Abstract
Head and neck cancer (HNC) currently affects approximately 11 200 people in the UK, with an increasing proportion known to be caused by the human papillomavirus (HPV). We undertook a systematic review of studies measuring the psychosocial impact of HPV-related HNC and also studies measuring knowledge about the link between HPV and HNC among different populations. Searches were conducted on MEDLINE, Embase, PsycINFO, CINAHL Plus and Web of Science, with reference and forward citation searches also carried out on included studies. Studies were selected if they (i) were original peer-reviewed research (qualitative or quantitative), (ii) mentioned HPV and HNC, (iii) measured an aspect of the psychosocial impact of the diagnosis of HPV-related HNC as the dependent variable and/or (iv) measured knowledge of the association between HPV and HNC. In total, 51 papers met the inclusion criteria; 10 measuring psychosocial aspects and 41 measuring knowledge of the link between HPV and HNC. Quality of life in those with HPV-positive HNC was found to be higher, lower or equivalent to those with HPV-negative HNC. Longitudinal studies found quality of life in patients was at its lowest 2–3 months after diagnosis and some studies found quality of life almost returned to baseline levels after 12 months. Knowledge of the link between HPV and HNC was measured among different populations, with the lowest knowledge in the general population and highest in medical and dental professionals. Due to the limited studies carried out with patients measuring the psychosocial impact of a diagnosis of HPV-positive HNC, future work is needed with the partners of HPV-positive HNC patients and health professionals caring for these patients. The limited knowledge of the association between HPV and HNC among the general population also indicates the need for research to explore the information that these populations are receiving.
Key words: Head and neck cancer, HPV, human papillomavirus, knowledge, quality of life
Statement of Search Strategies Used and Sources of Information
We searched MEDLINE, Embase, PsycINFO, CINAHL Plus and Web of Science using relevant search terms for the overview. There were no language or date restrictions applied to the search. All references were reviewed against the inclusion and exclusion criteria. Additional relevant papers were found through searching the reference lists of included studies and carrying out forward citation searches on included studies.
Introduction
Head and neck cancer (HNC) affects about 11 200 people in the UK each year [1], [2], [3], [4] and there are currently 62 500 survivors of the disease [5]. Diagnosis is associated with well-recognised psychosocial sequelae, including difficulties with communication with the partner, functioning in the family, and social and interpersonal relationships [6]. Patients have also been found to isolate themselves from their friends and family due to the disfiguring impact of treatment on their appearance [7], and many feel stigmatised [8]. Practical issues, such as problems with speaking, can also interfere with communication, and poorer communication has been found to result in greater distress [9]. Negative psychological consequences tend to be worse for patients undergoing surgery than those receiving alternative methods of treatment [10], [11], [12].
It is now clear that an increasing proportion of HNC cases are caused by the human papillomavirus (HPV) [13], [14], [15], [16], which has long been associated with cervical and other anogenital cancers [17]. The incidence of HPV-related HNC is rising [18], with numbers in the USA set to surpass the numbers of cervical cancer cases by 2020 if the current trend continues [19]. Patients with HPV-positive HNC are typically younger than those with HPV-negative disease, and tend to be white, male, married, educated and employed [20]. Risk factors are thought to be a greater lifetime number of sexual and oral sex partners [20], [21], [22], [23] due to greater exposure to HPV.
We know from the cervical cancer literature that the sexually transmitted nature of HPV can lead to additional psychological challenges to those associated with the cancer itself. Women have felt stigma, anxiety, concern about their relationships and worry about disclosure of their test results to others, following a diagnosis of HPV in the context of cervical screening [24]. As the link between HPV and HNC has been established, there has been increasing recognition of the need for guidance on how to discuss HPV with patients [25], [26], [27]. Behavioural and psychological science has made a significant contribution to understanding and addressing psychosocial issues associated with both HNC and HPV. One study measuring the supportive care needs of HNC patients found overwhelming evidence of unmet psychological needs [28] and a Cochrane review paper found a number of randomised controlled trials implementing psychosocial interventions for HNC patients, but concluded there was not enough evidence to conclude on their effectiveness [29]. It is also important to assess knowledge of the association between HPV and HNC among different populations, to identify gaps in knowledge and inform communication strategies.
Research has started to explore what the public know about HPV and HNC and how an HPV diagnosis affects patients. Knowledge of HPV appears to have increased following the introduction of the HPV vaccination, which is now offered to adolescents in most developed countries [30]. In the context of cervical cancer, an online survey of adults in the UK, USA and Australia following the introduction of the HPV vaccination showed 61% reported having heard of HPV [31]. By contrast, public awareness of the signs and risk factors for HNC has been shown to be poor [32]. In the clinical context, few resources currently exist to answer patients' concerns about how, when and why they got their cancer [27], the answers to which can have implications both for the patient and their past, present or future partners.
This review is timely in drawing together findings from the emerging literature and identifying priorities for a behavioural science research agenda in this field. The evidence from both the cervical cancer and HNC literature suggests that there may be greater psychological distress in these patients due to the combination of both a diagnosis of cancer and of HPV. The review aims to answer two questions:
-
(i)
What is the psychological impact of an HPV diagnosis in the context of HNC?
-
(ii)
What is known about HPV-related HNC in different population groups?
Materials and Methods
Search Methods for Identification of Studies
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for this systematic review [33]. Search terms were developed in consultation with a librarian at UCL and through extracting key terms from previous review papers and relevant primary research. Terms covered (i) the health condition of interest (e.g. HPV, human papillomavirus, head and neck cancer), (ii) psychosocial outcomes of interest (e.g. psychosocial, depression, anxiety, quality of life) and (iii) knowledge (e.g. knowledge, awareness). The full search strategies for each database can be found in the Supplementary Material. Initial search terms were later refined based on common text words from relevant articles retrieved from the search. MEDLINE, Embase and PsycINFO databases were accessed through Ovid databases and were searched from inception to present in December 2014. Search terms were adapted for CINAHL Plus and Web of Science. These databases were chosen based on previous review papers in this field and because all databases will complement each other and allow a broader scope of coverage. There were no language or date restrictions applied to the search. The reference lists of included studies were searched (RD) for additional relevant papers. The grey literature was also searched using OPENSIGLE (opensigle.inist.fr). Results of the literature search were downloaded into Endnote with duplicate articles removed.
Inclusion and Exclusion Criteria
Studies were included if they (i) were original peer-reviewed research (qualitative or quantitative), (ii) mentioned HPV and HNC, (iii) measured an aspect of the psychosocial impact of the diagnosis of HPV-related HNC as the dependent variable and/or (iv) measured knowledge of the association between HPV and HNC. Studies were excluded if they were not written in English, did not report original research or were conference abstracts.
Selection Procedure and Quality Assessment
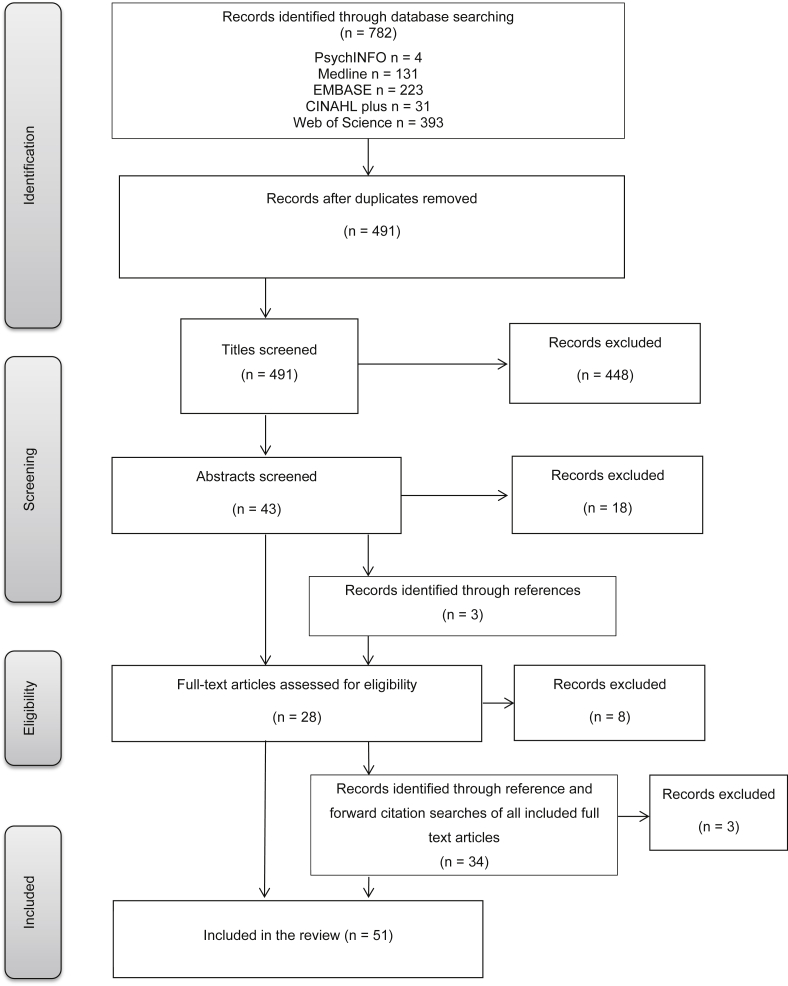
Article titles were screened by two authors (RD, JW) and were excluded if they were not written in the English language or were clearly irrelevant to the review. Two members of the review team (RD, LM) then screened the abstracts of the remaining articles, looking more specifically for articles mentioning HPV. Authors of conference abstracts that appeared to meet the eligibility criteria were contacted to request a copy of the full paper if it was available. Those not submitted or published in peer-review journals were excluded and one author did not reply after multiple attempts to contact them, so this paper was also excluded. Disagreements were resolved by discussion and reasons for inclusion/exclusion were noted. Articles that appeared to meet the inclusion criteria based on the title and abstract screen were obtained for full-text review (Figure 1). Copies of articles that could not be assessed for relevance based on the title and abstract screen were also obtained to determine eligibility based on full-text review.
Fig 1.
Flow diagram of study selection (adapted from [33]).
A full-text eligibility review was conducted by two members of the review team (RD, LM) with reasons for exclusion recorded. Reasons for excluding studies included the article not containing any original data relevant to our eligibility criteria, not mentioning HPV or not measuring our outcomes of interest as dependent variables. Forward citation searches were conducted for all papers obtained for full-text review and included those published up to August 2015. Eligibility of articles found through forward citation and reference searching was confirmed by a second screener (LM). A summary of the data from all full-text articles was extracted (Table 1, Table 2) into Microsoft Excel and the quality of the studies was assessed using an amended version of the National Institute for Health and Care Excellence (NICE) quality appraisal checklists for quantitative and qualitative studies [34]. This considered a range of factors, which included whether the source population was well described, whether the outcome measures were reliable and relevant, whether the analytical methods were appropriate and whether the findings could be generalised to the source population.
Table 1.
Psychosocial studies included in the systematic review
| Reference Country | Sample | HPV/p16 positive | Study design | Outcomes/measures | Relevant findings |
|---|---|---|---|---|---|
| [35] USA | 10 male survivors of HPV-OSCC between 1 and 5 years treatment completion | 100% (HPV+) |
Qualitative semi-structured interviews | Exploring the communication, comprehension and psychologic impact of a diagnosis of HPV-related oropharyngeal cancer |
|
| [36] Switzerland | 98 survivors of oropharyngeal cancer a median of 67 months after treatment | 89% (p16+) |
Follow-up survey (postal) | Quality of life: EORTC QLQ-C30 and EORTC QLQ-H&N35 |
|
| [37] USA | 22 patients at first head and neck cancer clinic visit; 2 females, 20 males | 80.9% (HPV+) 95.2% (p16+) |
Longitudinal study: baseline (preoperatively), 3 weeks, 3 months, 6 months and 12 months | Quality of life: Head and Neck Cancer Inventory |
|
| [38] USA | 87 patients at first new patient referral visit: 81 included in analysis | 63% (HPV+) 74% (p16+) |
Prospective cohort study | Quality of life: Head and Neck Cancer Inventory |
|
| [39] USA | 162 medical records – patients with locally advanced OSCC, known p16 status and treated by chemoradiation or primary surgery followed by adjuvant radiation therapy; 142 men, 20 women | 69% (p16+) |
Audit of medical records | Prevalence of anxiety disorder and major depression in patients with HPV+ and HPV– tumours |
|
| [40] USA | 177 patients with HNSCC and known HPV/p16 status diagnosed between 2006 and 2012 | 45% (p16+) |
Longitudinal study: baseline, 2 months, 6 months, 1 year and 1–3 years | Quality of life: UWQOL |
|
| [41] Italy | 79 patients with HNSCC; 17 women, 62 men Control group: healthy subjects matched for gender and disease proportion attending same clinic for non-pathological reasons |
100% (HPV+) |
Multicentre, observational and retrospective study | Perception of their health conditions: EQ-5D Measurement of utility loss considering patient's perspective |
|
| [42] USA | 62 newly diagnosed HPV+ patients initiating radiotherapy | 98% (p16+) 89% (HPV+) |
Cross-sectional survey | Self-reported:
|
|
| [43] USA | 228 patients diagnosed with primary OSCC between 2003 and 2010 Group 1: (n = 162) HPV– and low-risk HPV Group 2: (n = 66) High-risk HPV+ |
29% (HPV+) |
Longitudinal study: pre-treatment, immediate post-treatment and 1 year post-treatment | Quality of life: UWQOL |
|
| [44] USA | 40 head and neck cancer survivors >2 years after treatment: 34 men, 6 women | 98% (HPV+) |
Follow-up survey (postal) | Health-related quality of life: HNQOL, UWQOL, SF-36 |
|
HPV, human papillomavirus; HNSCC, head and neck squamous cell carcinoma; EORTC, European Organization for Research and Treatment of Cancer; UWQOL, University of Washington Quality of Life; HNQOL, Head and Neck Quality of Life; SF-36, Short Form 36; OSCC, Oropharyngeal squamous cell carcinoma.
Table 2.
Included studies measuring knowledge of human papillomavirus (HPV) and head and neck cancer
| Reference Country | Sample | Response rate | Study design | Outcomes/measures | Relevant findings |
|---|---|---|---|---|---|
| [45] Jordan | 112 newly graduated medical and dental SHO level; 49% dental degree, 51% medical | Not reported | Survey (in person) | Knowledge of risk factors for oral cancer (e.g. Which of the following factors is considered an increased factor for oral cancer: Human papillomavirus as a response option) |
|
| [46] USA | 651 dental hygienists from North Carolina State Board of Dental Examiners | 53% | Survey (postal) | Knowledge of risk factors for oral cancer (e.g. In the United States, which of the following factors places an individual at high risk for oral cancer? Human papillomavirus as yes/no/don't know option) |
|
| [47]∗ USA | 609 men aged 18–59 years from population-based panel of US households: Men's Health Study | 70% | Survey (online) | Awareness and knowledge (e.g. Which of the following do you think might increase the chances of getting oral cancer? Infection with a virus as a response option) Beliefs about causes of HPV-related disease (e.g. Do you think HPV can cause oral cancer? Yes/no/don't know) |
|
| [48] USA | 248 1st, 2nd, 3rd and 4th year dental students at University of Maryland Baltimore College of Dental Surgery | 59.6% | Cross-sectional survey (in person and postal) | Knowledge of oral cancer risk factors |
|
| [49] USA | 163 dental students, Medical University of South Carolina | 79.1% | Survey (in person) | Knowledge of oral cancer risk factors |
|
| [50] Canada | 670 dentists, British Columbia and Nova Scotia | 55.2% | Survey (postal) | Knowledge of oral cancer risk factors |
|
| [51] Puerto Rico | 206 Men in sexually transmitted disease clinic | Not reported | Survey (in person) | HPV awareness, HPV knowledge (e.g. HPV is associated with oral cancer: true/false/don't know) |
|
| [52] USA | 17 dentists in 2 focus groups, 21 dental hygienists in 2 focus groups | Not reported | Qualitative focus groups | Assess awareness of oral health providers regarding the HPV-oral cancer link Elicit attitudes and perceived role in screening for HPV-oral cancer lesions and discussing HPV as a contributing factor for oral cancer |
|
| [53] Ireland | 254 dentists | Not reported | Cross-sectional survey (online) | Knowledge of oral cancer risk factors |
|
| [54] USA | 93 community members | 32% | Survey (telephone) | Knowledge of oral cancer risk factors |
|
| [55] Romania | 192 1st–6th year dental students; 139 female, 53 male | 100% | Cross-sectional survey (in person) | Knowledge of oral cancer risk factors |
|
| [56] USA | 205 American Indian community members recruited via two community events; 70% female | Not reported | Survey (in person) | Knowledge of the risk factors of head and neck cancer including HPV (e.g. Do you think that HPV can cause head and neck cancer? yes/no/don't know) |
|
| [57] Jordan | 330 primary healthcare professionals | 87% | Survey (face-to-face interview) | Knowledge of oral cancer risk factors |
|
| [58]† Germany | 306 dentists in Schleswig-Holstein | 14% | Survey (postal) | Knowledge of oral cancer risk factors (e.g. Which of the following factors places an individual at high risk for oral cancers? Human papillomavirus as yes/no/don't know option) |
|
| [59] Germany | 394 dentists in Schleswig-Holstein | 17% | Survey (postal) | Knowledge of oral cancer risk factors (e.g. Which of the following factors places an individual at high risk for oral cancers? Human papillomavirus as yes/no/don't know option) |
|
| [60]† Germany | 306 dentists in Schleswig-Holstein; 1000 members of the public | 14% | Survey (postal and telephone) | Knowledge of oral cancer risk factors (e.g. Which of the following factors places an individual at high risk for oral cancers? Human papillomavirus as yes/no/don't know option) |
|
| [61] Germany | 388 medical practitioners in Schleswig-Holstein | 13% | Survey (postal) | Knowledge of oral cancer risk factors (e.g. Which of the following factors places an individual at high risk for oral cancers? Human papillomavirus as yes/no/don't know option) |
|
| [62]∗ USA | 609 men: 312 gay and bisexual, 296 heterosexual | 70% | Survey (online) | Knowledge of HPV (e.g. Do you think HPV can cause oral cancer? Yes/no/don't know) |
|
| [63] Saudi Arabia | 236 healthcare professionals | Not reported | Cross-sectional survey | Knowledge of oral cancer risk factors |
|
| [64] Saudi Arabia | 167 undergraduate medical students (all students in years 4–6) | 100% | Cross-sectional survey (in person) | Knowledge of oral cancer risk factors (e.g. Which of the following factors places an individual at high risk for oral cancers? Human papillomavirus as yes/no/don't know option) |
|
| [65] Saudi Arabia | 479 undergraduate dental students (all students in years 4–6) | 87.1% | Cross-sectional survey (in person) | Knowledge of oral cancer risk factors |
|
| [66] Canada | 176 males at postsecondary institutions in Greater Vancouver | Not reported | Survey (in person) | Knowledge of HPV |
|
| [67] USA | 2126 US adults from Harris Interactive online panel | Not reported | Cross-sectional survey (online) | Awareness (e.g. Did you know that the virus HPV (human papillomavirus) that causes cervical cancer is also associated with throat cancer?) Knowledge (e.g. How knowledgeable are you about oral, head, and neck cancer? Likert scale not at all to extremely knowledgeable) |
|
| [68] USA | 297 American Head and Neck Society head and neck surgeons | 27.5% | Survey (online) | Assess clinical practices Assess attitudes Assess knowledge regarding HPV-related cancer of the head and neck |
|
| [69] USA | 619 dentists in Maryland | 53.6% | Survey (postal) | Knowledge of oral cancer risk factors |
|
| [70] USA | 303 drag racers (28.3%) and fans (70%), vendors (1.7%) attending annual United Black Drag Racers drag racing event in St Louis | Not reported | Survey (in person) | Knowledge of HPV and head and neck cancer (e.g. Please indicate whether you think that each of these things may or may not increase a person's chance of getting head and neck cancer: Human papillomavirus infection; certain types of HPV can lead to oral cancer: True) |
|
| [71] USA | 584 licensed dentists in North Carolina | 52% | Survey (postal) | Knowledge of oral cancer risk factors (e.g. In the United States, which of the following factors places an individual at high risk for oral cancer? Human papillomavirus listed as an option) |
|
| [72] Italy | 1000 lesbian, gay and bisexual men and women | 86.8% | Cross-sectional survey (in person) | Know that HPV can cause oropharyngeal cancer |
|
| [73] USA | 62 senior citizens | 66% | Survey (in person) | Knowledge of oral cancer risk factors |
|
| [74] USA | 450 medical students, South Carolina | 78.8% | Cross-sectional survey (in person) | Knowledge of oral cancer risk factors |
|
| [75] USA | 269 dentists, 19 oral surgeons, 221 physicians | 57% dentists 76% oral surgeons 45% physicians |
Cross-sectional survey (postal) | Knowledge of oral cancer risk factors (e.g. Rank (high, medium, low) the association of known high-risk factors (Human papillomavirus) with oral cancer) |
|
| [76]∗ USA | 609 men aged 18–59 from national panel of US households | 70% | Cross-sectional survey (online) | Knowledge of HPV (e.g. Do you think HPV can cause oral cancer? Yes/no/don't know) |
|
| [77]∗ USA | 306 men self-identified as gay or bisexual aged 18–59 from national panel of US households | 70% | Cross-sectional survey (online) | Knowledge of HPV (e.g. Do you think HPV can cause oral cancer? Yes/no/don't know) |
|
| [78] USA | 2393 general population from rural areas | Not reported | Survey (telephone) | Knowledge of risk factors for mouth and throat cancer |
|
| [79] Malaysia | 362 dentists | 41.7% | Survey (in person) | Knowledge of oral cancer risk factors |
|
| [80] USA | 267 parents of sons eligible to receive HPV vaccination | Not reported | Cross-sectional survey (in person) | Parents' knowledge of HPV in oropharyngeal cancer |
|
| [81] Malaysia | 147 final year medical and dental undergraduates of Universiti Sains Malaysia | 73.5% | Survey (in person) | Aetiology of oral cancer |
|
| [82] USA | 68 male African American college students, St Louis | Not reported | Cross-sectional survey (online) | Knowledge of HPV |
|
| [83] USA | 361 freshman students at Texas State University | 10.7% | Survey (online) | Knowledge:
|
|
| [84] USA | 179 men self-identified as gay and bisexual aged 18–29 from student organisations and social networking sites | Not reported | Survey (online) | Knowledge of HPV |
|
| [85] USA | 491 NASCAR fans, 158 medical students, 186 undergraduate students | Not reported | Survey (in person) | Awareness of relationship between HPV and head and neck cancer (e.g. How much do you agree that HPV increases the risk of head and neck cancer?) |
|
These four papers used the data from one study.
These two papers used the data from one study.
Analysis
Data from all included articles were recorded using a data extraction form. The results from articles measuring psychosocial outcomes and knowledge are reported descriptively with comparisons drawn where appropriate. Qualitative findings are described separately.
Results
Search Results
The initial search returned 782 articles, which was reduced to 491 after the removal of duplicates; 448 were excluded on the basis of their title, leaving 43 abstracts to be reviewed. Once the articles had been screened by title and abstract, 25 were obtained for full-text review. An additional 37 articles were included after searching the reference lists, relevant review papers found through the search and searching forward citations of those already obtained for full-text review. Eleven articles were excluded during full-text review, leaving 51 papers included in the final analysis. Figure 1 shows the study selection process. All the authors agreed on the final papers included in the review.
Studies Assessing the Psychosocial Impact of Human Papillomavirus-related Head and Neck Cancer
Ten of the studies measured psychosocial outcomes [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]. Of these, eight were conducted in the USA [35], [37], [38], [39], [40], [42], [43], [44], one was conducted in Switzerland [36] and one in Italy [41]. Quantitative studies used survey-based methods [36], [37], [38], [40], [41], [42], [43], [44] and conducted an audit on medical records [39]. One article collected qualitative data from individual interviews [35]. All articles were published between 2012 and 2014. In some studies, p16 expression was used as a marker of HPV status, but for simplicity we refer to patients as HPV-positive throughout the review.
The psychosocial impact of HPV-related HNC was measured in patients [35], [36], [37], [38], [39], [40], [41], [42], [43], [44] at different time points in their care continuum from newly diagnosed [36], [38], [40], [42], [43], to up to 5 years post treatment completion [35].
Quality of Life Measures
Quality of life was the main outcome measure used in seven studies and was measured using a number of different tools. Six of the studies measuring quality of life used at least one HNC-specific measure (Table 3). Two studies used the Head and Neck Cancer Inventory (HNCI) [37], [38], which is a validated 30-item survey measuring patient-reported quality of life status in speech, eating, aesthetics and social disruption. Three studies used the University of Washington Quality of Life (UWQOL) measure [40], [43], [44], which is a validated HNC-specific quality of life questionnaire including 12 domains, with two subscales of physical and social-emotional functioning. One study used the European Organization for Research and Treatment of Cancer (EORTC) HNC-specific version (QLQ-H&N35) [36], which includes seven scales measuring pain, swallowing, senses, speech, social eating, social contact and sexuality. One study used Head and Neck Quality of Life (HNQOL) [44], which measures the four domains of eating, communication, pain and emotion. Generic measures were also used in three studies and included the Medical Outcome Study Short Form 36 (SF-36) [44], which is a 36-item generic measure of health status split into 10 domains, the EQ-5D [41], which has five dimensions of measuring quality of life, and the EORTC general core questionnaire (QLQ-C30) [36] measuring activity, physical and social functioning. Other psychosocial measures used were the National Comprehensive Cancer Network's distress thermometer, which uses a scale from 0 to 10 for patients to indicate how much distress they have been experiencing in the last week, and the Glinder and Compas one-item measure of behavioural blame [42] (i.e. ‘How much do you blame yourself for the kinds of things you did, that is, for any behaviours that may have led to your cancer?’).
Table 3.
Scores from psychosocial measures in human papillomavirus (HPV)-related head and neck patients in nine∗ studies
| Reference | Measure | HPV+ | HPV– | Significant difference |
|---|---|---|---|---|
| [36] | Quality of life - EORTC QLQ-C30 (median score; scale range 0–100) | |||
| Emotional | 91.67 | 83.33 | NS | |
| Social | 100 | 100 | NS | |
| Global health | 83.33 | 79.17 | NS | |
| [37] | Quality of life - HNCI (mean score; scale range 0–100) | |||
| Baseline | 94 | 75 | NS | |
| 3 weeks | 79 | 88 | NS | |
| 3 months | 48 | 58 | NS | |
| 6 months | 63 | 83 | NS | |
| 12 months | 88 | / | NS | |
| [38] | Quality of life - HNCI (mean score; scale range 0–100) | |||
| 12 months | 75 | 78 | NS | |
| [40] | Quality of life - UWQOL (mean score across 12 domains; scale range 0–100) | |||
| Baseline | 76 | 50 | 0.008 | |
| 2 months | 57 | 51 | NS | |
| 6 months | 67 | 59 | 0.034 | |
| 12 months | 69 | 64 | NS | |
| >12 months | 82 | 65 | 0.013 | |
| [43] | Quality of life - UWQOL (mean score across 12 domains; scale range 0–100) | |||
| Pre-treatment | 86 | 79 | 0.015 | |
| Immediate post-treatment | 63 | 73 | NS | |
| Post-treatment | 75 | 77 | NS | |
| [39] | Major depression | 9% | 10% | NS |
| Anxiety disorder | 6% | 12% | NS | |
| [42] | Distress (mean; scale range) | 3.38 (0–9) | / | |
| Self-blame (mean; scale range) | 2.27 (1–4) | / | ||
| [41] | EQ-5D (mean utility values) | |||
| Women | 0.7 | / | ||
| Men | 0.8 | / | ||
| [44] | Quality of life - UWQOL (mean score; scale range 0–100) | |||
| Pre-treatment | 10 | / | ||
| 24 months | 15.2 | / | ||
| Long term | 16.5 | / | ||
| HNQOL (mean score; scale range 0–100) | ||||
| Pre-treatment | 15.1 | / | ||
| 24 months | 9.5 | / | ||
| Long term | 11.9 | / | ||
EORTC, European Organization for Research and Treatment of Cancer; HNCI, Head and Neck Cancer Inventory; UWQOL, University of Washington Quality of Life; HNQOL, Head and Neck Quality of Life.
One reference not included as used qualitative methodology [35].
Studies with Human Papillomavirus-positive Patients Only
Three studies did not include a comparison between HPV-positive patients and HPV-negative patients [35], [42], [44]. The one qualitative study with HPV-positive HNC survivors reported that 3/10 cancer survivors felt a sense of stigma or shame associated with their diagnosis [35]. The second study measured distress and self-blame in newly diagnosed HPV-positive patients [42]. Distress levels were found to be moderate (mean 3.38, range 0–9), with 30% showing clinically meaningful scores (scores above or equal to 4). Self-blame levels were found to be low (mean 2.27, range 1–4). The third study measured quality of life using the UWQOL, HNQOL and SF-36 and found summary scores remained stable between 2 years and long-term follow-up (median of 78 months after the completion of treatment) [44]. Clinically meaningful (≥10 point change) declines in quality of life measured using the UWQOL was found in 14% of patients, whereas 11% of patients reported clinically meaningful improvements. Summary scores on this measure between pre-treatment and long-term follow-up were significantly worse. Clinically meaningful (≥10 point change) declines in quality of life measured using the HNQOL were found in 8% of patients, with 8% of patients reporting clinically meaningful improvements. Summary scores on this measure remained stable between pre-treatment and long-term follow-up. Scores on the SF-36 for long-term physical and mental health were comparable with the US population norms [44].
Cross-sectional Studies with a Comparison Group
One study compared quality of life in HPV-positive patients and HPV-negative patients using the EORTC QLQ-C30 [36]. Patients with HPV-positive tumours were found to score significantly better on physical and role functions of the scale [36], but there were no significant differences between the groups in the emotional, social and global health functions of the scale. In an audit of medical records, Hess and colleagues [39] found that there was a higher prevalence of anxiety in HPV-negative patients, but rates of major depression or anxiety disorder did not differ significantly between the HPV-positive and HPV-negative groups. Another study compared quality of life scores measured using the EQ-5D between HPV-positive patients and healthy subjects [41]. Overall quality of life was significantly lower in patients than in healthy subjects.
Longitudinal Studies with a Comparison Group
Four studies compared quality of life between HPV-positive and HPV-negative patients at more than one time point [37], [38], [40], [43]. One study measuring quality of life using the UWQOL [40], found that overall quality of life scores were better at each time point for HPV-positive patients than for HPV-negative patients, with the differences being significant at baseline, 6 months and after 12 months. Another study measuring quality of life using the UWQOL [43] found that pre-treatment quality of life scores were significantly higher in HPV-positive patients compared with HPV-negative patients, lower (but not significantly) immediately after treatment and similar at 1 year after treatment. Quality of life measured using the HNCI in one study found that HPV-positive patients had a higher quality of life at baseline, but then a lower quality of life at 3 weeks, 3 months and 6 months compared with HPV-negative patients [37]. Another study using the HNCI found that HPV status was not associated with quality of life outcomes at 12 months [38]. Using data from the whole sample, clinically meaningful declines were found from baseline to 12 months in speech function, aesthetic attitude, eating function and attitude [38].
Overall, these longitudinal studies found inconsistent results when comparing quality of life in HPV-positive patients and HPV-negative patients. Some reported HPV-positive patients with a combination of both higher and lower quality of life scores than HPV-negative patients depending on the time points (n = 3), with differences only significant when the quality of life scores were higher in HPV-positive patients. Others found no significant differences between the groups at any time point (n = 2).
Studies Assessing Knowledge of Human Papillomavirus-related Head and Neck Cancer
Forty-one papers from 37 studies assessed knowledge about HPV and HNC [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85]. Over half (n = 23) were conducted in the USA [46], [47], [48], [49], [52], [54], [56], [62], [67], [68], [69], [70], [71], [73], [74], [75], [76], [77], [78], [80], [82], [83], [84], [85], with others from Germany (n = 4) [58], [59], [60], [61], Saudi Arabia (n = 3) [63], [64], [65], Canada (n = 2) [50], [66], Malaysia (n = 2) [79], [81], Jordan (n = 2) [45], [57], Italy (n = 1) [72], Puerto Rico (n = 1) [51], Romania (n = 1) [55] and Ireland (n = 1) [53]. All were published between 2002 and 2015. Quantitative studies (n = 40) [45], [46], [47], [48], [49], [50], [51], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85] used survey-based data collection methods and one qualitative study collected data using focus groups [52].
Studies assessing knowledge of HPV and HNC included samples of dental students [48], [49], [55], [65], [81], medical students [64], [74], [81], [85], general undergraduate students [66], [82], [83], [85], oral health providers (dentists and dental hygienists) [46], [50], [52], [53], [58], [59], [60], [69], [71], [75], [79], head and neck surgeons [68], healthcare professionals [57], [61], [63], [75], a population-based sample of US men [47], [76] and a population-based sample of US adults [54], [67], [73], [78], [80]. Some specific sample populations were included, such as American Indian community members [56], bisexual and homosexual populations [62], [72], [77], [84] and National Association for Stock Car Auto Racing (NASCAR) fans [85].
Knowledge of the association between HPV and HNC varied across study populations and the questions asked (Table 4). All the questions involved recognition of HPV as either a cause or a risk factor for oral cancer, with no studies requiring participants to recall HPV as a risk factor for oral cancer. For example, Hertrampf and colleagues [58], [59], [60], [61] asked 'Which of the following factors places an individual at high risk for oral cancers?' with HPV listed as a response option and Cólon-López and colleagues [51] asked participants to respond true or false to the statement 'HPV is associated with oral cancer'. Knowledge of HPV as a risk factor for oral cancer ranged from 26 to 91% in medical or dental professional samples [45], [46], [50], [53], [57], [58], [59], [60], [61], [63], [68], [69], [71], [75], [79] compared with between 1 and 44% in samples of members of the general population [51], [54], [56], [60], [62], [67], [70], [72], [73], [76], [77], [80], [84], [47], [78]. Knowledge among students ranged from 18% in general undergraduate students to 84% in undergraduate dental students [48], [49], [55], [64], [65], [66], [74], [81], [82], [83].
Table 4.
Knowledge about human papillomavirus (HPV) and oral cancer reported in 35∗ studies
| Question | % (reference) | Sample population |
|---|---|---|
| Heard of HPV … (closed question) | 70.6% [47] | General population men (USA) |
| 61% [76] | General population men (USA) | |
| 79% [77] | General population men (USA) | |
| 93% [84] | General population men (USA) | |
| 60.6% [72] | General population (Italy) | |
| 59% [56] | General population (USA) | |
| 80% [66] | College students (Canada) | |
| 85% [82] | College students (USA) | |
| HPV as a risk factor for oral cancer was known by … (closed question) | 53.1% [50] | Dentists (Canada) |
| 60% [71] | Dentists (USA) | |
| 57.8% [58] | Dentists (Germany) | |
| 26% [75] | Dentists (USA) | |
| 61.2% [59] | Dentists (Germany) | |
| 60% [53] | Dentists (Ireland) | |
| 57.8% [60] | Dentists (Germany) | |
| 88% [69] | Dentists (USA) | |
| 67.2% [79] | Dentists (Malaysia) | |
| 47.1% [46] | Dental hygienists (USA) | |
| 79.8% [49] | Dental students (USA) | |
| 66.5% [48] | Dental students (USA) | |
| 54% [55] | Dental students (Romania) | |
| 83.7% [65] | Dental students (Saudi Arabia) | |
| 34% [45] | Newly graduated medical and dental personnel (Jordan) | |
| 37% [75] | Physicians (USA) | |
| 39.1% [63] | Healthcare professionals (Saudi Arabia) | |
| 91% [68] | Head and neck surgeons (USA) | |
| 50–82% [61] | Medical practitioners (Germany) | |
| 43.3% [57] | Healthcare professionals (Jordan) | |
| 61.4% [74] | Medical students (USA) | |
| 65.7% [64] | Medical students (Saudi Arabia) | |
| 44% [72] | General population (Italy) | |
| 29% [60] | General population (Germany) | |
| 32% [54] | General population (USA) | |
| 40.2% [78] | General population (USA) | |
| 0.8% [67] | General population (USA) | |
| 29.5% [73] | General population (USA) | |
| 29.9% [70] | General population (USA) | |
| 18% [80] | General population (USA) | |
| Knew HPV can cause oral cancer/head and neck cancer | 23.3% [47] | General population men (USA) |
| 21% [76] | General population (USA) | |
| 25% [77] | General population (USA) | |
| 39% [84] | General population men (USA) | |
| 21–25% [62] | General population men (USA) | |
| 27.4% [51] | General population men (Puerto Rico) | |
| 36% [56] | General population (USA) | |
| 12.8% [67] | General population (USA) | |
| 18.2% [83] | College students (USA) | |
| 59.6% [81] | Medical Students (Malaysia) | |
| 75.6% [81] | Dental Students (Malaysia) | |
| 32.9% [66] | College students (Canada) | |
| 60.2% in men [82] | College students (USA) | |
| 61.7% in women [82] | College students (USA) | |
| Knew HPV is strongly associated with oropharyngeal cancer | 51.6% [83] | College students (USA) |
Quality Assessment
Based on the NICE quality appraisal checklists for the quantitative studies, 27 studies were designed or conducted in a way that minimised bias, nine studies were partly designed or conducted to minimise bias and had aspects of the study design that were unclear, and 13 studies were either unclear on aspects of the study reported or may not have addressed all potential sources of bias. No studies were assessed as having significant sources of bias across all aspects of the study design. Most studies described the source population well, used reliable and valid outcome measures, measured outcomes that were relevant and used appropriate analytical methods. Of 25 studies in which it was relevant to carry out a power calculation, only eight did so. Many of the studies had small samples and so could not be generalised to the source population. For the two qualitative studies, both were clear in the purpose of the study, carried out the data collection appropriately, were clear on the context in which the study was carried out, conducted reliable analysis, provided convincing findings and drew relevant conclusions. Both studies were unclear about whether the relationship between the researcher and participants had been considered. Both studies were considered to be designed to have minimised bias.
Discussion
This review is the first to draw together the emerging literature on the psychosocial implications of an HPV-related HNC diagnosis and awareness of the link between HPV and HNC. Quality of life was measured in the HPV-related HNC patient population, with inconsistent results found. Quality of life in those with HPV-positive HNC was found to be higher, lower or equivalent to those with HPV-negative HNC. In longitudinal studies, irrespective of the instrument used, quality of life in patients was at its lowest 2–3 months after diagnosis. In some studies, quality of life almost returned to baseline levels after 12 months. The UWQOL was the instrument used in three of the 10 studies included in this review. This scale is specific to HNC and measures 12 different domains as single-item questions. To allow for comparisons across studies, it would be ideal to have a well-validated, standardised measure that could be used in all studies. As previously reported, it is difficult to make generalised statements about quality of life that can aid in clinical decision making, due to inconsistencies in the design of quality of life instruments for HNC and a lack of unified reporting standards [86].
Use of other psychosocial measures was limited, with only one other primary research study measuring domains other than quality of life. This one study found clinically meaningful levels of distress in 30% of patients, but relatively low levels of self-blame [42], suggesting there may be a need for interventions that may help alleviate distress levels. In the one qualitative study, a few survivors of HPV-positive HNC reported feelings of stigma and embarrassment about their diagnosis and this affected their sexual relationships, consistent with findings from the cervical cancer literature [24] and previous research with health professionals documenting concerns of HPV-positive HNC patients [87]. It is therefore difficult to draw conclusions based on the limited research that has been conducted around the psychosocial impact of HPV-related HNC. Future work is needed to explore the psychosocial impact of this diagnosis on the patient group, as well as their partners, the general population and health professionals.
The relationship between HPV and HNC is not well-known across most populations in the studies included here. The most knowledgeable group about HPV as a risk factor for HNC were second year dental students, dentists and head and neck surgeons (>85%), compared with one study finding that less than 1% of US adults knew that HPV is a risk factor for HNC. Awareness levels ranged across a variety of samples of the general population, dentists, students and specific sexually orientated groups, from 18 to 67%. Almost half the studies included dentists, dental hygienists or dental students, suggesting that the role dentists have to play in HPV and HNC is being increasingly recognised and educating them about HPV as a risk factor is important. All the questions were recognition questions rather than recall and so may not represent the true knowledge of participants as previous studies have found awareness to be higher in participants when responding to recognition questions when compared with recall [88], [89]. One study assessing knowledge in medical practitioners in Ireland found that when asked to list the risk factors they would associate with oral cancer, HPV was not listed [90]. There was also no standardised question assessing knowledge of the link between HPV and HNC, some asking it as a risk factor, whereas others were more specific (e.g. HPV is associated with some head and neck cancers). None of these studies were conducted in the UK, so no conclusions can be drawn about the level of knowledge in the UK. These studies were mainly from the USA, indicating a wide range of knowledge across different population subgroups, but that generally, there is a need for greater awareness.
Strengths and Limitations
Adhering to PRISMA guidelines ensured this review was carried out systematically. By including quantitative and qualitative studies in the review, we avoided exclusion of any eligible and relevant studies. As a number of different instruments were used to measure quality of life and at different points in the patient care continuum, it was difficult to compare across studies.
Conclusions
A limited number of studies have measured the psychosocial impact of a diagnosis of HPV-positive HNC and those few that have, have only measured this in patient populations. Future work is needed with the partners of HPV-positive HNC patients and health professionals caring for these patients. The limited knowledge of the association between HPV and HNC among the public also indicates the need for research to explore the information that these populations are receiving. The development of collaborations between behavioural scientists and clinicians in this field will help to ensure that awareness of the role of HPV in HNC is raised, and that the adverse psychological consequences associated with diagnosis are understood and minimised.
Acknowledgements
This work was funded by a Medical Research Council Studentship (MR/K501268/1). Cancer Research UK (C7492/A17219) funds Dr Jo Waller and Dr Laura Marlow.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.clon.2016.02.012.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Office of National Statistics. Cancer Registration Statistics 2013. Available at: http://www.ons.gov.uk/ons/datasets-and-tables/index.html?pageSize=50&sortBy=none&sortDirection=none&newquery=+Cancer+Registration+Statistics&content-type=Reference+table&content-type=Dataset. [accessed 15.07.15].
- 2.ISD Scotland. Cancer Incidence in Scotland 2013. Available at: http://www.isdscotland.org/Health-Topics/Cancer/Publications/data-tables.asp?id=1387#1387. [accessed 15.07.15].
- 3.Welsh Cancer Intelligence and Surveillance Unit. Incidence Trends 2013. Available at: http://www.wcisu.wales.nhs.uk/officical-statistics-exel-files-of-trend. [accessed 15.07.15].
- 4.Northern Ireland Cancer Registry. Cancer Data 2013. Available at: http://www.qub.ac.uk/research-centres/nicr/CancerStatistics/OnlineStatistics/HeadandNeck/. [accessed 15.07.15].
- 5.National Cancer Intelligence Network. Cancer Prevalence UK Summary Table. 20-Year Cancer Preval UK by Cancer Site 2015. Available at: http://www.ncin.org.uk/item?rid=2955. [accessed 15.07.15].
- 6.Rapoport Y., Kreitler S., Chaitchik S., Algor R., Weissler K. Psychosocial problems in head-and-neck cancer patients and their change with time since diagnosis. Ann Oncol. 1993;4:69–73. doi: 10.1093/oxfordjournals.annonc.a058365. [DOI] [PubMed] [Google Scholar]
- 7.Dhooper S.S. Social work with laryngectomees. Health Soc Work. 1985;10:217–227. doi: 10.1093/hsw/10.3.217. [DOI] [PubMed] [Google Scholar]
- 8.Strauss P. Psychosocial maxillofacial responses to oral and neck cancer. J Oral Maxillofac Surg. 1989;47:343–348. doi: 10.1016/0278-2391(89)90334-0. [DOI] [PubMed] [Google Scholar]
- 9.Vickery L.E., Latchford G., Hewison J., Bellew M., Feber T. The impact of head and neck cancer and facial disfigurement on the quality of life of patients and their partners. Head Neck. 2003;25:289–296. doi: 10.1002/hed.10206. [DOI] [PubMed] [Google Scholar]
- 10.De Boer M.F., Pruyn J.F.A., Van den Borne B., Knegt P.P., Ryckman R.M., Verwoerd C.D.A. Rehabilitation outcomes of long-term survivors treated for head and neck cancer. Head Neck. 1995;17:503–515. doi: 10.1002/hed.2880170608. [DOI] [PubMed] [Google Scholar]
- 11.Taylor J.C., Terrell J.E., Ronis D.L. Disability in patients with head and neck cancer. Arch Otolaryngol – Head Neck Surg. 2004;130:764–769. doi: 10.1001/archotol.130.6.764. [DOI] [PubMed] [Google Scholar]
- 12.Allal A.S., Nicoucar K., Mach N., Dulguerov P. Quality of life in patients with oropharynx carcinomas: assessment after accelerated radiotherapy with or without chemotherapy versus radical surgery and postoperative radiotherapy. Head Neck. 2003;25:833–839. doi: 10.1002/hed.10302. discussion 839–840. [DOI] [PubMed] [Google Scholar]
- 13.Rietbergen M.M., Leemans C.R., Bloemena E. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. 2013;132:1565–1571. doi: 10.1002/ijc.27821. [DOI] [PubMed] [Google Scholar]
- 14.Gillison M.L., Broutian T., Pickard R.K.L. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehanna H., Beech T., Nicholson T., El-hariry I., McConkey C. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer – systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 16.Näsman A., Attner P., Hammarstedt L. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz N., Castellsagué X., de González A.B., Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24:1–10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 18.Chaturvedi A.K., Engels E.A., Anderson W.F., Gillison M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi A.K., Engels E., Pfeiffer R.M. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillison M.L., D’Souza G., Westra W. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 21.D’Souza G., Kreimer A.R., Viscidi R. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 22.Blomberg M., Nielsen A., Munk C., Kjaer S.K. Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;129:733–741. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza G., Agrawal Y., Halpern J., Bodison S., Gillison M.L. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCaffery K., Waller J., Nazroo J., Wardle J. Social and psychological impact of HPV testing in cervical screening: a qualitative study. Sex Transm Infect. 2006;82:169–174. doi: 10.1136/sti.2005.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu A., Genden E., Posner M., Sikora A. A patient-centered approach to counseling patients with head and neck cancer undergoing human papillomavirus testing: a clinician's guide. Oncologist. 2013;18:180–189. doi: 10.1634/theoncologist.2012-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnigan J.P., Sikora A.G. Counseling the patient with potentially HPV-related newly diagnosed head and neck cancer. Curr Oncol Rep. 2014;16:1–8. doi: 10.1007/s11912-013-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakhry C., D’Souza G. Discussing the diagnosis of HPV-OSCC: common questions and answers. Oral Oncol. 2013;49:863–871. doi: 10.1016/j.oraloncology.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry M., Habib L.-A., Morrison M. Head and neck cancer patients want us to support them psychologically in the posttreatment period: survey results. Palliat Support Care. 2014;12:481–493. doi: 10.1017/S1478951513000771. [DOI] [PubMed] [Google Scholar]
- 29.Semple C.J., Parahoo K., Norman A., McCaughan E., Humphris G., Mills M. Psychosocial interventions for patients with head and neck cancer. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD009441.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly B.J., Leader A.E., Mittermaier D.J., Hornik R.C., Cappella J.N. The HPV vaccine and the media: how has the topic been covered and what are the effects on knowledge about the virus and cervical cancer? Patient Educ Couns. 2009;77:308–313. doi: 10.1016/j.pec.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marlow L.A.V., Zimet G.D., McCaffery K.J., Ostini R., Waller J. Knowledge of human papillomavirus (HPV) and HPV vaccination: an international comparison. Vaccine. 2013;31:763–769. doi: 10.1016/j.vaccine.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 32.Warnakulasuriya K.A., Harris C.K., Scarrott D.M. An alarming lack of public awareness towards oral cancer. Br Dent J. 1999;187:319–322. doi: 10.1038/sj.bdj.4800269. [DOI] [PubMed] [Google Scholar]
- 33.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence. NICE. Methods for the Development of NICE Public Health Guidance (third edn 2012). Available at: http://publications.nice.org.uk/methods-for-the-development-of-nice-public-health-guidance-third-edition-pmg4/appendix-h-quality-appraisal-checklist-qualitative-studies. [accessed 15.07.15]. [PubMed]
- 35.Baxi S.S., Shuman A.G., Corner G.W. Sharing a diagnosis of HPV-related head and neck cancer: the emotions, the confusion, and what patients want to know. Head Neck. 2012;35:1–8. doi: 10.1002/hed.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broglie M.A., Soltermann A., Haile S.R. Quality of life of oropharyngeal cancer patients with respect to treatment strategy and p16-positivity. Laryngoscope. 2013;123:164–170. doi: 10.1002/lary.23622. [DOI] [PubMed] [Google Scholar]
- 37.Durmus K., Patwa H.S., Gokozan H.M. Functional and quality-of-life outcomes of transoral robotic surgery for carcinoma of unknown primary. Laryngoscope. 2014;124:2089–2095. doi: 10.1002/lary.24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziegielewski P.T., Teknos T.N., Durmus K. Transoral robotic surgery for oropharyngeal cancer: long term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg. 2013;139:1099–1108. doi: 10.1001/jamaoto.2013.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hess C.B., Rash D.L., Daly M.E. Competing causes of death and medical comorbidities among patients with human papillomavirus-positive vs human papillomavirus-negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140:312–316. doi: 10.1001/jamaoto.2013.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maxwell J.H., Mehta V., Wang H. Quality of life in head and neck cancer patients: impact of HPV and primary treatment modality. Laryngoscope. 2013;4:1–6. doi: 10.1002/lary.24508. [DOI] [PubMed] [Google Scholar]
- 41.Marcellusi A., Capone A., Favato G. Health utilities lost and risk factors associated with HPV-induced diseases in men and women: the HPV Italian Collaborative Study Group. Clin Ther. 2014;37 doi: 10.1016/j.clinthera.2014.11.002. 156–167.e4. [DOI] [PubMed] [Google Scholar]
- 42.Milbury K., Rosenthal D.I., El-Naggar A., Badr H. An exploratory study of the informational and psychosocial needs of patients with human papillomavirus-associated oropharyngeal cancer. Oral Oncol. 2013;49:1067–1071. doi: 10.1016/j.oraloncology.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma A., Méndez E., Yueh B. HPV-positive oral cavity and oropharyngeal cancer patients do not have better QOL trajectories. Otolaryngol Neck Surg. 2012;146:739–745. doi: 10.1177/0194599811434707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vainshtein J., Moon D., Feng F., Chepeha D., Eisbruch A., Stenmark M. Long-term quality of life after swallowing and salivary sparing chemo-IMRT in survivors of HPV-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91:925–933. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alami A.Y., El Sabbagh R.F., Hamdan A. Knowledge of oral cancer among recently graduated medical and dental professionals in Amman, Jordan. J Dent Educ. 2013;77:1356–1364. [PubMed] [Google Scholar]
- 46.Ashe T.E., Elter J.R., Southerland J.H., Strauss R.P., Patton L.L. North Carolina dental hygienists ’ oral cancer knowledge and opinions: implications for education. J Cancer Educ. 2006;21:151–156. doi: 10.1207/s15430154jce2103_13. [DOI] [PubMed] [Google Scholar]
- 47.Brewer N.T., Ng T.W., McRee A.-L., Reiter P.L. Men's beliefs about HPV-related disease. J Behav Med. 2010;33:274–281. doi: 10.1007/s10865-010-9251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boroumand S., Garcia A.I., Selwitz R.H., Goodman H.S. Knowledge and opinions regarding oral cancer among Maryland dental students. J Cancer Educ. 2008;23:85–91. doi: 10.1080/08858190701821238. [DOI] [PubMed] [Google Scholar]
- 49.Cannick G.F., Horowitz A.M., Drury T.F., Reed S.G., Day T.A. Assessing oral cancer knowledge among dental students in South Carolina. J Am Dent Assoc. 2005;136:373–378. doi: 10.14219/jada.archive.2005.0180. [DOI] [PubMed] [Google Scholar]
- 50.Clovis J.B., Horowitz A.M., Poel D.H. Oral and pharyngeal cancer: knowledge and opinions of dentists in British Columbia and Nova Scotia. J Can Dent Assoc. 2002;68:415–420. [PubMed] [Google Scholar]
- 51.Colón-López V., Ortiz A.P., Del Toro-Mejías L.M., García H., Clatts M.C., Palefsky J. Awareness and knowledge of human papillomavirus (HPV) infection among high-risk men of Hispanic origin attending a sexually transmitted infection (STI) clinic. BMC Infect Dis. 2012;12:346. doi: 10.1186/1471-2334-12-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daley E., DeBate R., Dodd V. Exploring awareness, attitudes, and perceived role among oral health providers regarding HPV-related oral cancers. J Public Health Dent. 2011;71:136–142. doi: 10.1111/j.1752-7325.2011.00212.x. [DOI] [PubMed] [Google Scholar]
- 53.Decuseara G., MacCarthy D., Menezes G. Oral cancer: knowledge, practices and opinions of dentists in Ireland. J Ir Dent Assoc. 2011;57:209–214. [PubMed] [Google Scholar]
- 54.Dodd V.J., Riley J.L., Logan H.L. Developing an oropharyngeal cancer (OPC) knowledge and behaviors survey. Am J Health Behav. 2012;36:589–601. doi: 10.5993/AJHB.36.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumitrescu L., Ibric S., Ibric-Cioranu V. Assessing oral cancer knowledge in Romanian undergraduate dental students. J Cancer Educ. 2014;29:506–513. doi: 10.1007/s13187-014-0659-1. [DOI] [PubMed] [Google Scholar]
- 56.Dwojak S., Deschler D., Sargent M., Emerick K., Guadagnolo B.A., Petereit D. Knowledge and screening of head and neck cancer among American Indians in South Dakota. Am J Public Health. 2014;105:1155–1160. doi: 10.2105/AJPH.2014.302177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassona Y., Scully C., Shahin A., Maayta W., Sawair F. Factors influencing early detection of oral cancer by primary health-care professionals. J Cancer Educ. 09 April 2015 doi: 10.1007/s13187-015-0823-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Hertrampf K., Wiltfang J., Koller M., Klosa K., Wenz H.J. Dentists’ perspectives on oral cancer: a survey in Northern Germany and a comparison with international data. Eur J Cancer Prev. 2010;19:144–152. doi: 10.1097/CEJ.0b013e3283362a69. [DOI] [PubMed] [Google Scholar]
- 59.Hertrampf K., Wenz H.J., Koller M., Grund S., Wiltfang J. The oral cancer knowledge of dentists in Northern Germany after educational intervention. Eur J Cancer Prev. 2011;20:431–437. doi: 10.1097/CEJ.0b013e3283481df3. [DOI] [PubMed] [Google Scholar]
- 60.Hertrampf K., Wenz H.J., Koller M., Wiltfang J. Comparing dentists’ and the public's awareness about oral cancer in a community-based study in Northern Germany. J Cranio-Maxillofacial Surg. 2012;40:28–32. doi: 10.1016/j.jcms.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Hertrampf K., Wenz H.J., Koller M., Ambrosch P., Arpe N., Wiltfang J. Knowledge of diagnostic and risk factors in oral cancer: results from a large-scale survey among non-dental healthcare providers in Northern Germany. J Cranio-Maxillofacial Surg. 2014;42:1160–1165. doi: 10.1016/j.jcms.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert P., Brewer N.T., Reiter P.L., Ng T.W., Smith J.S. HPV vaccine acceptability in heterosexual, gay, and bisexual men. Am J Mens Health. 2011;5:297–305. doi: 10.1177/1557988310372802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaber L., Shaban S., Hariri D. Oral cancer prevention and early detection: knowledge and practice among Saudi Arabian healthcare practitioners. Int J Health Care Qual Assur. 2012;25:64–74. doi: 10.1108/09526861211192412. [DOI] [PubMed] [Google Scholar]
- 64.Kujan O., Abuderman A., Azzegahiby S., Alenzi F.Q., Idrees M. Assessing oral cancer knowledge among Saudi Medical undergraduates. J Cancer Educ. 2013;28:717–721. doi: 10.1007/s13187-013-0527-4. [DOI] [PubMed] [Google Scholar]
- 65.Kujan O., Alzoghaibi I., Azzeghaiby S. Knowledge and attitudes of Saudi Dental undergraduates on oral cancer. J Cancer Educ. 2014;29:735–738. doi: 10.1007/s13187-014-0647-5. [DOI] [PubMed] [Google Scholar]
- 66.Little K., Ogilvie G., Mirwaldt P. Human papillomavirus awareness, knowledge, and vaccination status in a diverse population of male postsecondary students in Greater Vancouver. B C Med J. 2015;57:64–69. [Google Scholar]
- 67.Luryi A.L., Yarbrough W.G., Niccolai L.M. Public awareness of head and neck cancers: a cross-sectional survey. JAMA Otolaryngol Head Neck Surg. 2014;140:639–646. doi: 10.1001/jamaoto.2014.867. [DOI] [PubMed] [Google Scholar]
- 68.Malloy K.M., Ellender S.M., Goldenberg D., Dolan R.W. A survey of current practices, attitudes, and knowledge regarding human papillomavirus-related cancers and vaccines among head and neck surgeons. JAMA Otolaryngol Head Neck Surg. 2013;139:1037–1042. doi: 10.1001/jamaoto.2013.4452. [DOI] [PubMed] [Google Scholar]
- 69.Maybury C., Horowitz A.M., Yan A.F., Green K.M., Wang M.Q. Maryland dentists’ knowledge of oral cancer prevention and early detection. J Calif Dent Assoc. 2012;40:341–350. [PubMed] [Google Scholar]
- 70.Osazuwa-Peters N., Wang D.D., Namin A. Sexual behavior, HPV knowledge, and association with head and neck cancer among a high-risk group. Oral Oncol. 2015;51:452–456. doi: 10.1016/j.oraloncology.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Patton L.L., Elter J.R., Southerland J.H., Strauss R.P. Knowledge of oral cancer risk factors and diagnostic concepts among North Carolina dentists: implications for diagnosis and referral. J Am Dent Assoc. 2005;136:602–610. doi: 10.14219/jada.archive.2005.0231. [DOI] [PubMed] [Google Scholar]
- 72.Pelullo C.P., Di Giuseppe G., Angelillo I.F. Human papillomavirus infection: knowledge, attitudes, and behaviors among lesbian, gay men, and bisexual in Italy. PLoS One. 2012;7:e42856. doi: 10.1371/journal.pone.0042856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Posorski E., Boyd L., Giblin L.J., Welch L. Oral cancer awareness among community-dwelling senior citizens in Illinois. J Community Health. 2014;39:1109–1116. doi: 10.1007/s10900-014-9862-6. [DOI] [PubMed] [Google Scholar]
- 74.Reed S.G., Duffy N.G., Walters K., Day T.A. Oral cancer knowledge and experience: a survey of South Carolina medical students in 2002. J Cancer Educ. 2005;20:135–142. doi: 10.1207/s15430154jce2003_6. [DOI] [PubMed] [Google Scholar]
- 75.Reed S.G., Cartmell K.B., Duffy N.G. Oral cancer preventive practices of South Carolina dentists and physicians. J Cancer Educ. 2010;25:166–173. doi: 10.1007/s13187-009-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reiter P.L., Brewer N.T., Smith J.S. Human papillomavirus knowledge and vaccine acceptability among a national sample of heterosexual men. Sex Transm Infect. 2010;86:241–246. doi: 10.1136/sti.2009.039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reiter P.L., Brewer N.T., McRee A.-L., Gilbert P., Smith J.S. Acceptability of HPV vaccine among a national sample of gay and bisexual men. Sex Transm Dis. 2010;37:197–203. doi: 10.1097/OLQ.0b013e3181bf542c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riley J.L., Pomery E.A., Dodd V.J., Muller K.E., Guo Y., Logan H.L. Disparities in knowledge of mouth or throat cancer among rural Floridians. J Rural Health. 2013;29:294–303. doi: 10.1111/jrh.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saleh A., Kong Y.H., Vengu N., Badrudeen H., Zain R.B., Cheong S.C. Dentists’ perception of the role they play in early detection of oral cancer. Asian Pacific J Cancer Prev. 2014;15:229–237. doi: 10.7314/apjcp.2014.15.1.229. [DOI] [PubMed] [Google Scholar]
- 80.Schuler C.L., Coyne-Beasley T. Has their son been vaccinated? Beliefs about other parents matter for human papillomavirus vaccine. Am J Mens Health. 15 January 2015 doi: 10.1177/1557988314567324. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 81.Sitheeque M., Ahmad Z., Saini R. Awareness of oral cancer and precancer among final year medical and dental students of Universiti Sains Malaysia (USM), Malaysia. Arch Orofac Sci. 2014;9:53–64. [Google Scholar]
- 82.Sledge J.A. The male factor: human papillomavirus (HPV) and HPV4 vaccine acceptance among African American young men. J Community Health. 2015;40:834–842. doi: 10.1007/s10900-015-0007-3. [DOI] [PubMed] [Google Scholar]
- 83.Trad M., Reardon R.F., Caraveo D. Understanding HPV and the future: implications of contracting the virus. Radiol Technol. 2013;84:457–466. [PubMed] [Google Scholar]
- 84.Wheldon C.W., Daley E.M., Buhi E.R., Nyitray A.G., Giuliano A.R. Health beliefs and attitudes associated with HPV vaccine intention among young gay and bisexual men in the southeastern United States. Vaccine. 2011;29:8060–8065. doi: 10.1016/j.vaccine.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 85.White L.J., Creighton F.X., Jr., Wise J.C., Hapner E.R. Association between HPV and head and neck cancer: differences in understanding among three distinct populations. Am J Cancer Prev. 2014;2:14–19. [Google Scholar]
- 86.Ojo B., Genden E.M., Teng M.S., Milbury K., Misiukiewicz K.J., Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012;48:923–937. doi: 10.1016/j.oraloncology.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dodd R.H., Marlow L.A.V., Waller J. Discussing a diagnosis of human papillomavirus oropharyngeal cancer with patients: an exploratory qualitative study of health professionals. Head Neck. 2016;38:394–401. doi: 10.1002/hed.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Low E.L., Simon A.E., Lyons J., Romney-Alexander D., Waller J. What do British women know about cervical cancer symptoms and risk factors? Eur J Cancer. 2012;48:3001–3008. doi: 10.1016/j.ejca.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 89.Marlow L.A.V., Waller J., Wardle J. Public awareness that HPV is a risk factor for cervical cancer. Br J Cancer. 2007;97:691–694. doi: 10.1038/sj.bjc.6603927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni Riordain R., McCreary C. Oral cancer – current knowledge, practices and implications for training among an Irish general medical practitioner cohort. Oral Oncol. 2009;45:958–962. doi: 10.1016/j.oraloncology.2009.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.