Abstract
Minocycline-based combination therapy has been suggested to be a possible choice for the treatment of infections caused by minocycline-susceptible Acinetobacter baumannii, but its use for the treatment of infections caused by minocycline-resistant A. baumannii is not well established. In this study, we compared the efficacy of minocycline-based combination therapy (with colistin, cefoperazone-sulbactam, or meropenem) to that of colistin in combination with meropenem for the treatment of minocycline-resistant A. baumannii infection. From 2006 to 2010, 191 (17.6%) of 1,083 A. baumannii complex isolates not susceptible to minocycline from the Taiwan Surveillance of Antimicrobial Resistance program were collected. Four representative A. baumannii isolates resistant to minocycline, amikacin, ampicillin-sulbactam, ceftazidime, ciprofloxacin, cefepime, gentamicin, imipenem, levofloxacin, meropenem, and piperacillin-tazobactam were selected on the basis of the diversity of their pulsotypes, collection years, health care setting origins, and geographic areas of origination. All four isolates had tetB and overexpressed adeABC, as revealed by quantitative reverse transcription-PCR. Among all minocycline-based regimens, only the combination with colistin produced a fractional inhibitory concentration index comparable to that achieved with meropenem combined with colistin. Minocycline (4 or 16 μg/ml) in combination with colistin (0.5 μg/ml) also synergistically killed minocycline-resistant isolates in time-kill studies. Minocycline (50 mg/kg of body weight) in combination with colistin (10 mg/kg) significantly improved the survival of mice and reduced the number of bacteria present in the lungs of mice compared to the results of monotherapy. However, minocycline (16 μg/ml)-based therapy was not effective at reducing biofilm-associated bacteria at 24 or 48 h when its effectiveness was compared to that of colistin (0.5 μg/ml) and meropenem (8 μg/ml). The clinical use of minocycline in combination with colistin for the treatment of minocycline-resistant A. baumannii may warrant further investigation.
INTRODUCTION
Extensively drug-resistant Acinetobacter baumannii has emerged worldwide. These bacteria mostly cause pneumonia, bloodstream infections, urinary tract infections, and biofilm-associated device infections. In addition to conventional antimicrobial agents, they also develop resistance to sulbactam, tigecycline, and colistin (1). Because of limited drug choices, intravenous minocycline has been proposed for the treatment of drug-resistant A. baumannii on the basis of its high degree of susceptibility to this drug and the favorable pharmacokinetic profile of minocycline (2, 3). The average rate of susceptibility of A. baumannii to minocycline is approximately 80% worldwide, and only the rate of susceptibility to colistin is better (4). Minocycline has a long half-life, which is not affected by renal or liver impairment (5, 6). Although the clinical experience with minocycline is limited and it is often used in combination with other antibiotics, accumulating data reveal that it has high treatment success rates and good tolerability (2).
However, A. baumannii is notorious for its rapid acquisition of resistance following the introduction of new antibiotics (7), and approximately 20% of A. baumannii isolates have been found to be nonsusceptible to minocycline. Efflux pumps are the main determinants of minocycline resistance, and the genes that code for efflux pumps are often carried by mobile elements, suggesting that minocycline resistance can be easily spread (8). For instance, plasmid-borne tetB::ISCR2 led to the emergence of minocycline resistance in A. baumannii isolates in Argentina (9). Despite the emergence of minocycline-resistant isolates, the efficacy of combination therapies encompassing minocycline has not been evaluated. In this study, we compared the synergy of minocycline plus colistin, cefoperazone-sulbactam, or meropenem against isolates with resistance to multiple antibiotics, including minocycline. Additionally, for comparison the combination of meropenem plus colistin was included in the study because a meta-analysis has shown that the combination of polymyxins and carbapenems has a persistently high rate of synergy in vitro (10). Both free-living and biofilm-embedded A. baumannii isolates were examined.
MATERIALS AND METHODS
Selection of drug-resistant bacterial isolates.
Bacterial isolates were collected from 11 medical centers and 15 regional hospitals during 2006, 2008, and 2010 under the Taiwan Surveillance of Antimicrobial Resistance program (7). A total of 1,083 A. baumannii complex isolates were identified by the use of either Vitek I (2006) or Vitek II (2008 and 2010) GN cards (bioMérieux, Marcy l'Etoile, France). A. baumannii was identified to the species level by molecular methods (11). The MICs of the bacteria were determined by broth microdilution methods following the guidelines of the Clinical and Laboratory Standards Institute (CLSI). A. baumannii isolates that were resistant to amikacin, ampicillin-sulbactam, ceftazidime, ciprofloxacin, cefepime, gentamicin, imipenem, levofloxacin, meropenem, minocycline, and piperacillin-tazobactam were selected for pulsed-field gel electrophoresis (PFGE) to determine their clonality, as previously described (12). DNA restriction patterns were interpreted according to the criteria of Tenover et al. (13). The stained gel was photographed and analyzed by BioNumerics software (Applied Maths) to generate a dendrogram of relatedness among these isolates. Isolates showing more than three DNA fragment differences and a similarity of <85% following dendrogram analysis were considered to represent different pulsotypes.
Resistance mechanism.
One of the most common minocycline resistance mechanisms is the overexpression of efflux pumps. The presence of tetA, tetB, tetM, and tet39 was tested by PCR. The transcript levels of adeB, adeJ, and adeG were measured by quantitative reverse transcription-PCR (qRT-PCR). ATCC 17978 and clinical isolates were grown to mid-log phase in Luria-Bertani (LB) broth, and RNA was extracted with the RNAprotect Bacteria reagent and an RNeasy minikit (Qiagen, Valencia, CA, USA). RNase-free DNase was used to remove residual genomic DNA. cDNAs were reverse transcribed with random hexamers and Moloney murine leukemia virus reverse transcriptase (Epicenter, Madison, WI, USA). The genes of interest were subsequently quantified by real-time PCR amplification. The housekeeping gene rpoB was used as an internal control. All experiments were conducted using an ABI 7500 Fast real-time PCR system (Applied Biosystems, Inc., Carlsbad, CA, USA). Expression levels were standardized relative to the transcript levels of the rpoB gene for each isolate and relative to the expression level in ATCC 17978 (by the 2−ΔΔCT threshold cycle [CT] method). The experiments were performed in triplicate.
Checkerboard synergy assay.
The MIC of each antibiotic alone or the MICs of the antibiotics in combination were tested in Mueller-Hinton broth (Difco, Detroit, MI, USA) in a broth microdilution checkerboard procedure (14). A two-dimensional checkerboard with 2-fold dilutions of each antibiotic was set up for the combined treatments. Each microwell contained 100 μl of a bacterial inoculum of about 105 CFU/ml. Synergism was determined by use of the fractional inhibitory concentration index (FICI) as previously described (14). Combinations with FICIs of ≤0.5, >0.5 to ≤4, and >4 were defined as synergistic, indifferent, and antagonistic, respectively (10).
Time-kill assay.
Time-kill assays were performed as previously described (15). Each isolate was prepared by suspension of bacteria from a logarithmic-phase culture in Mueller-Hinton broth, and the bacteria were adjusted to a final concentration of approximately 105 CFU/ml in a final volume of 100 ml. Combinations of different antimicrobial agents were added to the broth. Because sulbactam is commonly used with cefoperazone in clinical settings, cefoperazone-sulbactam was used instead of sulbactam alone. The concentrations of meropenem (8 μg/ml; Sigma), minocycline (4 or 16 μg/ml; Sigma), and sulbactam (16 μg/ml) were based on the resistance breakpoints noted by the CLSI. Sulbactam combined with cefoperazone in a 1:1 ratio was purchased from TTY Biopharm, Taiwan, and the cefoperazone/sulbactam concentration ratio used was 16:16 μg/ml. Colistin (Sigma) was used at 0.5 μg/ml due to the high degree of susceptibility of clinical isolates. The mixture of bacteria and antibiotics was then incubated at 37°C. One-milliliter aliquots were taken at 0, 6, and 24 h, serially diluted in normal saline, and spread on Mueller-Hinton agar plates. Bacterial colonies were counted after 24-h overnight cultures. A >2-log reduction in the number of CFU for a given combination compared to the number of CFU obtained with the most active single agent was defined as synergy, and a >2-log increase was defined as antagonism (10).
Colony biofilm assay.
Bacterial counts after 24-h and 48-h incubations with antibiotics were measured using a colony biofilm assay as described previously (16). An inoculum of 2.0 × 105 CFU/ml of each isolate was sprayed in 96-well culture plates. The cultures were subsequently grown in LB broth supplemented with 1% d-glucose with shaking (180 rpm) for 24 h at 30°C to let the biofilms form. After the formation of biofilms, LB broth alone, each antimicrobial agent alone, or the combination of antimicrobial agents was added to the cultures for 24 or 48 h (constituting the 24-h and 48-h treatment groups, respectively). For the 48-h treatment group, the antimicrobial-containing medium was replaced with fresh medium containing antibiotics 24 h after the administration of antibiotics to ensure drug efficacy. To quantify the bacterial counts, the biofilms on the surface were scratched with a 10-μl loop and suspended in medium. Samples were serially diluted and plated for viable cell counting after overnight culture at 30°C. The reduction of the bacterial load at 24 or 48 h was calculated by subtracting the number of bacterial biofilm cells growing after incubation with the different regimens by the number growing in LB broth alone. All tests were performed in triplicate. The criteria for synergy and antagonism were similar to those defined above for the time-kill assay.
Mouse pneumonia model.
The animal study was approved by the Institutional Animal Care and Use Committee of the National Health Research Institutes. Clinical isolates were grown in 30 ml LB broth with shaking at 37°C to reach the mid-logarithmic phase. The precipitate obtained by centrifugation at 4,000 × g for 15 min was dissolved in phosphate-buffered saline and mixed with mucin from the porcine stomach (type 3; Sigma-Aldrich, Taiwan) to a final concentration of 5% mucin. The mice were anesthetized with 2% tribromoethanol (Avertin; 0.18 ml/10.0 g body weight) administered intraperitoneally. An aliquot of 3 × 108 CFU/20 μl was inoculated intratracheally into the mice to induce pneumonia. At 2 h after inoculation, the mice were injected peritoneally with colistin (10 mg/kg of body weight), minocycline (50 mg/kg), or both drugs every 12 h (17). In preliminary experiments, mice had a high survival rate when they were given colistin intraperitoneally at a dose of 16 mg/kg. Finally, we adopted a dose of 10 mg/kg in accordance with that used by Bowers et al. (17). At 24 h after infection, 12 mice (4 in each group) were sacrificed and their lungs were homogenized. Bacterial counts were determined by plating the mixture in serial 10-fold dilutions on LB agar. Thirty or 36 mice (10 or 12 in each group) were observed for 7 days to compare survival under monotherapy with that under combination therapy. The difference in survival among the groups was assessed by Kaplan-Meier survival analysis.
RESULTS AND DISCUSSION
During the study period, 82.4% (892/1,083) of the isolates of the A. baumannii complex were susceptible to minocycline. The rates of susceptibility to minocycline were 75%, 86%, and 85% in 2006, 2008, and 2010, respectively, which is better than those in most regions in other parts of the world, except Latin American (rate of susceptibility, 91.7%) (4). The rate of susceptibility was only less than that to polymyxin (99.9%, 1,082/1,083) and tigecycline (≤2 μg/ml; 97.9%, 1,060/1,083). We identified 17 A. baumannii isolates resistant to all antibiotics tested in our study except colistin or tigecycline. Among them, four representative isolates were selected on the basis of the diversity of their pulsotypes, years of collection, health care setting origins, and geographic areas of origination (Table 1; see also Fig. S1 in the supplemental material). Whole-genome sequencing is increasingly being used in epidemiology and outbreak studies (18–20) owing to its greater accuracy and reliability than PFGE. However, PFGE remains an easy and economical way to determine clonality.
TABLE 1.
Characteristics of minocycline-resistant Acinetobacter baumannii isolatesa
| Isolate | Clinical data |
Genetic determinants (efflux pump) associated with minocycline resistance | MIC (μg/ml) |
FICI |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yr of isolation | Hospital type | Region | Specimen type | Patient age (yr) | Patient location | MIN | MP | CPZ/SB | CS | MIN and MP | MIN and CPZ/SB | MIN and CS | CS and MP | ||
| 2006S136 | 2006 | RH | Central | CVC | 77 | Inpatient | tetB, adeB | 16 | 8 | 16/16 | 0.5 | 1 | 0.75 | 0.38 | 0.38 |
| 2006Z174 | 2006 | MC | Eastern | Respiratory | 66 | ICU | tetB, adeB | 16 | 64 | 16/16 | 0.5 | 1 | 1.5 | 0.56 | 0.53 |
| 2008V462 | 2008 | MC | Central | Respiratory | 62 | ICU | tetB, adeB | 32 | 128 | 64/64 | 0.5 | 0.75 | 1.5 | 0.19 | 0.53 |
| 2010Y134 | 2010 | MC | Northern | Respiratory | 90 | Inpatient | tetB, adeB | 32 | 32 | 64/64 | 0.5 | 2 | 1 | 0.38 | 0.38 |
RH, regional hospital; MC, medical center; CVC, central venous catheter; FICI, fractional inhibitory concentration index; ICU, intensive care unit; MIN, minocycline; MP, meropenem; CPZ/SB, cefoperazone-sulbactam; CS, colistin.
The tetB gene was present in all four clinical isolates but not in ATCC 17978. Among the adeB, adeJ, and adeG genes, only the transcript levels of adeB in the clinical isolates were consistently higher than those in ATCC 17978 (see Fig. S2 in the supplemental material). The contribution of tetB to minocycline resistance has been reported (9). The role of adeABC overexpression is controversial; the overexpression of adeABC has been associated with minocycline resistance (21), but it has also been reported in minocycline-susceptible isolates (9).
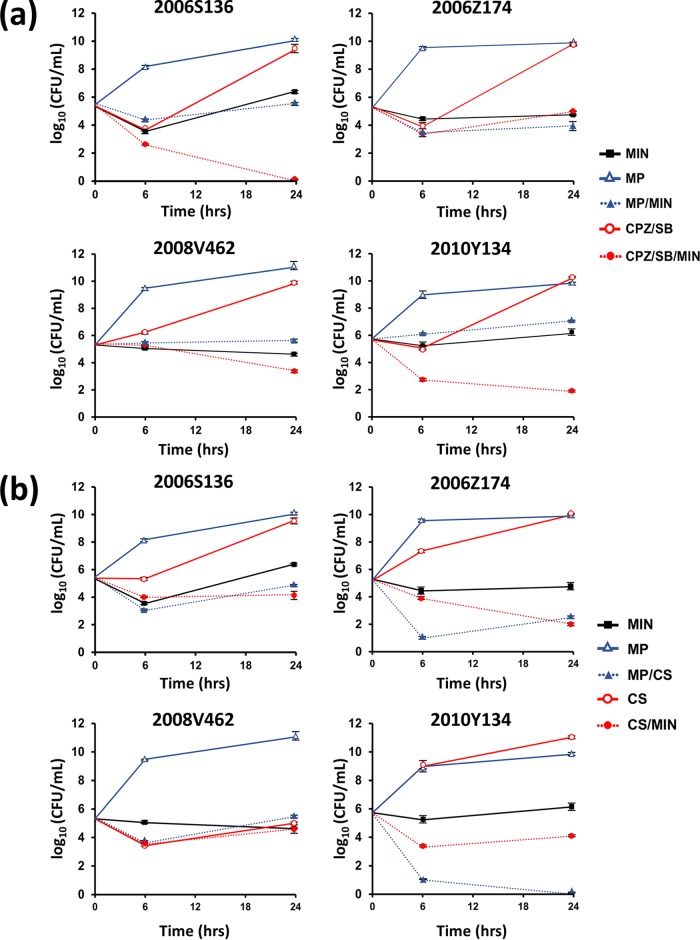
A checkerboard synergy assay (Table 1) revealed that, among the minocycline-based treatments, minocycline in combination with colistin was more likely to produce lower FICIs; the levels were similar to those observed with a combination of colistin and meropenem. The FICIs of minocycline combined with colistin ranged from 0.19 to 0.56 for four minocycline-resistant isolates. The concentrations of colistin that were able to lower the minocycline MICs to the susceptible level varied among the isolates (see Fig. S3 in the supplemental material). Time-kill curves obtained using high (16 μg/ml) and low (4 μg/ml) concentrations of minocycline are shown in Fig. 1 and Fig. S4 and S5 in the supplemental material. Briefly, high-dose minocycline with colistin reduced the bacterial load of three of the minocycline-resistant isolates (2006S136, 2006Z174, and 2010Y134), whereas the low-dose treatment resulted in a synergistic effects against all four isolates. Combinations of high- and low-dose minocycline with cefoperazone-sulbactam demonstrated synergy against some clinical isolates (2006S136 and 2010Y134 with the high dose and 2008V462 and 2010Y134 with the low dose). The results obtained with these combinations were comparable to those obtained with a combination of meropenem and colistin, but neither high- nor low-dose minocycline with meropenem produced a synergistic effect against any isolate.
FIG 1.
Time-kill assays for minocycline-resistant Acinetobacter baumannii using a combination of minocycline and meropenem (a), cefoperazone-sulbactam (a), colistin (b), and meropenem and colistin (b). MIN, minocycline (16 μg/ml); MP, meropenem (8 μg/ml); CPZ/SB, cefoperazone-sulbactam (16/16 μg/ml); CS, colistin (0.5 μg/ml).
Previous studies highlighted the good in vitro efficacy of combinations of minocycline and polymyxin B against minocycline-susceptible isolates (17, 22, 23). However, few studies have addressed the efficacy of minocycline-based combination therapies against minocycline-resistant isolates. The results of both the checkerboard and time-kill assays demonstrated that among the minocycline-based combination therapies, colistin combined with minocycline was more effective against minocycline-resistant isolates than the other combinations and was as effective as meropenem-colistin therapy, whereas minocycline combined with cefoperazone-sulbactam was effective against only some isolates. The results of our studies of synergistic effects were not always consistent with those of the checkerboard assay, as has been seen in other studies (24, 25). The checkerboard assay is a bacteriostatic method that reflects clinical MICs. Meanwhile, the time-kill assay is a bactericidal method used to examine the extent of bacterial killing over time and thus may provide more information about the nature of the interaction (26).
The mechanism of synergy is not well understood. Different antibiotics may target distinct bacterial subpopulations (subpopulation synergy) and different metabolic pathways (mechanistic synergy) (27). Additional reasons why minocycline combined with colistin exhibits better synergy than the other regimens may be as follows. First, colistin may further increase membrane permeability and the intracellular level of the second antibiotic (17, 28). Second, minocycline, being a protein synthesis inhibitor, may prevent the induction of colistin resistance genes. Third, alteration of the function of the efflux pump, for example, TetB and, possibly, AdeABC, to export minocycline by disruption of the cell membrane using polymyxins may also be a mechanism (17). One study showed the better efficacy of minocycline-based combination therapy in isolates without tetB (29). In the current study, all four isolates carried tetB, and therefore, the effect of minocycline combined with antimicrobials other than colistin may be underestimated.
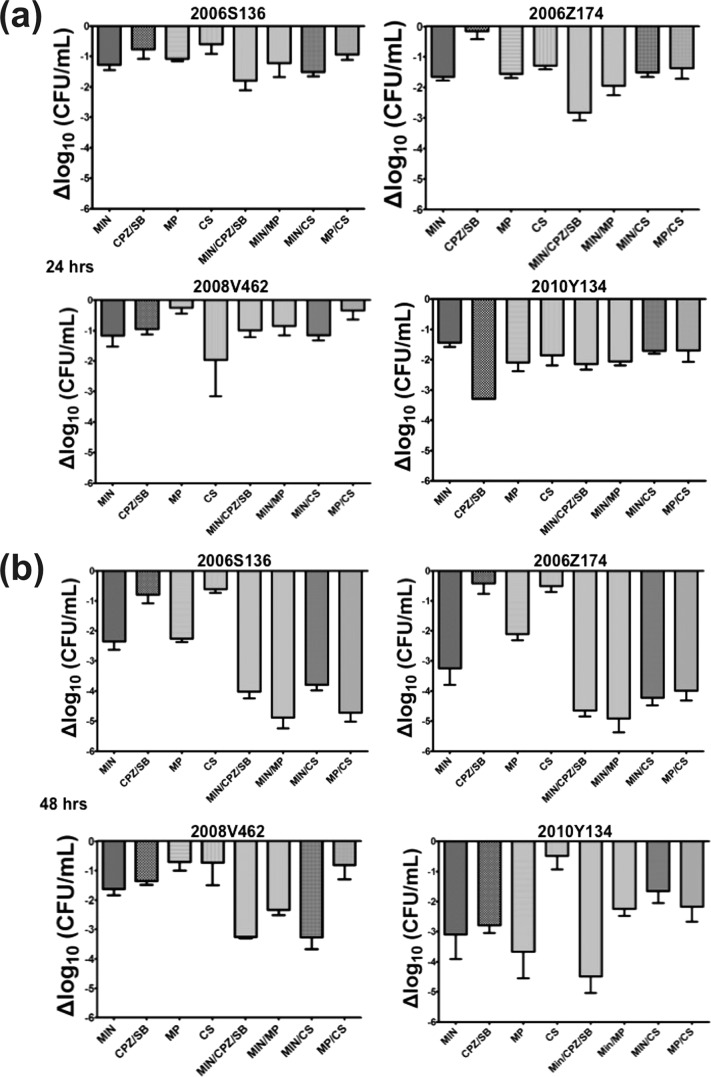
Many A. baumannii infections are associated with biofilms. These include catheter-related urinary tract and bloodstream infections as well as wound and bone infections (30). To the best of our knowledge, assessment of the synergistic effects of minocycline or colistin against Acinetobacter biofilms has not been fully investigated (31, 32). Our study showed that at 24 h after biofilm formation, none of the minocycline-based combination treatments resulted in a ≥100-fold reduction in the bacterial load compared to that achieved with the most effective single agent (Fig. 2a), indicating that they did not have synergistic effects within 24 h. After 48 h of incubation, minocycline with colistin was still an ineffective treatment against all isolates embedded in the biofilm. Meropenem combined with minocycline was effective only against isolate 2006S136, whereas meropenem combined with colistin was synergistically active against the 2006S136 and 2006Z174 isolates (Fig. 2b). Thus, our study showed that the addition of other antibiotics to minocycline is of little benefit; however, the addition of meropenem may increase the poor antibiofilm effect of colistin for some isolates.
FIG 2.
Bacterial load reduction in biofilms. Biofilms of the minocycline-resistant Acinetobacter baumannii isolates were treated for 24 (a) and 48 h (b) with the indicated antibiotics. The reduction of the bacterial load at 24 or 48 h was calculated by subtracting the number of bacterial biofilm cells incubated with the indicated antibiotics by the number of bacterial biofilm cells incubated with LB alone. MIN, minocycline (16 μg/ml); CPZ/SB, cefoperazone-sulbactam (16/16 μg/ml); MP, meropenem (8 μg/ml); CS, colistin (0.5 μg/ml).
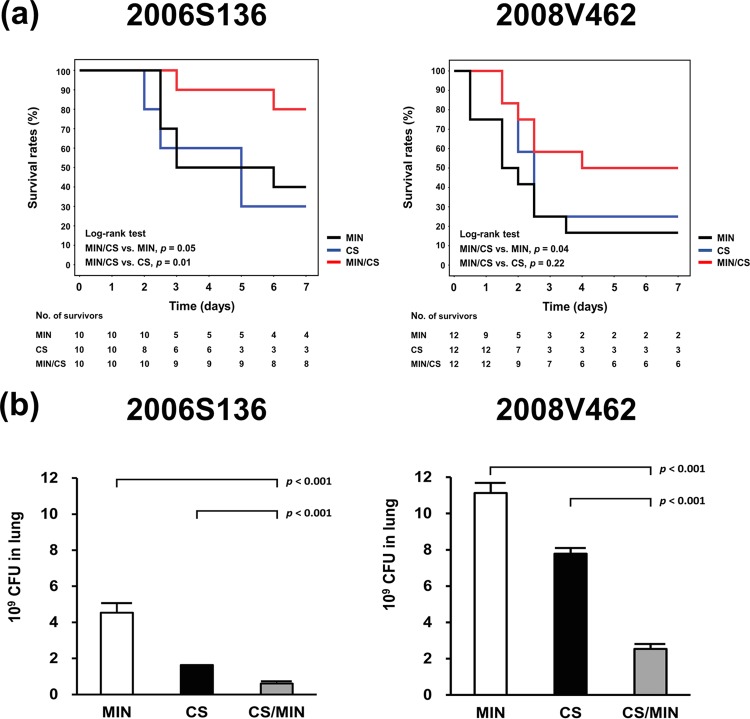
Considering the relatively beneficial effects of minocycline combined with colistin when treating isolate 2006S136 in the checkerboard test, the time-kill assay, and the biofilm assay, this clinical isolate was used for in vivo experiments. Figure 3a shows that the 7-day survival rates for mice receiving combination therapy, minocycline, or colistin were 80%, 40%, and 30%, respectively (for combination therapy versus monotherapy with minocycline, P = 0.01; for combination therapy versus monotherapy with colistin, P = 0.05). Combination therapy improved the rate of survival compared to that achieved with monotherapy. The number of bacteria present in the lungs of mice treated with minocycline and colistin was significantly lower than that observed when each agent was used individually (Fig. 3b). For mice infected with isolate 2008V462, against which no synergy of high-dose minocycline (16 μg/ml)-based combinations was observed, there was a trend toward a better survival rate and a greater number of bacteria being reduced in the lungs of mice treated with minocycline and colistin (Fig. 3a and b). However, the previous study showed that minocycline in combination with colistin may not be effective against minocycline-resistant isolates (17). A variation in the susceptibility to different regimens between our isolate and the isolates in the previous study may explain this discrepancy. We provided evidence that minocycline in combination with colistin might be effective in mice infected with minocycline-resistant isolates. Considering the in vitro and in vivo efficacy of carbapenem and colistin that has been translated into clinical practice, minocycline combined with colistin may be worth further investigation for patients infected with minocycline-resistant isolates.
FIG 3.
Survival (a) and bacterial load in the lungs (b) of mice that received different antibiotics. Beginning at 2 h after infection, mice were injected peritoneally with minocycline (50 mg/kg), colistin (10 mg/kg), or both antibiotics every 12 h. For calculation of the bacterial load in lungs, the mice were sacrificed at 24 h after infection. MIN, minocycline; CS, colistin.
In conclusion, this study demonstrated that minocycline-based combinations (especially minocycline in combination with colistin) have synergistic effects comparable to those observed with meropenem and colistin against some minocycline-resistant A. baumannii isolates. In vivo synergistic efficacy was also observed. However, the in vitro synergistic efficacy of minocycline-based combination therapies for the treatment of biofilm infections was not obvious even after prolonged periods of time. Thus, further clinical study of the activity of minocycline-based treatments against infections without an association with biofilms may be warranted.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Tri-Service General Hospital (TSGH-C103-125, TSGH-C104-119, TSGH-105-113, and DV104-09), the Ministry of Science and Technology (104-2314-B-075-043-MY3, 103-2314-B-016-039, and 104-2314-B-016-051), and the National Health Research Institutes (IV-102-PP-01 and IV-104-PP-17), Taiwan.
We declare that we have no competing interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02994-15.
REFERENCES
- 1.Taneja N, Singh G, Singh M, Sharma M. 2011. Emergence of tigecycline & colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India. Indian J Med Res 133:681–684. [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchie DJ, Garavaglia-Wilson A. 2014. A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin Infect Dis 59(Suppl 6):S374–S380. doi: 10.1093/cid/ciu613. [DOI] [PubMed] [Google Scholar]
- 3.Goff DA, Bauer KA, Mangino JE. 2014. Bad bugs need old drugs: a stewardship program's evaluation of minocycline for multidrug-resistant Acinetobacter baumannii infections. Clin Infect Dis 59(Suppl 6):S381–S387. doi: 10.1093/cid/ciu593. [DOI] [PubMed] [Google Scholar]
- 4.Castanheira M, Mendes RE, Jones RN. 2014. Update on Acinetobacter species: mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clin Infect Dis 59(Suppl 6):S367–S373. doi: 10.1093/cid/ciu706. [DOI] [PubMed] [Google Scholar]
- 5.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 6.Brogden RN, Speight TM, Avery GS. 1975. Minocycline: a review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 9:251–291. doi: 10.2165/00003495-197509040-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kuo SC, Chang SC, Wang HY, Lai JF, Chen PC, Shiau YR, Huang IW, Lauderdale TL, TSAR Hospitals. 2012. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis 12:200. doi: 10.1186/1471-2334-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyne S, Courvalin P, Perichon B. 2011. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother 55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilacoba E, Almuzara M, Gulone L, Traglia GM, Figueroa SA, Sly G, Fernandez A, Centron D, Ramirez MS. 2013. Emergence and spread of plasmid-borne tet(B)::ISCR2 in minocycline-resistant Acinetobacter baumannii isolates. Antimicrob Agents Chemother 57:651–654. doi: 10.1128/AAC.01751-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen TL, Siu LK, Wu RC, Shaio MF, Huang LY, Fung CP, Lee CM, Cho WL. 2007. Comparison of one-tube multiplex PCR, automated ribotyping and intergenic spacer (ITS) sequencing for rapid identification of Acinetobacter baumannii. Clin Microbiol Infect 13:801–806. doi: 10.1111/j.1469-0691.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang LY, Chen TL, Lu PL, Tsai CA, Cho WL, Chang FY, Fung CP, Siu LK. 2008. Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin Microbiol Infect 14:1010–1019. doi: 10.1111/j.1469-0691.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 13.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornsey M, Longshaw C, Phee L, Wareham DW. 2012. In vitro activity of telavancin in combination with colistin versus Gram-negative bacterial pathogens. Antimicrob Agents Chemother 56:3080–3085. doi: 10.1128/AAC.05870-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen PJ, Labthavikul P, Jones CH, Bradford PA. 2006. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemother 57:573–576. doi: 10.1093/jac/dki477. [DOI] [PubMed] [Google Scholar]
- 16.Hengzhuang W, Ciofu O, Yang L, Wu H, Song Z, Oliver A, Hoiby N. 2013. High beta-lactamase levels change the pharmacodynamics of beta-lactam antibiotics in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 57:196–204. doi: 10.1128/AAC.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowers DR, Cao H, Zhou J, Ledesma KR, Sun D, Lomovskaya O, Tam VH. 2015. Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrob Agents Chemother 59:2720–2725. doi: 10.1128/AAC.04110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzpatrick MA, Ozer EA, Hauser AR. 2016. Utility of whole-genome sequencing in characterizing Acinetobacter epidemiology and analyzing hospital outbreaks. J Clin Microbiol 54:593–612. doi: 10.1128/JCM.01818-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuter S, Ellington MJ, Cartwright EJ, Koser CU, Torok ME, Gouliouris T, Harris SR, Brown NM, Holden MT, Quail M, Parkhill J, Smith GP, Bentley SD, Peacock SJ. 2013. Rapid bacterial whole-genome sequencing to enhance diagnostic and public health microbiology. JAMA Intern Med 173:1397–1404. doi: 10.1001/jamainternmed.2013.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright MS, Haft DH, Harkins DM, Perez F, Hujer KM, Bajaksouzian S, Benard MF, Jacobs MR, Bonomo RA, Adams MD. 2014. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio 5:e00963-13. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rumbo C, Gato E, Lopez M, Ruiz de Alegria C, Fernandez-Cuenca F, Martinez-Martinez L, Vila J, Pachon J, Cisneros JM, Rodriguez-Bano J, Pascual A, Bou G, Tomas M, Spanish Group of Nosocomial Infections and Mechanisms of Action and Resistance to Antimicrobials (GEIH-GEMARA), Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC), Spanish Network for Research in Infectious Diseases (REIPI). 2013. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 57:5247–5257. doi: 10.1128/AAC.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang W, Liu XF, Huang J, Zhu DM, Li J, Zhang J. 2011. Activities of colistin- and minocycline-based combinations against extensive drug resistant Acinetobacter baumannii isolates from intensive care unit patients. BMC Infect Dis 11:109. doi: 10.1186/1471-2334-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan TY, Ng LS, Tan E, Huang G. 2007. In vitro effect of minocycline and colistin combinations on imipenem-resistant Acinetobacter baumannii clinical isolates. J Antimicrob Chemother 60:421–423. doi: 10.1093/jac/dkm178. [DOI] [PubMed] [Google Scholar]
- 24.Gordon NC, Png K, Wareham DW. 2010. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 54:5316–5322. doi: 10.1128/AAC.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng WH, Wang JT, Li SY, Lin YC, Cheng A, Chen YC, Chang SC. 2011. Comparative in vitro antimicrobial susceptibilities and synergistic activities of antimicrobial combinations against carbapenem-resistant Acinetobacter species: Acinetobacter baumannii versus Acinetobacter genospecies 3 and 13TU. Diagn Microbiol Infect Dis 70:380–386. doi: 10.1016/j.diagmicrobio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 26.March GA, Bratos MA. 2015. A meta-analysis of in vitro antibiotic synergy against Acinetobacter baumannii. J Microbiol Methods 119:31–36. doi: 10.1016/j.mimet.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Lora-Tamayo J, Murillo O, Bergen PJ, Nation RL, Poudyal A, Luo XL, Yu HY, Ariza J, Li J. 2014. Activity of colistin combined with doripenem at clinically relevant concentrations against multidrug-resistant Pseudomonas aeruginosa in an in vitro dynamic biofilm model. J Antimicrob Chemother 69:2434–2442. doi: 10.1093/jac/dku151. [DOI] [PubMed] [Google Scholar]
- 28.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. 2015. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents 45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez CH, Nastro M, Vay C, Famiglietti A. 2015. In vitro activity of minocycline alone or in combination in multidrug-resistant Acinetobacter baumannii isolates. J Med Microbiol 64:1196–1200. doi: 10.1099/jmm.0.000147. [DOI] [PubMed] [Google Scholar]
- 30.Gaddy JA, Actis LA. 2009. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 4:273–278. doi: 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamal MA, Rosenblatt JS, Hachem RY, Ying J, Pravinkumar E, Nates JL, Chaftari AM, Raad II. 2014. Prevention of biofilm colonization by Gram-negative bacteria on minocycline-rifampin-impregnated catheters sequentially coated with chlorhexidine. Antimicrob Agents Chemother 58:1179–1182. doi: 10.1128/AAC.01959-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozbek B, Mataraci E. 2013. In vitro effectiveness of colistin, tigecycline and levofloxacin alone and combined with clarithromycin and/or heparin as lock solutions against embedded Acinetobacter baumannii strains. J Antimicrob Chemother 68:827–830. doi: 10.1093/jac/dks472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.