Abstract
For treatment of multidrug-resistant tuberculosis (MDR-TB), there is a scarcity of antituberculosis drugs. Co-trimoxazole is one of the available drug candidates, and it is already frequently coprescribed for TB-HIV-coinfected patients. However, only limited data are available on the pharmacokinetic (PK) and pharmacodynamic (PD) parameters of co-trimoxazole in TB patients. The objective of this study was to evaluate the PK parameters and in vitro PD data on the effective part of co-trimoxazole: sulfamethoxazole. In a prospective PK study in patients infected with drug-susceptible Mycobacterium tuberculosis (drug-susceptible TB patients) (age, >18), sulfamethoxazole-trimethoprim (SXT) was administered orally at a dose of 960 mg once daily. One-compartment population pharmacokinetic modeling was performed using MW\Pharm 3.81 (Mediware, Groningen, The Netherlands). The area under the concentration-time curve for the free, unbound fraction of a drug (ƒAUC)/MIC ratio and the period in which the free concentration exceeded the MIC (fT > MIC) were calculated. Twelve patients received 960 mg co-trimoxazole in addition to first-line drugs. The pharmacokinetic parameters of the population model were as follows (geometric mean ± standard deviation [SD]): metabolic clearance (CLm), 1.57 ± 3.71 liters/h; volume of distribution (V), 0.30 ± 0.05 liters · kg lean body mass−1; drug clearance/creatinine clearance ratio (fr), 0.02 ± 0.13; gamma distribution rate constant (ktr_po), 2.18 ± 1.14; gamma distribution shape factor (n_po), 2.15 ± 0.39. The free fraction of sulfamethoxazole was 0.3, but ranged between 0.2 and 0.4. The median value of the MICs was 9.5 mg/liter (interquartile range [IQR], 4.75 to 9.5), and that of theƒAUC/MIC ratio was 14.3 (IQR, 13.0 to 17.5). The percentage of ƒT > MIC ranged between 43 and 100% of the dosing interval. The PK and PD data from this study are useful to explore a future dosing regimen of co-trimoxazole for MDR-TB treatment. (This study has been registered at ClinicalTrials.gov under registration no. NCT01832987.)
INTRODUCTION
Tuberculosis (TB) still accounts annually for millions of cases of active disease and a significant number of deaths worldwide. Among the patients who were reported to have TB in 2013, there were 1.1 million new cases of TB among HIV-positive patients and 480,000 cases of multidrug-resistant TB (MDR-TB) (1). The prevalence of MDR-TB has reached epidemic levels and is increasing in Africa, Asia, and Eastern Europe (2), and the majority of cases are not treated according to the WHO recommendations.
Standard MDR-TB treatment includes first-line drugs, to which the causative strain appears susceptible, plus an aminoglycoside and a fluoroquinolone, with additional drugs from groups 4 and 5 to complete the regimen (3). Unfortunately, the use of second-line drugs, including injectables, such as aminoglycosides (4), is inconvenient in high-prevalence areas, requiring parenteral administration (5). In addition, there are other disadvantages of second-line drugs compared to the two first-line drugs, isoniazid and rifampin, such as their cost and toxicity.
Co-trimoxazole, an antimicrobial drug that has been on the market since the late 1960s and is cheap and relatively safe, is not registered for treatment of TB, but it could be active against MDR-TB (6). Co-trimoxazole, a combination of sulfamethoxazole and trimethoprim (SXT), is widely used for the prophylaxis and treatment of a range of other infectious diseases (7). Also, in TB patients coinfected with the human immunodeficiency virus (HIV), 41% reduction in mortality was reported among patients receiving 960 mg co-trimoxazole in a randomized controlled trial in South Africa (8). Another study in Switzerland confirmed that co-trimoxazole decreased the risk for development of TB in HIV-TB-coinfected patients who did not receive combined antiretroviral therapy (cART) and, to a lesser extent, in cART-treated patients (9). The rates of occurrence of side effects after receiving 960 mg co-trimoxazole were similar in placebo- and co-trimoxazole-treated groups of HIV-TB patients (10, 11).
Recently, in vitro studies and observational clinical data showed promising antimicrobial activity of sulfamethoxazole against Mycobacterium tuberculosis strains, revealing a MIC range of 4.75 to ≤38 mg/liter and inactivity of trimethoprim against the bacteria (12–16).
The renal excretion of unchanged sulfamethoxazole is limited to about 20%. Sulfamethoxazole is also acetylated by N-acetyltransferase into sulfamethoxazole-N-acetyl, which increases its solubility. The renal excretion of sulfamethoxazole-N-acetyl is the major pathway of sulfamethoxazole removal (17, 18).
The pharmacodynamic (PD) properties of SXT are not completely clarified, but a few reports in the literature favor the ratio of the area under the concentration-time curve for the free, unbound fraction of a drug from 0 to 24 h (ƒAUC0–24) to the MIC and the amount of time a free-drug concentration remains above the MIC (fT > MIC) as potentially predictive pharmacokinetic (PK)/PD indices for determining the efficacy of sulfamethoxazole (11, 19).
In a previous retrospective study evaluating 8 MDR-TB patients receiving sulfamethoxazole at a dose of 400 to 800 mg once daily, the pharmacokinetic parameters showed little variability (15).
Based on a target ƒAUC0–24/MIC ratio of 25 derived from other bacterial infections (20) and the safety data from studies in HIV-TB patients (8, 11, 21), supplemented with earlier data on pharmacokinetics from MDR-TB patients (15) and MIC values (19), we postulated that a dose of 960 mg once daily may serve as a suitable starting point for dose selection for MDR-TB treatment.
To explore if the PK/PD target was met, a prospective open-label study evaluating co-trimoxazole at 960 mg once daily in drug-sensitive TB patients was performed.
MATERIALS AND METHODS
Study design.
This study was a prospective, open-label, single-arm study and was performed at the TB unit of the University Medical Center Groningen located in Beatrixoord in Haren, The Netherlands. It was estimated that a sample size of 12 patients was sufficient to explore PK/PD target attainment after administration of SXT at 960 mg once daily. The study was approved by the medical ethical committee (METc 2013/195) and registered at ClinicalTrials.gov (NCT01832987). The patients in the study received co-trimoxazole in addition to their standard TB treatment (rifampin, isoniazide, pyrazinamide, or ethambutol) in a dose of 960 mg orally for 4 to 6 days (in order to prevent blood sampling during weekends) to reach steady state, since the half-life is approximately 10 h (22).
Patients.
The subjects eligible for inclusion were culture-confirmed TB patients aged 18 years and older. Patients were enrolled in this study after they provided written informed consent. The patients were excluded if they had shown hypersensitivity to sulfonamides or trimethoprim, were pregnant or breastfeeding, or had preexisting renal dysfunction (serum creatinine clearance of ≤15 ml/min) or gastrointestinal complaints like diarrhea and vomiting. Patients receiving angiotensin-converting enzyme inhibitors, potassium-sparing diuretics, methotrexate, dofetilide, phenytoin, sulfonylureas (glibenclamide, gliclazide, glimepiride, and tolbutamide), or procainamide hydrochloride were also excluded from study participation. TB patients, concomitantly receiving treatment with a vitamin K antagonist (acenocoumarol) were also excluded from participation in the study.
Additionally, patients who had experienced an adverse effect of co-trimoxazole or similar antimicrobial drugs and patients with HIV or AIDS; severe damage to the liver parenchyma characterized by elevation of alanine-aminotransferase (ALT) (normal value, <45 U/liter) and/or aspartate-aminotransferase (ASAT) (normal value, <40 U/liter) to three times the normal values; or hematological disorders, mainly anemia (hemoglobin level, <5.5 mmol/liter), thrombocytopenia (leukocyte count, >6 × 109/liter), and agranulocytosis (granulocyte count, <2 × 109/liter) were also excluded.
Study procedures.
Evaluation of the medical charts of the TB patients, including demographic characteristics, underlying disease, and localization of TB, was done on day 1 of the study (baseline).
Co-trimoxazole at 960 mg (Sandoz; Salutas Pharma GmbH, Barleben, Germany) was given orally in a single daily dose after a light breakfast. Blood samples were collected before administration and at 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 8 h, and 24 h after co-trimoxazole administration after at least 4 days of treatment (i.e., at steady state) (22, 23).
The concentrations of sulfamethoxazole in human plasma samples were analyzed by validated liquid chromatography-tandem mass spectrometry (LS–MS-MS) as described in detail previously (15). Measurement of sodium, potassium, and creatinine levels and platelet counts of the participants was done at baseline.
Drug susceptibility testing (DST) was performed at the National Mycobacterial Reference Laboratory (National Institute for Public Health and the Environment [RIVM], Bilthoven, The Netherlands) by the Middlebrook 7H10 agar dilution method (24). As recommended by the European Committee on Antimicrobial Susceptibility Testing guidelines, the MIC of co-trimoxazole is expressed as trimethoprim/sulfamethoxazole at a ratio of 1:19.
The unbound concentration of sulfamethoxazole in the plasma ultrafiltrate was measured in a sample at 3 time points (2 h, 4 h, and 24 h). Individual pharmacokinetic parameters were calculated using the MW\Pharm 3.81 KinFit module with a one-compartment model with lag time and a fixed-estimated bioavailability of 1.
Population pharmacokinetics and model validation.
The pharmacokinetic parameters were calculated using one-compartment analysis (MW\Pharm 3.81; Mediware, Groningen, The Netherlands), utilizing an iterative two-stage Bayesian approach (25). Based on these calculated parameters, a one-compartment population model was developed based on 800 mg sulfamethoxazole. The creatinine clearance was estimated using the Cockcroft-Gault formula (26). The volume of distribution (V) was normalized to the lean body mass (LBM). The model was optimized to fit the curves and to minimize the calculated Akaike information criterion (AIC) value.
A two-compartment model showed no significant improvement in fitting the sulfamethoxazole concentration-time curves. The clearance (CL) was calculated as follows: CL = metabolic clearance (CLm) (liters/h) × body surface area (BSA) (m2)/1.85 + the drug clearance/creatinine clearance (CLcr) (liters/h) ratio (fr) × CLcr.
Other descriptors in the formulas, such as body weight, lean body mass, and free fat mass, did not improve the model fit based on the calculated AIC value. Also, the use of an allometric component, b (standardized at 0.75), did not improve the model fit. The model was built using the transit absorption rate with initial gamma distribution rate constant (ktr_po) and gamma distribution shape factor (n_po) values of 2 ± 0.5 (27, 28). The bioavailability (F) was fixed at 1. The parameters of the population model were assumed to be log-normally distributed, and the variability in the pharmacokinetic parameters was calculated using bootstrap analysis (n = 1,000). The assay error was assumed to be normally distributed and was estimated at 0.1 + 0.1 × C, where C is the concentration. Covariate analysis was performed with MW\Pharm, assessing the influence of age, weight, height, BSA, lean body mass, and CLcr with the CLm, fr, V, and absorption constant (Ka).
The population pharmacokinetic model was cross-validated using the n − 1 method, where a model with one omitted patient was repeatedly used to calculate the AUC0–24 of the omitted patient (29).
Furthermore, the model was externally validated using the PK data for a cohort of MDR-TB patients (n = 8) using SXT at 480 to 960 mg once daily (sulfamethoxazole dose, 400 to 800 mg) (15).
The unbound concentrations of sulfamethoxazole were measured and divided by the total concentration to find the free fractions of sulfamethoxazole, which were assumed to be comparable regardless of the concentration. Consequently, the unbound AUC0–24 was calculated by multiplying the total AUC0–24 by the average free fraction. In turn, the fAUC0–24/MIC ratio was also determined based on the individual observations of the AUC0–24 multiplied by the average free fraction measured in 3 different samples of a particular patient. The period in which the free concentration exceeded the MIC (fT > MIC) of sulfamethoxazole was calculated. The maximum plasma concentration (Cmax) and minimum plasma concentration (Cmin) were assessed directly from the plasma concentration data.
Statistical analysis.
The difference between the population pharmacokinetic data and the observed data was tested by calculating the root mean square error (RMSE) and by constructing a Bland-Altman plot. Furthermore, the n − 1 model was also compared with the observed data using both techniques. The predictive value of the model for the earlier population was evaluated using the RMSE. The pharmacokinetic parameters were compared using Wilcoxon signed rank tests with SPSS 20 (SPSS, Chicago, IL).
RESULTS
Patient characteristics.
A total of 12 patients (10 males and 2 females) with a median age of 30 (interquartile range [IQR], 25 to 50) years were enrolled in the study. They had a median body mass index of 20.2 (IQR, 18.7 to 21.9) kg/m2. All the patients received 960 mg of co-trimoxazole once daily, which was equal to a median dose of 13 (IQR, 11.8 to 14.2) mg/kg of body weight. The diagnosis of TB was confirmed by culture and/or molecular tests; 10 patients were diagnosed with pulmonary TB, and 1 patient was diagnosed with spinal (extrapulmonary) TB and 1 with both pulmonary and pleural and spinal (extrapulmonary) TB. The baseline characteristics of the TB patients are shown in Table 1.
TABLE 1.
Baseline characteristics of TB patients receiving SXT (n = 12)
| Parameter | Value [median (IQR)] |
|---|---|
| Age (yr) | 30 (25–50) |
| Gender (male/female) | 10/2 |
| Body mass index (kg/m2) | 20.2 (18.7–22) |
| Ethnicity | |
| Europe | 6 |
| Africa | 3 |
| America | 1 |
| Middle East | 1 |
| Western Pacific region | 1 |
| Co morbidity | |
| Smoking | 6 |
| Alcohol abuse | 4 |
| Illicit drug | 1 |
| Diabetes mellitus | 1 |
| Anemia | 1 |
| Anorexia | 1 |
| Localization of TB | |
| Pulmonary | 11a |
| Extrapulmonary (pleural and spinal) | 1 |
| Other anti-TB drugs | Isoniazid, rifampin, pyrazinamide, ethambutol, moxifloxacin |
| Sodium level (mmol/liter) | 140 (139–141.2) |
| Potassium level (mmol/liter) | 4 (3.7–4.2) |
| Creatinine clearance (ml/min/1.73 m2) | 103.2 (106.0–112.0) |
| Platelet count (× 109/liter) | 292 (235.5–394.7) |
One of the 12 patients was diagnosed with both pulmonary and extrapulmonary TB.
Observed kinetic parameters.
The observed pharmacokinetic parameters, as calculated using a one-compartment model with lag time and a fixed bioavailability of 1, are shown in Table 2. A large interindividual deviation in the rate and onset of absorption was observed, which explains the large Ka variation. The elimination phase (elimination rate constant [kel]), however, was consistent and showed only a little variation (median, 0.09 h−1; IQR, 0.08 to 0.14 h−1). The median distribution volume per lean body mass varied and was calculated at 0.025 (IQR, 0.020 to 0.028) liters/kg.
TABLE 2.
Observed pharmacokinetic parameters of sulfamethoxazole (800 mg) calculated using a one-compartment model with lag time
| Parametera | Median (IQR) |
|---|---|
| AUC (mg/liter · h)b | 566.6 (360.8–658.1) |
| CL (liters/h) | 1.34 (1.19–2.04) |
| V (liters) | 14.53 (11.82–16.82) |
| V/BW (liter/kg) | 0.23 (0.19–0.30) |
| kel (h−1) | 0.09 (0.08–0.14) |
| Ka (h−1) | 1.34 (0.72–4.49) |
| Lag time (h) | 0.46 (0.17–0.63) |
| F | 1 (fixed) |
BW, body weight.
Calculated using the trapezium rule.
Pharmacokinetics, model validation, and pharmacodynamics.
The pharmacokinetic parameters of the population model are shown in Table 3.
TABLE 3.
Pharmacokinetic parameters of the population model
| Parameter | Mean (95% CI)a | SD (95% CI) | Shrinkage |
|---|---|---|---|
| CLm (liters/h/1.85 m2) | 1.57 (1.01–2.04) | 3.71 (0.26–3.46) | −0.8 |
| V (liters · kg lean body mass−1) | 0.30 (0.25–0.39) | 0.05 (0.02–0.11) | 0.20 |
| fr | 0.02 (0.00–0.10) | 0.13 (0.00–0.33) | 0.08 |
| Ktr_po (h−1) | 2.26 (1.21–6.36) | 1.05 (0.28–2.57) | −0.02 |
| N_po | 2.12 (1.00–5.84) | 0.73 (0.17–3.44) | 0.51 |
The 95% confidence intervals (CI) were obtained by bootstrap analysis.
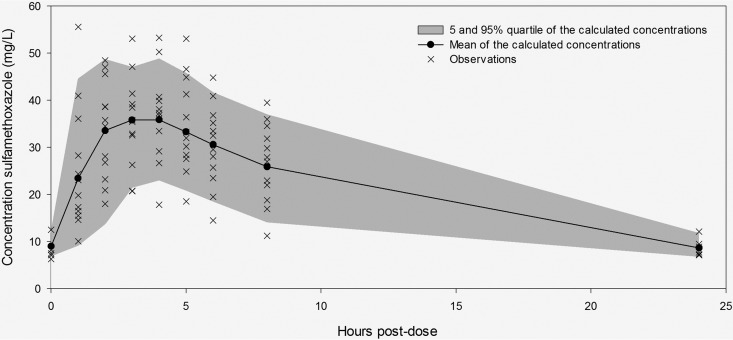
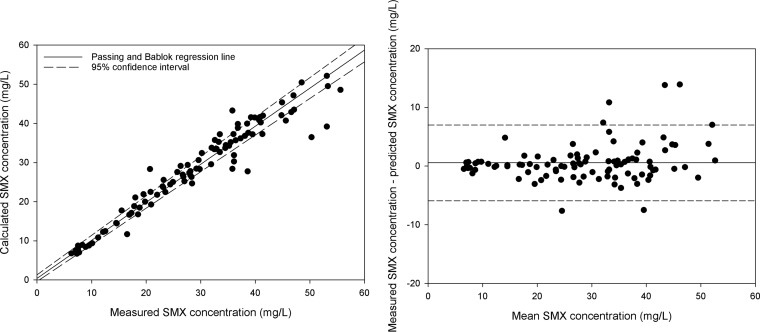
Curves fitted to the population pharmacokinetic model resulted in a median AUC0–24 of 458.35 mg/liter · h (IQR, 380.65 to 553.9 mg/liter · h). The fitted curve with the 5% and 95% percentiles and the observations are shown in Fig. 1. The observed and model calculated sulfamethoxazole concentrations are shown in Fig. 2, left, and a Bland-Altman plot of the concentrations is shown in Fig. 2, right. The mean value of the free fraction of sulfamethoxazole that is responsible for antimicrobial activity was 0.3 (range, 0.2 to 0.4).
FIG 1.
Sulfamethoxazole concentrations predicted by the model (line) and observations.
FIG 2.
(Left) Passing and Bablok regression of the observed and model calculated sulfamethoxazole concentrations (dashed lines, 95% confidence interval). (Right) Bland-Altman plot of the measured concentrations versus the predicted concentrations. The coordinates of each point (x, y) are as follows: (measured concentration + predicted concentration)/2 and measured concentration − predicted concentration.
Covariate analysis indicated that none of the tested parameters (CLm, fr, V, ktr_po, and n_po) was significantly correlated with age, weight, height, gender, BSA, lean body mass, or CLcr (P > 0.05).
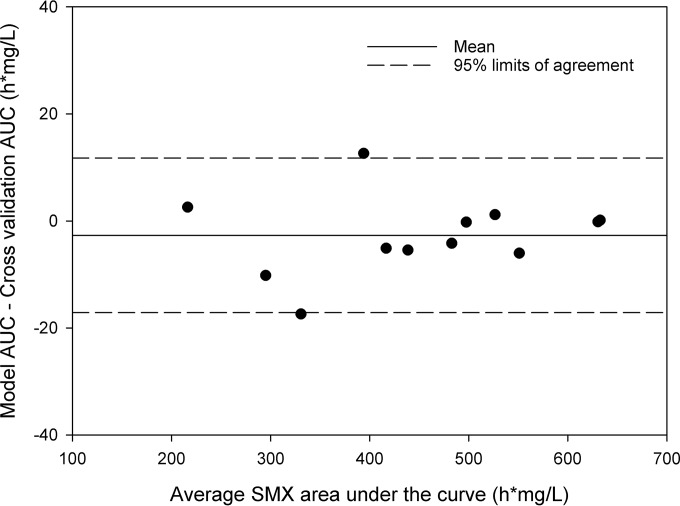
Thereafter, the model was cross-validated using the n − 1 validation procedure. The RMSE in the AUC was calculated at 7.6 h · mg/liter, with a coefficient of variation (CV) of the RMSE of 1.7%. The pharmacokinetic parameters resulting from the model and the n − 1 validation were statistically equal (P < 0.05), except for the fr (P = 0.038). However, the difference between the fr from all individuals fitted to the model and the fr resulting from the n − 1 validation was relatively small (median fr, 0.00 versus 0.17). A Bland-Altman plot assessing the difference in the AUC0–24 between the model and cross-validation calculations is shown in Fig. 3.
FIG 3.
Bland-Altman plot cross-validation population pharmacokinetic model. Each dot represents a patient, where the coordinates of each point (x, y) are as follows: [modeled AUC + (n − 1 AUC)]/2 and modeled AUC – (n − 1 AUC).
An additional model validation, in order to validate the model structure, was carried out by using the curves of eight patients as published previously (15). The clearance and volume of distribution were calculated based on these eight curves and compared to the pharmacokinetic parameters in the model of the earlier retrospective study (15). The calculated values and their corresponding reported values are shown in Table 4.
TABLE 4.
Retrospective validation
| Parametera | Mean (±SD) |
||
|---|---|---|---|
| Model | Alsaad et al. (15) | Pb | |
| CL (liters/h) | 1.18 ± 0.52 | 1.21 ± 0.43 | 0.674 |
| V (liter · kg lean body mass−1) | 0.30 ± 0.07 | 0.25 ± 0.04 | 0.050 |
CL, clearance; V, volume of distribution.
Wilcoxon signed rank test (two tailed).
Drug susceptibility testing revealed that all of the M. tuberculosis isolates from 11 patients were susceptible to sulfamethoxazole, with a median value for the MICs of 9.5 (IQR, 4.75 to 9.5) mg/liter. The median values of the AUC/MIC and ƒAUC0–24/MIC ratios after receiving 800 mg sulfamethoxazole were 51.2 (IQR, 35.7 to 66.6) h · mg/liter and 14.3 (IQR, 13.0 to 17.5) h.mg/liter, respectively. Thus, none of the patients had an ƒAUC0–24/MIC ratio of sulfamethoxazole greater than 25. The percentage of free T > MIC ranged between 43% and 100% of the dosing interval.
DISCUSSION
In our study, we investigated the PK/PD parameters of sulfamethoxazole in patients infected with drug-susceptible M. tuberculosis (drug-susceptible TB patients) receiving SXT in addition to first-line drugs. This is relevant, as it can be considered to be one of the first steps in exploring SXT as a potential alternative drug in the treatment of MDR-TB (6, 15).
The observed pharmacokinetic parameters were calculated using a one-compartment model with lag time and a fixed bioavailability of 1 (15, 20). The Ka was shown to be variable, with a large deviation, which might indicate that the absorption could be influenced by food intake. For this reason, we added a Bayesian simulated lag time to the population pharmacokinetic model to reduce Ka variability. Nevertheless, there was high variability in the observed pharmacokinetic absorption constant. Therefore, we can conclude that the time to the maximum blood concentration was not homogeneous in our population.
The model was also validated by refitting a new population model using the curves collected during a retrospective study. The differences in CL and V of both models were not statistically significant (P ≥ 0.05) (Table 4). However, the Ka found in our report was higher than that in the retrospective study by Alsaad et al. (15). This can be explained by the fact that a one-compartment model without lag time was used, which may have caused the difference in the absorption constant.
Our results show that the median values of exposure (AUC0–24) in drug-susceptible TB patients are lower than in previously reported data obtained from eight MDR-TB patients (15). The lower AUC0–24 might be explained by drug-drug interaction with rifampin, as this drug reduced the AUC0–24 of sulfamethoxazole by 23% in a previous study (30, 31) when coadministered to HIV patients. Interestingly, this percentage is comparable to the difference (22.2%) in AUCs between drug-susceptible TB patients in this study and MDR-TB patients from our retrospective study (15).
Moreover, the free (unbound) fraction of sulfamethoxazole is responsible for the antimicrobial activity (32). The unbound fraction of sulfamethoxazole in TB patients was comparable to that in healthy human subjects in an earlier study (32). The similarity in the free fractions between TB patients and healthy subjects, therefore, cannot explain the difference in AUCs between healthy subjects and our patients.
In our study, we measured the concentrations of the drug only in serum rather than in epithelial lining fluid (ELF). For pulmonary infections, the concentration of drug in ELF and alveolar macrophages may represent the antibiotic activity at the infection site. Unfortunately, sampling by bronchoscopic bronchoalveolar lavage without a clinical indication to collect ELF was not feasible. Therefore, the concentration of free drug in serum is the most reliable for the time being, as it is correlated with patient outcome (33).
The MIC values of sulfamethoxazole against clinical isolates of M. tuberculosis were similar to the MICs in previous studies (12–16). We have previously shown that the MIC of sulfamethoxazole is not significantly different in MDR-TB patients versus drug-susceptible TB patients (16). The MICs reported in this study are therefore representative of the sensitivity of multidrug-resistant M. tuberculosis.
The percentage of ƒT > MIC in our study is more than 43% of the dosing interval. This is comparable with other drugs with anti-TB activity, like pyrazinamide, in TB patients (34).
Because of the lack of data on the clinically validated values of ƒAUC/MIC and the percentage of free T > MIC in TB patients, these parameters in our patients could be used as a starting point to evaluate the efficacy of sulfamethoxazole.
This study has several limitations. The PK parameters of sulfamethoxazole were determined while sulfamethoxazole was administered in combination with rifampin, which likely resulted in a 23% lower exposure (23, 24). Another limitation is that the absolute bioavailability could not be calculated, as the sulfamethoxazole exposure after oral administration was not compared with the administration of an intravenous dose. Additionally, the study was not designed to assess the efficacy/outcome of sulfamethoxazole, and therefore, the PK/PD target index could not be evaluated in this study.
One of the possibilities to learn more about the PK/PD index, including fAUC/MIC and T > MIC, in relation to efficacy is to test sulfamethoxazole in a hollow-fiber infection model. Based on that target, the dose selection for an explorative phase II study in MDR-TB patients can be performed.
In summary, this is the first report evaluating the PK/PD parameters of sulfamethoxazole in drug-susceptible TB patients. The established PK, encouraging antimicrobial activity of sulfamethoxazole against M. tuberculosis strains in vitro, and safety profiles in humans make this drug a suitable therapeutic option for treatment of MDR-TB in the near future.
ACKNOWLEDGMENTS
We thank the nursing staff of Tuberculosis Center Beatrixoord (Haren, The Netherlands), who assisted in data collection from patients, and also the patients who participated in this study and volunteered to help obtain the data. We particularly thank Hans Proost for his suggestions and aid in PK analysis in the study.
Stichting Beatrixoord Noord Nederland financially supported the study.
We declare no conflict of interest.
Funding Statement
This work was funded by Stichting Beatrixoord Noord Nederland (210.146).
REFERENCES
- 1.World Health Organization. 2014. Tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Daley CL, Caminero JA. 2013. Management of multidrug resistant tuberculosis. Semin Respir Crit Care Med 34:44–59. doi: 10.1055/s-0032-1333546. [DOI] [PubMed] [Google Scholar]
- 3.Zumla A, Raviglione M, Hafner R, von Reyn CF. 2013. Tuberculosis. N Engl J Med 368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 4.Furin J. 2007. The clinical management of drug-resistant tuberculosis. Curr Opin Pulm Med 13:212–217. doi: 10.1097/MCP.0b013e3280f3c0b2. [DOI] [PubMed] [Google Scholar]
- 5.Sturdy A, Goodman A, Jose RJ, Loyse A, O'Donoghue M, Kon OM, Dedicoat MJ, Harrison TS, John L, Lipman M, Cooke GS. 2011. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother 66:1815–1820. doi: 10.1093/jac/dkr221. [DOI] [PubMed] [Google Scholar]
- 6.Alsaad N, Wilffert B, van Altena R, de Lange WC, van der Werf TS, Kosterink JG, Alffenaar JW. 2014. Potential antimicrobial agents for the treatment of multidrug-resistant tuberculosis. Eur Respir J 43:884–897. doi: 10.1183/09031936.00113713. [DOI] [PubMed] [Google Scholar]
- 7.DeAngelis DV, Woolley JL, Sigel CW. 1990. High-performance liquid chromatographic assay for the simultaneous measurement of trimethoprim and sulfamethoxazole in plasma or urine. Ther Drug Monit 12:382–392. doi: 10.1097/00007691-199007000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Grimwade K, Sturm AW, Nunn AJ, Mbatha D, Zungu D, Gilks CF. 2005. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS 19:163–168. doi: 10.1097/00002030-200501280-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hasse B, Walker AS, Fehr J, Furrer H, Hoffmann M, Battegay M, Calmy A, Fellay J, Di Benedetto C, Weber R, Ledergerber B, Swiss HIV Cohort Study. 2014. Cotrimoxazole prophylaxis is associated with reduced risk of incident tuberculosis in participants in the Swiss HIV Cohort Study. Antimicrob Agents Chemother 58:2363–2368. doi: 10.1128/AAC.01868-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, Djomand G, Ackah A, Domoua K, Kadio A, Yapi A, Combe P, Tossou O, Roels TH, Lackritz EM, Coulibaly D, De Cock KM, Coulibaly IM, Greenberg AE. 1999. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet 353:1469–1475. doi: 10.1016/S0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 11.Boeree MJ, Sauvageot D, Banda HT, Harries AD, Zijlstra EE. 2005. Efficacy and safety of two dosages of cotrimoxazole as preventive treatment for HIV-infected Malawian adults with new smear-positive tuberculosis. Trop Med Int Health 10:723–733. doi: 10.1111/j.1365-3156.2005.01433.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang TS, Kunin CM, Yan BS, Chen YS, Lee SS, Syu W Jr. 2012. Susceptibility of Mycobacterium tuberculosis to sulfamethoxazole, trimethoprim and their combination over a 12 year period in Taiwan. J Antimicrob Chemother 67:633–637. doi: 10.1093/jac/dkr501. [DOI] [PubMed] [Google Scholar]
- 13.Forgacs P, Wengenack NL, Hall L, Zimmerman SK, Silverman ML, Roberts GD. 2009. Tuberculosis and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother 53:4789–4793. doi: 10.1128/AAC.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong W, Sievers A, Leslie DE. 2010. Mycobacterium tuberculosis and sulfamethoxazole susceptibility. Antimicrob Agents Chemother 54:2748. doi: 10.1128/AAC.00029-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsaad N, van Altena R, van Soolingen D, Lange WCM, van der Werf T, Kosterink JGW, Alffenaar JC. 2013. Evaluation of co-trimoxazole in treatment of multidrug-resistant tuberculosis. Eur Respir J 43:504–512. doi: 10.1183/09031936.00114812. [DOI] [PubMed] [Google Scholar]
- 16.Alsaad N, van der Laan T, van Altena R, Wilting KR, van der Werf TS, Stienstra Y, van Soolingen D, Alffenaar JW. 2013. Trimethoprim/sulfamethoxazole susceptibility of Mycobacterium tuberculosis. Int J Antimicrob Agents 42:472–474. doi: 10.1016/j.ijantimicag.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Cribb AE, Spielberg SP, Griffin GP. 1995. N4-hydroxylation of sulfamethoxazole by cytochrome P450 of the cytochrome P4502C subfamily and reduction of sulfamethoxazole hydroxylamine in human and rat hepatic microsomes. Drug Metab Dispos 23:406–414. [PubMed] [Google Scholar]
- 18.Lavergne SN, Kurian JR, Bajad SU, Maki JE, Yoder AR, Guzinski MV, Graziano FM, Trepanier LA. 2006. Roles of endogenous ascorbate and glutathione in the cellular reduction and cytotoxicity of sulfamethoxazole-nitroso. Toxicology 222:25–36. doi: 10.1016/j.tox.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Alsaad N, van Soolingen D, van der Werf TS, Alffenaar JW. 2013. Is 960 mg of co-trimoxazole a suitable dose for future studies against TB?, abstr 23 Sixth Int Workshop Clin Pharmacol TB Drugs, Denver, CO. [Google Scholar]
- 20.Cheng AC, McBryde ES, Wuthiekanun V, Chierakul W, Amornchai P, Day NP, White NJ, Peacock SJ. 2009. Dosing regimens of cotrimoxazole (trimethoprim-sulfamethoxazole) for melioidosis. Antimicrob Agents Chemother 53:4193–4199. doi: 10.1128/AAC.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anglaret X, Messou E, Ouassa T, Toure S, Dakoury-Dogbo N, Combe P, Mahassadi A, Seyler C, N′Dri-Yoman T, Salamon R, ANRS 1203 Study Group . 2003. Pattern of bacterial diseases in a cohort of HIV-1 infected adults receiving cotrimoxazole prophylaxis in Abidjan, Cote d'Ivoire. AIDS 17:575–584. doi: 10.1097/00002030-200303070-00013. [DOI] [PubMed] [Google Scholar]
- 22.Varoquaux O, Lajoie D, Gobert C, Cordonnier P, Ducreuzet C, Pays M, Advenier C. 1985. Pharmacokinetics of the trimethoprim-sulphamethoxazole combination in the elderly. Br J Clin Pharmacol 20:575–581. doi: 10.1111/j.1365-2125.1985.tb05114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak A, Klimowicz A, Kadykow M. 1985. Distribution of trimethoprim and sulphamethoxazole in blood during treatment with co-trimoxazole. Eur J Clin Pharmacol 29:231–234. doi: 10.1007/BF00547428. [DOI] [PubMed] [Google Scholar]
- 24.van Klingeren B, Dessens-Kroon M, van der Laan T, Kremer K, van Soolingen D. 2007. Drug susceptibility testing of Mycobacterium tuberculosis complex by use of a high-throughput, reproducible, absolute concentration method. J Clin Microbiol 45:2662–2668. doi: 10.1128/JCM.00244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proost JH, Eleveld DJ. 2006. Performance of an iterative two-stage Bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res 23:2748–2759. doi: 10.1007/s11095-006-9116-0. [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 27.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 28.Rousseau A, Leger F, Le Meur Y, Saint-Marcoux F, Paintaud G, Buchler M, Marquet P. 2004. Population pharmacokinetic modeling of oral cyclosporin using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit 26:23–30. doi: 10.1097/00007691-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Dijkstra JA, van Altena R, Akkerman OW, de Lange WC, Proost JH, van der Werf TS, Kosterink JG, Alffenaar JW. 2015. Limited sampling strategies for therapeutic drug monitoring of amikacin and kanamycin in patients with multidrug-resistant tuberculosis. Int J Antimicrob Agents 46:332–337. doi: 10.1016/j.ijantimicag.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Ribera E, Fernandez-Sola A, Juste C, Rovira A, Romero FJ, Armadans-Gil L, Ruiz I, Ocana I, Pahissa A. 1999. Comparison of high and low doses of trimethoprim-sulfamethoxazole for primary prevention of toxoplasmic encephalitis in human immunodeficiency virus-infected patients. Clin Infect Dis 29:1461–1466. doi: 10.1086/313515. [DOI] [PubMed] [Google Scholar]
- 31.Ribera E, Pou L, Fernandez-Sola A, Campos F, Lopez RM, Ocana I, Ruiz I, Pahissa A. 2001. Rifampin reduces concentrations of trimethoprim and sulfamethoxazole in serum in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:3238–3241. doi: 10.1128/AAC.45.11.3238-3241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wijkstrom A, Westerlund D. 1983. Plasma protein binding of sulphadiazine, sulphamethoxazole and trimethoprim determined by ultrafiltration. J Pharm Biomed Anal 1:293–299. doi: 10.1016/0731-7085(83)80041-7. [DOI] [PubMed] [Google Scholar]
- 33.Mandell L, Woodhead M, Ewig S, Torres A (ed), 2006. Respiratory infections. Taylor and Francis, Milton Park, United Kingdom. [Google Scholar]
- 34.Sahota T, Della Pasqua O. 2012. Feasibility of a fixed-dose regimen of pyrazinamide and its impact on systemic drug exposure and liver safety in patients with tuberculosis. Antimicrob Agents Chemother 56:5442–5449. doi: 10.1128/AAC.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]