Abstract
Background and purpose
The enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) hydrolyze endogenous cannabinoids (eCBs), N-arachidonoyl ethanolamine (AEA) and 2-arachidonoyl glycerol (2-AG), respectively. These enzymes also metabolize eCB analogs such as lipoamines and 2-acyl glycerols, most of which are not ligands at CB1. To test the hypothesis that deleting eCB hydrolyzing enzymes and CB1 shifts lipid metabolism more broadly and impacts more families of eCB structural analogs, targeted lipidomics analyses were performed on FAAH KO, MAGL KO, and CB1 KO mice and compared to WT controls in 8 brain regions.
Experimental approach
Methanolic extracts of discrete brain regions (brainstem, cerebellum, cortex, hippocampus, hypothalamus, midbrain, striatum and thalamus) were partially purified on C-18 solid-phase extraction columns. Over 70 lipids per sample were then analyzed with HPLC/MS/MS.
Key results
AEA and 2-AG were unaffected throughout the brain in CB1 KO mice; however, there was an increase in the arachidonic acid (AA) metabolite, PGE2 in the majority of brain areas. By contrast, PGE2 and AA levels were significantly reduced throughout the brain in the MAGL KO corresponding to significant increases in 2-AG. No changes in AA or PGE2 were seen throughout in the FAAH KO brain, despite significant increases in AEA, suggesting AA liberated by FAAH does not contribute to steady state levels of AA or PGE2. Changes in the lipidome were not confined to the AA derivatives and showed regional variation in each of the eCB KO models.
Conclusions and implications
AEA and 2-AG hydrolyzing enzymes and the CB1 receptor link the eCB system to broader lipid signaling networks in contrasting ways, potentially altering neurotransmission and behavior independently of cannabinoid receptor signaling.
Keywords: Endogenous cannabinoid, lipidomics, FAAH, MAGL, CB1, arachidonic acid
Graphical Abstract
1. Introduction
1.1 CB1 cannabinoid receptor
Although the behavioral effects of delta-9-tetrahydrocannabinol (THC), the primary psychoactive component of the cannabis plant, were documented upon its isolation, its main mechanism of action was elucidated more than 20 years later, when a specific cannabinoid receptor was identified in the rat brain [1]. Cloned by Matsuda and colleagues, this cannabinoid receptor is a G-protein coupled receptor (GPCR) frequently signaling via Gi/o G proteins [2]. Subsequently, Munro and colleagues isolated a clone for a second cannabinoid receptor, also a GPCR, the highest levels of which were found in macrophage cell lines and spleen; they did not detect mRNA for this second cannabinoid receptor in the brain [3]. Later, the brain-enriched cannabinoid receptor was named CB1 and the peripherally-enriched cannabinoid receptor was designated CB2. THC acts as a low efficacy agonist at both of these receptors [4, 5]. The endogenous agonists for CB1 and CB2 receptors are lipids and are referred to as endogenous cannabinoids (eCBs) [6]. The eCBs, N-arachidonoyl ethanolamine (AEA) [7], also known as anandamide, and 2-arachidonoyl glycerol (2-AG) [8, 9] are derived from arachidonic acid (AA) containing lipids. The activation of cannabinoid receptors is implicated in many processes, such as the regulation of appetite [10, 11], pain perception [12], motivation [13, 14], inflammation [15], and learning and memory [16].
1.2 Fatty acid amide hydrolase (FAAH) is a ubiquitous enzyme with a wide range of substrates
AEA belongs to a larger structural family of N-acyl ethanolamines (NAEs); whereas AEA is formed from N-arachidonoyl phosphatidylethanolamine, in which its structure represents the conjugation of AA and ethanolamine, other NAEs are formed from N-acyl phosphatidylethanolamine with other fatty acids (i.e. acyl groups). The first NAE to be identified was N-palmitoyl ethanolamine (PEA), the conjugate of palmitic acid and ethanolamine. Kuehl and colleagues found PEA in soybeans, peanuts, and egg yolks and determined that PEA had anti-inflammatory effects [17].
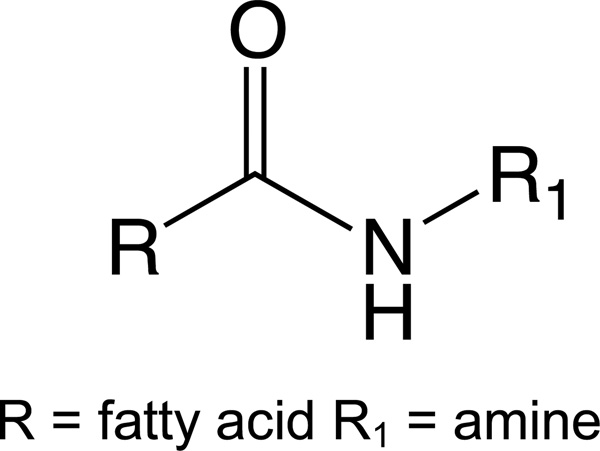
Lipoamines, also known as N-acyl amides (i.e. fatty acid amides), are molecules derived from the conjugation of amines (e.g. amino acids) with fatty acids (Figure 1). Lipoamines are all structurally analogous to the NAEs [18]. A theoretical computation of 7 common fatty acids in mammalian systems (arachidonic, stearic, docosahexaenoic, oleic, palmitic, linolenic, and linoleic) with 20 amino acids, and 4 common amines (ethanolamine, dopamine, GABA, and taurine), yields 168 possible lipoamine combinations in mammals [19]. Lipoamines are a rapidly growing class of signaling molecules that are produced throughout the brain and body [20–22]. Most lipoamines do not activate cannabinoid receptors, so they are not categorized as eCBs; however, some do activate other receptors, such as GPR18 [23], GPR119 [24] as well as transient receptor potential (TRP) channels [25].
Figure 1.
Generic structure of a lipoamine (also known as an N-acyl amide), the conjugation of a fatty acid and an amine via an amide bond.
FAAH is hypothesized to be responsible for the vast majority of AEA hydrolysis, initially producing ethanolamine and AA [26, 27]. In the mammalian brain, FAAH is particularly highly expressed in the cerebellum, hippocampus and cortex. Even though FAAH is best known for its role in AEA hydrolysis, it can also hydrolyze other lipoamines [28]. Earlier work demonstrated that N-arachidonoyl glycine (NAGly) and N-arachidonoyl dopamine (NADA) were significantly reduced in the rat striatum when FAAH was inhibited, suggesting FAAH participates in their synthesis, and not solely as a degradative enzyme for NAEs [29, 30].
1.3 Monoacylglycerol lipase (MAGL) is the primary hydrolytic enzyme for 2-AG
Hydrolyzing 2-AG into AA and glycerol, monoacylglycerol lipase (MAGL) is hypothesized to hydrolyze 50–85% of the brain’s 2-AG. Enzymes such as α/β hydrolase domain-6 (ABHD6) and α/β hydrolase domain-12 (ABHD12) are hypothesized to be responsible for most of the remaining hydrolysis in brain [31], with a small portion of 2-AG metabolism catalyzed by FAAH and cyclooxygenase (COX) enzymes [32]. In cultured hippocampal neurons, cerebellar slices, and hippocampal slices, MAGL helps set the duration of depolarization induced suppression of excitation/inhibition (DSE/DSI) [33–35], a classic property of 2-AG signaling [36], suggesting it is an important 2-AG hydrolyzing enzyme at many synapses. MAGL mRNA is widely expressed in the brain throughout the rat cortex, hippocampus, cerebellum, thalamus, striatum, and pontine nuclei [37]. Using immunohistochemistry, the same study revealed that MAGL protein was localized in axons and co-localized with CB1 in the rat amygdala, hippocampus, and cerebellum. MAGL’s localization in axons contrasts with the expression of FAAH, which is most abundant in dendrites and soma [38].
In the same manner as NAEs, endogenous structural analogs of 2-AG likewise exist wherein additional fatty acids are conjugated to glycerols to make a range of 2-acyl glycerols, such as 2-oleoyl glycerol (2-OG) and 2-linoleoyl glycerol (2-LG) [39]. Although 2-LG and 2-OG do not directly activate CB1 or CB2 [40], they are agonists at some of the orphan GPCRs that are members of the extended cannabinoid receptor family [41], such as GPR119, the same receptor activated by N-oleoyl ethanolamine (OEA) to reduce appetite [42]. Another proposed mechanism of action of 2-LG is that it potentiates the binding of 2-AG to CB1 and inhibits 2-AG degradation in neuronal cells. This effect has been called the “entourage effect” [40, 43], though molecular evidence for these interactions is sparse. Moreover, AA is a product of AEA and 2-AG degradation and is a substrate for prostaglandin (PG) biosynthesis, linking the eCB system to broader lipid signaling including signaling through PG receptors [44].
1.4 Mice lacking FAAH, MAGL and CB1 have distinctive behavioral phenotypes
Illustrating an analgesic phenotype, FAAH knockout (KO) mice demonstrate prolonged response latency in the tail immersion and hot plate tests compared to wild-type (WT) mice. Showing that other types of pain sensitivity are also reduced in FAAH KO mice, pain behavior was attenuated in the formalin test [27]. Consequently, FAAH became a therapeutic target for chronic pain; however, limited analgesic efficacy of a FAAH inhibitor was found in a human clinical trial [45], which may be explained by the observation that FAAH deletion actually enhances some forms of pain [46]. Similarly to FAAH, MAGL is a potential drug target to treat pain. Although acute treatment with MAGL blockers was effective at reducing cold and mechanical allodynia in the chronic constriction injury mouse model of chronic pain, prolonged, complete blockade of MAGL desensitized CB1, leading to analgesic tolerance [47, 48]. MAGL KO mice demonstrated improved performance over WT in a hippocampal-dependent learning task [49] and were somewhat protected from different models of neurodegeneration, independent of CB receptors and likely through alterations in AA levels and PG production [50].
Many studies have examined mice lacking cannabinoid receptors as an indirect approach to understanding the function of these receptors. The behavioral characteristics of CB1 KO mice have been well-documented: one prominent facet involves a protective phenotype against addiction. For example, CB1 KO mice had reduced voluntary alcohol consumption compared to WT littermates and appear to find drugs of abuse less rewarding. CB1 KO mice failed to release dopamine, measured by cyclic voltammetry, into the nucleus accumbens (NAc) after consuming alcohol, a chemical event that is believed to drive the rewarding effect of a drug [51]. Further studies showed that CB1 activation was required for dopamine release into the NAc in response to nicotine and cocaine, as well as alcohol [52].
Highlighting the effect of CB1 deletion on metabolism, CB1 KO mice are much leaner, resistant to diet-induced obesity, and demonstrated enhanced sensitivity to leptin compared to WT mice [11]. Signaling via CB1 is hypothesized to inhibit the HPA-axis to reduce levels of stress hormones. Consistent with this fact, the HPA is overactive in CB1 KO mice, under both basal and stressful conditions [53]. In addition, CB1 KO mice are very resistant to the extinction of fear conditioning, as CB1 activation in the basolateral amygdala seems to be crucial to extinguish aversive memories [16].
1.5 What are the effects of deleting eCB-hydrolyzing enzymes on the brain’s eCB-related lipidome?
The biochemical changes contributing to the phenotypes of each of the eCB enzyme KO mice are not only likely increases in eCB signaling, but broader changes in levels of eCB analogs due to alterations in lipid metabolism. To test the hypothesis that deleting an enzyme that metabolizes AEA (FAAH), an enzyme that metabolizes 2-AG (MAGL), and the brain’s main eCB receptor (CB1) has differential effects on the eCB-related lipidome, we compared levels of NAEs and over 60 structurally analogous N-acyl amides, 2-AG and its structural analogs, and two species of PGs, PGE2 and PGF2α, in a targeted lipidomics screen in 8 distinct brain regions: brainstem, cerebellum, cortex, hippocampus, hypothalamus, midbrain, striatum and thalamus. Deleting these different enzymes or the CB1 receptor impacted the production of a wide-range of lipids in ways that went well beyond changes in levels of an enzyme’s substrate or a receptor’s ligand. Reflecting the link between eCBs and PGs as AA derivatives, both deletions in MAGL and CB1 affected levels of PGs, whereas deletions in FAAH affected levels of AA-derived lipoamines, but not AA or PGs. Therefore, disrupting the hydrolysis of eCBs and related lipids has potential consequences for signaling in the brain independent of cannabinoid receptor activation.
2 Methods
2.1 Mice and tissue collection
For the FAAH KO study, 6 WT mice were compared to 6 FAAH KO mice from the C57BL6/J strain. Similarly, for the MAGL KO study, 6 WT mice were compared to 6 FAAH KO mice from the C57BL6/J strain. To compare CB1 KO to WT, 6 KO and 6 WT mice from the CD1 strain were used. The mice for the CB1 KO versus WT screenings and the MAGL KO versus WT screenings were all male. The remaining groups were of mixed sex: FAAH KO 2 female/4 male with 2 male/4 female WT. MAGL KO and FAAH KO mice were a generous gift from Ben Cravatt. CB1 heterozygous mice used to found a CB1 knockout colony were a generous gift from Catherine Ledent. The mice were 2 months old at the time of sacrifice. All protocols involving mice were approved by the Indiana University Institutional Animal Care and Use Committee and ARRIVE guidelines.
Brain tissue was collected and dissected as previously described [54]. In summary, all mice were sacrificed via rapid cervical dislocation on the same day and brains were immediately removed, flash frozen, and stored in a −80°C freezer until dissections were performed. Brains were dissected while semi-frozen on an ice-cold plate into the following regions: striatum (STR), hippocampus (HIPP), cerebellum (CER), thalamus (THAL), cortex (CTX), hypothalamus (HYP), midbrain (MID), and brainstem (STEM). Each dissected area was immediately placed in liquid nitrogen and stored at −80°C until used for lipid extraction.
2.2 Lipid extraction
Tissue extracts were performed as previously described [22, 55]. In brief, to begin the lipid extraction, samples were shock frozen in liquid nitrogen, and weighed before being transferred to a centrifuge tube. The mass of the largest sample was multiplied by 50 to determine how many milliliters of HPLC-grade methanol (Avantor Performance Materials, Inc., Center Valley PA) to be added to the centrifuge tube. Then, samples were spiked with 500 picomols deuterium-labeled NAGly (d8NAGly; Cayman Chemical, Ann Arbor, MI, USA) as an internal standard to determine extraction efficiency. Samples were placed on ice in darkness for 2 hours then individually homogenized. Homogenates were centrifuged at 19,000g for 20 minutes at 20°C. Supernatants were decanted and diluted with HPLC water (purified in house) to make a 75:25 water to supernatant solution. Partial purification was achieved using C-18 solid phase extraction columns (Agilent, Palo Alto, CA, USA). A series of 4 elutions with 1.5 mL of 60%, 75%, 85%, and 100% methanol were collected for analysis [54]. Vials of eluants were stored at −80°C until they were ready for analysis.
2.3 High pressure liquid chromatography coupled with tandem mass spectrometry (HPLC/MS/MS)
Samples were analyzed using an Applied Biosystems API 3000 triple quadrupole mass spectrometer with electrospray ionization (Foster City, CA, USA). 20μL from each elution were chromatographed using XDB-C18 reversed phase HPLC analytical column (Agilent) and optimized mobile phase gradients. Mobile phase A: 20% methanol, 80% water (v/v) and 1 mM ammonium acetate (Sigma, St. Louis, MO, USA). Mobile phase B: 100% methanol, 1 mM ammonium acetate. Two Shimadzu 10ADvp pumps (Columbia, MD, USA) provided the pressure for gradient elution. Every method run began with 0% mobile phase B, reached a state of 100% mobile phase B flowing at 0.2 mL per minute, and gradually returned to 0% mobile phase B.
Levels of each compound were determined by running each sample using a multiple reactions monitoring method tailored for each amide family of compounds (Figure 2). Analysis of the HPLC/MS/MS data was performed using Analyst software (Applied Biosystems, Framingham, MA, USA) as previously described [22, 55]. Chromatograms were generated by determining the retention time of analytes with a [M−1] or [M+1] parent peak and a fragmentation peak corresponding to the programmed values.
Figure 2.
Lipids in HPLC/MS/MS screening library with parent ion and fragment ion masses. Lipids are grouped by amide family and are screened in a multiple reactions monitoring (MRM) method. Negative ionization mode, resulting in a [M − H]− parent ion, is used for all methods except the N-acyl ethanolamine and 2-acyl glycerol methods, which uses positive ionization and generates a [M + H]+ parent ion. The parent ion is then fragmented into the collision chamber and an abundant fragment can be selected as the fragment ion.
The retention time was then compared to the retention time of a standard for the suspected compound. If the retention times matched, then the concentration of the compound was determined by calculating the area under the curve for the unknown and comparing it to the calibration curve obtained from the standards. Therefore, unknown lipids are matched to known standards according to retention time from the analytical column and according to their mass fingerprint.
2.4 Data analysis and statistical procedures
Extraction efficiency was calculated with the d8NAGly spiked recovery vial as a standard as previously described [22, 25, 54, 55]. For each individual lipid in each brain region, concentrations in moles per gram adjusted for percent recovery from the KO animals were compared to WT concentrations using a one-way ANOVA. All statistical tests were carried out using SPSS Statistics (IBM, Armonk, NY, USA). Statistical significance was defined as p < .05 and a trending effect was defined as .05<p≤.10.
Analysis of such large data sets continues to be problematic for both the implementation and the interpretation of such data. The hypothesis that we are testing is simply that the WT values for each individual lipid differs from the KO values in each brain region analyzed. There would be the expectation that the values would be evenly distributed and a T-type comparison would yield an analysis of the means by which this could be determined. However, theoretical constructs of using T-test analyses on large data sets suggest the potential to generate false positives with a series of T-tests; therefore, the use of corrective analyses allows for a more conservative approach to these types of calculations. To this end, we have grouped data for analyses by lipid species based on either the amine group for the N-acyl amides, free fatty acids, 2-acyl glycerols, or PGs. This separation also extends to discrete analyses being performed on these categories only within a brain region. In this configuration there is no expectation that one specific lipid category has an influence on the other; therefore, the post-hoc adjustments for multiple comparisons are limited to the specific subcategories listed. We do recognize that there may be potential predictive analyses that can be performed between brain areas and categories of lipid species; however, those types of analyses fall into a realm of modeling that is beyond the scope of the questions being asked of these initial data sets. An example of a categorical analysis is that of all NAE species (6 total) within a discrete brain region would be considered a selection of values with the most likelihood of having the same direction and magnitude of effects by the deletion of a gene given their strong structural homology and their regional specificity (i.e. a discreet brain region). Therefore, all of these lipids were analyzed as a unit with an overall ANOVA and then a corrected post-hoc analysis using Fishers Least Significant Difference was performed in the event that the overall ANOVA determined an interaction.
3 Results
3.1 HPLC/MS/MS signal detection of targeted lipidome in WT and KO mice
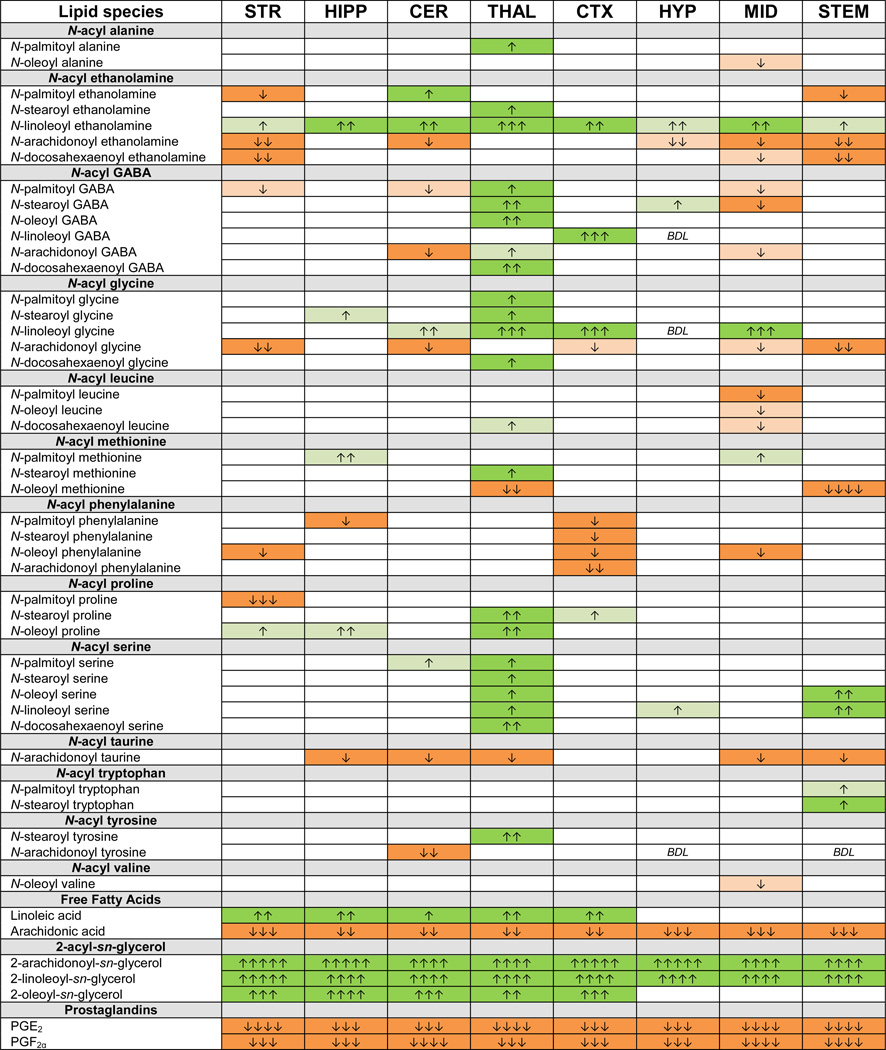
Of the 76 lipoamines in our screening library, over 60 were detected in most brain regions in the WT, FAAH, MAGL, and CB1 KO mice, with more lipids being detected in the larger brain regions such as the cortex (CTX) and brainstem (STEM) and the fewest being detected in the hypothalamus (HYP). Each of the PGs, 2-acyl glycerol, and free fatty acid species analyzed here were detected in all regions of the brain. This level of analysis constitutes ~23000 data points; therefore, the detailed list of levels of analytes detected in WT and KO mice and the statistical analyses of each are available in the Supplemental Tables. In order to summarize the analyses, we have presented the data using a schematic representation of the following output: 1) significance from WT within specific brain regions, 2) magnitude of change and 3) direction of change (Figure 3).
Figure 3.
Key for Figures 4–7. The arrow color (left column) indicates the direction of a significant result relative to wild-type. Green colors represent increases and orange color represents decreases in a lipid’s concentration. The number of arrows (right columns) indicates the magnitude of the difference between the wild-type and KO mice. To determine the magnitude change and therefore the number of arrows to assign each significant or trending difference, the mean level of a particular lipid in a specific region of the KO mice was divided by that same lipid’s mean level in the same brain region of the corresponding WT mice. For decreases the process was very similar: the mean level in the KO was divided by the mean level in the WT; however, the reciprocal of the decimal was taken to express a fold decrease (if the level in the KO mouse is ½ of the WT level then that is a 2 fold decrease, if the level in the KO mouse is ¼ of the WT level then that is a 4 fold decrease, etc.).
3.2 FAAH deletion has contrasting effects on brain lipoamines
3.2.1 Levels of all NAEs in all brain regions are significantly higher in FAAH KO mice
Of the 6 NAEs in the screening library, all were significantly increased by FAAH deletion, including the eCB AEA, in all brain regions assayed (Figure 4). The increases in polyunsaturated fatty acid derivatives like N-linoleoyl ethanolamine (LEA), N-docosahexaenoyl ethanolamine (DEA) and AEA were of a lower magnitude than the increases in saturated derivatives, PEA and N-stearoyl ethanolamine (SEA), or the increase in levels of monounsaturated derivatives like OEA. Notably, the NAEs were the only family of lipids screened in FAAH KO mice whose every member increased in all regions.
Figure 4.
Significant differences in brain lipidome in FAAH KO compared to WT. Only those lipids with changes from WT are shown here. Up arrows and green shading denote significant increases; whereas down arrows and orange shading denote significant decreases. See Figure 3 and Methods for more detailed description of analysis.
3.2.2 AA derivative lipoamines are particularly sensitive to FAAH deletion
Of the lipids measured in this screen, those with AA as part of their molecular structure showed a wide-range in their levels following FAAH deletion (Figure 5). Although several AA-derived lipoamines were detected throughout the brain in WT and FAAH KO mice, only AEA was significantly increased in all brain regions. The only other AA-derived lipoamine whose levels increased in FAAH KO was N-arachidonoyl serine (A-Ser), with region-specific increases in the striatum (STR), thalamus (THAL), and STEM. By contrast, levels of 7 additional N-arachidonoyl amides were decreased in FAAH KO mice. In all 8 brain regions, NAGly and N-arachidonoyl GABA (A-GABA) were significantly reduced in FAAH KO mice. The next most prominent decrease was in N-arachidonoyl taurine (A-Taur), whose levels fell in all areas except the HYP. The concentration of N-arachidonoyl alanine was significantly lower in the hippocampus (HIPP), cerebellum (CER), and THAL of FAAH KO. In FAAH KO mice, N-arachidonoyl methionine levels were trending lower in CER and significantly lower in the CTX. N-arachidonoyl phenylalanine levels (A-Phe) were significantly lower in the CER and trending lower in the HIPP, THAL, and midbrain (MID), and N-arachidonoyl tyrosine (A-Tyr) levels were trending lower in the THAL and CTX of FAAH KO mice. NADA levels were drastically reduced in the STR of FAAH KO mice, which was the only region where NADA was reliably detected.
Figure 5.
Comparison of effects of FAAH, MAGL and CB1 deletion on levels of arachidonic acid derivatives
3.2.3 Levels of 2-AG change in a region-specific manner in FAAH KO mice
Compared to WT, levels of 2-AG were significantly lower in the CER, THAL, and CTX but were unchanged in the STR, HIPP, HYP, MID, and STEM in FAAH KO mice. The magnitude of the decrease in 2-AG was largest in the CER. The 2-AG analog 2-OG was changed in some areas of the FAAH KO mice: it was increased in the STR, CER, and MID, but the changes in 2-OG was not correlated with those of 2-AG. 2-LG was the only 2-acyl glycerol screened that was not affected in any brain region by FAAH deletion.
3.2.4 Free AA and PG levels are insensitive to FAAH deletion
Given that the primary function of FAAH is reported to be the hydrolysis of fatty acid amides such as AEA into free AA and ethanolamine [26], it was predicted that levels of AA should be significantly reduced in FAAH KO mice. However, there were no changes in the amount of free AA in any brain region of FAAH KO (Figure 5). Therefore, none of the changes in AA derivatives were accompanied by a significant change in in free AA, suggesting FAAH feeds into a metabolic pathway that does not lead to accumulation of free AA. Likewise, no changes in PGE2 levels were measured in FAAH KO mice.
3.3 MAGL deletion has regionally specific effects on lipoamines
3.3.1 Increases in 2-acyl glycerols and reductions in PGs and AA after MAGL deletion
Replicating previous finding and supporting the hypothesis that MAGL is a hydrolytic enzyme that releases (and increases) free AA [56], MAGL KO mice had higher levels of 2-AG in all brain regions and corresponding decreases in AA (Figures 5 and 6). Levels of 2-AG were 10-fold greater than those seen in WT in the STR, HIPP, CTX, and HYP and were over 5 times WT levels in the CER, THAL, HYP and MID. 2-LG increased to a similar extent in MAGL KO mice. Unlike 2-AG and 2-LG, levels of 2-OG did not increase in every region with MAGL KO. Instead, 2-OG levels were higher than WT in the STR, HIPP, CER, THAL, and CTX but were the same as WT in the HYP, MID and STEM. Even where 2-OG increased, the increases were of a smaller magnitude than those of 2-AG and 2-LG. In contrast to increases in levels of 2-AG, levels of PGE2, PGF2α, and free AA were significantly lower in all 8 brain regions of MAGL KO mice. Levels of PGs were at least fifty percent lower in the MAGL KO mice relative to WT.
Figure 6.
Significant differences in brain lipidome in MAGL KO compared to WT. Only those lipids with changes from WT are shown here. Up arrows and green shading denote significant increases; whereas down arrows and orange shading denote significant decreases. See Figure 3 and Methods for more detailed description of analysis.
3.3.2 AA-derived lipoamines decrease in MAGL KO mice
Similarly to FAAH KO mice, levels of AA-derived lipoamines were decreased throughout the brain (Figure 5). In contrast to the FAAH KO mice, MAGL deletion caused decreases in AA-derived lipoamines that were very region-specific. In MAGL KO mice, levels of the eCB AEA were significantly lower than WT in the STR, CER, MID and STEM and trending lower than WT in the HYP. Levels of the AEA metabolite NAGly were significantly lower in the STR, CER, and STEM and trending lower in the CTX and MID of MAGL KO mice. Levels of A-GABA decreased relative to WT in the CER and MID and were unaffected in the STR, HIPP, CTX, HYP and STEM of MAGL KO mice. MAGL KO mice had lower levels of A-Phe in the CTX only and lower levels of A-Tyr in the CER only. A-Taur concentrations were significantly lower in the HIPP, CER, THAL, MID, and STEM of MAGL KO mice.
3.3.3 Levels of linoleic acid and specific linoleic acid-derived lipoamines increase in MAGL KO mice
Reductions in AA and AA-derived lipoamines appeared to be contrasted by increases in linoleic acid and its lipoamine derivatives. Importantly, the AEA analog LEA was increased in all 8 regions of MAGL KO. Increases in levels of other linoleic acid lipoamine derivatives were region dependent. For example, levels of N-linoleoyl GABA were higher in the CTX, levels of N-linoleoyl glycine were significantly higher in the THAL, CTX, MID and trending higher in the CER, and levels of N-linoleoyl serine were significantly higher in THAL, STEM and trending higher in the HYP of MAGL KO mice relative to WT.
3.3.4 Changes in specific lipoamine subfamilies are highly region dependent in the MAGL KO
Specific brain regions appeared to be more sensitive to changes in lipoamines in the MAGL KO mice. For example, the THAL had increases in 5 out of the 6 members of the N-acyl GABA and N-acyl serine families, whereas, other regions only had changes in at most a couple of members of these families. The THAL stands out because it contained many more significant increases in levels of lipoamines than decreases (the only N-acyl amides to be decreased in the THAL of MAGL KO were N-oleoyl methionine and A-Taur). In contrast, other regions like MID or STR contained more decreases than increases in levels of N-acyl amides in MAGL KO mice.
Of all 8 brain regions assayed, the HYP was the least affected by MAGL deletion in terms of the number of lipids affected and the magnitudes of the changes.
3.4 Effects of CB1 KO on a targeted brain lipidome
3.4.1 Deletion of CB1 does not affect eCB levels
Notably, in all 8 brain regions assayed there were no significant changes in either AEA or 2-AG in the CB1 KO mice (Figure 5 and Figure 7). Also, NADA, a ligand at CB1 whose expression is restricted to the STR [30], levels did not change in CB1 KO mice. Only 2 NAE AEA analogs were regionally affected by CB1 deletion, in that SEA increased in the THAL and STEM and in LEA in the CER. The AEA metabolite NAGly was both regionally and differentially affected by CB1 deletion, with a significant decrease in the STEM of KO mice and with a trending increase in the HIPP.
Figure 7.
Significant differences in brain lipidome in CB1 KO compared to WT. Only those lipids with changes from WT are shown here. Up arrows and green shading denote significant increases; whereas down arrows and orange shading denote significant decreases. See Figure 3 and Methods for more detailed description of analysis.
3.4.2 All differential changes in the lipidome in CB1 KO mice were regionally specific
There was no single lipid in the screening library that changed in all 8 brain regions of the CB1 KO mice thereby highlighting the importance of examining individual brain regions (Figure 7). Interestingly, in the CER, none of the significant or trending changes involved decreases relative to WT but in the other 7 regions, levels of at least one lipid decreased relative to WT. In fact, all changes in the STR were decreases with the exception of the change in PGF2α. Likewise, all changes in HYP were decreases except the increase in PGE2. In general, N-acyl serines were one of the more impacted families of lipids in CB1 KO with every member of the family changing concentration in at least one region. However, levels of any N-acyl serine failed to change in the STR and HYP.
Contrast this with the THAL, where levels of N-palmitoyl serine, N-stearoyl serine, N-linoleoyl serine and N-docosahexaenoyl serine rose in CB1 KO, whereas there was a decrease in A-Ser. Therefore, effects of CB1 deletion are highly region-dependent, which suggests region-specific adaptions in lipid biosynthesis and metabolism as a result of a lack of CB1 signaling.
3.4.3 Prostaglandin levels are elevated in many brain regions of CB1 KO mice
Compared to WT, levels of PGE2 were significantly elevated in the HIPP, THAL, CTX, HYP, MID, and STEM of CB1 KO mice (Figure 5 and Figure 7). Levels of PGE2 were additionally trending higher in the CER. In STR, the concentration of PGE2 did not differ significantly between KO and WT mice. Levels of PGF2α were also increased relative to WT; however, often in different regions. PGF2α levels were significantly higher in the HIPP, THAL, CTX, MID and STEM and trending higher in the STR of CB1 KO mice and were unchanged in the CER and HYP. The magnitude difference in PG levels between WT and CB1 KO was highest in the HIPP, with PGE2 levels being twice as high in the KO mice and PGF2α levels being over 1.5 times higher that WT levels. Despite the increases in PGs, free AA levels increased in only one region, which is in contrast to the dynamics of AA and PG reported for the MAGL KO [50]. AA levels were higher in the CER and were unchanged in the other 7 regions.
4 Discussion and conclusions
Cannabinoid research has tended to focus on the eCB ligand substrates AEA and 2-AG, overlooking a host of related compounds that are often co-produced or proximal metabolites of these lipids. Data here provide a broader window into the long-term effects of deletion of each of these eCB-related proteins. Our findings highlight the importance of considering the lipidome as a dynamic, interconnected system. Unraveling how these connections play a role in cell signaling will allow for a deeper understanding of cellular communication.
4.1 Functional specialization of lipid signaling in specific brain regions
Our results demonstrate the importance of evaluating multiple brain areas. For instance, a previous study of FAAH KO mice relying on whole-brain analysis revealed no changes in 2-AG [27]. In this multi-area study, we show that there are region-dependent decreases in 2-AG in FAAH KO mice. Similarly, our finding of decreased AEA levels in 5 brain regions of MAGL KOs is novel, as whole brain studies did not observe any changes in AEA [50] (Figure 5). Although a study by Imperatore and colleagues did not find any differences in AEA in the PFC, amygdala, HIPP or CER of MAGL KO, they did find that desensitization of CB1 was brain region dependent. The desensitization of CB1 in the HIPP and amygdala of MAGL KO was of particular importance, because it was linked to anxiety-like behavior [57]. This means that adaptations to a missing enzyme are not uniform across the brain, and elevations in 2-AG signaling have different consequences for neurotransmission and behavior depending on which neural circuitry is affected.
Most studies that use CB1 KO mice looked at either the whole brain, or focus on brain areas where CB1 receptor expression was high, like the HIPP [58]. Here, by examining effects of CB1 deletion in 8 different brain regions, the STR appears to be most resistant to the consequences of receptor deletion that result in changes in lipid levels. For example, PGE2 levels are the same as WT in the STR of CB1 KO, whereas, levels of that lipid were higher in CB1 KO in the other 7 regions. Interestingly, the STR was the last region to show desensitization of CB1 after chronic THC treatment in the rat brain, whereas CB1 in HIPP quickly desensitized and to a much larger degree [59, 60]. The HIPP was one of the only regions where deleting the 2-AG synthesizing enzyme diacylglycerol lipase affected the activity of CB1 [61]. Indeed, the HIPP was one of the most affected areas in CB1 KO mice. The disruption of CB1 signaling in the HIPP is hypothesized to impair discriminative memory and is associated with anxiety-like behaviors [57]. Therefore, it appears that the STR is a more resilient brain area when it comes to maintaining homeostatic lipid metabolism after challenges to CB1 signaling.
4.2 Synergistic effects of deleting eCB hydrolyzing enzymes on N-acyl amides
Notably, AEA can also activate TRPV1, a receptor involved in pain perception [62]. Other NAEs such as OEA [63], LEA and DEA also activate TRPV1 [25]. NAEs are not the only TRPV agonists in the screening library: A-Taur activates TRPV1 and TRPV4 receptors [64]. We have also demonstrated a wider variety in TRPV activation with lipoamines [25]. At TRPV1, in addition to LEA and DEA, N-linoleoyl GABA, A-GABA, N-docosahexaenoyl GABA, N-docosahexaenoyl aspartic acid, N-docosahexaenoyl glycine, and N-docosahexaenoyl serine all demonstrated agonist activity at low micromolar concentrations. These ligands are also synergistic when applied in combination, perhaps through allosterism, since their EC50 declines into the nanomolar range when they are applied together [19, 25]. This may be especially important in the case of FAAH KO, where all members of the NAE family are robustly elevated across all brain regions, or in the case of acute pharmacological FAAH inhibition [65].
4.3 FAAH-mediated AEA hydrolysis is an important source of AA for other N-arachidonoyl amides but not for free AA
Data here suggest that effects of FAAH deletion on NAEs are not translatable to all other lipoamines in that many other members of this family of lipids were not upregulated in response to this deletion. In fact, levels of most AA-derived lipoamines measured here were lower in FAAH KO mice, such as NAGly, which was lower in all 8 regions. NAGly is an agonist at GPR18 [23, 66], drives microglial migration [67], and is antinociceptive and anti-inflammatory in several animal models of pain [18, 68, 69]. In mammalian cells, NAGly can be formed from AEA via two distinct pathways. The first is a direct oxidation of AEA by alcohol dehydrogenase, and the second is via the conjugation of AA and glycine using AA released by the hydrolysis of AEA by FAAH [29]. If the second pathway is more important, then we would predict that as AEA levels increase in the absence of FAAH, then NAGly levels will decrease, as less AEA is being metabolized as a source of AA for NAGly. Consistent with the latter pathway, levels of NAGly were lower in all 8 brain regions of FAAH KO mice. Furthermore, FAAH-mediated hydrolysis of AEA is a source for NADA production [30], and we found that FAAH KO mice had lower levels of NADA in STR. Our reports of lower NAGly and A-GABA levels in FAAH KO mice are consistent with another study examining the consequences of FAAH blockade on AA-derived signaling molecules, although that study used URB597 to inhibit FAAH [70]. These results suggest that the FAAH-mediated metabolism of AEA is necessary to maintain levels of other AA-derived lipoamines. Importantly, contrary to our current understanding of the metabolic pathways of AEA through FAAH, levels of free AA in the FAAH KO mice remained unchanged throughout the brain. The meaning of these data are unclear; however, it does suggest that the current understanding of the direct hydrolyses of AEA into free AA and ethanolamine is in question. It is possible that any AA freed by FAAH is so rapidly conjugated into another lipid that the flux of AA through FAAH is much higher than suggested by the levels of AA.
4.4 2-AG as a stable storage form of AA
Free AA is very easily oxidized by environmental and enzymatic influences. For example, AA is oxidized by COX enzymes to form PGs [44]. Therefore, one could make an evolutionary argument that it is advantageous to “store” AA in a more stable form, until it can be liberated for oxidation as part of the AA cascade. As noted above, a previous study of MAGL deletion saw dramatically lowered levels of AA alongside rises in 2-AG [50], a finding consistent with the hypothesis that 2-AG is a stable precursor hydrolyzed on-demand, after which the unstable AA is promptly metabolized. Replicating and expanding upon these previous findings we found that levels of AA were significantly lower in all brain regions of MAGL KO mice. Moreover, the reduction in free AA has consequences for other AA-derivative lipid signaling molecules, as demonstrated by a brain-wide decrease in PG production in MAGL KO, presumably because less AA is available for COX-2. The magnitude of the drop in PGs was relatively consistent across the 8 regions, suggesting that 2-AG hydrolysis by MAGL is a major source of metabolic liberation for AA throughout the brain. Therefore, it is likely that MAGL hydrolysis of 2-AG is a significant source of AA for PG synthesis. In contrast, our work suggests that AEA is not a major storage form for free AA: despite a greater than 5 fold increases in AEA in FAAH KO mice, levels of AA and PGs are not significantly altered. Instead, these data suggest that AEA serves as a more directed substrate for AA-derived lipoamines.
4.5 Opposing effects of MAGL and CB1 on PGs
PGE2 and PGF2α are potent mediators of inflammation [44]; however, PGE2 in the brain has also been shown to be an important signaling molecule involved in the development of dendritic spines in some brain regions [71–73]. In addition, activation of PG receptors on microglia has been implicated as important for protection against neurodegenerative diseases [74, 75]. Therefore, a change in brain PGs may alter key aspects of neurodevelopment and effect both neuronal and immune functions in the brain. In MAGL KO mice, levels of PGE2 and PGF2α were strongly attenuated in all brain regions, replicating findings of lowered PGs in whole-brain of MAGL KOs [50]. Behaviorally, MAGL KO mice perform better than WT littermates on HIPP-dependent tasks that test spatial memory. The improved memory in these animals is hypothesized to be due to desensitization of CB1 in the HIPP that occurs after prolonged exposure to elevated 2-AG [49]. However, PG production in the HIPP has been recently linked to learning and memory. In one study, upregulated COX-2 expression in the HIPP was correlated with memory deficits in rats. Treatment with COX inhibitors ‘rescued’ the phenotype of memory impairment [76]. Therefore, lowering PG levels via COX-2 inhibition is hypothesized to improve memory performance after CB1 activation, raising the possibility that reduced PG levels are contributing to heightened spatial memory performance in MAGL KO.
Although MAGL KO is hypothesized to desensitize CB1, genetically deleting CB1 produced opposing effects on levels of PGs, with increases in PGs in many brain regions of CB1 KO. Thus, differences may exist in how the brain’s lipid metabolism responds to desensitization versus deletion. Our study is not the first to report a basal neuroinflammatory state in CB1 KO mice: Cutando and colleagues found increased mRNA levels of COX-2 in the cerebellum on CB1KO mice. This was accompanied by upregulation of the cytokine interleukin 1 beta (IL-1β), which by itself can induce COX-2 expression, and upregulation of mRNA for tumor necrosis factor alpha and chemokine (C-X-C motif) ligand 2 (CXCL2). Furthermore, mRNA levels for CB2 were significantly higher in the cerebellum of CB1 KO. The same study also reported evidence of microglial activation in the cerebellum of CB1 KO: the microglial marker CD11b was upregulated and the number of CD11b positive cells was higher in CB1 KO. Reducing the number of microglia with minocycline treatment significantly reduced the neuroinflammation, and levels of mRNA for COX-2, IL-1β, CXCL2 and CB2 all significantly fell in CB1 KO after minocycline treatment [77]. A follow-up study will examine changes in COX-2 expression as a potential mechanism for the increased PG levels in CB1 KO. These studies will further detail interactions between eCB and PG signaling.
The link between reducing CB1 expression and increasing inflammation may be relevant for cannabis use in humans. Tolerance to THC’s effects develops with chronic administration, which is hypothesized to be due to downregulation of CB1 and CB1 signaling [78, 79]. PET studies showed a decrease in CB1 density in the brains of cannabis smokers, especially in cortical regions, that returned to the same levels as healthy controls after 4 weeks of supervised abstinence [80]. In mice, attenuations in CB1 expression were found 5 days after THC treatment [77], as well as upregulated expression of the markers of microglial activation CBD11b and IL-1β in the cerebellum [77]. The specific effects of THC on CB1 levels are dose and brain-region dependent, but in general THC reduces CB1 levels [60]. Thus, reducing CB1 expression, THC possibly dysregulates PG signaling.
Given the role of eCBs in brain development, the effects of THC may be longer lasting and more severe in adolescence [81]. After 3 weeks of escalating THC treatment in adolescent rats, CB1 binding was reduced in all 4 brain regions assayed: the hippocampus, caudate putamen, substantia nigra and the cingulate gyrus [82]. A previous study highlighted a persistent neuroinflammatory state after chronic THC treatment in adolescent female rats that correlated with depressive-like behaviors, especially in the pre-frontal cortex, accompanied by upregulation of markers of inflammation such as COX-2 and a downregulation of the anti-inflammatory marker IL-10 [83]. Taken together our data suggest that disrupting CB1 drives up PG production which may contribute to an inflammatory phenotype and which could be causing a dysregulation of microglial activity.
4.6 Conclusion
The lipids profiled in this study are not static, and instead can be considered as part of a complex fluvial network. Through metabolism, they ‘flow’ from one form to another. Deprived of upstream precursors, the downstream pathways dry up, while a dammed pathway (as in enzymatic blockade) raises the levels of upstream lipids, perhaps to the point of overflowing into other, perhaps unexpected, pathways. These dynamic processes are inherently difficult to study because we are viewing snapshots in time. By systematically comparing deletion mutants of two enzymes that hydrolyze eCBs and one receptor protein we have been afforded windows into these dynamic relationships. We have observed dramatic shifts in CNS lipid metabolism that vary considerably both by enzyme, receptor, and by brain region. FAAH-mediated hydrolysis of AEA appears to be important for maintaining levels of AA-derived lipoamines but not for free AA. In contrast, 2-AG hydrolysis by MAGL appears to be important for maintaining levels of free AA and downstream synthesis of PGs. The lipid profiles of FAAH KO, MAGL KO, and CB1 KO mice are a great model to demonstrate the interconnectedness of biochemical pathways that metabolize eCBs with the broader AA cascade. They also offer insight into the compartmentalization of lipid signaling in specific brain regions that likely have functional implications. Altered lipid profiles may have consequences for other receptor classes such as the TRP family since several of the lipids studied here are agonists at those receptors. The consequences of FAAH and MAGL deletion for the lipid profile were more striking, often impacting lipids that were far ‘from the tree’. Illuminating the distant consequences of deleting a receptor for eCBs on lipid signaling, That CB1 deletion upregulates PG signaling but does not affect levels of eCBs is another example of an “off target” effect that is not easily predicted. Our results highlight the benefits of systematic side-by-side comparisons such as those undertaken in this study. These results also point to the sometimes unexpected consequences of perturbing the flow of the CNS lipid stream, consequences that must be considered in development and clinical use of blockers for these eCB proteins.
Supplementary Material
Acknowledgments
This work was funded by NIH grants: EY024625, DA011322, and DA021696.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- 2-LG
2-linoleoyl glycerol
- 2-OG
2-oleoyl glycerol
- AA
arachidonic acid
- ABHD6
α/β hydrolase domain-6
- ABHD12
α/β hydrolase domain-12
- AEA
N-arachidonoyl ethanolamine
- A-GABA
N-arachidonoyl GABA
- A-Phe
N-arachidonoyl phenylalanine
- A-Ser
N-arachidonoyl serine
- A-Taur
N-arachidonoyl taurine
- A-Tyr
N-arachidonoyl tyrosine
- CER
cerebellum
- COX
cyclooxygenase
- CTX
cortex
- CXCL2
chemokine (C-X-C motif) ligand 2
- DEA
N-docosahexaenoyl ethanolamine
- DSE/I
depolarization induced suppression of excitation/inhibition
- eCB
endogenous cannabinoid
- FAAH
fatty acid amide hydrolase
- GPCR
G-protein coupled receptor
- HIPP
hippocampus
- HPLC/MS/MS
high pressure liquid chromatography coupled with tandem mass spectrometry
- HYP
hypothalamus
- IL-1β
interleukin 1 beta
- KO
knockout
- LEA
N-linoleoyl ethanolamine
- MAGL
monoacylglycerol lipase
- MID
midbrain
- NADA
N-arachidonoyl dopamine
- NAc
nucleus accumbens
- NAE
N-acyl ethanolamine
- NAGly
N-arachidonoyl glycine
- OEA
N-oleoyl ethanolamine
- PEA
N-palmitoyl ethanolamine
- PG
prostaglandin
- SEA
N-stearoyl ethanolamine
- STEM
brainstem
- STR
striatum
- THAL
thalamus
- THC
delta-9-tetrahydrocannabinol
- TRP
transient receptor potential
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author, Heather B Bradshaw, of this manuscript is on the Advisory Board for Phytecs and consults on how endogenous cannabinoids function in the central nervous system. Phytecs had no financial contribution to the current work. The other authors have no conflicts of interest to declare.
References
- 1.Devane WA, Dysarz Fr, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Molecular pharmacology. 1988;34:605–613. [PubMed] [Google Scholar]
- 2.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cdna. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid cb1 and cb2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 5.Pertwee RG. The diverse cb1 and cb2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British journal of pharmacology. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (grac), 5th edition. British journal of pharmacology. 2011;164(Suppl 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochemical and biophysical research communications. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical pharmacology. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 10.Palomba L, Silvestri C, Imperatore R, Morello G, Piscitelli F, Martella A, Cristino L, Di Marzo V. Negative regulation of leptin-induced ros formation by cb1 receptor activation in hypothalamic neurons. Journal of Biological Chemistry. 2015 doi: 10.1074/jbc.M115.646885. jbc. M115. 646885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 12.Woodhams SG, Sagar DR, Burston JJ, Chapman V. The role of the endocannabinoid system in pain. Pain control: Springer. 2015:119–143. doi: 10.1007/978-3-662-46450-2_7. [DOI] [PubMed] [Google Scholar]
- 13.Leishman E, Kokesh KJ, Bradshaw HB. Lipids and addiction: How sex steroids, prostaglandins, and cannabinoids interact with drugs of abuse. Annals of the New York Academy of Sciences. 2013;1282:25–38. doi: 10.1111/nyas.12081. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG. Handbook of cannabis. Oxford University Press; 2014. [Google Scholar]
- 15.Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. Journal of leukocyte biology. 2015 doi: 10.1189/jlb.3RU0115-021R. jlb. 3RU0115-0021R. [DOI] [PubMed] [Google Scholar]
- 16.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 17.Kuehl FA, Jacob TA, Ganley OH, Ormond RE, Meisinger MAP. The identification of n-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J Am Chem Soc. 1957;79:5577–5578. [Google Scholar]
- 18.Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. The Journal of biological chemistry. 2001;276:42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- 19.Bradshaw HB, Raboune S, Hollis JL. Opportunistic activation of trp receptors by endogenous lipids: Exploiting lipidomics to understand trp receptor cellular communication. Life sciences. 2012 doi: 10.1016/j.lfs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, Mo F-M, Offertaler L, Pacher P, Kunos G. N-arachidonoyl l-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smoum R, Bar A, Tan B, Milman G, Attar-Namdar M, Ofek O, Stuart JM, Bajayo A, Tam J, Kram V. Oleoyl serine, an endogenous n-acyl amide, modulates bone remodeling and mass. Proceedings of the National Academy of Sciences. 2010;107:17710–17715. doi: 10.1073/pnas.0912479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tortoriello G, Rhodes BP, Takacs SM, Stuart JM, Basnet A, Raboune S, Widlanski TS, Doherty P, Harkany T, Bradshaw HB. Targeted lipidomics in drosophila melanogaster identifies novel 2-monoacylglycerols and n-acyl amides. PloS one. 2013;8:e67865. doi: 10.1371/journal.pone.0067865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHugh D, Page J, Dunn E, Bradshaw HB. Delta(9)-tetrahydrocannabinol and n-arachidonyl glycine are full agonists at gpr18 receptors and induce migration in human endometrial hec-1b cells. British journal of pharmacology. 2012;165:2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu ZL, Carroll C, Chen R, Alfonso J, Gutierrez V, He H, Lucman A, Xing C, Sebring K, Zhou J, Wagner B, Unett D, Jones RM, Behan DP, Leonard J. N-oleoyldopamine enhances glucose homeostasis through the activation of gpr119. Mol Endocrinol. 2010;24:161–170. doi: 10.1210/me.2009-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raboune S, Stuart JM, Leishman E, Takacs SM, Rhodes B, Basnet A, Jameyfield E, McHugh D, Widlanski T, Bradshaw HB. Novel endogenous n-acyl amides activate trpv1-4 receptors, bv-2 microglia, and are regulated in brain in an acute model of inflammation. Frontiers in cellular neuroscience. 2014;8:195. doi: 10.3389/fncel.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 27.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulder AM, Cravatt BF. Endocannabinoid metabolism in the absence of fatty acid amide hydrolase (faah): Discovery of phosphorylcholine derivatives of n-acyl ethanolamines. Biochemistry. 2006;45:11267–11277. doi: 10.1021/bi061122s. [DOI] [PubMed] [Google Scholar]
- 29.Bradshaw HB, Rimmerman N, Hu SS, Benton VM, Stuart JM, Masuda K, Cravatt BF, O'Dell DK, Walker JM. The endocannabinoid anandamide is a precursor for the signaling lipid n-arachidonoyl glycine by two distinct pathways. BMC biochemistry. 2009;10:14. doi: 10.1186/1471-2091-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu SS, Bradshaw HB, Benton VM, Chen JS, Huang SM, Minassi A, Bisogno T, Masuda K, Tan B, Roskoski R, Jr, Cravatt BF, Di Marzo V, Walker JM. The biosynthesis of n-arachidonoyl dopamine (nada), a putative endocannabinoid and endovanilloid, via conjugation of arachidonic acid with dopamine. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81:291–301. doi: 10.1016/j.plefa.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chemistry & biology. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straiker A, Wager-Miller J, Hu SS, Blankman JL, Cravatt BF, Mackie K. Cox-2 and fatty acid amide hydrolase can regulate the time course of depolarization-induced suppression of excitation. British journal of pharmacology. 2011;164:1672–1683. doi: 10.1111/j.1476-5381.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, Mackie K. Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol. 2009;76:1220–1227. doi: 10.1124/mol.109.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straiker A, Mackie K. Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience. 2009;163:11. doi: 10.1016/j.neuroscience.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, Liu QS. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (jzl184) enhances retrograde endocannabinoid signaling. The Journal of pharmacology and experimental therapeutics. 2009;331:591–597. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 37.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. The European journal of neuroscience. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 39.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–621. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- 40.Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: Nomenclature and pharmacological principles. Progress in neuro-psychopharmacology & biological psychiatry. 2012;38:4–15. doi: 10.1016/j.pnpbp.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godlewski G, Offertaler L, Wagner JA, Kunos G. Receptors for acylethanolamides-gpr55 and gpr119. Prostaglandins & other lipid mediators. 2009;89:105–111. doi: 10.1016/j.prostaglandins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-oleoyl glycerol is a gpr119 agonist and signals glp-1 release in humans. The Journal of clinical endocrinology and metabolism. 2011;96:E1409–E1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 44.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. The Journal of biological chemistry. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 45.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor pf-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 46.Carey LM, Slivicki RA, Leishman E, Cornett B, Mackie K, Bradshaw HB, Hohmann AG. A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase. Molecular Pain. 2016 doi: 10.1177/1744806916649192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature neuroscience. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, Lu P, Piesla MJ, Zhang MY, Bingham B, Uveges A, Kowal D, Garbe D, Kouranova EV, Ring RH, Bates B, Pangalos MN, Kennedy JD, Whiteside GT, Samad TA. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- 49.Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid cb1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. Journal of neurochemistry. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 52.Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barna I, Zelena D, Arszovszki AC, Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: In vivo and in vitro studies in cb1 receptor knockout mice. Life sciences. 2004;75:2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Stuart JM, Paris JJ, Frye C, Bradshaw HB. Brain levels of prostaglandins, endocannabinoids, and related lipids are affected by mating strategies. International journal of endocrinology. 2013;2013:436252. doi: 10.1155/2013/436252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- 56.Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochemical Journal. 1997;322:671. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imperatore R, Morello G, Luongo L, Taschler U, Romano R, De Gregorio D, Belardo C, Maione S, Di Marzo V, Cristino L. Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor cb(1)r signaling and anxiety-like behavior. Journal of neurochemistry. 2015;135:799–813. doi: 10.1111/jnc.13267. [DOI] [PubMed] [Google Scholar]
- 58.Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: Evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhuang S, Kittler J, Grigorenko EV, Kirby MT, Sim LJ, Hampson RE, Childers SR, Deadwyler SA. Effects of long-term exposure to delta9-thc on expression of cannabinoid receptor (cb1) mrna in different rat brain regions. Brain research Molecular brain research. 1998;62:141–149. doi: 10.1016/s0169-328x(98)00232-0. [DOI] [PubMed] [Google Scholar]
- 60.McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. The Journal of pharmacology and experimental therapeutics. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aaltonen N, Riera Ribas C, Lehtonen M, Savinainen JR, Laitinen JT. Brain regional cannabinoid cb(1) receptor signalling and alternative enzymatic pathways for 2-arachidonoylglycerol generation in brain sections of diacylglycerol lipase deficient mice. Eur J Pharm Sci. 2014;51:87–95. doi: 10.1016/j.ejps.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 62.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 63.Ahern GP. Activation of trpv1 by the satiety factor oleoylethanolamide. The Journal of biological chemistry. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- 64.Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A faah-regulated class of n-acyl taurines that activates trp ion channels. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- 65.Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, Petrosino S, De Petrocellis L, Cristino L, Przewlocka B, Di Marzo V. Full inhibition of spinal faah leads to trpv1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PloS one. 2013;8:e60040. doi: 10.1371/journal.pone.0060040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME. Activation of gpr18 by cannabinoid compounds: A tale of biased agonism. British journal of pharmacology. 2014;171:3908–3917. doi: 10.1111/bph.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, Bradshaw HB. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through gpr18, the putative abnormal cannabidiol receptor. BMC neuroscience. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bradshaw HB, Rimmerman N, Hu SS, Burstein S, Walker JM. Novel endogenous n-acyl glycines identification and characterization. Vitamins and hormones. 2009;81:191–205. doi: 10.1016/S0083-6729(09)81008-X. [DOI] [PubMed] [Google Scholar]
- 69.Rimmerman N, Bradshaw HB, Hughes HV, Chen JS, Hu SS, McHugh D, Vefring E, Jahnsen JA, Thompson EL, Masuda K, Cravatt BF, Burstein S, Vasko MR, Prieto AL, O'Dell DK, Walker JM. N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol Pharmacol. 2008;74:213–224. doi: 10.1124/mol.108.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han B, Wright R, Kirchhoff AM, Chester JA, Cooper BR, Davisson VJ, Barker E. Quantitative lc-ms/ms analysis of arachidonoyl amino acids in mouse brain with treatment of faah inhibitor. Analytical biochemistry. 2013;432:74–81. doi: 10.1016/j.ab.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amateau SK, McCarthy MM. Induction of pge2 by estradiol mediates developmental masculinization of sex behavior. Nature neuroscience. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 72.Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM. Prostaglandin e2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. The European journal of neuroscience. 2012;35:1218–1229. doi: 10.1111/j.1460-9568.2012.08032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, Brown HD, Mhatre SD, Loui T, Andreasson KI. Prostaglandin signaling suppresses beneficial microglial function in alzheimer's disease models. J Clin Invest. 2015;125:350–364. doi: 10.1172/JCI77487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chu CH, Chen SH, Wang Q, Langenbach R, Li H, Zeldin D, Chen SL, Wang S, Gao H, Lu RB, Hong JS. Pge2 inhibits il-10 production via ep2-mediated beta-arrestin signaling in neuroinflammatory condition. Mol Neurobiol. 2015;52:587–600. doi: 10.1007/s12035-014-8889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen R, Zhang J, Fan N, Teng ZQ, Wu Y, Yang H, Tang YP, Sun H, Song Y, Chen C. Delta9-thc-caused synaptic and memory impairments are mediated through cox-2 signaling. Cell. 2013;155:1154–1165. doi: 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cutando L, Busquets-Garcia A, Puighermanal E, Gomis-Gonzalez M, Delgado-Garcia JM, Gruart A, Maldonado R, Ozaita A. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest. 2013;123:2816–2831. doi: 10.1172/JCI67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid cb₁ receptor desensitization in female adolescent rats following repeated delta-tetrahydrocannabinol exposure. British journal of pharmacology. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazenka MF, David BG, Lichtman AH, Nestler EJ, Selley DE, Sim-Selley LJ. Delta fosb and ap-1-mediated transcription modulate cannabinoid cb(1) receptor signaling and desensitization in striatal and limbic brain regions. Biochemical pharmacology. 2014;91:380–389. doi: 10.1016/j.bcp.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid cb1 receptors in chronic daily cannabis smokers. Molecular psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu CS, Jew CP, Lu HC. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011;6:459–480. doi: 10.2217/fnl.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS. Cannabidiol potentiates delta(9)-tetrahydrocannabinol (thc) behavioural effects and alters thc pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011;218:443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- 83.Zamberletti E, Gabaglio M, Prini P, Rubino T, Parolaro D. Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. Eur Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.