Abstract
Two colepid ciliates, Coleps amphacanthus Ehrenberg, 1833 and Levicoleps biwae jejuensis Chen et al., 2016, were first recorded in China. Their living morphology, infraciliature and small subunit (SSU) rRNA gene sequences were determined using standard methods. The improved diagnosis of Coleps amphacanthus is as follows:cell size about 100×50 μm in vivo, barrel-shaped; 22-28 ciliary rows each composed of about 14-21 monokinetids and two perioral dikinetids; 5-10 caudal cilia; and one terminal contractile vacuole. Levicoleps biwae jejuensis was also investigated, with an improved diagnosis given based on previous and present work. The phylogenetic analyses based on SSU rRNA gene sequences revealed that all Coleps species were grouped together, except for Coleps amphacanthus, which was grouped into a clade of the genus Levicoleps.
Keywords: Ciliate, Coleps, Levicoleps, New record, Phylogeny, SSU rRNA, Taxonomy
INTRODUCTION
Colepid ciliates are commonly found in a wide range of habitats, including benthic, pelagic and marine psammobiotic. They are one of the main components of the microbial community and play an important role in the function of the microbial food webs (Finlay & Fenchel, 1996; Song et al., 2009) . Since the first species, Coleps hirtus (Müller, 1786) Nitzsch, 1827 , was reported two centuries ago, more than 40 species of this family have been found and recorded. They are characterized by cylindrical or barrel-shaped bodies covered by unique calcified cuirasses (armored plates with small lateral plate processes arranged in longitudinal rows) , sometimes with anterior and/or posterior spines. In general, their caudal cilia are clearly longer than other somatic cilia (Corliss, 1979; Dragesco & Dragesco-Kernéis, 1991; Foissner, 1983; Kahl, 1930; Noland, 1925; Obolkina, 1995) . Individual taxa may be characterized by a variety of morphological features including the number of armor tiers, structure of plates, presence or absence of spines, and number of adoral organelles (Foissner et al., 2008) . According to these taxonomic standards, some new genera have been established in recent years, including the construction of Levicoleps due to the absence of spines and armor tiers with hirtus-type plates and Kotinia characterized by spiny armor composed of eight tiers and five adoral organelles (Chen et al., 2009, 2010, 2012, 2016; Foissner et al., 2008; Obolkina, 1995).
The present paper provides a morphological description of a poorly known species, Coleps amphacanthus Ehrenberg, 1833, and a new record of Levicoleps biwae jejuensis Chen et al., 2016, in China. Phylogenetic analyses based on SSU rRNA gene sequences were also performed.
MATERIALS AND METHODS
Sample collection, observation, and identification
Coleps amphacanthus Ehrenberg, 1833 was collected from the brackish water of Hangzhou Bay, Ningbo (N30°22'; E121°12') , China, on 22 July, 2014. The water temperature was about 25℃ and salinity was about 2‰. Samples with decaying plants were collected from the surface of the sediment using a plastic dropper, then diluted with untreated water from the collection site.
Levicoleps biwae jejuensis Chen et al., 2016 was collected on 22 June, 2015, from a freshwater pond in Ningbo (N29°55'; E121°39') . The water temperature was about 23 ℃. Samples were collected by 20 µm mesh plankton nets from the upper layer of the water.
The behavior of the organisms was studied in Petri dishes under a dissecting microscope. Living morphology was investigated under a compound microscope equipped with a high-power oil immersion objective as well as differential interference contrast optics (Olympus BH-2, Olympus Optical Co., Ltd, Tokyo, Japan) . The infraciliature was revealed via protargol impregnation (Wilbert, 1975) . Counts and measurements of the morphological characteristics of stained specimens were performed at a magnification of ×1 250. Drawings were made with the help of a camera lucida. Terminology used is primarily in accordance with Foissner et al.(2008)and Chen et al.(2010).
DNA extraction, gene amplification, and sequencing
Genomic DNA was extracted from cells using the Dneasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The PCR amplification of the SSU rRNA gene sequences was performed using universal eukaryotic primers 18S-F (5'-AACCTGGTTGATCCTGCCAGT-3') and 18S-R (5'-TGATCCTTCTGCAGGTTCACCTAC-3') (Medlin et al., 1988) . PCR products were purified using an EasyPure® Quik Gel Extraction Kit (TransGen, EG101, Beijing, China), then cloned using a pEASY®-T1 Cloning kit (TransGen, CT101, Beijing, China) . Sequencing was performed bidirectionally on an ABI 3700 sequencer (GENEWIZ Biotechnology Co., Ltd., Beijing, China) .
Phylogenetic analyses
The two newly characterized SSU rRNA gene sequences and the sequences of another 45 species/populations obtained from the NCBI GenBank database were used for phylogenetic analyses. Sequences were aligned using Bioedit v7.1.3.0. (Hall, 1999) with the ClustalW algorithm. The resulting alignments were manually refined by trimming both ends. Final alignment included 1 767 characters and 47 taxa. Paramecium tetraurelia, Ichthyophthirius multifiliis, and Tetrahymena pyriformis were selected as outgroup taxa for phylogenetic analyses. Bayesian inference (BI) and maximum likelihood(ML)analyses were performed online using the CIPRES Science Gateway v3.3 (http://www.phylo.org/portal2) . BI analysis was performed with MrBayes on XSEDE v3.2.6 (Ronquist & Huelsenbeck, 2003) using the GTR+I+G model as selected by MrModeltest v2.2 (Nylander, 2004) . The chain length of the Bayesian analyses was 10 000 000 generations, sampled every 100 generations. The first 10 000 sampled trees were considered as burn-in. ML analysis was performed with RAxML-HPC2 on XSEDE v8.2.4 (Stamatakis et al., 2008) using the GTR+I+G model as selected by Modeltest v3.4 (Posada & Crandall, 1998) . The reliability of ML internal branches was assessed using a nonparametric bootstrap method with 1 000 replicates. MEGA v5.0 (Tamura et al., 2011) was used to view and edit tree topologies. Systematic classification mainly followed Lynn(2008)and Yi et al.(2010).
Hypothesis testing
To test the hypothesis that the morphologically-defined genera are monophyletic groups, RAxML (Shimodaira, 2002; Stamatakis et al., 2008) was used to generate ML trees with enforced topological constraints. For all constraints, internal relationships within the constrained groups and among the remaining taxa were unspecified. The site-wise likelihoods for the resulting constrained topologies and the non-constrained ML topologies were calculated using PAUP (Swofford, 2002) . Approximately unbiased (AU) tests (Shimodaira, 2002) were performed using CONSEL v 0.1 (Shimodaira & Hasegawa, 2001) to obtain P-values.
RESULTS
Coleps amphacanthusEhrenberg, 1833 (Table 1; Figure 1 and Figure 2)
Table 1.
Morphometric data on Coleps amphacanthus (first line) and Levicoleps biwae jejuensis (second line)
| Characters | Min | Max | M | Mean | SD | SE | CV | n |
| Body length | 82 | 98 | 90.0 | 89.0 | 4.9 | 2.0 | 5.5 | 8 |
| 85 | 103 | 94.0 | 95.1 | 5.2 | 1.3 | 5.5 | 15 | |
| Body width | 39 | 51 | 45.0 | 46.0 | 5.0 | 2.0 | 10.1 | 8 |
| 37 | 57 | 47.0 | 45.7 | 6.6 | 1.7 | 14.4 | 15 | |
| Somatic kineties, number | 24 | 26 | 25.0 | 25.0 | 0.8 | 0.0 | 3.2 | 8 |
| 21 | 24 | 22.5 | 23.1 | 0.8 | 0.2 | 3.5 | 15 | |
| Transverse ciliary rows, number | 18 | 18 | 18.0 | 18.0 | 0.0 | 0.0 | 0.0 | 9 |
| 15 | 15 | 15.0 | 15.0 | 0.0 | 0.0 | 0.0 | 15 | |
| Macronucleus length | 25 | 32 | 28.5 | 29.3 | 2.6 | 0.9 | 8.9 | 8 |
| 21 | 38 | 29.5 | 27.7 | 4.8 | 1.2 | 17.3 | 15 | |
| Macronucleus width | 16 | 29 | 22.5 | 23.8 | 4.5 | 1.6 | 18.9 | 8 |
| 17 | 32 | 24.5 | 21.2 | 3.9 | 1.0 | 18.4 | 15 |
Data based on protargol-impregnated specimens. All measurements in µm. CV: coefficient of variation in %, Max: maximum, Mean: arithmetic mean, M: median, Min: minimum, n: number of specimens analysed, SD: standard deviation, SE: standard error. *: Excluding perioral dikinetids.
Figure 1.
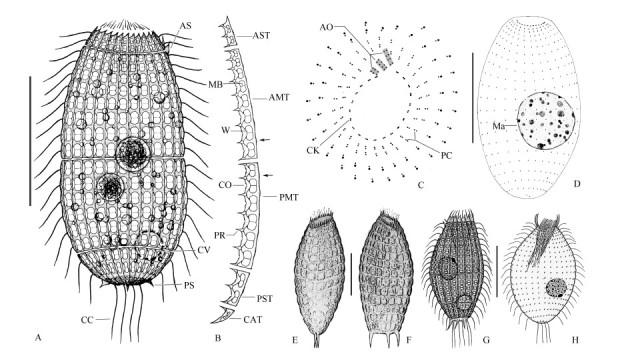
Morphology and infraciliature of Coleps amphacanthus
Figure 2.
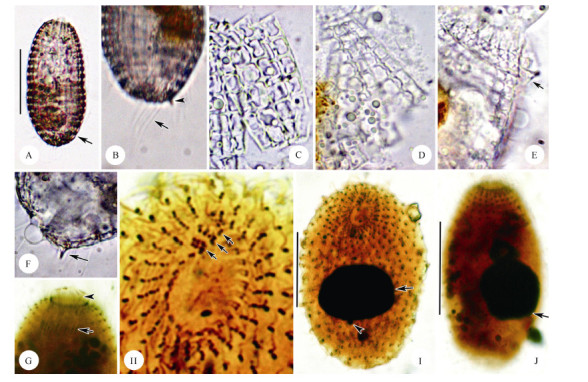
Microphotographs of living Coleps amphacanthus and after protargol impregnation
Coleps amphacanthus is a poorly known and rarely reported species (Foissner & O’onoghue, 1990; Huttenlauch, 1986, 1987; Huttenlauch & Bardele, 1987; Kreutz & Foissner, 2006; Noland, 1925) . According to these historic studies, the morphological features are not particularly stable and most descriptions lack the infraciliature. Therefore, re-description is necessary to reduce confusion and clarify intraspecific variations.
Improved diagnosis. Cells about (80-110) µm× (40-60) µm in vivo, barrel shaped. On average 22-28 ciliary rows, each composed of about 14-21 monokinetids and two perioral dikinetids. Anterior and posterior main plates each with 5-8 and 5-6 windows, respectively; anterior and posterior secondary plates with 2-3 windows, respectively. Five to ten caudal cilia. One subterminal contractile vacuole. Fresh-or brackish-habitat.
Voucher slide. A voucher slide with protargol-impregnated specimens was deposited in the Laboratory of Protozoology, Ocean University of China (registration number: LU-20140722-04-01).
Morphological description based on Chinese population. Body size (90-110) µm× (40-60) µm in vivo, usually about 100 µm×50 µm, barrel-shaped, with a slight wave-like arch at each window caused by sieve domes, usually slightly narrowed in the mid-body where the main armor plates abut. Ratio of body length to width about 2: 1. Anterior end broad, transversely truncated and crown-like due to triangle-shaped secondary tier plates; posterior end moderately rounded (Figure 1A; Figure 2A) . Macronucleus spherical to broadly ellipsoidal with an average length: width ratio of 1.3, usually located at the equatorial level and near the periphery of the cell. Micronucleus globular and approximately 2 µm in diameter, adherent to the macronucleus (Figure 1D; Figure 2I, J) . Contractile vacuole about 12 µm in diameter, positioned near the subterminal of the body (Figure 1A; Figure 2A) . Cytoplasm colorless, usually containing several food vacuoles (10-20 µm in diameter) and mass of refractive lipid droplets (1-4 µm across) (Figure 1A; Figure 2A) .
Armor composed of hirtus-type plates arranged in six tiers: circumoral, anterior secondary, anterior main, posterior main, posterior secondary, and caudal, with each tier consisting of about 25 rectangular plates (Figure 1A, B; Figure 2A) . Circumoral tiers hardly recognizable in vivo. Anterior secondary plates about 12 µm in length, with two windows, two ciliary outlets, three plate processes, and triangle-shaped anterior end; usually two of them with anterior spines located at the same side (Figure 1A, B; Figure 2E) . Anterior main plates approximately 40 µm long, with eight windows gradually enlarged from anterior to posterior, nine plate processes, and eight ciliary outlets(Figure 1B; Figure 2D) . Posterior main plates approximately 30 µm long, with six windows, seven plate processes, and six ciliary outlets (Figure 1B; Figure 2C) . Posterior secondary plates trapezoidal, about 12 µm long, with two elongated windows, three plate processes, and two ciliary outlets (Figure 1B) . Caudal tier visible only in oblique or posterior polar view, about 5 µm long, usually with three small and sharp posterior spines (Figure 1A, B; Figure 2F) . The fine structure of the armor plates is shown in Figure 1B; 2C-D: left margin generally smooth and slightly convex at the level of the bridge; sharp plate processes arranged alternately with windows on the right edge, connected with conspicuous bridges; windows '8' shaped with small radian sieve domes, separated in pairs by a midbar; generally, 2, 8, 6, 2 windows in the middle four tiers from the anterior to posterior (Figure 1A; Figure 2A) .
Oral opening at anterior end of cell. Circumoral kinety circular, composed of dikinetids, interrupted at the site of three adoral organelles. Adoral organelles short and obliquely arranged. Organelle 1 composed of three pairs of kinetosomes, organelles 2 and 3 each composed of four pairs of kinetosomes (Figure 1C; Figure 2H) . Internal and external oral basket in center of mouth, about 6 µm and 10 µm long, respectively, conspicuous in protargol-impregnated specimens (Figure 2G).
Somatic cilia about 15 µm long and regularly arranged, forming 18 transverse circles and 25 longitudinal rows on average (Figure 1D; Figure 2I, J) . Anterior two transverse circles with dikinetids closely arranged and forming perioral ciliature (Figure 1C; Figure 2H) . Five to ten significantly long caudal cilia (about 25 µm) (Figure 1A; Figure 2B) .
Levicoleps biwae jejuensis Chen et al., 2016 (Table 1; Figure 3 and 4)
Figure 3.
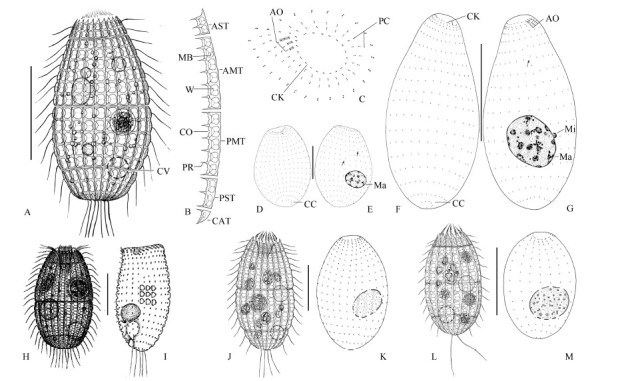
Morphology and infraciliature of the Chinese population (A-G) and South Korean population (J, K) of Levicoleps biwae jejuensis, L. biwae biwae (H, I) , and L. taehwae (L, M)
Figure 4.
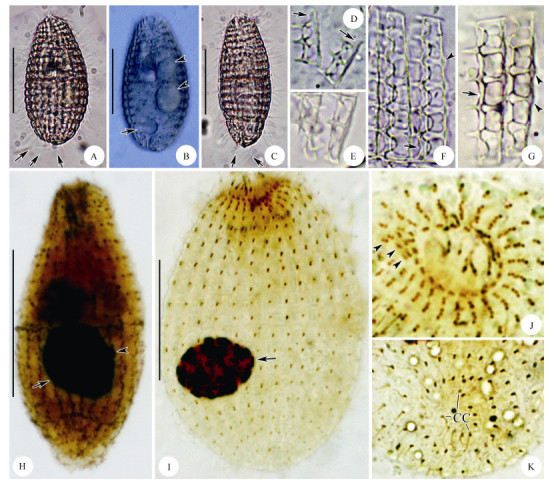
Microphotographs of the Chinese population of living Levicoleps biwae jejuensis and after protargol impregnation
The genus Levicoleps was established by Foissner et al.(2008)with Levicoleps biwae as the type species. Chen et al.(2016) reported a South Korean subspecies, L. biwae jejuensis, from Jeju Island. These two subspecies are very similar in morphological characters and infraciliature, except the number of caudal cilia. A Chinese population of L. biwae jejuensis is described here to clarify intraspecific variations.
Improved diagnosis. Size in vivo about (70-110) µm× (30-60) µm, barrel shaped. On average 21-24 ciliary rows, each composed of about 15-18 monokinetids and two perioral dikinetids; 4-10 caudal cilia. Anterior and posterior main plates each with six and five windows, respectively; anterior and posterior secondary plates each with two windows, respectively.
Voucher slides. Two voucher slides with protargol-impregnated specimens were deposited in the Laboratory of Protozoology, Ocean University of China(registration numbers: LU-20150622-02-01, 02).
Morphological description of Chinese population. Size about (85-110) µm× (40-60) µm in vivo, barrel-shaped body with a length to width ratio of about 2: 1, usually slightly narrowed in the mid-body where the main armor plates abut. Body margin conspicuously wavy due to window domes (Figure 3A; Figure 4A) . Anterior end transversely truncated and crown-like, posterior end moderately rounded to slightly pointed (Figure 3A; Figure 4A-C) . Macronucleus globular to slightly ellipsoidal with a length: width ratio of about 1.2, positioned near the mid-body and close to the cell periphery. Micronucleus spherical, about 2 µm in diameter, attached to macronucleus (Figure 3E, G; Figure 4H, I) . Contractile vacuole about 10 µm across, located near the subterminal of the body (Figure 3A; Figure 4B) . Cytoplasm colorless, usually containing several food vacuoles (5-15 µm in diameter) and numerous droplets (1-5 µm across) (Figure 3A; Figure 4A-C) .
Armor plates hirtus-type and composed of six tiers: circumoral, anterior secondary, anterior main, posterior main, posterior secondary and caudal, with each tier composed of 21-24 plates (Figure 3A, B; Figure 4A-G) . Circumoral tier hardly observed in vivo. Anterior secondary plates about 15 µm long, with triangle-shaped anterior end, two windows, three plate processes, and two ciliary outlets (Figure 3B; Figure 4D) . Anterior main plates about 35 µm long, with six windows gradually enlarged from the anterior to posterior, seven plate processes, and six ciliary outlets(Figure 3B; Figure 4F) . Posterior main plates about 30 µm long, with five windows, six plate processes, and five ciliary outlets (Figure 3B; Figure 4G) . Posterior secondary plates trapezoidal-shaped, about 12 µm long, with two elongated windows, three plate processes, and two ciliary outlets (Figure 3B; Figure 4E) . Caudal tier about 4 µm long with a single window (Figure 3B). The fine structure of the armor plates is shown in Figure 3B and Figure 4D-G: left margin generally smooth and slightly waved at the level of the bridge, plate processes on the right edge arranged alternately with windows and connected with inconspicuous bridges; bi-window with conspicuous radian sieve domes. In general, 2, 6, 5, 2 windows in the middle four tiers from the anterior to posterior (Figure 3A, B; Figure 4A-G).
Oral opening occupying the central region of the anterior pole. Circumoral kinety circular and composed of dikinetids (Figure 3C; Figure 4J) . Adoral organelles consisting of three parts, organelle 1 and 2 each composed of two pairs of kinetosomes, organelle 3 composed of three pairs of kinetosomes (Figure 3C; Figure 4J) . Internal and external oral basket about 8 µm and 12 µm long, respectively, in protargol-impregnated specimens (Figure 4H) .
Regularly arranged somatic cilia about 18 µm long, forming 15 transverse circles and 21-24 longitudinal rows (Figure 3D-G; Figure 4H, I) . Anterior two close dikinetids of each longitudinal ciliary row forming perioral ciliature. Six to ten caudal cilia about 25 µm long in vivo (Figure 3A, D, F; Figure 4A, C, K) .
SSU rRNAgene sequences and phylogenetic analyses
The SSU rRNA gene sequences of Coleps amphacanthus and Levicoleps biwae jejuensis were deposited in the GenBank database with accession numbers KU525296 and KU525297, respectively. The length and GC content of the SSU rRNA gene sequence of Coleps amphacanthus were 1 718 bp and 44.18%, respectively, while those of Levicoleps biwae jejuensis (Ningbo population) were 1 719 bp and 44.27%, respectively.
Forty-seven species/populations were included in the present phylogenetic analyses, containing three oligohymenophorean species as out-groups and all species from classes Prostomatea and Plagiopylea available for SSU rRNA gene sequences. In the class Prostomatea, there were 34species/populations representing 15 genera from seven families (Balanionidae, Colepidae, Holophryidae, Placidae, Plagiocampidae, Prorodontidae, and Urotrichidae) . The topologies of the ML and BI trees were basically congruent and, therefore, a single topology was presented based on the ML tree with support values from both algorithms indicated on the branches (Figure 5) . In the phylogenetic trees, Prostomatea was polyphyletic with all species grouped into three clades: Placidae formed one fully supported clade; Colepidae, Prorodontidae, and Pelagothrix alveolata (Holophryidae) formed the second clade (61% ML, 0.98 BI) ; Plagiocampidae, Urotrichidae, Balanionidae, and Cryptocaryon irritans (Holophryidae) formed the third clade (88% ML, 0.98 BI) , and is a sister to the class Plagiopylea (65% ML, 0.70 BI) . Within the family Colepidae, both genera Coleps and Levicoleps were grouped together with high support (96% ML, 1.00 BI) . All Coleps species were branched together, except for Coleps amphacanthus, which was grouped into the clade of the genus Levicoleps. All three Levicoleps biwae isolates formed a well-supported clade (97% ML, 1.00 BI) , with the two isolates ofLevicoleps biwae jejuensisgrouped together.
Figure 5.
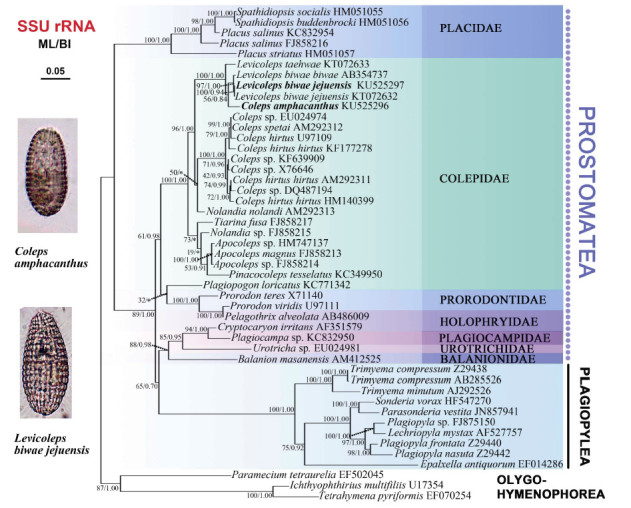
Maximum likelihood(ML)tree inferred from SSU rRNA gene sequences showing positions of Coleps amphacanthus and Levicoleps biwae jejuensis
Hypothesis testing
Neither the genus Coleps nor Levicoleps was a monophyletic group in the present phylogenetic tree, as Coleps amphacanthus was always nested within the Levicoleps cluster. However, the constrained topology that all species of Levicoleps formed a monophyletic clade was not rejected at the 5% significance level (P=0.127) . The hypothesized monophyly of the genus Coleps was rejected (P=8e-008) , which raises the question whether the current morphological classification of Coleps is appropriate. Additional molecular data from taxa with indistinct spines or several caudal cilia are needed to determine whether this genus should be further split (Table 2) .
Table 2.
Approximately unbiased test results
| Topology constraints | Log-likelihood (-lnL) | AU value (P) |
| Unconstrained | 14143.12163 | 0.967 |
| Coleps | 14157.50543 | 8e-008 |
| Levicoleps | 14251.18767 | 0.127 |
Significant differences (P<0.05) between the best maximum likelihood tree and constrained topologies are in bold.
DISCUSSION
Remarks on Coleps amphacanthus Ehrenberg, 1833
Coleps Nitzsch, 1827 is characterized by its spiny armor composed of six tiers with hirtus-type plates and three adoral organelles. Till now, only four species of Coleps have been investigated using silver staining methods: Coleps amphacanthus Ehrenberg, 1833, C. elongatus Ehrenberg, 1831, C. hirtus hirtus Nitzsch, 1827, C. hirtus viridis Ehrenberg, 1831, and C. spetai Foissner, 1984 (Foissner, 1984; Foissner & O'onoghue, 1990; Foissner et al., 1994, 1999) .
Coleps amphacanthus has been investigated several times based on living and fixed specimens (Ehrenberg, 1833; Huttenlauch, 1987; Hutternlauch & Bardele, 1987; Foissner & O'onoghue, 1990; Kreutz & Foissner, 2006; Noland, 1925) . However, standard taxonomic data were only provided by Huttenlauch (1987) and Foissner & O'onoghue (1990). Except for smallish spines (vs. conspicuous spines in the original description and subsequent re-descriptions) , the morphology of the present organism is identical with previous populations. The dissimilarity of the spines is possibly due to different environments (the definite conclusion can not be provided here, further studies on this character are necessary) , and hence should be considered as a population-dependent character.
Coleps amphacanthus differs from most congeners by its large body size and non-single caudal cilium. Only one species, namely C. elongatus, has more than one caudal cilium and should be compared with C. amphacanthus. The latter can be easily distinguished from the former by the number of somatic kineties (24-26 in C. amphacanthus vs. 14-18 in C. elongatus) , the number of windows in the anterior and posterior main plates (8, 6 in C. amphacanthus vs. 5, 4 in C. elongatus, respectively) , and the number of caudal cilia (5-10 in C. amphacanthus vs. 2 in C. elongatus) .
In the phylogenetic trees, Coleps amphacanthus was separated from the genus Coleps, and clustered in the genus Levicoleps (Figure 5) . Foissner et al.(2008) suggested that the presence or absence of armor spines is one of four principal generic features in the family Colepidae, with Levicoleps differing from Coleps mainly by its smooth armor without any spines. As previously described, Coleps amphacanthus has several obvious anterior and posterior spines in the original and subsequent re-descriptions (Ehrenberg, 1833; Noland, 1925; Huttenlauch, 1986; Foissner & O'onoghue, 1990) . The Ningbo population of C. amphacanthus has two anterior and three posterior inconspicuous smallish spines, and therefore should be assigned to Coleps according to the possession of spines. However, phylogenetic analyses did not support this, indicating that the presence or absence of armor spines might not be a good generic feature to separate Coleps and Levicoleps. Unfortunately, no molecular information currently exists in regards to historic populations. Additional molecular data will help reveal the correct phylogenetic position of this organism.
Remarks on Levicoleps biwae jejuensis Chen et al., 2016
Levicoleps biwae was originally discovered in a 4-million-year-old ancient freshwater lake (Lake Biwa) in Japan (Foissner et al., 2008) . Combined other biogeographic information, including the occurrence of Planicoleps only in Lake Tanganyika (Africa) (Dragesco & Dragesco-Kernéis, 1991) , the genera Baikalocoleps, Kotinia, Macrocoleps, and Tiarinella seem to be restricted to Lake Baikal (Obolkina, 1995) . Foissner et al.(2008) deduced that Levicoleps is a special genus from Lake Biwa. However, Chen et al.(2016) found a subspecies and other Levicoleps species, namely Levicoleps biwae jejuensis and L. taehwae, in South Korea. We also found a Chinese population of L. biwae jejuensis from a freshwater pond in Ningbo. In view of these isolations in South Korea and China, the genus Levicoleps does not appear to be endemic to Lake Biwa only, but is at least common to Asia.
Levicoleps is characterized by smooth armor composed of six tiers with hirtus-type plates, the absence of spines and three adoral organelles (Foissner et al., 2008) . We identified our form by basic characters of armor plate, absence of spines, three adoral organelles, and several caudal cilia. Except for the number of caudal cilia and windows of the main armor plates, the organism we isolated corresponded perfectly with L. biwae jejuensis. At the present state of knowledge, these differences should be considered as population-dependent characters in Levicoleps species (Foissner et al., 2008) . Moreover, this organism and L. biwae jejuensis (KT072632) were grouped together with strongly supported SSU rRNA trees (100% ML, 0.94 BI) , and their SSU rRNA gene sequences differed by only one nucleotide. Based on morphological and molecular information, our isolate should be considered a Chinese population of L. biwae jejuensis. In addition, the SSU rRNA gene sequence of our isolate differed by seven nucleotides from that of L. biwae biwae.
ACKNOWLEDGMENTS
We are grateful to the three anonymous reviewers for their thoughtful comments that led to improvements in the manuscript. Many thanks are due to Mr. Xiao CHEN,Ms. Chun-Di WANG and Teng-Teng ZHANG,graduate students at Ocean University of China,for their help with phylogenetic analyses and gene sequencing.
Funding Statement
This project was supported by the Natural Science Foundation of China (31572230) and the Ningbo Natural Science Foundation (2015A610263, 2015A610264)
REFERENCES
- 1. Chen XR, Warren A, Song WB. 2009. Taxonomic studies on a new marine ciliate, Apocoleps magnus gen. nov., spec. nov. (Ciliophora, Colepidae), isolated from Qingdao, China. Journal of Ocean University of China, 8 (4): 317- 321. [Google Scholar]
- 2. Chen XR, Wang YG, Long HA, Al-Rasheid KAS, Warren A, Song WB. 2010. Morphological studies on two marine colepid ciliates from Qingdao, China, Nolandia orientalis spec. nov. and Pinacocoleps similis (Kahl, 1933) comb. nov. (Ciliophora, Colepidae). European Journal of Protistology, 46 (4): 254- 262. [DOI] [PubMed] [Google Scholar]
- 3. Chen XR, Gao S, Liu WW, Song WB, Al-Rasheid KAS, Warren A. 2012. Taxonomic descriptions of three marine colepid ciliates, Nolandia sinica spec. nov., Apocoleps caoi spec. nov. and Tiarina fusa (Claparède & Lachmann, 1858) Bergh, 1881 (Ciliophora, Prorodontida). International Journal of Systematic and Evolutionary Microbiology, 62 (3): 735- 744. [DOI] [PubMed] [Google Scholar]
- 4. Chen XR, Shazib SUA, Kim JH, Shin MK. 2016. Morphological description and molecular phylogeny of two species of Levicoleps (Ciliophora, Prostomatida), L. taehwae nov. spec. and L. biwae jejuensis nov. subspec., collected in Korea. The Journal of Eukaryotic Microbiology, [DOI] [PubMed] [Google Scholar]
- 5. Corliss JO. 1979. The Ciliated Protozoa: Characterization, Classification and Guide to the Literature. 2nd ed., Oxford: Pergamon Press; [Google Scholar]
- 6. Dragesco J, Dragesco-Kernéis A. 1991. Free-living ciliates from the coastal area of Lake Tanganyika (Africa). European Journal of Protistology, 26 (3-4): 216- 235. [DOI] [PubMed] [Google Scholar]
- 7. Ehrenberg CG. 1833. Dritter Beitrag zur Erkenntniss grosser Organisation in der Richtung des kleinsten Raumes. Berlin:Abhandlungen der Preussischen Akademie der Wissenschaften (Berlin) aus den Jahre, [Google Scholar]
- 8. Finlay BJ, Fenchel T. 1996. Ecology:role of ciliates in the natural environment. In:Hausmann K, Bradbury PC. Ciliates:Cells As Organisms. Stuttgart:Gustav Fischer, [Google Scholar]
- 9. Foissner W. 1983. Taxonomische studien über die Ciliaten des Großglocknergebietes(Hohe Tauren, Österreich) I. Familien holophryidae, prorodontidae, plagiocampidae, colepidae, enchelyidae und lacrymariidae nov. fam. Annalen des Naturhistorischen Museums in Wien. Serie B für Botanik und Zoologie, 84B 49- 85. [Google Scholar]
- 10. Foissner W. 1984. Infraciliatur, Silberliniensystem und Biometrie einiger neuer und wenig bekannter terrestrischer, limnischer und mariner Ciliaten(Protozoa:Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora. Stapfia, 12 1- 165. [Google Scholar]
- 11. Foissner W, O'Donoghue PJ. 1990. Morphology and infraciliature of some freshwater ciliates (Protozoa:Ciliophora) from Western and South Australia. Invertebrate Systematics, 3 (6): 661- 696. [Google Scholar]
- 12. Foissner W, Berger H, Kohmann F. 1994. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems-Band III:Hymenostomata, Prostomatida, Nassulida. Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft, 1/94 1- 548. [Google Scholar]
- 13. Foissner W, Berger H, Schaumburg J. 1999. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft, 3/99 274- 300. [Google Scholar]
- 14. Foissner W, Kusuoka Y, Shimano S. 2008. Morphology and gene sequence of Levicoleps biwae n. gen., n. sp. (Ciliophora, Protstomatida), a proposed endemic from the ancient Lake Biwa, Japan. The Journal of Eukaryotic Microbiology, 55 (3): 185- 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall TA. 1999. BioEdit:a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41 95- 98. [Google Scholar]
- 16. Huttenlauch I. 1986. Morphologie und Morphogenese des Cortex von Coleps amphacanthus. Ph.D. thesis, University of Tübingen, Tübingen, 136 [Google Scholar]
- 17. Huttenlauch I. 1987. Ultrastructural aspects of the somatic and buccal infraciliature of Coleps amphacanthus Ehrenberg 1833. Protoplasma, 136 (2-3): 191- 198. [Google Scholar]
- 18. Huttenlauch I, Bardele CF. 1987. Light and electron microscopical observations on the stomatogenesis of the ciliate Coleps amphacanthus Ehrenberg, 1833. The Journal of Protozoology, 34 (2): 183- 192. [Google Scholar]
- 19. Kahl A. 1930. Urtiere oder Protozoa I:Wimpertiere oder Ciliata (Infusoria) 1. Allgemeiner Teil und Prostomata. Die Tierwelt Deutschlands, 18 1- 180. [Google Scholar]
- 20. Kreutz M, Foissner W. 2006. The Sphagnum ponds of Simmelried in Germany:a biodiversity hot-spot for microscopic organisms. Protozoological Monographs, 3 1- 267. [Google Scholar]
- 21. Lynn DH. 2008. The Ciliated Protozoa:Characterization, Classification and Guide to the Literature. 3rd ed, Dordrecht: Springer Science; [Google Scholar]
- 22. Medlin L, Elwood HJ, Stickel S, Sogin ML. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene, 71 (2): 491- 499. [DOI] [PubMed] [Google Scholar]
- 23. Noland LE. 1925. A review of the genus Coleps with descriptions of two new species. Transactions of the American Microscopical Society, 44 (1): 3- 13. [Google Scholar]
- 24. Nylander JAA. 2004. MrModeltest Version 2, Uppsala: Evolutionary Biology Centre, Uppsala University; [Google Scholar]
- 25. Obolkina LA. 1995. New species of the family Colepidae (Prostomatida, Ciliophora) from Lake Baikal. Zoologlchesky Zhurnal, 74 (9): 3- 19. [Google Scholar]
- 26. Posada D, Crandall KA. 1998. Modeltest:testing the model of DNA substitution. Bioinformatics, 14 (9): 817- 818. [DOI] [PubMed] [Google Scholar]
- 27. Ronquist F, Huelsenbeck JP. 2003. MrBayes 3:Bayesian phylogenetic inference under mixed models. Bioinformatics, 19 (12): 1572- 1574. [DOI] [PubMed] [Google Scholar]
- 28. Shimodaira H. 2002. An approximately unbiased test of phylogenetic tree selection. Systematic Biology, 51 492- 508. [DOI] [PubMed] [Google Scholar]
- 29. Shimodaira H, Hasegawa M. 2001. CONSEL:for assessing the confidence of phylogenetic tree selection. Bioinformatics, 17 (12): 1246- 1247. [DOI] [PubMed] [Google Scholar]
- 30. Song WB, Warren A, Hu XZ. 2009. Free-living Ciliates in the Bohai and Yellow Seas, China, Beijing: Science Press; [Google Scholar]
- 31. Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Systematic Biology, 57 (5): 758- 771. [DOI] [PubMed] [Google Scholar]
- 32.Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. http: //www.paup.csit.fsu.edu/.
- 33. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5:Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28 (10): 2731- 2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilbert N. 1975. Eine verbesserte technik der protargol-imprägnierung für ciliaten. Mikrokosmos, 64 (6): 171- 179. [Google Scholar]
- 35. Yi ZZ, Dunthorn M, Song WB, Stoeck T. 2010. Increasing taxon sampling using both unidentified environmental sequences and identified cultures improves phylogenetic inference in the Prorodontida (Ciliophora, Prostomatea). Molecular Phylogenetics and Evolution, 57 (2): 937- 941. [DOI] [PubMed] [Google Scholar]


