Abstract
PURPOSE
We assessed a novel Food and Drug Administration–approved hydrogel, synthesized as absorbable iodinated particles, in gynecologic-cancer patients undergoing computed tomography (CT) or magnetic resonance (MR) based brachytherapy after external beam radiation.
METHODS AND MATERIALS
Nineteen patients underwent CT-guided (n = 13) or MR-guided (n = 6) brachytherapy for gynecologic cancers. Seventy-seven hydrogel injections were placed. The hydrogel material was injected into gross residual disease and/or key anatomic landmarks in amounts ranging from 0.1 to 0.4 mL. The visibility of the tracer was scored on CT and on MR images using a 5-point scoring scale. A Cohen’s kappa statistic was calculated to assess interobserver agreement. To assess the unadjusted effects of baseline parameters on hydrogel visibility, we modeled visibility using a linear mixed-effect model.
RESULTS
Injections were without complication. The kappa statistic was 0.77 (95% confidence interval [CI], 0.68–0.87). The volume of hydrogel injected was significantly associated with visibility on both CT (p = 0.032) and magnetic resonance imaging (p = 0.016). We analyzed visibility by location, controlling for amount. A 0.1-cc increase in volume injected was associated with increases of 0.54 (95% CI = 0.05–1.03) in the CT visibility score and 0.83 (95% CI = 0.17–1.49) in the MR visibility score. Injection of 0.4 cc or more was required for unequivocal visibility on CT or MR. No statistically significant correlation was found between tumor type, tumor location, or anatomical location of injection and visibility on either CT or magnetic resonance imaging.
CONCLUSIONS
In this first report of an injectable radiopaque hydrogel, targets were visualized to assist with three-dimensional–based brachytherapy in gynecologic malignancies. This marker has potential for several applications, is easy to inject and visualize, and caused no acute complications.
Keywords: Fiducial, Radiopaque polymer hydrogel, Brachytherapy
Introduction
Fiducial markers are composed of radiopaque materials, most commonly gold or a metal alloy, and are used for the clinical and radiographic localization of normal and malignant tissues. These markers have been used in gynecologic radiation therapy to confirm daily setup position, assess target motion during external beam radiation therapy (EBRT), and to identify the tumor or cervix location during brachytherapy (1–3). Fiducial markers have also been used to assess intrafraction organ motion during therapy (4). In the modern era of three-dimensional (3D) image-guided therapy (5), accurate delineation of target volumes (6) is feasible and allows a greater understanding of organ- and tumor-motion management and allows the assessment of intrinsic patient setup uncertainty. These advancements have led to dose escalation of the primary tumor and dose reduction to nearby organs at risk (7–9).
Traditionally, fiducial markers have been composed of inert metals (2). The ability to successfully implant fiducial markers into gynecologic organs is known (1–3). Novel fiducial-marker gel compounds may offer advantages over metallic markers including decreased image artifact, decreased migration in tissue, absorbability, and the ability to create 3D structures of varying sizes (10).
The novel fiducial marker used in this series was a Food and Drug Administration (FDA)-approved polyethylene glycol (PEG) hydrogel micro particles containing covalently bound iodine (TraceIT Tissue Marker; Augmenix, Waltham, MA). PEG hydrogels are well-suited as fiducial markers as they are well-tolerated with minimal immunogenicity (11). A small risk of side effects is noted in drug delivery (12, 13), medical sealants and barriers (14–16) that use hydrogels. Additionally, because of its high water and iodine content, the hydrogel can be visualized using magnetic resonance imaging (MRI), computed tomography (CT), and/or ultrasound. The hydrogel is water-soluble and is visible for 3 months, after which the gel is slowly absorbed into the body over approximately 6 months, and subsequently excreted through the renal filtration system (10). We performed a prospective clinical evaluation of this novel hydrogel fiducial marker, assessing its visibility in patients receiving brachytherapy for gynecologic malignancies.
Methods and materials
Nineteen patients with gynecologic cancers treated with high-dose-rate brachytherapy were prospectively enrolled in this protocol, which was approved by the institutional review board.
Clinical information
The 19 patients underwent brachytherapy for primary or recurrent cervical or endometrial carcinoma. Twelve patients had primary cervical cancer, three had primary vaginal cancer, three had recurrent endometrial cancer at the vaginal cuff, and one had recurrent cervical cancer. All patients underwent 3D image-based brachytherapy. Seven patients had CT-planned tandem and ring, six had CT-planned interstitial, four had MR-planned interstitial, and two had MR-planned tandem and ring.
Imaging and implantation of fiducial markers
Before EBRT, all 19 patients had a diagnostic CT and eight also had a diagnostic MRI. After external beam, patients were placed under anesthesia. With the patient in the lithotomy position before brachytherapy applicator insertion, the hydrogel material was injected using a transvaginal approach through an 18-gauge spinal needle into the target with direct visualization of the injection site. The injection marked gross residual disease and/or key anatomic landmarks. The injected amount ranged from 0.1 to 0.4 mL. Post-implantation CT imaging was completed on all patients. The six patients whose procedure was done in the MR suite also underwent T1-and/or T2-weighted MRI (Siemens Magnetom Verio 3T) after implantation.
Materials
The Food and Drug Administration–approved hydrogel was synthesized as microbeads impregnated with iodine contrast material (TraceIT Tissue Marker; Augemenix, Co). This polymer is an absorbable tissue marker that remains visible for approximately three months. It is composed primarily of water and iodinated cross-linked PEG.
Interobserver variability
Two physicians separately scored the visibility of the radiopaque hydrogel on CT and on MRI using a 5-point scoring scale. Physicians had pretreatment imaging and postinjection CT and/or MR imaging available for review. At the time of scoring, neither physician had access to the other’s scores nor was scoring performed under time constraints. The values were predetermined as follows: (1) not visualized; (2) faint or trace visibility (shadow or haze); (3) visible but indistinct borders (definable entity, not just haze); (4) partially distinct border, partial haze; and (5) clearly visualized, unequivocal. Cohen’s Kappa statistic and the associated standard error were calculated to assess interobserver agreement (17). Standard nomenclature for the agreement levels associated with the kappa values was used: poor (kappa < 0), slight (kappa 0.01–0.2), fair (kappa 0.21–0.3), moderate (kappa 0.41–0.6), substantial (kappa 0.61–0.8), and almost perfect (kappa 0.81–1).
Statistical analysis
All statistical analysis (including the computation of the kappa statistics) was performed using R version 3.0.1. To assess the unadjusted effect of baseline parameters of anatomic differences and their relationship to hydrogel visibility, we fit a series of linear mixed-effect models to predict the visibility score based on the volume. Volume was treated as a fixed effect, and patient ID was treated as a random effect. Separate models were fit for CT visibility and MRI visibility. A second version of each model was also calculated that included a second fixed effect for tumor type (classified as cervical, vaginal, or endometrial). The coefficient for each model was used to estimate the increase in visibility for a 0.1-cc increase in the volume injected.
Results
Successful submucosal implantation of the radiopaque gel was accomplished without significant bleeding or interprocedure loss of the hydrogel marker. Ensuring minimal movement after insertion of the needle was critical to prevent the gel from tracking or smearing in and around the track of the needle. The weighted Cohen’s kappa statistic was 0.77 (95% confidence interval [CI], 0.68–0.87) demonstrating substantial interobserver agreement. The volume of hydrogel injected was significantly associated with visibility on both CT (p = 0.032) and MRI (p = 0.016) (Table 1). A 0.1-cc increase in the volume injected was associated with increases of 0.54 (95% CI = 0.05–1.03) in the CT visibility score and 0.83 (95% CI = 0.17–1.49) in the MR visibility score. No statistically significant correlation was found between tumor type (primary vs. recurrent), tumor location or anatomic location, and visibility on either CT or MRI. Our analysis did reveal a trend toward statistical significance (p = 0.11) with hydrogel visibility within the cervical stroma compared with other anatomic sites. Visualization of the hydrogel marker was incorporated into the radiation treatment planning system. Fig 1 demonstrates the use of hydrogel implant in a 42-year-old female with primary cervix cancer. The hydrogel is easily identifiable and well delineated (score 5). Separately, Fig 2, from the case of a 64-year-old female with primary cervical cancer, demonstrates possible diffusion of the marker into surrounding tissue with adjacent edema seen on MR at the level of the cervix with the use of hydrogel (score 3).
Table 1.
Visibility scores by disease site and injection amount for gynecologic-cancer patients at the time of brachytherapy having CT and/or MR imaging after hydrogel injection
| Disease site | Mean CT score | Median CT score | Mean MRI score | Median MRI score | Range |
|---|---|---|---|---|---|
| Recurrent endometrial | 3.38 | 3.50 | 4.17 | 5.0 | 1–5 |
| Recurrent cervical | 3.20 | 4.0 | NA | NA | 1–5 |
| Primary cervical | 3.08 | 3.0 | 2.83 | 2.5 | 1–5 |
| Primary vaginal | 4.0 | 4.0 | 3.67 | 4 | 3–5 |
| Volume injected: | |||||
| 0.1 mL | 2.19 | 1.5 | 2.38 | 2.0 | 1–5 |
| 0.2 mL | 3.19 | 3.0 | 2.83 | 2.5 | 1–5 |
| 0.3 mL | 3.25 | 4.0 | 2.89 | 3.0 | 1–5 |
| 0.4 mL | 5.0 | 5.0 | 4.50 | 4.50 | 4–5 |
CT = computed tomography; MRI = magnetic resonance imaging; NA = not applicable.
Note. Visibility scoring was on a 5-point scale of: 1—not visualized; 2—faint or trace visibility (shadow or haze); 3—visible but indistinct borders (definable entity, not just haze); 4—partially distinct border, partial haze; and 5—clearly visualized, unequivocal.
Fig. 1.
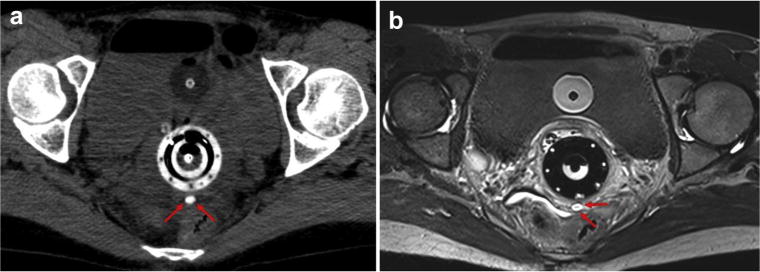
(a) Axial nonenhanced CT scan demonstrating a tandem and with the hydrogel fiducial marker immediately posterior to the ring (red arrows). (b) Axial T1-weighted MRI without contrast demonstrating superior soft-tissue delineation with the fiducial marker, seen as a hyperintense well-defined oval (red arrows) posterior to the cervical ring. Packing with an intravaginal balloon is visible between the bladder and the ring. CT = computed tomography; MRI = magnetic resonance imaging. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
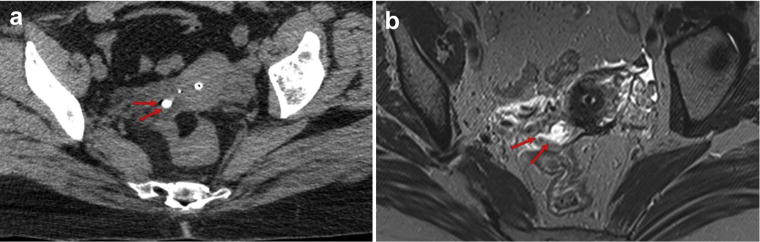
(a) Axial noncontrast-enhanced CT scan demonstrating a hyperdense oval representing the injectable hydrogel marker with some diffusion at the level of the cervix (red arrows). The tandem is located anterior to the fiducial marker. (b) Axial T1-weighted MRI without contrast demonstrating the hydrogel marker with surrounding edema (red arrows) and a lack of clearly identifiable fiducial marker at the level of the cervix. CT = computed tomography; MRI = magnetic resonance imaging. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Discussion
This is the first reported series of the use of an injectable radiopaque hydrogel (TraceIT Tissue Marker) to visualize targets on 3D imaging during brachytherapy for cervical, endometrial, or vaginal cancer. Our experience demonstrates the technical feasibility and the variation in radiographic visibility of this novel fiducial marker.
Injectable radiopaque hydrogel has more potential applications than traditional fiducial markers. Injectable hydrogel is absorbable in tissue, in contrast to metal fiducial markers that are placed permanently. Injecting a hydrogel marker directly into a tumor allows radiation targeting with greater precision than without targeting, given the limitations of 3D imaging alone, particularly at the time of brachytherapy. The hydrogel has the distinct advantage of having an undefined shape; it can conform to particular anatomic locations or be injected into a particular geometric form based on necessity. In our analysis, we examined specific anatomic targets and the visibility of the hydrogel based on these locations. We found a trend toward significance with hydrogel visibility within the cervical stroma compared with other sites. This finding suggests that tissue-density considerations are important when determining which tissues are optimal candidates for hydrogel implantation, with increasing tissue density leading to more reliable hydrogel implantation and visualization. Additionally, as the marker is in liquid form, it can be introduced with a smaller caliber needle than traditional fiducial markers, allowing specific targeting of the material. This difference in needle caliber could translate into less patient discomfort in patients with gynecologic malignancies as well as those with other disease sites. Using traditional needles, usually 18- or 19-gauge, fiducial marker placement within the lung has been shown to be feasible; however, injection resulted in a pneumothorax 33%–68% of the time (10,18–21). A previous experience using human cadaveric models demonstrated that injectable hydrogel fiducials could be effectively injected using 22-gauge needles and seen on imaging. The use of this smaller gauge needle would likely lower the incidence of pneumothorax in this high-risk patient population (10); additional studies are necessary to provide definitive evidence.
Furthermore, this technology offers an array of additional applications. The hydrogel could be placed at the time of surgery at a known positive margin, in the area at highest risk of disease recurrence, or in a concerning lymph node, thereby accurately identifying the postoperative target for the radiation oncologist without image artifact obscuring the target. A similar application for the hydrogel could be used to improve demarcation of tumor extent through more precise delineation of the anatomic boundaries of the disease. This application would be effective in the setting of esophageal cancer, as pathology series have demonstrated that CT imaging is only able to correctly identify the anatomic boundaries of the tumor in ~30% of esophageal cases (22), and accurate endoscopy reports identifying the incisor-to-tumor distance are often not available or inaccurate (23).
Another application of hydrogels in general is as a spacer between the prostate and rectum in the treatment of prostate cancer to distance the organs at risk from the target high-dose region. This application is used in prostate-cancer patients treated with EBRT (24, 25) and brachytherapy (26) and has also been used in patients with recurrent gynecologic malignancies (27). As with any nascent technology, additional studies are needed to further explore additional potential applications and the most effective clinical implementation strategies. Our results using this hydrogel for gynecologic cancers involving the cervix, endometrium, and vagina to assist in the delineation of the extent of disease at the time of brachytherapy are encouraging and show promise for use during treatment to assist in treatment planning and tracking of the disease.
Conclusion
This study provides an initial step in understanding the feasibility of using an injectable radiopaque hydrogel to delineate targets. Our experience suggests the need to inject ≥0.4 cc of the hydrogel into the desired anatomic location to achieve unequivocal visibility on CT or MRI.
Acknowledgments
The authors thank Barbara Silver for reviewing this manuscript and Nicole Cimbak for data management.
Financial disclosure: Dr. A.N.V. receives support from the NIH R21 CA167800 (PI: Viswanathan) and the Boerner Family Fund. The TraceIT Tissue Marker was supplied by Augmenix, Inc., (Waltham, MA).
Footnotes
The authors report no conflicts of interest.
References
- 1.Katee RS, Olofsen MJ, Verstraate MB, et al. Detection of organ movement in cervix cancer patients using fluoroscopic electronic portal imaging device and radiopaque markers. Int J Radiat Oncol Biol Phys. 2002;54:576–583. doi: 10.1016/s0360-3016(02)02953-x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto R, Yonesaka A, Nishioka S, et al. High dose three dimensional conformal boost (3DCB) using an orthogonal X-ray set-up for patients with gynaecological malignancy: a new application of real-time tumor-tracking system. Radiother Oncol. 2004;73:219–222. doi: 10.1016/j.radonc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Lee CM, Shrieve DC, Gaffney DK. Rapid involution and mobility of carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2004;58:625–630. doi: 10.1016/j.ijrobp.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 4.Tyagi N, Lewis JH, Yashar CM, et al. Daily online cone beam computed tomography to assess interfractional motion in patients with intact cervical cancer. Int J Radiat Oncol Biol Phys. 2011;80:273–280. doi: 10.1016/j.ijrobp.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan AN, Kirisits C, Erickson B, Potter R, editors. Gynecologic Radiation Therapy: Novel Approaches to Image-Guidance and Management. Berlin/Heidelberg, Germany: Springer-Verlag; 2011. [Google Scholar]
- 6.Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Georg P, Lang S, Dimopoulos JC, et al. Dose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:356–362. doi: 10.1016/j.ijrobp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104–109. doi: 10.1016/j.ijrobp.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan AN, Szymonifka J, Tempany-Afdhal CM, et al. A prospective trial of real-time magnetic resonance-guided catheter placement in interstitial gynecologic brachytherapy. Brachytherapy. 2013;12:240–247. doi: 10.1016/j.brachy.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 10.de Souza Lawrence L, Ford E, Gilbert C, et al. Novel applications of an injectable radio-opaque hydrogel tissue marker for management of thoracic malignancies. Chest. 2013;143:1635–1641. doi: 10.1378/chest.12-1691. [DOI] [PubMed] [Google Scholar]
- 11.Kozlowski A, Charles SA, Harris JM. Development of pegylated interferons for the treatment of chronic hepatitis C. BioDrugs. 2001;15:419–429. doi: 10.2165/00063030-200115070-00001. [DOI] [PubMed] [Google Scholar]
- 12.Koumenis IL, Shahrokh Z, Leong S, et al. Modulating pharmacokinetics of an anti-interleukin-8F(ab’)(2) by amine-specific PEGylation with preserved bioactivity. Int J Pharm. 2000;198:83–95. doi: 10.1016/s0378-5173(99)00458-5. [DOI] [PubMed] [Google Scholar]
- 13.Luxon BA, Grace M, Brassard D, et al. Pegylated interferons for the treatment of chronic hepatitis C infection. Clin Ther. 2002;24:1363–1383. doi: 10.1016/s0149-2918(02)80042-x. [DOI] [PubMed] [Google Scholar]
- 14.Cosgrove GR, Delashaw JB, Grotenhuis JA, et al. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J Neurosurg. 2007;106:52–58. doi: 10.3171/jns.2007.106.1.52. [DOI] [PubMed] [Google Scholar]
- 15.Fargen KM, Hoh BL, Mocco J. A prospective randomized single-blind trial of patient comfort following vessel closure: extravascular synthetic sealant closure provides less pain than a self-tightening suture vascular compression device. J Neurointerv Surg. 2011;3:219–223. doi: 10.1136/jnis.2010.003988. [DOI] [PubMed] [Google Scholar]
- 16.Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine. 2011;36:1906–1912. doi: 10.1097/BRS.0b013e3181fdb4db. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]
- 18.Collins BT, Erickson K, Reichner CA, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39. doi: 10.1186/1748-717X-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhagat N, Fidelman N, Durack JC, et al. Complications associated with the percutaneous insertion of fiducial markers in the thorax. Cardiovasc Intervent Radiol. 2010;33:1186–1191. doi: 10.1007/s00270-010-9949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kothary N, Heit JJ, Louie JD, et al. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20:235–239. doi: 10.1016/j.jvir.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Yousefi S, Collins BT, Reichner CA, et al. Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin Lung Cancer. 2007;8:252–256. doi: 10.3816/CLC.2007.n.002. [DOI] [PubMed] [Google Scholar]
- 22.Drudi FM, Trippa F, Cascone F, et al. Esophagogram and CT vs endoscopic and surgical specimens in the diagnosis of esophageal carcinoma. Radiol Med. 2002;103:344–352. [PubMed] [Google Scholar]
- 23.Rice PF, Crosby TL, Roberts SA. Variability of the carina–incisor distance as assessed by endoscopic ultrasound. Clin Oncol. 2003;15:383–385. doi: 10.1016/s0936-6555(03)00115-8. [DOI] [PubMed] [Google Scholar]
- 24.Hatiboglu G, Pinkawa M, Vallée JP, et al. Application technique: placement of a prostate–rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012;110:e647–652. doi: 10.1111/j.1464-410X.2012.11373.x. [DOI] [PubMed] [Google Scholar]
- 25.Pinkawa M, Piroth MD, Holy R, et al. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother Oncol. 2013;106:220–224. doi: 10.1016/j.radonc.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Prada PJ, Jimenez I, González-Suárez H, et al. High-dose-rate interstitial brachytherapy as monotherapy in one fraction and transperineal hyaluronic acid injection into the perirectal fat for the treatment of favorable stage prostate cancer: treatment description and preliminary results. Brachytherapy. 2012;11:105–110. doi: 10.1016/j.brachy.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan AN, Damato AL, Nguyen PL. Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol. 2013;31:e446–e447. doi: 10.1200/JCO.2012.47.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]


