037. [DOI] [PubMed] [Google Scholar]
- Shillito P, Molenaar PC, Vincent A, Leys K, Zheng W, Van den Berg R, Plomp JJ, Van Kempen GT, Chauplannaz G, Wintzen AR. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol. 1995;38:714–722. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- Shin YW, Lee STSKST, Shin JW, Moon J, Lim JAA, Byun JII, Kim TJ, Lee KJ, Kim YS, Park KI, Jung KH, Lee SK, Chu K. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol. 2013;265:75–81. doi: 10.1016/j.jneuroim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Shutoh F, Katoh A, Kitazawa H, Aiba A, Itohara S, Nagao S. Loss of adaptability of horizontal optokinetic response eye movements in mGluR1 knockout mice. Neurosci Res. 2002;42:141–145. doi: 10.1016/s0168-0102(01)00308-x. [DOI] [PubMed] [Google Scholar]
- Sillevis Smitt PA, Manley GT, Posner JB. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology. 1995;45:1873–1878. doi: 10.1212/wnl.45.10.1873. [DOI] [PubMed] [Google Scholar]
- Sillevis Smitt P, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, Henzen-Logmans S, Vecht C, De Zeeuw C, Sekiyama N, Nakanishi S, Shigemoto R. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342:21–27. doi: 10.1056/NEJM200001063420104. [DOI] [PubMed] [Google Scholar]
- Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 2014;82:24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Solimena M, Folli F, Denis-Donini S, Comi GC, Pozza G, De Camilli P, Vicari AM. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N Engl J Med. 1988;318:1012–1020. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- Sommer C, Weishaupt A, Brinkhoff J, Biko L, Wessig C, Gold R, Toyka KV. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet. 2005;365:1406–1411. doi: 10.1016/S0140-6736(05)66376-3. [DOI] [PubMed] [Google Scholar]
- Spatola M, Stojanova V, Prior JO, Dalmau J, Rossetti AO. Serial brain 18FDG-PET in anti-AMPA receptor limbic encephalitis. J Neuroimmunol. 2014;271:53–55. doi: 10.1016/j.jneuroim.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Stemmler N, Rohleder K, Malter MP, Widman G, Elger CE, Beck H, Surges R. Serum from a patient with GAD65 antibody-associated limbic encephalitis did not alter GABAergic neurotransmission in cultured hippocampal networks. Front Neurol. 2015;6:1–7. doi: 10.3389/fneur.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell. 1991;67:325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- Thomas L, Mailles A, Desestret V, Ducray F, Mathias E, Rogemond V, Didelot A, Marignier S, Stahl JP, Honnorat J, Vuillemet F. Autoimmune N-methyl-D-aspartate receptor encephalitis is a differential diagnosis of infectious encephalitis. J Infect. 2014;68:419–425. doi: 10.1016/j.jinf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin WO, Lennon VA, Komorowski L, Probst C, Clardy SL, Appendino JP. DPPX potassium channel antibody frequency, clinical accompaniments, and outcomes in 20 patients. Neurology. 2014;83:1797–1803. doi: 10.1212/WNL.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter JL, Hendin BA, Osterland CK. Cerebellar degeneration with Hodgkin disease. An immunological study. Arch Neurol. 1976;33:660–661. doi: 10.1001/archneur.1976.00500090066014. [DOI] [PubMed] [Google Scholar]
- Turner MR, Irani SR, Leite MI, Nithi K, Vincent A, Ansorge O. Progressive encephalomyelitis with rigidity and myoclonus: glycine and NMDA receptor antibodies. Neurology. 2011;77:439–443. doi: 10.1212/WNL.0b013e318227b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüzün E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–743. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaccoz A, Desestret V, Ducray F, Picard G, Cavillon G, Rogemond V, Antoine JC, Delattre JY, Honnorat J. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82:556–563. doi: 10.1212/WNL.0000000000000126. [DOI] [PubMed] [Google Scholar]
- Wei YC, Liu CH, Lin JJ, Lin KJ, Huang KL, Lee TH, Chang YJ, Peng TI, Lin KL, Chang TY, Chang CH, Kuo HC, Chang KH, Cheng MY, Huang CC. Rapid progression and brain atrophy in anti-AMPA receptor encephalitis. J Neuroimmunol. 2013;261:129–133. doi: 10.1016/j.jneuroim.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Woodhall M, Coban A, Waters P, Ekizoğlu E, Kürtüncü M, Shugaiv E, Türkoğlu R, Akman-Demir G, Eraksoy M, Vincent A, Tüzün E. Glycine receptor and myelin oligodendrocyte glycoprotein antibodies in Turkish patients with neuromyelitis optica. J Neurol Sci. 2013;335:221–223. doi: 10.1016/j.jns.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Wuerfel E, Bien CG, Vincent A, Woodhall M, Brockmann K. Glycine receptor antibodies in a boy with focal epilepsy and episodic behavioral disorder. J Neurol Sci. 2014;343:180–182. doi: 10.1016/j.jns.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tanaka K, Sun P, Nakata M, Yamamoto R, Sakimura K, Matsui M, Kato N. Suppression of synaptic plasticity by cerebrospinal fluid from anti-NMDA receptor encephalitis patients. Neurobiol Dis. 2012;45:610–615. doi: 10.1016/j.nbd.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Zuliani L, Sabater L, Saiz A, Baiges JJ, Giometto B, Graus F. Homer autoimmunity in subacute idiopathic cerebellar ataxia. Neurology. 2007;68:239–240. doi: 10.1212/01.wnl.0000251308.79366.f9. [DOI] [PubMed] [Google Scholar]
- Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MA, Ekici AB, Reis A, Schenck A, Rauch A. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]