Abstract
The Wnt/β-catenin signaling pathway plays a crucial role in the embryonic development of metazoans. Although the pathway has been studied extensively in many model animals, its function in amphioxus, the most primitive chordate, remains largely uncharacterized. To obtain basic data for functional analysis, we identified and isolated seven genes (Lrp5/6, Dvl, APC, CkIα, CkIδ, Gsk3β, and Gro) of the Wnt/β-catenin signaling pathway from the amphioxus (Branchiostoma floridae) genome. Phylogenetic analysis revealed that amphioxus had fewer members of each gene family than that found in vertebrates. Whole-mount in situ hybridization showed that the genes were maternally expressed and broadly distributed throughout the whole embryo at the cleavage and blastula stages. Among them, Dvl was expressed asymmetrically towards the animal pole, while the others were evenly distributed in all blastomeres. At the mid-gastrula stage, the genes were specifically expressed in the primitive endomesoderm, but displayed different patterns. When the embryo developed into the neurula stage, the gene expressions were mainly detected in either paraxial somites or the tail bud. With the development of the embryo, the expression levels further decreased gradually and remained only in some pharyngeal regions or the tail bud at the larva stage. Our results suggest that the Wnt/β-catenin pathway might be involved in amphioxus somite formation and posterior growth, but not in endomesoderm specification.
Keywords: Wnt/β-catenin signaling pathway, Gene expression, Amphioxus, Whole-mount in situ hybridization, Embryo
INTRODUCTION
The Wnt/β-catenin signaling pathway acts as a major route passing signals from the outside to the inside of a cell. The signaling is initiated by binding of Wnt lig and s to Frizzled receptors and Lrp5/6 co-receptors, which in turn results in recruitment of Axin and Dvl to the cell membrane and succedent disassembly of β-catenin from the Apc-Axin-Gsk3β-CkI complex. After that, the released β-catenin proteins translocate to the nucleus and interact with the Tcf/Lef transcription factor to activate target gene expression(Logan & Nusse, 2004; Rao & Kuhl, 2008). Comparison among available genome sequences shows that members of this pathway are conserved throughout all metazoan clades, but not among fungi, plants, or unicellular eukaryotes(Holstein, 2012).
The conservation of the Wnt/β-catenin signaling pathway indicates its important role in metazoic embryogenesis. Indeed, functional studies have demonstrated that the signaling controls many aspects of metazoic development, including germ layer specification, axis patterning, and posterior growth(Hikasa & Sokol, 2013; Martin & Kimelman, 2009; Petersen & Reddien, 2009). In vertebrates, the signaling plays an early role in dorsalventral(D-V)axis determination and a late role in anteriorposterior(A-P)axis development and posterior growth regulation(Hikasa & Sokol, 2013; Martin & Kimelman, 2009). In invertebrate deuterostomes, such as Ciona, sea urchins, and hemichordates, the signaling is essential for both endomesoderm specification and A-P axis development(Darras et al., 2011; Imai et al., 2000; Logan et al., 1999; McCauley et al., 2015; Momose et al., 2008; Momose & Houliston, 2007; Wikramanayake et al., 1998, 2003). In protostomes, the function of this signaling pathway exhibits considerable divergence among taxonomic groups. For example, in Caenorhabditis elegans and Drosophila melanogaster, the signaling pathway regulates cell asymmetric division(Mizumoto & Sawa, 2007), but in nemertean Cerebratulus lacteus it participates in endoderm and A-P axis formation(Henry et al., 2008) and in short-germ b and insects and spiders, it plays an essential role in posterior growth.
It appears that the function of the Wnt/β-catenin signaling pathway evolved distinctively during metazoan evolution, but questions about this evolvement have not yet been fully addressed. To increase our underst and ing of this pathway, we analyzed the expression profiles of eight genes involved in Wnt signaling at various developmental stages in amphioxus embryos. Amphioxus occupies a key position in the phylogeny of chordates(Delsuc et al., 2006), and its embryogenesis shows similarities to that of invertebrates(before the gastrula stage) and vertebrates(after the neurula stage). Importantly, amphioxus embryos have a clear A-P axis(Holland & Holland , 2007) and a posterior growth zone(tail bud)(Schubert et al., 2001), making them ideal research models to clarify the above questions. Previous studies have indicated that Wnt signaling is likely involved in amphioxus A-P axis determination, but not in D-V specification(Holland et al., 2005; Onai et al., 2009, 2012). However, whether signaling regulates amphioxus endomesoderm specification and posterior growth still remains to be elucidated.
MATERIALS AND METHODS
Animals and embryos
Originally provided from Jr-Kai Yu's lab by Dr. Zhang in 2011, amphioxus Branchiostoma floridae was introduced to our lab and cultured as described previously for B. belcheri (Li et al., 2012, 2013). Mature males and females with well-developed gonads were induced to spawn via thermal shock(Li et al., 2013). The eggs were fertilized in vitro and raised in dishes. Embryos at different developmental stages, including one-cell, two-cell, four-cell, blastula, mid-gastrula, early neurula(neural plate), mid-neurula(5-6 somites), late neurula(10-15 somites), and early larva(mouth open), were collected and fixed in 4% paraformaldehyde(PFA)dissolved in 4-morpholinepropanesulfonic acid(MOPS)buffer for whole-mount in situ hybridization(WISH).
Gene identification and cDNA cloning
Sequences of amphioxus Lrp5/6, Dvl, APC, CkIα, CkIδ, Gsk3β, and Gro genes were retrieved from the NCBI database using the Tblastn program with mouse homologous sequences as queries, and further verified with our transcriptome dataset. Primer pairs for each target gene were then designed(Table 1) and used to amplify the gene fragments using cDNA templates derived from gastrulae and neurulae. The amplified fragments were separately purified using a gel extraction kit(Omega, USA), subcloned into pGEM-T easy vector(Promega, USA), and verified by sequencing analysis.
Table 1.
Primer sequences used for cDNA cloning and vector-construction
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| Lrp5/6 | GACCGATGCCTAGCCAAGT | CTTCTCGCCAGCAGGAGGATT |
| Dvl | ATGGAGGAGACGAAAATCATT | TCACATGACGTCCACGAAG |
| Axin | ATGAGTATTGGTGTACAGGAATAC | TCACTCTATGCGCTCCACCTT |
| Apc | AGCTCGGTTGCTACAGGAGATAG | GTGCCGACAAGTTCCACAAA |
| CkIα | GGTACCCTTTGGGACAGAATGGCGAGC | ACTAGTCACCTCAGCGTCCTTTAC |
| CkIδ | AGAGCCGAGATGGAGTTGA | CTCCGACTACTTCTTGATG |
| Gsk3β | ATGAGCGGAAGACCACGGACAAC | TTATGTCCCCCCAGAGGCTGATGCG |
| Gro | ATGTATCCTCAAAACAGAC | TCAGTAGATGACTTCGTAAAG |
Phylogenetic analysis
For each target gene, homologous sequences from Homo sapiens, Mus musculus, Gallus gallus, Xenopus tropicalis, Danio rerio, Ciona intestinalis, Saccoglossus kowalevskii, and Strongylocentrotus purpuratus were downloaded from public databases and aligned using the ClustalW program in MEGA5 software(Tamura et al., 2011). The alignment files were then used to construct neighbor-joining(NJ)trees using MEGA5 software with the Poisson model and 500 bootstrap replications.
Whole-mount in situ hybridization
Digoxigenin-labeled(Roche, USA)sense and antisense probes for target genes were synthesized using Sp6 or T7 RNA polymerase from the templates of linearized recombination pGEM-T easy vectors, and WISH was performed according to previously reported methods(Yu & Holland , 2009), with slight modification. Before the WISH experiments, envelopes of unhatched embryos were removed with a slim glass pin to facilitate reagent penetration. After proper staining, the embryos were fully washed with PBS, and were then mounted in 80% glycerol and photographed under an inverted microscope.
RESULTS
Identification of Wnt/β-catenin signaling components in amphioxus
Several genes encoding the components in the Wnt/β-catenin signaling pathway have been described in amphioxus in previous research(Holland, 2002; Lin et al., 2006; Qian et al., 2013; Yu et al., 2007). In the present study, we isolated another seven genes, including Lrp5/6, Dvl, APC, CkIα, CkIδ, Gsk3β, and Gro, from the genome of Branchiostoma floridae. Results indicated that amphioxus possesses all genes involved in the Wnt/β-catenin signaling pathway, but fewer homologous genes than that observed in vertebrates(Supplementary Figure 1-7 and Supplementary Table 1). For example, two Wnt co-receptor genes(Lrp5 and Lrp6)exist in the vertebrate genome, but a single ortholog(Lrp5/6)was found in the amphioxus genome; three Dvl genes exist in the genomes of most vertebrate species, but only one counterpart was detected in amphioxus. These observations are consistent with the hypothesis of a preduplicated genome status in amphioxus, indicating that components of the Wnt/β-catenin signaling pathway have not experienced lineage-specific expansion and should be much simpler than that in vertebrates.
Supplementary Figure 1.
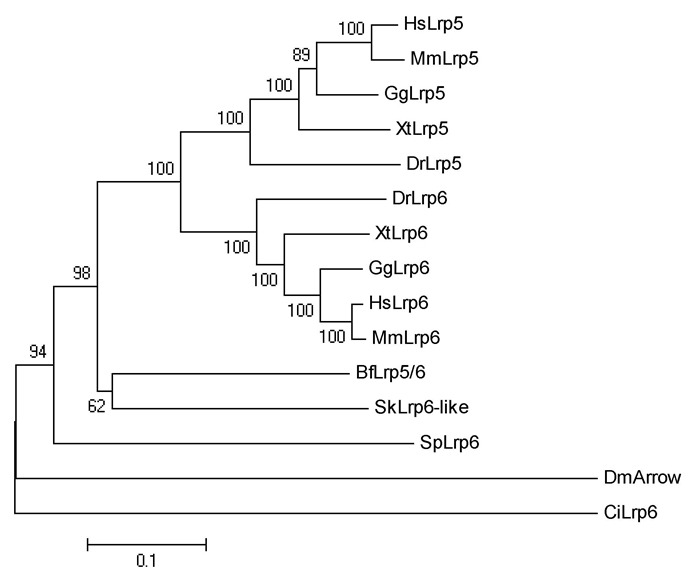
Phylogenetic relationship of Lrp5 and Lrp6 proteins
Supplementary Figure 7.
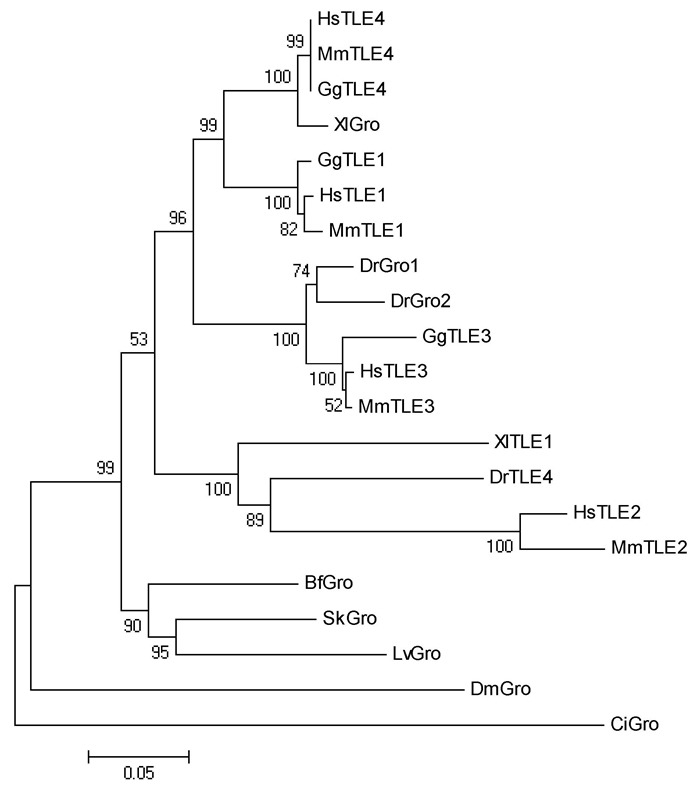
Phylogenetic relationship of Gro proteins
Supplementary Table 1.
Numbers of seven Wnt/β-catenin signaling genes in different animals
| Human Mouse Chicken Frog Zebrafish Ciona Amphioxus Hemichordate Sea urchin Fruitfly | ||||||||||
| Lrp5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Lrp6 | 1 | 1 | 1 | 1 | 1 | |||||
| Dvl1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Dvl2 | 1 | 1 | 0 | 1 | 1 | |||||
| Dvl3 | 1 | 1 | 1 | 1 | 2 | |||||
| APC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| APC2 | 1 | 1 | 1 | 1 | 1 | |||||
| CkIα | 1 | 1 | 1 | 1 | 1 | 2 | 1 | ? | 1 | 4 |
| CkIα-like | 1 | |||||||||
| CkIδ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ? | 1 | 1 |
| Gsk3α | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 |
| Gsk3β | 1 | 1 | 1 | 1 | 2 | |||||
| TLE1 | 1 | 1 | 1 | ? | 1 | 1 | 1 | 1 | ? | 1 |
| TLE2 | 1 | 1 | ? | ? | 1 | |||||
| TLE3 | 1 | 1 | 1 | ? | ? | |||||
| TLE4 | 1 | 1 | 1 | ? | 1 | |||||
Gene numbers for human, mouse, chicken, frog, zebrafish, ciona and fruitfly are according to Ensembl Release 84, and for amphioxus, hemichordate and sea urchin are determined by searching their genome database at JGI or NCBI.
Expression pattern of genes involved in the Wnt/β-catenin signaling pathway
To investigate the function of the Wnt/β-catenin signaling pathway during amphioxus embryogenesis, we further determined the expression patterns of the seven newly identified genes using the WISH method. The Axin gene was included in the present analysis since its expression at early development stages has not been determined in previous studies(Beaster-Jones et al., 2008). Figures 1, 2, and 3 are overviews of the gene expression patterns. From the one-cell to late blastula stages, the transcripts of Lrp5/6, Axin, APC, CkIα, CkIδ, Gsk3β, and Gro appeared evenly throughout the developing embryos, but that of Dvl was asymmetrically localized towards the animal pole(Figure 1, column A to D), which was denoted by the Vasa gene expression at the opposite vegetal pole(Figure 1A2, B2). At the gastrula stage, the expressions of the genes became undetectable in the ectoderm and conspicuously appeared in the primitive endomesoderm(Figure 1, column E and F). Interestingly, these genes were differentially expressed in the endomesoderm, with Lrp5/6 mainly expressed in the dorsal and lateral regions, Dvl and Gro primarily expressed in the dorsal-anterior region, APC and two CkIs expressed in the dorsal region, Axin expressed in the blastopore rim, and Gsk3β evenly expressed throughout the endomesoderm. These patterns were typically maintained until the early neurula stage, except that the signal in the dorsal mesoderm became more conspicuous than that in other tissue(Figure 2, column A to C). At the mid and late neurula stages, the expressions of these genes were commonly detected in the paraxial mesoderm(somites)(Figure 2, column D and E; Figure 3, column A and B). Other expression sites were also detected for the Axin gene in the tail bud, Dvl and Gro in the anterior endoderm diverticulum, and Gsk3β in the gut. At the larva stage, the expressions of the genes decreased and weak signals remained in some pharyngeal regions or the tail bud only(Figure 3, column C). We also synthesized sense probes of Gsk3β, Gro, and APC genes and performed side-by-side in situ hybridization experiments using both sense and antisense probes. No signal was observed in the embryos hybridized with the sense probes, but a strong specific staining was observed in the embryos hybridized with the antisense probes(Supplementary Figure 8).
Figure 1.
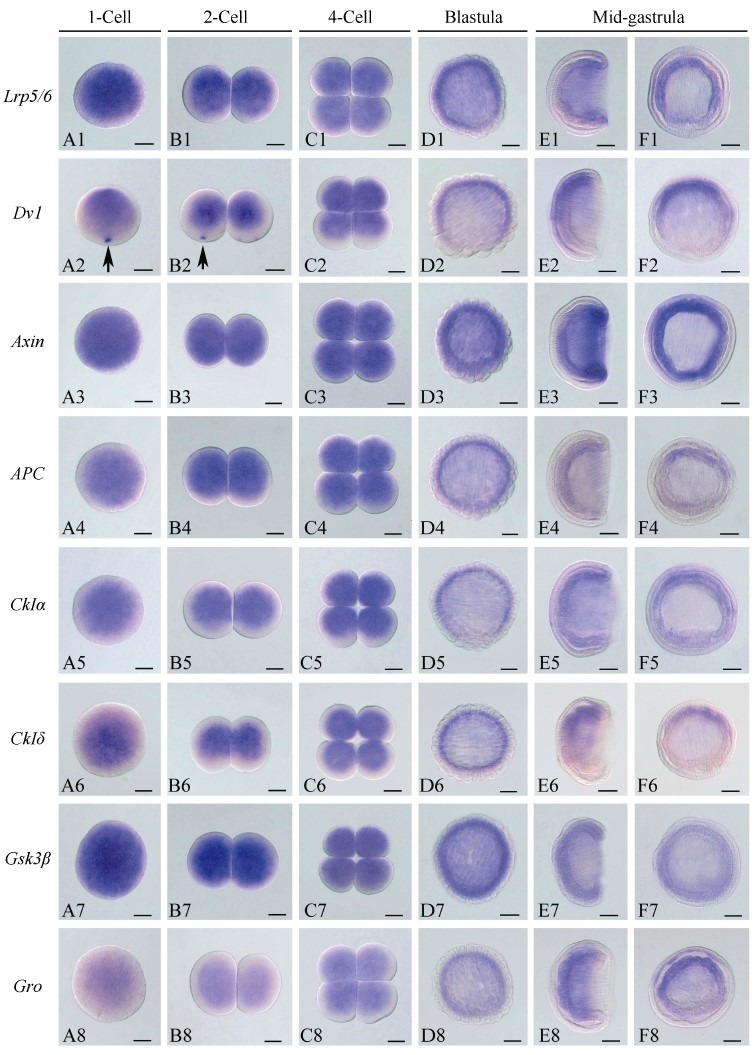
Overview of the expression patterns of eight Wnt/β-catenin signaling genes in one-cell to mid-gastrula stages of amphioxus embryos
Figure 2.
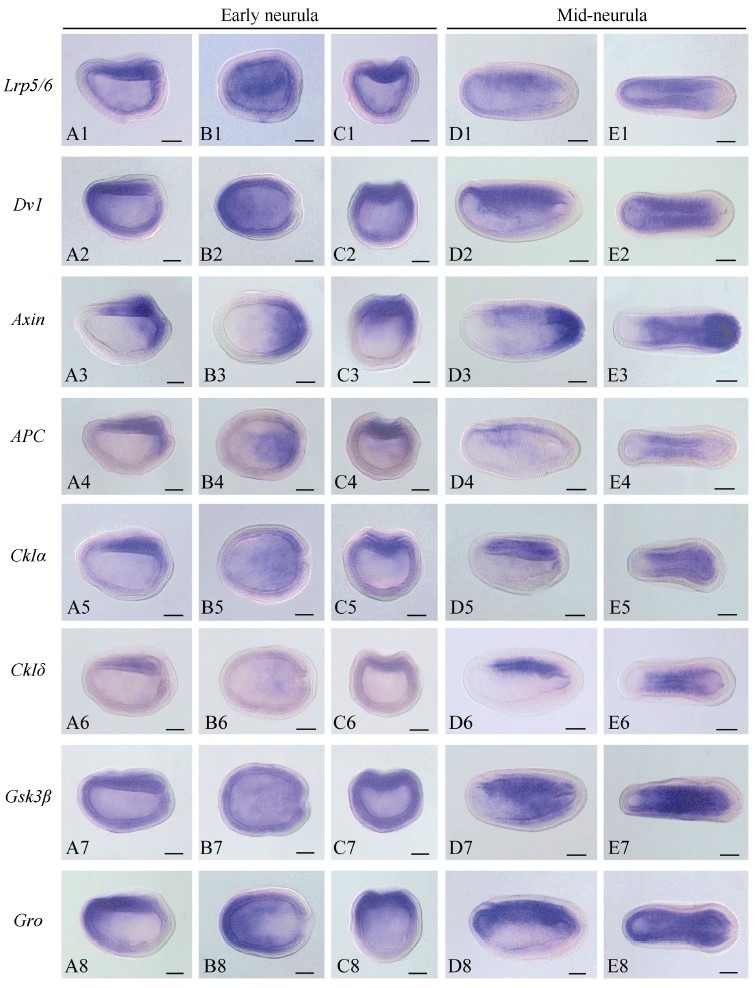
Overview of the expression patterns of eight Wnt/β-catenin signaling genes in early and mid-neurula stages of amphioxus embryos
Figure 3.
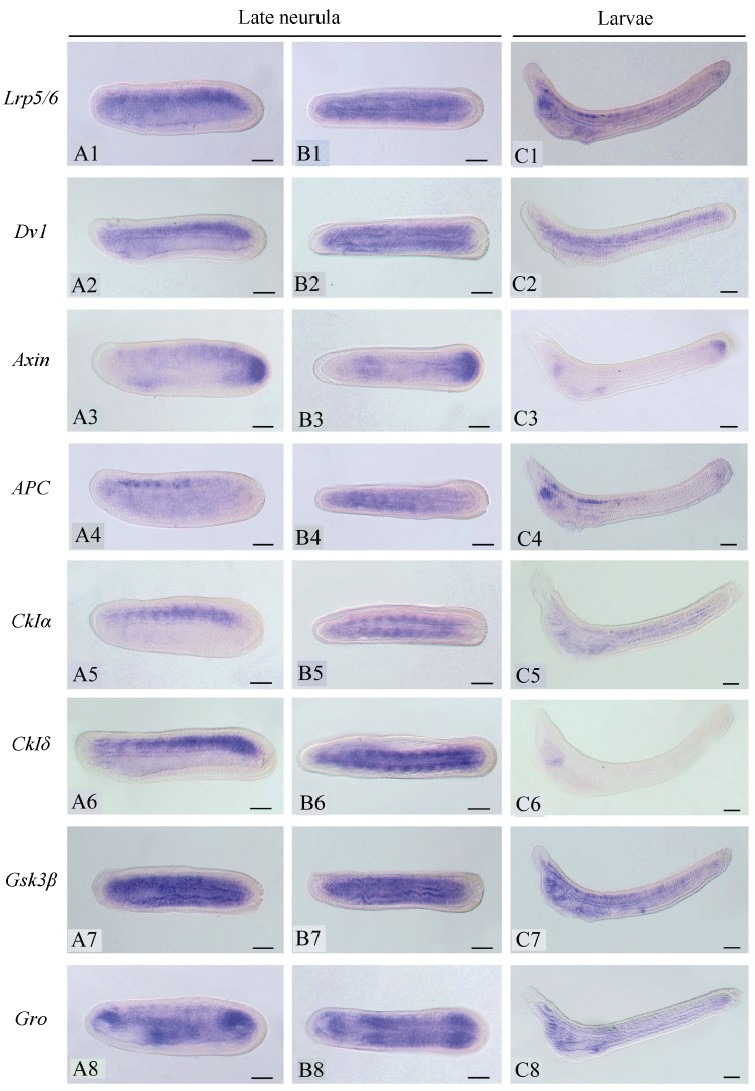
Overview of the expression patterns of eight Wnt/β-catenin signaling genes at late neurula and early larva stages
Supplementary Figure 8.
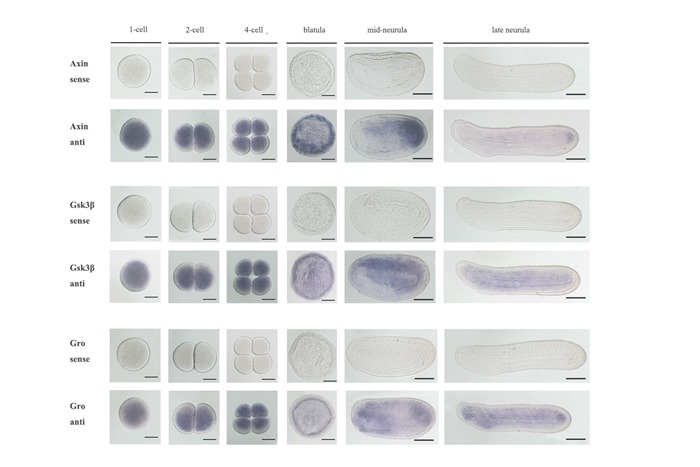
Whole mount in situ analysis in amphioxus embryos with sense and antisense probes of Gsk3β, Axin and Gro genes
DISCUSSION
Function of the Wnt/β-catenin signaling pathway in amphioxus early embryogenesis
The activation of Wnt/β-catenin signaling on one side of a cleavage-stage embryo is a crucial process in metazoan embryonic axis patterning and germ layer specification. In animals such as sea urchins(Logan et al., 1999), hemichordates(Darras et al., 2011), and Ciona (Imai et al., 2000), early regionalized Wnt signaling is localized to the vegetal pole and associated with endomesoderm specification. In cnidarians, the signaling is involved in endomesoderm specification(Martindale, 2005), although it is activated in the animal pole(Wikramanayake et al., 2003). In vertebrates, however, the signaling activity is enriched in the dorsal region, where it is crucial for Spemann-Mangold organizer formation and subsequent D-V axis determination(Hikasa & Sokol, 2013; Niehrs, 2004). This early regionalized Wnt signaling center is generally activated by localized expression of upstream signaling components such as Wnt ligands(Tao et al., 2005) and /or Dvl(Miller et al., 1999; Weitzel et al., 2004; Tadjuidje et al., 2011). To date, ten Wnt genes have been identified in amphioxus and eight of their developmental expression patterns have been analyzed by in situ hybridization; however, none of these genes have shown a detectable expression before the gastrula stage(Holl and , 2002). Using RT-qPCR, five Wnt genes, including Wnt1, 6, 9, 10, and 11, were found to be maternally expressed, although expression levels were relatively low(Qian et al., 2013). However, it is not clear whether these genes are expressed asymmetrically at one pole of cleavage-stage embryos. In the present study, the amphioxus Dvl gene was asymmetrically expressed toward the animal pole in the amphioxus embryo from the one-cell to late blastula stages. This expression pattern is the same as that of amphibian Dvl(Miller et al., 1999; Tadjuidje et al., 2011), but different from that of sea urchin Dvl, which is enriched in the vegetal pole(Weitzel et al., 2004). Thus, it appears unlikely that amphioxus embryos could form a regionalized Wnt signaling center at the early stages via the asymmetric expression of the Dvl gene since the downstream effector β-catenin can localize nuclei of all embryonic blastomeres from the 16-cell to late blastula stages(Holland et al., 2005). This possibility is further strengthened by the ubiquitous expression of Lrp5/6, Axin, APC, CkIα, CkIδ, Gsk3β, and Gro(present study) and four Frizzled genes(Qian et al., 2013), and the undetectable expression of Wnt/β-catenin signaling antagonists such as Dkks, sFrps, and Ceberus in amphioxus embryos before the gastrula stage(Yu et al., 2007; Onai et al., 2010, 2012; unpublished data). Results suggest that Wnt signaling is probably not involved in endomesoderm specification or D-V axis determination in amphioxus(Holland et al., 2005). Consistent with this assumption, the upregulation of Wnt signaling through the inhibition of Gsk3β activity by lithium chloride before the midblastula stage has no effect on mesendoderm specification or D-V axis development(Holland et al., 2005). The evidence supporting amphioxus having a regionalized Wnt/β-catenin signaling center in the cleavage-stage embryo is the asymmetrical expression of the Tcf gene in the animal pole of early stage embryos(Lin et al., 2006). It would be interesting to perform functional study on the amphioxus Tcf gene via overexpression and knockdown experiments in the future.
Function of Wnt/β-catenin signaling in amphioxus posterior growth and somitogenesis
Body elongation via posterior growth is an important feature during the late stage of vertebrate embryogenesis. This elongation is accomplished by means of continuously adding segmented somite blocks to the posterior region(tail bud)at the neurula stage. Wnt signaling plays an essential role during the process by coordinating mesoderm formation and segmentation(Dunty et al., 2008). Vertebrate embryos lacking Wnt signaling components like Wnt3a or β-catenin display posterior axis truncations and form only head structures and anterior parts of the trunk(Dunty et al., 2008; Takada et al., 1994). Posterior growth does not exist in laboratory non-invertebrate metazoan animals such as sea urchins, Ciona, Drosophila, and Caenorhabditis elegans. This makes determining whether Wnt signaling regulates posterior growth in animals outside of vertebrates elusive. However, several studies on short germb and insects, including the red flour beetle and cricket, have indicated a conserved role for Wnt signaling in posterior growth(Bolognesi et al., 2008; Miyawaki et al., 2004), although the genetic network underlying it is relatively different between insects and vertebrates(Martin & Kimelman, 2009). Amphioxus embryos have a posterior growth zone(tail bud)from which posterior somites can continuously bud off in a one-left-oneright manner(Schubert et al., 2001). Based on the expressions of Wnt and target genes, such as Brachyury and Caudal, within or around the tail bud of amphioxus embryos, Holl and (2002)speculated that the Wnt signaling pathway should play a critical role in amphioxus posterior growth. Studies on other amphioxus Wnt signaling components, including β-catenin (Holland et al., 2005), Tcf (Lin et al., 2006), Frizzleds (Qian et al., 2013), Lrp5/6, Dvl, Axin, Cks, Gsk3β, and Gro (present study), have further strengthened this speculation as these genes were transcribed within or around the amphioxus tail bud region. These results have also implicated that posterior growth mediated by Wnt/β-catenin signaling might be an ancient characteristic in metazoans and the genetic toolkit underlying this process would be relatively complete in amphioxus.
The Wnt/β-catenin signaling pathway is also essential for early stages of somitogenesis in vertebrates by maintaining the expression of myogenesis gene MyoD and inducing axial mesoderm inhibitor genes Vent/Vox (Hoppler & Moon, 1998; Ramel & Lekven, 2004). However, after the commitment of paraxial mesoderm to somatic fate, Wnt signaling is rapidly downregulated and not required for late myogenesis(Tian et al., 1999). Preliminary analysis on Wnt8 expression patterns indicated a similar role for Wnt signaling in amphioxus early somitogenesis(Schubert et al., 2000). It also revealed that amphioxus Wnt8 was continuously transcribed in some of the differentiated somites, indicating an essential role for Wnt/β-catenin signaling in both early stage somite formation and somite differentiation(Schubert et al., 2000). Consistent with this observation, we found that amphioxus Lrp5/6, Dvl, Axin, APC, Cks Gsk3β, and Gro were all expressed in most differentiated somites in the mid to late neurulae. Further functional experiments are necessary to clarify this hypothesis.
Supplementary Figure 2.
Phylogenetic relationship of Dvl proteins
Supplementary Figure 3.
Phylogenetic relationship of APC proteins
Supplementary Figure 4.
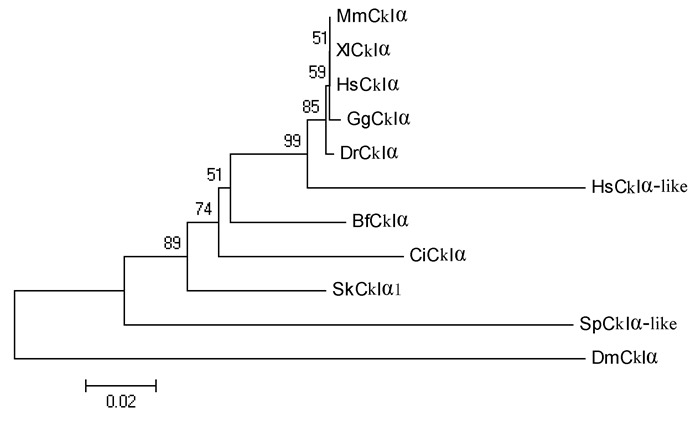
Phylogenetic relationship of CkIα proteins
Supplementary Figure 5.
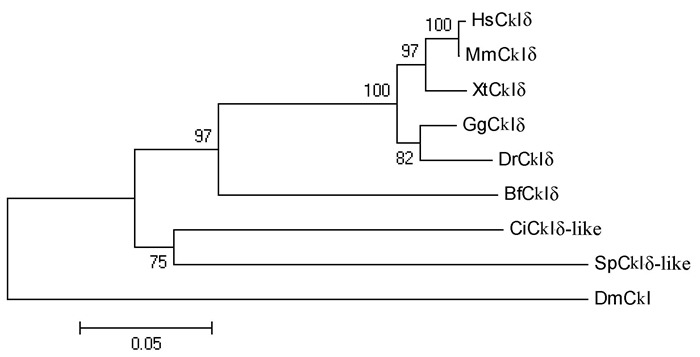
Phylogenetic relationship of CkIδ proteins
Supplementary Figure 6.
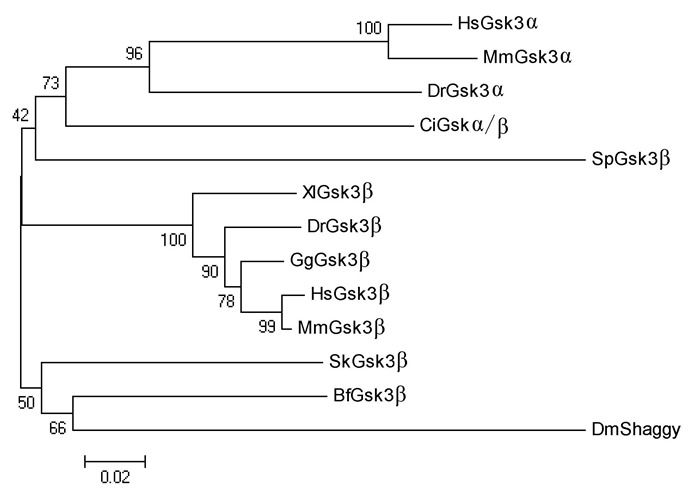
Phylogenetic relationship of Gsk3β proteins
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (31372188, 31471986) and the Science and Technology Innovation Commission of Shenzhen Municipality (CXZZ20120614164555920)
REFERENCES
- 1. Beaster-Jones L, Kaltenbach SL, Koop D, Yuan SC, Chastain R, Holland LZ. 2008. Expression of somite segmentation genes in amphioxus: a clock without a wavefront. Development Genes and Evolution, 2018 (11-12): 599- 611. [DOI] [PubMed] [Google Scholar]
- 2. Bolognesi R, Farzana L, Fischer TD, Brown SJ. 2008. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Current Biology, 18 (20): 1624- 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Darras S, Gerhart J, Terasaki M, Kirschner M, Lowe CJ. 2011. β-Catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development, 138 (5): 959- 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delsuc F, Brinkmann H, Chourrout D, Philippe H. 2006. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature, 439 (7079): 965- 968. [DOI] [PubMed] [Google Scholar]
- 5. Dunty WC, J r., Biris KK, Chalamalasetty RB, Taketo MM, Lewandoski M, Yamaguchi TP. 2008. Wnt3a/β-Catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development, 135 (1): 85- 94. [DOI] [PubMed] [Google Scholar]
- 6. Henry JQ, Perry KJ, Wever J, Seaver E, Martindale MQ. 2008. β-Catenin is required for the establishment of vegetal embryonic fates in the nemertean, Cerebratulus lacteus. Developmental Biology, 317 (1): 368- 379. [DOI] [PubMed] [Google Scholar]
- 7. Hikasa H, Sokol SY. 2013. Wnt signaling in vertebrate axis specification. Cold Spring Harbor Perspectives in Biology, 5 (1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holland LZ. 2002. Heads or tails? Amphioxus and the evolution of anteriorposterior patterning in deuterostomes. Developmental Biology, 241 (2): 209- 228. [DOI] [PubMed] [Google Scholar]
- 9. Holland LZ, Holland ND. 2007. A revised fate map for amphioxus and the evolution of axial patterning in chordates. Integrative and Comparative Biology, 47 (3): 360- 372. [DOI] [PubMed] [Google Scholar]
- 10. Holland LZ, Panfilio KA, Chastain R, Schubert M, Holland ND. 2005. Nuclear β-Catenin promotes non-neural ectoderm and posterior cell fates in amphioxus embryos. Developmental Dynamics, 233 (4): 1430- 1443. [DOI] [PubMed] [Google Scholar]
- 11. Holstein TW. 2012. The evolution of the Wnt pathway. Cold Spring Harbor Perspectives in Biology, 4 (7): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoppler S, Moon RT. 1998. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mechanisms of Development, 71 (1-2): 119- 129. [DOI] [PubMed] [Google Scholar]
- 13. Imai K, Takada N, Satoh N, Satou Y. 2000. β-Catenin mediates the specification of endoderm cells in ascidian embryos. Development, 127 (14): 3009- 3020. [DOI] [PubMed] [Google Scholar]
- 14. Li G, Shu ZH, Wang YQ. 2013. Year-round reproduction and induced spawning of Chinese amphioxus, Branchiostoma belcheri, in laboratory. PLoS One, 8 (9): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li G, Yang X, Shu ZH, Chen XY, Wang YQ. 2012. Consecutive spawnings of Chinese amphioxus, Branchiostoma belcheri, in captivity. PLoS One, 7 (12): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin HC, Holland LZ, Holland ND. 2006. Expression of the AmphiTcf gene in amphioxus:insights into the evolution of the TCF/LEF gene family during vertebrate evolution. Developmental Dynamics, 235 (12): 3396- 3403. [DOI] [PubMed] [Google Scholar]
- 17. Logan CY, Nusse R. 2004. The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology, 20 781- 810. [DOI] [PubMed] [Google Scholar]
- 18. Logan CY, Miller JR, Ferkowicz MJ, Mcclay DR. 1999. Nuclear β-Catenin is required to specify vegetal cell fates in the sea urchin embryo. Development, 126 (2): 345- 357. [DOI] [PubMed] [Google Scholar]
- 19. Martin BL, Kimelman D. 2009. Wnt signaling and the evolution of embryonic posterior development. Current Biology, 19 (5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martindale MQ. 2005. The evolution of metazoan axial properties. Nature Reviews Genetics, 6 (12): 917- 927. [DOI] [PubMed] [Google Scholar]
- 21. McCauley BS, Akyar E, Saad HR, Hinman VF. 2015. Dose-dependent nuclear β-Catenin response segregates endomesoderm along the sea star primary axis. Development, 142 (1): 207- 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. 1999. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. The Journal of Cell Biology, 146 (2): 427- 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyawaki K, Mito T, Sarashina I, Zhang HJ, Shinmyo Y, Ohuchi H, Noji S. 2004. Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mechanisms of Development, 121 (2): 119- 130. [DOI] [PubMed] [Google Scholar]
- 24. Mizumoto K, Sawa H. 2007. Two βs or not two βs:regulation of asymmetric division by β-Catenin. Trends in Cell Biology, 17 (10): 465- 473. [DOI] [PubMed] [Google Scholar]
- 25. Momose T, Houliston E. 2007. Two oppositely localised frizzled RNAs as axis determinants in a cnidarian embryo. PLoS Biology, 5 (4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Momose T, Derelle R, Houliston E. 2008. A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development, 135 (12): 2105- 2113. [DOI] [PubMed] [Google Scholar]
- 27. Niehrs C. 2004. Regionally specific induction by the Spemann-Mangold organizer. Nature Reviews Genetics, 5 (6): 425- 434. [DOI] [PubMed] [Google Scholar]
- 28. Onai T, Takai A, Setiamarga DH, Holland LZ. 2012. Essential role of Dkk3 for head formation by inhibiting Wnt/β-Catenin and Nodal/Vg1 signaling pathways in the basal chordate amphioxus. Evolution & Development, 14 (4): 338- 350. [DOI] [PubMed] [Google Scholar]
- 29. Onai T, Yu JK, Blitz IL, Cho KW, Holland LZ. 2010. Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Developmental Biology, 344 (1): 377- 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Onai T, Lin HC, Schubert M, Koop D, Osborne PW, Alvarez S, Alvarez R, Holland ND, Holland LZ. 2009. Retinoic acid and Wnt/β-Catenin have complementary roles in anterior/posterior patterning embryos of the basal chordate amphioxus. Developmental Biology, 332 (2): 223- 233. [DOI] [PubMed] [Google Scholar]
- 31. Petersen CP, Reddien PW. 2009. Wnt signaling and the polarity of the primary body axis. Cell, 139 (6): 1056- 1068. [DOI] [PubMed] [Google Scholar]
- 32. Qian GH, Li G, Chen XY, Wang YQ. 2013. Characterization and embryonic expression of four amphioxus Frizzled genes with important functions during early embryogenesis. Gene Expression Patterns, 13 (8): 445- 453. [DOI] [PubMed] [Google Scholar]
- 33. Ramel MC, Lekven AC. 2004. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development, 131 (16): 3991- 4000. [DOI] [PubMed] [Google Scholar]
- 34. Rao TP, Kuhl M. 2008. An updated overview on Wnt signaling pathways:a prelude for more. Circulation Research, 106 (12): 1798- 1806. [DOI] [PubMed] [Google Scholar]
- 35. Schubert M, Holland LZ, Stokes MD, Holland ND. 2001. Three amphioxus Wnt genes (AmphiWnt3, AmphiWnt5, and AmphiWnt6) associated with the tail bud:the evolution of somitogenesis in chordates. Developmental Biology, 240 (1): 262- 273. [DOI] [PubMed] [Google Scholar]
- 36. Schubert M, Holland LZ, Panopoulou GD, Lehrach H, Holland ND. 2000. Characterization of amphioxus AmphiWnt8:insights into the evolution of patterning of the embryonic dorsoventral axis. Evolution & Development, 2 (2): 85- 92. [DOI] [PubMed] [Google Scholar]
- 37. Tadjuidje E, Cha SW, Louza M, Wylie C, Heasman J. 2011. The functions of maternal Dishevelled 2 and 3 in the early Xenopus embryo. Developmental Dynamics, 240 (7): 1727- 1736. [DOI] [PubMed] [Google Scholar]
- 38. Takada S, Stark KL, Shea MJ, Vassileva G, Mcmahon JA, Mcmahon AP. 1994. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes & Development, 8 (2): 174- 189. [DOI] [PubMed] [Google Scholar]
- 39. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28 (10): 2731- 2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tao QH, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin XH, Heasman J. 2005. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell, 120 (6): 857- 871. [DOI] [PubMed] [Google Scholar]
- 41. Tian Q, Nakayama T, Dixon MP, Christian JL. 1999. Post-transcriptional regulation of Xwnt-8 expression is required for normal myogenesis during vertebrate embryonic development. Development, 126 (15): 3371- 3380. [DOI] [PubMed] [Google Scholar]
- 42. Weitzel HE, Illies MR, Byrum CA, Xu RH, Wikramanayake AH, Ettensohn CA. 2004. Differential stability of β-Catenin along the animal-vegetal axis of the sea urchin embryo mediated by dishevelled. Development, 131 (12): 2947- 2956. [DOI] [PubMed] [Google Scholar]
- 43. Wikramanayake AH, Huang L, Klein WH. 1998. β-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proceedings of the National Academy of Sciences of the United States of America, 95 (16): 9343- 9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wikramanayake AH, Hong M, Lee PN, Pang K, Byrum CA, Bince JM, Xu RH, Martindale MQ. 2003. An ancient role for nuclear β-Catenin in the evolution of axial polarity and germ layer segregation. Nature, 426 (6965): 446- 450. [DOI] [PubMed] [Google Scholar]
- 45. Yu JK, Holland LZ. 2009. Amphioxus whole-mount in situ hybridization. Cold Spring Harbor Protocols, 2009 (9): [DOI] [PubMed] [Google Scholar]
- 46. Yu JK, Satou Y, Holland ND, Shin IT, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. 2007. Axial patterning in cephalochordates and the evolution of the organizer. Nature, 445 (7128): 613- 617. [DOI] [PubMed] [Google Scholar]


