Abstract
Purpose
To address gaps in understanding and treating lower urinary tract symptoms (LUTS), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) created the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN). The goals of LURN are to work collaboratively to 1) identify and explain the important subtypes of LUTS; 2) improve the measurement of patient experiences of LUTS; 3) disseminate novel findings to researchers, clinicians, and patients; and 4) generate data, research tools, and biological samples for future studies.
Methods
As a first step in understanding subtypes of LUTS, LURN will focus on disorders of urinary sensation (e.g., urgency) and their causes. These are being examined with respect to patient experience, organism or systemic factors, genitourinary organs and tissues, and cellular/molecular factors. This is being achieved via an observational cohort study that is currently enrolling patients with LUTS (target N = 1,000) and that will extensively characterize patients with LUTS. Future studies embedded within the observational cohort study will focus on neuroimaging and sensory testing, biomarkers, and organ-based factors. To advance the science of measurement of LUTS, LURN is also developing and evaluating a comprehensive set of self-report questions to provide more granular assessments of LUTS.
Conclusions
LURN has taken its first steps by developing a framework for studying LUTS subtypes, choosing an initial domain on which to focus (sensory experiences), and creating and executing protocols designed to improve measurement of self-reported symptoms and identifying patient subtypes.
Keywords: lower urinary tract, research network
INTRODUCTION
To hasten advances in assessing and treating lower urinary tract dysfunction, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) created the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN). The purpose of this paper is to introduce the motivation for LURN, its scientific objectives and study design, and lessons learned to date. The research and clinical communities are invited to provide comments, ideas, and proposals to interact with LURN.
Motivation for LURN
The Challenge of Understanding and Treating Lower Urinary Tract Symptoms (LUTS)
Patients present with a wide variety of symptoms putatively but not uniformly related to the lower urinary tract, including LUTS associated with urine storage, voiding, and post-micturition. Although symptoms are attributed to the lower urinary tract, their etiology may or may not have their source in the organs, structures, and functions of the lower urinary tract. For example, urinary incontinence may be entirely due to anxiety disorder1. When the cause of LUTS is definitively linked to one or more structures and/or functions of the lower urinary tract, the term lower urinary tract dysfunction (LUTD) is appropriately applied. The distinction between LUTS and LUTD is important; improvements in the diagnosis and management of patients with LUTS are predicated on better understanding of all potential etiologic and contributing factors, including but definitely not limited to LUTD alone.
LUTS are highly prevalent and occur in each sex to a similar extent: 51.3% and 59.2% of men and women, respectively, exhibit storage symptoms; 25.7% and 19.5%, respectively, exhibit voiding symptoms; and 16.9% and 14.2%, respectively, exhibit post-micturition symptoms2. Unfortunately, many patients who seek care for LUTS experience neither total nor permanent resolution of their symptoms with current management approaches3,4. The impact of LUTS is significant and wide-ranging5,6. LURN was developed to address the gaps in understanding and treating LUTS.
One of the barriers to improving diagnosis and management of patients with LUTS is incomplete knowledge and imprecise classification of subtypes of LUTS patients and their associated etiologies. Effective treatments cannot be determined without identifying clinically meaningful clusters of patients and their corresponding causes. There has been a growing appreciation that the causes of LUTS are perhaps more numerous than once thought. The original use of the term LUTS attached symptoms to a specific urologic organ, the prostate. In men over 50 years old, LUTS is usually attributed to urinary obstruction caused by an enlarged prostate (i.e., benign prostatic hyperplasia). However, research suggests that common pathophysiological changes, such as inflammation and fibrosis, and connective tissue, vascular, or neurologic factors in more than one urologic organ may be responsible for a group of symptoms7-9. Moreover, involvement of adjacent non-urological organs (e.g., colon), remote organs (e.g., brain), other diseases or conditions (e.g., diabetes mellitus), medications (e.g., diuretics), or lifestyle factors (e.g., fluid consumption habits) also contribute to the development or severity of LUTS. While LUTS was used for male symptoms associated with an enlarged prostate in the past, today it has been expanded to include urinary symptoms of women. In the US general population, women have a higher prevalence of urinary symptoms10, many of which are commonly considered a side effect of childbearing. But obesity, diabetes, metabolic syndrome, cardiovascular disease, sexual activity, and aging are also associated with increased risk of LUTS11. Thus, research is needed to understand how these and other factors determine the symptoms and experiences of important clinical subtypes of patients with LUTS related to LUTD and other etiologies.
Opportunity for Improved Self-Reported Measurements of Health in Patients with LUTS
The effort to identify subtypes of patients and explain their variants of LUTS will benefit from high quality measurement of all patient characteristics. This is especially true of patient characteristics that are typically self-reported, such as symptoms, bother, approaches to adaptation/coping, and functioning. Success in identifying and explaining important patient subtypes will rely on the comprehensiveness, validity, and precision of such self-reported concepts. In terms of comprehensiveness, the set of measures must address the symptoms and experiences deemed important by patients. Validity includes determination of the time period within which patients can accurately recall the measures being self-reported (e.g., prior seven days vs. prior month). For precision, the measures must have as little error as possible to allow sufficient distinctions between patients to define important clinical subtypes. Thus, improved self-reported measurements can advance person-centered approaches to classifying and treating patients with LUTS.
Development of LURN
In recognition of the preceding challenges and opportunities, NIDDK convened a Meeting on Measurement of Urinary Symptoms (MOMUS) in November 2011 to frame a discussion focused on identifying the research needed to advance the field of LUTS diagnosis and treatment (http://www.niddk.nih.gov/news/events-calendar/Pages/meeting-on-measurement-of-urinary-symptoms-momus.aspx). Based on the MOMUS recommendations, the NIDDK developed and funded LURN as an interdisciplinary consortium-based cooperative research network (RFA-DK-11-026) to catalyze the LUTS research community. Initially formed in 2012, and expanded in 2013 (RFA-DK-12-017), LURN is now a functioning research network (www.nih-lurn.org) comprising six clinical research sites and one data coordinating center (DCC). The long-term goals of LURN are to work collaboratively to increase understanding of LUTS, in order to achieve four objectives: 1) identify and explain the important subtypes of LUTS; 2) improve the measurement of patient experiences of LUTS; 3) disseminate novel findings to researchers, clinicians, and patients; and 4) generate data, research tools, and biological samples for future studies.
In the remainder of this article, we describe LURN's strategy to achieve these objectives and comment on some of the early lessons we have learned.
Objective 1: Identify and Explain Important Subtypes of LUTS
Conceptual Framework
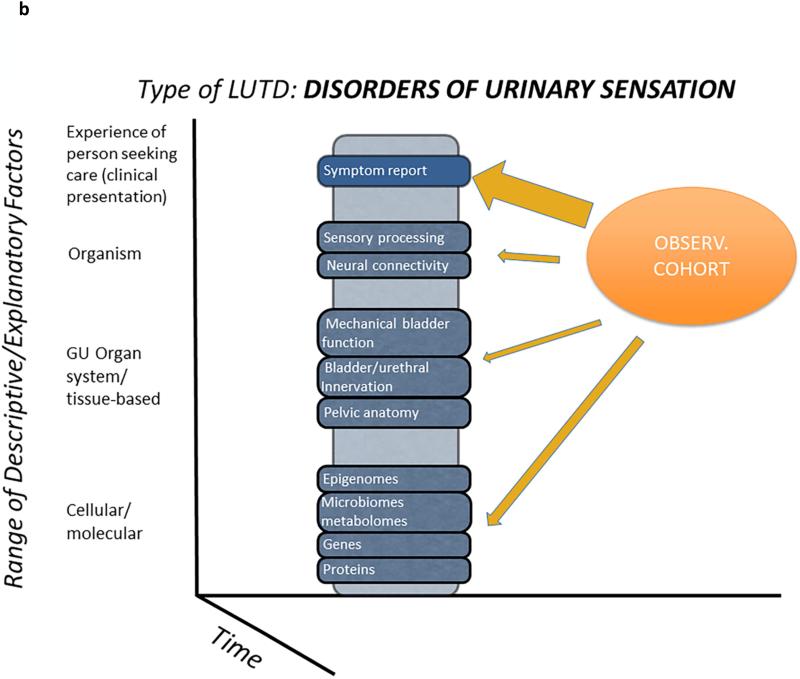
Given the complexity of trying to characterize meaningful and actionable subtypes of LUTS, we began with a conceptual framework within which all of our research efforts in LURN can be understood (Figure 1). The foundation of this framework is a cube representing all possible knowledge about LUTS—a cube that is now relatively unpopulated because the planned research program has not been completed (Figure 1a). The axes that define the cube are as follows: The y-axis is the range of descriptive or explanatory factors that define the etiology and maintenance of symptoms. The LURN investigators have selected four levels on which to focus: patient experience, organism or systemic factors, genitourinary organs and tissues, and cellular/molecular factors. Meaningful differences among patients may be identified at any of these levels. For example, patients experience a range of combinations of symptoms, such as urgency, nocturia, incontinence, and hesitancy. At the cellular/molecular level, patients may differ in their genetic predisposition for different subtypes of LUTS. The range across patients within a given level of descriptive/explanatory factors is indicated on the x-axis. The z-axis represents time. Combining all three dimensions, we can say, for example, that the top “slice” of this cube reflects all possible symptoms of LUTS and their changes over time, whether due to natural progression, prevention, or treatment. The middle slice of the cube reflects all possible states of the genitourinary organs and tissues and their changes over time.
Figure 1. Conceptual representation of LURN studies.
Figure 1a. Conceptual framework within which all of the LURN research efforts can be understood. The structure of this framework is a “knowledge space” imagined here as a cube, representing all possible knowledge about LUTD—a cube that is now only partially and irregularly filled because the majority of research done heretofore has been done in relative isolation. The work of LURN is a systematic effort to “fill in” this knowledge space.
Figure 1b. Components of LURN phenotyping studies. The LURN approach to phenotyping will be to start with a prospective observational cohort of subjects with LUTD. This cohort will also serve as the basis for further studies, which will allow more in-depth characterization of subsets of participants. These targeted studies will focus on sensory disorders of the urinary tract, and include neuroimaging and multimodal sensory testing, organ-based studies (i.e., testing of bladder and urethral function), and the evaluation of biological samples for biomarkers.
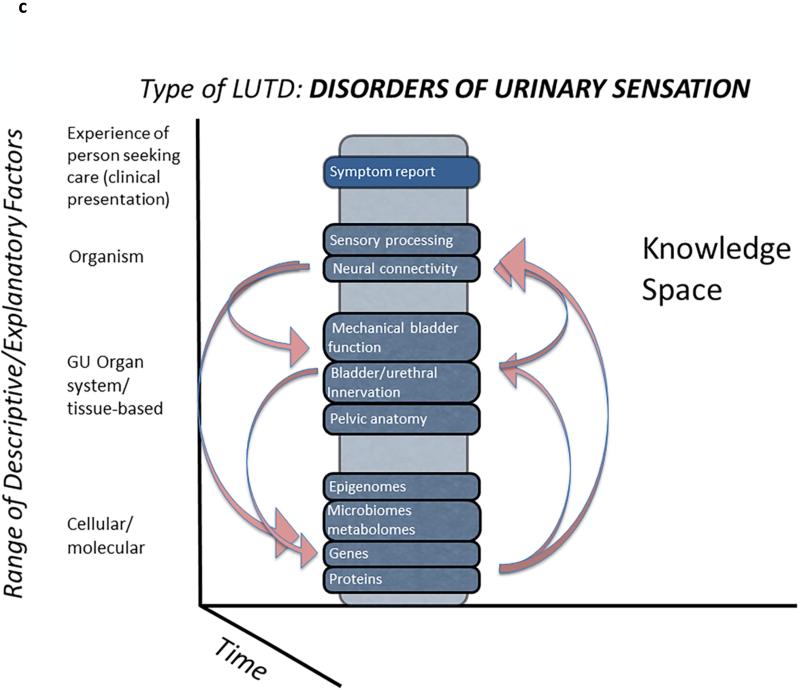
Figure 1c. Interaction of LURN studies. LURN is purposely and prospectively examining multiple factors looking for associations, rather than trying to isolate specific factors-- an entirely unique approach to the study of LUTD. The arrows designate the possible associations and links between the substudies, and they are not limited to the combinations shown here.
LURN is engaged in a systematic effort to populate this knowledge cube. Specifically, LURN seeks to first identify important differences among patients at a given level (Figure 1b, y-axis), whether it be in terms of patient experience, organism or systemic factors, genitourinary organs and tissues, or cellular/molecular factors. We then wish to explain why differences among patients exist at a given level (e.g., genitourinary organs and tissues) by identifying relationships between those characteristics and other levels (e.g., cells and molecules, Figure 1c). For example, patient symptoms can be linked to a particular matrix of organ physiology, environmental factors, or life experiences. These linkages will subsequently define and characterize subtypes of LUTS. Factors at multiple levels are grounded in mechanistic theories about biological, behavioral, and environmental influences on the person. These theories give rise to the hypotheses and research questions that motivate the specific LURN phenotyping protocols.
LURN Research Program to Identify Subtypes of LUTS
Our next step after specifying the conceptual framework was to identify the part of the cube that would be addressed first. LURN investigators agreed to focus on disorders of urinary sensation (e.g., urgency) and their causes, because most prior research has emphasized motor aspects of the urinary tract. To identify important patient subtypes of LUTS within the domain of sensory disorders, LURN is developing a series of four interrelated research components, described in Table 1. Collectively, these protocols will recruit and enroll research subjects whose data will be analyzed to identify new and potentially useful subtypes of patients with LUTS.
Table 1.
Objectives and Research Protocols of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
| LURN Objective 1: Identify subtypes of patients with LUTS | LURN Objective 2: Improve the measurement of patient experiences of LUTS | ||
|---|---|---|---|
| Protocol | Objective | Protocol | Objective |
| Observational Cohort Study | Characterize patients with LUTS; identify distinct LUTS subtypes; and describe treatment outcomes one year after enrollment | Item Development | Create self-report items that measure the full range of LUTS, are understandable to broad populations, and are easily translated into non-English languages. |
| Biomarker Substudy | Use banked biological samples to discover and validate associations of biomarkers with LUTS. | Evaluation of Recall Period | Compare how reliably patients can report LUTS experiences over given time periods (e.g., one week vs. one month). |
| Neuroimaging and Sensory Substudy | Use functional magnetic resonance imaging to look for connectivity differences in persons with and without LUTS. Test for an association between LUTS and altered central nervous system responses to auditory and pain stimuli. | ||
| Organ-Based Substudy | Conduct in-depth evaluations of the urethral and vesical components of the lower urinary tract to identify pathophysiological differences between patients with and without LUTS. | ||
The overarching research tool is a prospective observational cohort study that is designed to recruit a large group of incident men and women with LUTS presenting for the first time to LURN research site providers. The objectives of the study are to: 1) extensively characterize patients with LUTS; 2) identify distinct LUTS subtypes using cluster analysis and classification and regression trees; and 3) describe treatment outcomes one year after enrollment. We plan to enroll 1000 participants with LUTS exclusive of those with pain as a dominant symptom, since pain is a primary research objective of a separately-funded NIDDK Multidisciplinary Approach to Chronic Pelvic Pain (MAPP) Research Network (http://www.mappnetwork.org). Clinical data, health-related quality of life assessments, and self-reported measurement data at baseline will be extensively characterized. Enrollment is anticipated to be completed within 12-15 months; change in LUTS (progression; response to treatment) will be measured with follow-up assessments over 12 months. Biological specimens (blood, urine, saliva, and microbiological specimens) will be collected at baseline and during follow-up. These materials will be used to establish a banked biospecimen collection from these extensively characterized LUTS study participants, and will be accessible for future studies. The observational cohort study is currently enrolling research subjects. The other three research components, each embedded under the observational cohort study, are described next.
The second planned component is a neuroimaging and sensory testing study that will examine the hypothesis that characteristic central nervous system features can be identified by functional magnetic resonance imaging in patients with LUTS. Psychophysical testing procedures will also be conducted to test for an association between LUTS and altered central nervous system responses to auditory and pain stimuli. Study participants without LUTS will also be tested as a comparator group.
The third planned component is an embedded biomarker study, and is currently under development. Banked biological samples from study participants will be used to discover and validate associations of biomarkers with LUTS. Biological samples from study participants without LUTS will be collected for assay as a non-LUTS comparator group.
The fourth planned embedded component is an organ-based testing study. In-depth, novel evaluations of the urethral and vesical components of the lower urinary tract will be conducted with the aim to reveal pathophysiological differences between patients with LUTS and control participants without LUTS.
These four studies will generate rich and comprehensive data across multiple domains that will provide opportunities for cross-cutting analyses above and beyond those envisioned in each of the individual components. As noted above, the connections across studies will be used to further define and characterize subtypes of LUTS.
Additionally, LURN investigators recognized early on that it is critically important to incorporate measurements of non-urologic factors related to LUTS, which we have defined as those factors distinct from the lower urinary tract, e.g., fluid consumption habits, psychosocial factors. We reasoned that knowledge of some non-urologic factors could contribute significantly to the identification of important patient subtypes. A separate workgroup took on the task of reviewing the literature on non-urologic factors. This proved to be a challenging task due to the large number of potential non-urologic factors and the generally poor quality of published research on these factors. At present, this literature review is still under way. Our challenge is to make decisions about incorporating certain non-urologic factors into LURN research protocols under development, while at the same time trying to process the literature to date on these factors to inform better research strategies in the future.
Objective 2: Improve the Measurement of Patient Experiences of LUTS
The main goals of this aspect of LURN are to (1) compile a set of best-in-class, self-report measurement tools for patients with LUTS for use in efforts to identify subtypes of LUTS; (2) create a publically available resource for best-in-class measures of LUTS; and (3) lay the groundwork for the future adaptations of the LURN self-report measures into “fit for purpose” study endpoints, as well as clinical tools for screening, triage, and symptom monitoring. Toward the first goal, LURN is currently conducting one protocol and developing a second, each of which will lead to a set of items for measuring LUTS for the purpose of subtyping studies, known as the LURN Comprehensive Assessment of Self-reported Urinary Symptoms (LURN CASUS; see Table 1).
Self-reported measures of patient experiences may play varied roles throughout the LURN research program. We are currently focused on discovery of clinically relevant patient subtypes, and rich symptom reports are an important part of that investigation. Thus, the first self-reported tool LURN will produce—the LURN CASUS--is intended to facilitate the discovery of these patient subtypes. Developing self-reported measures for use in clinical subtyping studies is different than developing measures for use as patient-reported outcome measures (PROMs) in clinical trials or measures for screening patients into different diagnostic categories. Most extant guidelines12 for self-reported measure development are specifically for designing measures of study endpoints. But the demands of a measure for use in subtyping studies are somewhat different. For example, PROMs are generally brief and focus on those aspects of the health condition that matter most to patients, i.e., key symptoms and their impact on functioning. Furthermore, PROMs typically result in a small number of scale scores, because a larger number of endpoints in a clinical trial require more care to protect against Type 1 error. However, LURN network discussions that included investigators focused on the development of the self-report tools and those focused on the design of protocols to identify clinical patient subtypes suggested a different set of considerations for designing a self-report measure that would be useful for identifying patient subtypes—namely, granularity and comprehensiveness of symptom assessments. And, since Type 1 error is less important in this context than obtaining useful information for identifying subtypes, the self-report measure can have a separate score for every specific aspect of symptom experience. At this early stage of research on patient subgroups, the pitfall to avoid is having measurements that are too broad to make fine-grained discriminations among patients.
Once the important subtypes of LUTS are identified, a new set of self-reported measurement tools will be developed to facilitate clinical research and clinical care based on these new subtypes. With respect to clinical research, it will be important to derive reliable and valid measures of patient-reported outcomes to evaluate the safety and efficacy of new and existing treatments for patients within each subtype of LUTS. Such patient-reported measures will require items addressing symptoms as well as impact on functioning. With respect to clinical care, self-reported tools can be developed to serve two functions. Self-report items could be included as part of efficient clinical screening tools to identify the subtype of a newly presenting LUTS patient. Such tools are likely to require far fewer items than will be needed in the discovery phase of self-reported measures used for LUTS subtyping. A second type of self-reported measure in clinical care is one that can be used to monitor changes in symptoms over time. Thus, the LURN self-reported measurement efforts will span the entire research program of LURN from discovery to dissemination into clinical care.
Objective 3: Disseminate Novel Findings
The studies undertaken by the LURN consortium are intended to change the way we study and treat patients with LUTS. To facilitate this change, LURN is considering a broad range of dissemination strategies to target the agents of change. The cornerstone of the dissemination strategy will be peer-reviewed manuscripts and presentations at professional conferences to establish the scientific validity of the findings. Beyond this, however, LURN investigators will make special efforts to share and discuss study findings with the leadership of relevant professional societies, public and commercial sponsors of research, and patient groups. Additionally, the study website (www.nih-lurn.org) offers publicly accessible information to interested parties, and will be used as a portal for patients, providers, researchers, and others to access research products of the network as they are produced.
Objective 4: Generate Reposited Data, Research Tools, and Biological Samples
NIDDK has established centralized data and biospecimen repositories to increase the impact of current and previously funded NIDDK studies by making these broadly available to the scientific community (www.niddkrepository.org). The DCC works closely with the NIDDK repositories to coordinate best practice procedures for integrated data and biosample acquisition, handling, shipment, storage, and use. The DCC is responsible for submission of research datasets to the data repository once the network's scientific activities are completed. This ensures that the investments in generating these unique and valuable resources can be leveraged by other researchers in the future. Biological samples housed at the NIDDK Repository will be made available to other researchers in a similar manner after completion of scientific work by the network. Additionally, while the LURN network is still active, data and/or biospecimens may be made available to qualified researchers who have a scientific connection to LURN through an Ancillary Studies Policy.
LURN Research Vision
It is a central premise of LURN that morbidity due to LUTS and associated adverse effects on the patient experience can be ameliorated as a result of scientifically sound elaboration of subtypes of patients with LUTS. LURN has taken its first steps by developing a framework for studying LUTS subtypes, choosing an initial domain on which to focus (sensory experiences), and creating protocols designed to improve measurement of self-reported symptoms and identifying patient subtypes. LURN sees itself as a part of the NIDDK's urologic research strategy to make advances in understanding and treating the major symptomatic conditions of the lower genitourinary tract. Our sister research networks include the MAPP Research Network, which has entered its second funding cycle, and the newly formed Prevention of Lower Urinary Tract Symptoms (PLUS) Consortium. Hopefully, the complementary efforts of these three networks will advance our understanding of normal and abnormal function of the lower urinary tract, and this comprehensive approach will lead to reduced morbidity from LUTS.
ACKNOWLEDGEMENTS
This is publication number 1 of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
This study is supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants DK097780, DK097772, DK097779, DK099932, DK100011, DK100017, DK097776).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Duke University, Durham, North Carolina (DK097780): PI: Cindy Amundsen, MD, Kevin Weinfurt, PhD; Co-Is: Kathryn Flynn, PhD, Matthew O. Fraser, PhD, Todd Harshbarger, PhD, Drew Peterson, MD, Xavier A. Preud'homme, MD, Nazema Siddiqui, MD, George Webster, MD, ChB, FRCS; Study Coordinators: Carrie Dombeck, MA, Robin Gilliam, MSW, Akira Hayes, Nicole Longoria, PA, Shantae McLean, MPH
University of Iowa, Iowa City, IA (DK097772): PI: Karl Kreder, MD, MBA, Catherine S Bradley, MD, MSCE, Co-Is: Bradley A. Erickson, MD, MS, Susan K. Lutgendorf, PhD, Vince Magnotta, PhD, Michael A. O'Donnell, MD, Vivian Sung, MD; Study Coordinators: Linda Moss, RN, BSN, CCRC, Andrea Lopez
Northwestern University, Chicago, IL (DK097779): PI: David Cella, PhD, Brian T. Helfand, MD, PhD; Co-Is: James Griffith, PhD, John C. Hairston, MD, Kimberly Kenton, MD, MS, Todd Parrish, PhD, Jennie Yu Fan Chan, MD; Study Coordinators: Alexandria Alverdy, Sarah Buono, Maria Corona, Jasmine Nero, Pooja Talaty, Veronica Venezuela
University of Michigan Health System, Ann Arbor, MI (DK099932): PI: J Quentin Clemens, MD, FACS, MSCI; Co-Is: Mitch Berger, MD, PhD, John DeLancey, MD, Dee Fenner, MD, Rick Harris, MD, Steve Harte, PhD, Anne Pelletier-Cameron, MD, John Wei, MD; Study Coordinators: Linda Drnek, Nina Dutta, Nicole Elmblad, Greg Mowatt, Julie Tumbarello
University of Washington, Seattle Washington (DK100011): PI: Claire Yang, MD; Co-I: John L. Gore, MD, MS; Study Coordinators: Sara Teller, Brenda Vicars, RN
Washington University in St. Louis, St. Louis Missouri (DK100017): PI: Gerald Andriole, MD, Henry Lai; Co-I: Joshua Shimony, MD; Study Coordinators: Aleksandra Klim, RN, MHS, CCRC, Susan Mueller, RN, BSN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Urology Clinical Research and Epidemiology, Bethesda, MD: Project Scientist: Ziya Kirkali MD; Project Officer: John Kusek, PhD, NIH Personnel: Tamara Bavendam, MD, Robert Star, MD, Jenna Norton
Arbor Research Collaborative for Health, Data Coordinating Center, Ann Arbor, MI (DK097776): PI: Robert Merion, MD, FACS; Co-Is: Brenda Gillespie, PhD, Victor Andreev, PhD, DSc; Project Manager: Suzanne Kapica, MA, LPC, CAADC; Clinical Study Process Manager: Peg Hill-Callahan, BS, LSW; Timothy Buck, BS, CCRP; Analyst: Jon Wiseman, MS; Project Associate: Julieanne Lock, MLitt, BA
This article outlines independent research commissioned by the National Institutes of Health (NIH). The views expressed in this article are those of the author(s) and are not necessarily those of the NIH, the NIDDK, or the Department of Health and Human Services.
STANDARD ABBREVIATIONS KEY
- DCC
data coordinating center
- LURN
Symptoms of Lower Urinary Tract Dysfunction Research Network
- LUTD
lower urinary tract dysfunction
- LUTS
lower urinary tract symptoms
- MAPP
Multidisciplinary Approach to Chronic Pelvic Pain
- MOMUS
Meeting on Measurement of Urinary Symptoms
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- PROMs
patient-reported outcome measures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bradley CS, Nygaard IE, Mengeling MA, et al. Urinary incontinence, depression and posttraumatic stress disorder in women veterans. Am J Obstet Gynecol. 2012;206:502, e1. doi: 10.1016/j.ajog.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Richter HE, Litman HJ, Lukacz ES, et al. Demographic and clinical predictors of treatment failure one year after midurethral sling surgery. Obstet Gynecol. 2011;117:913. doi: 10.1097/AOG.0b013e31820f3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh JS, Cho KJ, Kim HS, et al. Twelve-month medication persistence in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Int J Clin Pract. 2014;68:197. doi: 10.1111/ijcp.12241. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Sexton CC, Irwin DE, et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101:1388. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 6.Tennstedt SL, Chiu GR, Link CL, et al. The effects of severity of urine leakage on quality of life in Hispanic, white, and black men and women: the Boston community health survey. Urology. 2010;75:27. doi: 10.1016/j.urology.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013;10:546. doi: 10.1038/nrurol.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 9.Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn. 2011;30:673. doi: 10.1002/nau.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litwin MS, Saigal CS, editors. Urologic Diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; US Government Printing Office; Washington, DC: 2012. p. xi. Table 3. Introduction NIH PUblication No. 12-7865. [Google Scholar]
- 11.Newman DK, Cardozo L, Sievert KD. Preventing urinary incontinence in women. Curr Opin Obstet Gynecol. 2013;25:388. doi: 10.1097/GCO.0b013e328364a35d. [DOI] [PubMed] [Google Scholar]
- 12.DeWalt DA, Rothrock N, Yount S, et al. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45:S12. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]