Abstract
Ceftolozane-tazobactam (TOL-TAZ) is a novel cephalosporin/beta-lactamase inhibitor with activity against several Gram-negative pathogens. Daptomycin (DAP) has demonstrated synergistic activity with beta-lactams against methicillin-resistant Staphylococcus aureus (MRSA) isolates with reduced lipopeptide and glycopeptide susceptibilities. Our objective was to determine if DAP and TOL-TAZ possess synergy in hollow-fiber pharmacokinetic/pharmacodynamic (PK/PD) models. One isogenic pair of daptomycin-susceptible and daptomycin-nonsusceptible MRSA strains was evaluated. DAP, TOL-TAZ, and cefazolin (CFZ) MIC determinations were performed. DAP MIC determinations were also performed in the presence of subinhibitory concentrations of TOL-TAZ and CFZ. Ninety-six-hour in vitro models were run, simulating DAP at 10 mg/kg of body weight/day; TOL-TAZ at 1,500 mg every 8 h; TOL at 1,000 mg every 8 h; and DAP combined with TOL-TAZ (DAP+TOL-TAZ), DAP+TOL, DAP+TAZ, and DAP+CFZ at 2,000 mg every 8 h. DAP MICs were 0.5 and 4 μg/ml for strains R8845 and R8846, respectively. In the presence of CFZ, R8845 and R8846 DAP MICs were reduced 8-fold and 16-fold, respectively. TOL and TAZ had no effect on DAP MICs. PK/PD models demonstrated bactericidal activity with DAP+CFZ against both strains. The combination of DAP+TOL-TAZ was bactericidal against R8845 but was not bactericidal against daptomycin-nonsusceptible strain R8846. DAP+TOL and DAP+TAZ were not bactericidal. No other regimens were bactericidal. DAP+TOL-TAZ did not demonstrate synergistic activity against daptomycin-nonsusceptible S. aureus but prevented daptomycin-nonsusceptible MRSA emergence. Because DAP+TOL or TAZ alone did not prevent daptomycin-nonsusceptible MRSA emergence, the combination TOL-TAZ may be necessary for synergy with DAP. DAP+CFZ demonstrated enhancement against both strains. The combination of DAP+CFZ warrants further clinical study.
INTRODUCTION
Staphylococcus aureus is the leading cause of hospital-associated infections in the United States, and methicillin-resistant S. aureus (MRSA) strains comprise up to 50% of isolates (1). More problematic is the emergence of MRSA isolates with reduced susceptibility to vancomycin, a drug which has been a mainstay of MRSA therapy for more than 40 years (2). Treatment options against such isolates are limited. Among them is daptomycin (DAP), a lipopeptide antibiotic with bactericidal activity against Gram-positive pathogens (3). Daptomycin maintains activity against MRSA isolates with reduced vancomycin susceptibility, and nonsusceptibility to daptomycin is rare, occurring in <0.05% of a sampling of >9,000 S. aureus isolates (4). Daptomycin nonsusceptibility has been reported, however, and creative therapies are needed to combat this problem (5–10). Recent data have demonstrated that beta-lactam antibiotics in combination with daptomycin are efficacious in both preventing daptomycin nonsusceptibility and providing bactericidal activity (6, 8, 10–13).
Because antibiotic resistance among Gram-negative bacteria is as much, if not more, of a problem as resistance among Gram-positive bacteria, several agents are under development or have recently been approved to combat Gram-negative infections. Ceftolozane (TOL) is a novel cephalosporin with activity against a broad range of Gram-negative pathogens, including excellent activity against Pseudomonas aeruginosa (14). When partnered with the beta-lactamase inhibitor tazobactam (TAZ), it possesses activity against expanded-spectrum-beta-lactamase-producing Gram-negative bacilli as well (14). The combination of ceftolozane and tazobactam has recently been approved for the treatment of complicated intra-abdominal infections in combination with metronidazole as well as for the treatment of complicated urinary tract infections, including pyelonephritis (15, 16). Ceftolozane on its own lacks intrinsic Gram-positive activity, limiting its use against S. aureus. However, in critically ill patients, coinfection with several organisms is not uncommon, and coinfection by resistant Pseudomonas aeruginosa and MRSA isolates would provide a scenario in which ceftolozane may be administered concomitantly with daptomycin. Therefore, it is important to determine if exposure to ceftolozane-tazobactam enhances or antagonizes daptomycin activity. The goal of our study was to compare the activity of daptomycin alone to those of daptomycin in combination with ceftolozane alone, tazobactam alone, the combination of ceftolozane-tazobactam, or cefazolin (CFZ) in a pharmacokinetic/pharmacodynamic (PK/PD) model.
MATERIALS AND METHODS
Bacterial strains.
One clinical isogenic pair of MRSA strains, one a daptomycin-susceptible strain (R8845) and the other a daptomycin-nonsusceptible strain (R8846), were selected for this study. These strains were chosen from our library at the Anti-Infective Research Laboratory.
Antimicrobials.
Daptomycin was purchased commercially from Cubist Pharmaceuticals (Lexington, MA). Ceftolozane and tazobactam were obtained from Cubist Pharmaceuticals for the purpose of this study. Cefazolin was purchased commercially from Sigma Chemical Co. (St. Louis, MO).
Susceptibility testing and time-kill experiments.
MIC values of the studied antimicrobials were determined in duplicate by broth microdilution at ∼106 CFU/ml in Mueller-Hinton broth (MHB; Difco, Detroit, MI) supplemented with 50 μg/ml calcium according to CLSI guidelines (17). Ceftolozane-tazobactam MICs were obtained at fixed concentrations of both 4 μg/ml (CLSI standard) and 23.8 μg/ml (maximum free-drug concentration in serum [fCmax]) of tazobactam to determine any possible effect of the tazobactam concentration on the ceftolozane MIC against these strains. Daptomycin MIC determinations were also performed in the presence of ceftolozane, tazobactam, ceftolozane-tazobactam, and cefazolin at 0.5× the MIC of the respective organisms or the fCmax of the antibiotic, whichever value was lower, to determine the effects of these antimicrobials on daptomycin MIC reduction. Time-kill experiments were also performed against both strains with daptomycin, ceftolozane-tazobactam, cefazolin, and the combinations of daptomycin and ceftolozane-tazobactam and daptomycin and cefazolin. The initial bacterial inoculum was ∼106 CFU/ml; daptomycin concentrations were 0.5× MIC (0.25 μg/ml for R8845 and 1 μg/ml for R8846); ceftolozane and tazobactam concentrations represented the biological free peaks of 55 μg/ml and 23.8 μg/ml, respectively, against R8845 and 0.5× MIC of ceftolozane (8 μg/ml) with 23.8 μg/ml tazobactam against R8846; and cefazolin concentrations were 0.5× MIC (16 μg/ml for R8845 and 1 μg/ml for R8846). The ceftolozane concentration against R8846 was used based on the MIC obtained for ceftolozane of 16 μg/ml in the presence of 23.8 μg/ml tazobactam. Samples were collected at 0, 4, 8, 24, and 48 h, and colony counts (log10 CFU per milliliter) were compared among the regimens at 48 h. Synergy was defined as a combination regimen possessing a >100-fold increase in bacterial killing compared to the most active single constituent, with one of the single constituents demonstrating no important effect on organism growth (18).
In vitro PK/PD model.
An in vitro, hollow-fiber, pharmacokinetic/pharmacodynamic model with a 400-ml capacity and inflow and outflow ports was used. The target starting inoculum was ∼108 CFU/ml. Fresh medium (MHB) was continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) at an appropriate rate to simulate the average human clearance and half-lives (t1/2) of the antimicrobials. The antimicrobial regimens evaluated were simulations of daptomycin at 10 mg/kg of body weight every 24 h (targeted fCmax of 11.3 μg/ml, t1/2 of 8 h, 92% protein binding, and free-drug area under the concentration-time curve [AUC] over 24 h [fAUC0–24] of 114.8 μg · h/ml) (19), ceftolozane-tazobactam at 1,500 mg every 8 h (targeted fCmax of 55 μg/ml, t1/2 of 2.7 h, and 20% protein binding for ceftolozane and targeted fCmax of 8 μg/ml, t1/2 of 2.7 h, and 30% protein binding for tazobactam) (20), daptomycin in combination with ceftolozane-tazobactam, daptomycin in combination with ceftolozane at 1,000 mg every 8 h without tazobactam, and daptomycin in combination with cefazolin at 2,000 mg every 8 h (targeted fCmax of 28 μg/ml, t1/2 of 2.7 h, and 93% protein binding) (21). A regimen of daptomycin in combination with tazobactam at 500 mg every 8 h was also run against R8845. The tazobactam pharmacokinetics were adjusted from an fCmax of 23.8 μg/ml and a t1/2 of 1 h to an fCmax of 8.8 μg/ml and a t1/2 of 2.7 h, with both regimens achieving identical AUC values. A regimen of daptomycin in combination with either ceftolozane or tazobactam alone was performed to determine if either component could be responsible for a potential synergistic effect with daptomycin. The models were performed in duplicate to ensure reproducibility. Supplemental daptomycin was added at an appropriate rate to ceftolozane, tazobactam, ceftolozane-tazobactam, and cefazolin to simulate the clearances of these beta-lactams (22).
Pharmacodynamic analysis.
Samples from each model were collected at 0, 4, 8, 24, 32, 48, 72, and 96 h in duplicate. Colony counts were determined by spiral plating of appropriate dilutions using an automatic spiral plater (Wasp; DW Scientific, West Yorkshire, England). Colonies were counted by using a laser colony counter (ProtoCOL; Synoptics Limited, Frederick, MD). Bacteria were plated onto tryptic soy agar (TSA; Difco, Detroit, MI). These methods have a lower limit of reliable detection of 2 log10 CFU/ml. The total reduction in log10 CFU per milliliter over 96 h was determined by plotting model time-kill curves based on the number of remaining organisms over the 96-h time period. Bactericidal activity was defined as a ≥3-log10 CFU/ml (99.9%) decrease in colony counts from the initial inoculum. Bacteriostatic activity was defined as a <3-log10 CFU/ml reduction in colony counts from the initial inoculum. Enhancement of combinations was defined as a ≥2-log10 CFU/ml reduction at 96 h for the combination compared to the colony counts with either agent alone.
Pharmacokinetic analysis.
Ceftolozane and cefazolin concentrations were determined by a bioassay using Escherichia coli ATCC 25922. Each sample was tested in duplicate by placing antibiotic-permeated disks of known, standard antibiotic concentrations and disks permeated with unknown antibiotic concentrations from predetermined time points on agar plates (antibiotic medium number 11; Difco, Detroit, MI) inoculated with a suspension of the test organisms at a 0.5 McFarland standard. Ceftolozane and cefazolin MIC values for E. coli ATCC 25922 were determined in the presence of 11.3 μg/ml daptomycin to ensure that there was no synergistic effect of daptomycin on the bioassay, and time-kill experiments were also performed with both agents in combination with daptomycin to further test for any synergistic activity (see the supplemental material). Daptomycin concentrations were determined by using standard high-performance liquid chromatography (HPLC) (23). The half-lives, AUC values, and peak concentrations of the antibiotics were determined by the trapezoidal method using PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
Resistance.
Emergence of resistance was evaluated at 96 h by plating 100 μl of samples from the models onto plates containing brain heart infusion agar (BHIA; Difco, Detroit, MI) supplemented with 50 mg/liter calcium and daptomycin at a concentration 3 times the daptomycin MIC for the tested organism. Resistant colonies growing on screening plates were evaluated by broth microdilution methods to determine the daptomycin MIC.
Statistical analysis.
Changes in CFU per milliliter at 96 h were compared by one-way analysis of variance (ANOVA) for in vitro models. A P value of ≤0.05 was considered significant. All statistical analyses were performed by using SPSS statistical software (release 22; SPSS, Inc., Chicago, IL).
RESULTS
Susceptibility testing and time-kill experiments.
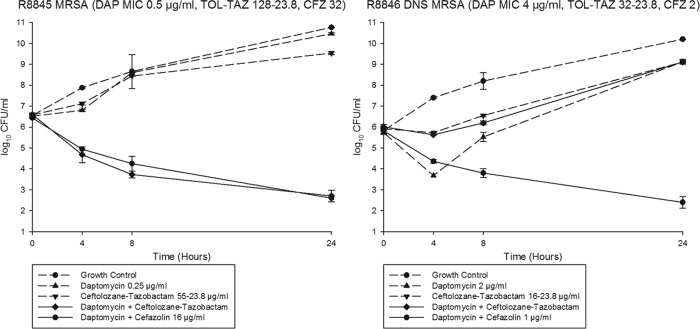
MIC values of all antimicrobials against R8845 and R8846 are listed in Table 1. R8845 was susceptible to daptomycin, with an MIC of 0.5 μg/ml, and R8846 was nonsusceptible, with an MIC of 4 μg/ml. Daptomycin MIC values in the presence of the beta-lactams are also listed in Table 1. In the time-kill experiments with daptomycin, ceftolozane-tazobactam, and cefazolin against strain R8845, the combination of daptomycin and ceftolozane-tazobactam was bactericidal, synergistic, and statistically more effective at 24 h than the growth control, daptomycin alone, and ceftolozane-tazobactam alone (P < 0.001) (Fig. 1). Daptomycin in combination with cefazolin was also bactericidal, synergistic, and statistically superior to each single-drug exposure (P < 0.001) (Fig. 1). The combinations of daptomycin plus cefazolin and daptomycin plus ceftolozane-tazobactam were statistically similar at 24 h.
TABLE 1.
MICs of antibiotics for tested organisms and DAP MICs in combination with TOL-TAZ, TOL, TAZ, or CFZ
| Strain | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| DAP | TOL-TAZ-4a | TOL-TAZ-23.8a | TOL | CFZ | DAP+TOL-TAZ-4b | DAP+TOLb | DAP+TAZb | DAP+CFZb | |
| R8845 | 0.5 | 128/4 | 128/23.28 | 128 | 32 | 0.25 | 0.5 | 0.5 | 0.06 |
| R8846 | 4 | 32/4 | 32/23.8 | 32 | 2 | 4 | 4 | 4 | 0.25 |
Ceftolozane-tazobactam MICs were obtained with static tazobactam concentrations of both 4 μg/ml (TAZ-4) (CLSI standard) and 23.8 μg/ml (TAZ-23.8) (fCmax of tazobactam).
For combination MICs, TOL-TAZ, TOL, and CFZ were present in broth at 0.5× the reported MIC for each organism.
FIG 1.
Twenty-four-hour time-kill experiments. The combination of daptomycin at 0.5× the MIC and ceftolozane-tazobactam at free biologic peaks was synergistic and bactericidal against R8845 at 48 h. DNS, daptomycin nonsusceptible.
In time-kill experiments against strain R8846, daptomycin alone, ceftolozane-tazobactam alone, and daptomycin in combination with ceftolozane-tazobactam displayed no appreciable activity at 24 h (Fig. 1). In contrast, daptomycin in combination with cefazolin was bactericidal, synergistic, and statistically superior to all other regimens tested (P < 0.001) (Fig. 1).
In vitro PK/PD models.
The average observed fCmax for daptomycin was 10.8 μg/ml (target, 11.3 μg/ml), the average fAUC0–24 was 110.8 μg · h/ml (target, 114.8 μg · h/ml), and the average t1/2 was 7.7 h (target, 8 h) (19). The average observed fCmax for ceftolozane was 53.9 μg/ml (target, 55 μg/ml), and the average t1/2 was 2.5 h (target, 2.7 h) (20). The average observed fCmax for cefazolin was 29.3 μg/ml (target, 28 μg/ml), and the average t1/2 was 2.83 h (target, 2.7 h) (21). Time-kill and MIC data suggested that there was no synergistic activity between daptomycin and ceftolozane-tazobactam against reference E. coli strain ATCC 25922 (see the supplemental material).
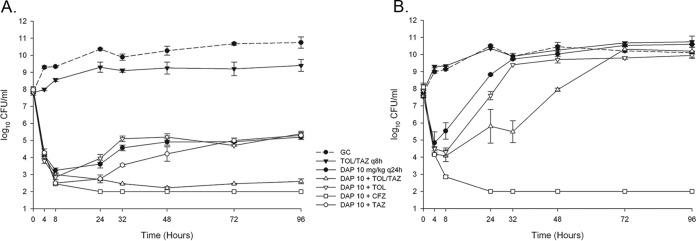
Daptomycin alone was initially bactericidal against daptomycin-susceptible MRSA strain R8845 (Table 2 and Fig. 2A) but demonstrated regrowth at 32 h and did not maintain bactericidal activity at 96 h. At 96 h, the daptomycin MIC increased from 0.5 μg/ml to 2 μg/ml, representing a shift from daptomycin susceptibility to daptomycin nonsusceptibility. The fAUC0–24/MIC ratio of daptomycin for this organism based on the initial isolate MIC of the isolate was 221.6 μg · h/ml. Ceftolozane-tazobactam, ceftolozane alone, and cefazolin demonstrated little to no activity as single agents. Daptomycin in combination with ceftolozane-tazobactam (fAUC0–24/MIC ratio based on an MIC of 443.2 μg · h/ml for daptomycin plus ceftolozane-tazobactam) was bactericidal at 96 h and demonstrated significantly greater activity than did daptomycin alone, ceftolozane-tazobactam alone, or daptomycin in combination with ceftolozane (fAUC0–24/MIC ratio of 221.6 μg · h/ml) or tazobactam (fAUC0–24/MIC ratio of 221.6 μg · h/ml) alone (P < 0.001). Daptomycin in combination with cefazolin (fAUC0–24/MIC ratio of 1,772.8 μg · h/ml) was bactericidal by as soon as 4 h and remained bactericidal throughout the regimen, displaying statistically significantly more activity than any other regimen, including daptomycin plus ceftolozane-tazobactam (P = 0.025), at 96 h.
TABLE 2.
In vitro activities of regimens against R8845 and R8846 at 96 h
| Regimen | Mean log10 CFU/ml at 96 h ± SD (mean change from baseline inoculum [log10 CFU/ml] ± SD)b |
|
|---|---|---|
| R8845 | R8846 | |
| DAP | 5.20 ± 0.14 (−2.60 ± 0.14) | 10.60 ± 0.02 (+3.05 ± 0.07) |
| TOL-TAZ | 9.40 ± 0.35 (+1.56 ± 0.33) | 10.68 ± 0.08 (+2.84 ± 0.19) |
| DAP+TOL-TAZ | 2.60 ± 0.14 (−5.29 ± 0.11) | 10.19 ± 0.01 (+2.55 ± 0.13) |
| DAP+TOL | 5.40 ± 0.14 (−2.40 ± 0.14) | 9.95 ± 0.11 (+2.05 ± 0.11) |
| DAP+TAZ | 5.33 ± 0.21 (−2.68 ± 0.23) | NA |
| DAP+CFZ | 2.00 ± 0.00 (−6.05 ± 0.00)a | 2.00 ± 0.00 (−6.10 ± 0.00)a |
| Growth control | 10.75 ± 0.33 (+2.91 ± 0.05) | 10.10 ± 0.38 (+2.37 ± 0.32) |
The limit of detection for the CFU counting methods is 2 CFU/ml.
NA, not applicable.
FIG 2.
Ninety-six-hour, hollow-fiber PK/PD models. Daptomycin (DAP) at 10 mg/kg/day in combination with cefazolin (CFZ) was bactericidal and demonstrated enhanced activity against both strains. The combination of DAP plus ceftolozane-tazobactam (TOL-TAZ) was bactericidal and demonstrated enhanced activity against only R8845. Activities of DAP+TOL and DAP+TAZ against R8845 were similar to those of DAP alone. (A) S. aureus R8845; (B) S. aureus R8846. GC, growth control; q24h, every 24 h.
Daptomycin alone displayed little to no activity against daptomycin-nonsusceptible MRSA strain R8846 throughout the regimen (Table 2 and Fig. 2B). The fAUC0–24/MIC ratio of daptomycin for this organism was 27.7 μg · h/ml. Daptomycin alone, ceftolozane-tazobactam alone, daptomycin in combination with ceftolozane-tazobactam (fAUC0–24/MIC ratio of 27.7 μg · h/ml), and daptomycin in combination with ceftolozane (fAUC0–24/MIC ratio of 27.7 μg · h/ml) were all statistically similar to the growth control at 96 h. However, the combination of daptomycin and cefazolin (fAUC0–24/MIC ratio of 443.2 μg · h/ml) was bactericidal throughout the 96-h time period and statistically superior to all other regimens at the end of the model run (P < 0.001). Cefazolin was the lone agent to increase the daptomycin fAUC0–24/MIC ratio against R8846 based on daptomycin MIC reduction.
DISCUSSION
Our study is the first to evaluate the novel cephalosporin ceftolozane in combination with daptomycin against resistant S. aureus isolates. Here, we have demonstrated that synergistic activity with daptomycin is not universal among all beta-lactam agents, as ceftolozane possessed little to no enhancement potential in these in vitro models. Interestingly, when tazobactam was paired with ceftolozane as in the clinical setting, enhancement of daptomycin activity against the daptomycin-susceptible strain was demonstrated. Converse to the combination of daptomycin and ceftolozane, the combination of daptomycin and cefazolin demonstrated bactericidal activity against both daptomycin-susceptible and daptomycin-nonsusceptible MRSA isolates.
The lack of synergistic activity between daptomycin and ceftolozane is disappointing if not altogether unexpected. Previously reported data demonstrated synergy between daptomycin and several beta-lactams against MRSA, but those beta-lactams all possessed at least some level of antistaphylococcal activity (6, 10–13, 24–27). Unlike those successful beta-lactams, ceftolozane lacks appreciable activity against Gram-positive organisms, which may be the reason for the lack of activity observed in our study. The differences in synergistic activity may involve differential penicillin-binding protein (PBP) binding. One paper, in particular, suggested that beta-lactams with PBP1 activity, such as cefazolin and meropenem, seem to possess the best synergistic activity with daptomycin against S. aureus (28). To date, there are no data describing the PBP-binding profile of ceftolozane against Gram-positive bacteria, although a lack of activity against PBP1 may be a factor.
When ceftolozane and tazobactam were given together with daptomycin, the results were suggestive of synergistic activity, at least against the daptomycin-susceptible parent MRSA strain. Previously reported in vitro data demonstrate a synergistic relationship between daptomycin and piperacillin-tazobactam or ampicillin-sulbactam against MRSA (29). These data, combined with the lack of synergistic activity demonstrated in our study when either ceftolozane or tazobactam was given alone with daptomycin, suggest that the interaction between a beta-lactam and a beta-lactamase inhibitor may be capable of enhancing the activity of daptomycin against MRSA. The strain pair used in our study was part of a clinical case in which daptomycin at 10 mg/kg/day was unable to clear an infection, so the fact that daptomycin simulated at 10 mg/kg/day led to the development of daptomycin nonsusceptibility in our in vitro study is not surprising. Potentially, the mechanism of prevention of daptomycin nonsusceptibility may be related to the reduction of the R8845 daptomycin MIC in the presence of ceftolozane-tazobactam. This daptomycin MIC-lowering effect increased the daptomycin fAUC0–24/MIC ratio. Previous studies demonstrated that the daptomycin fAUC0–24/MIC ratio plays an important role in both preventing daptomycin nonsusceptibility and establishing bactericidal activity (30, 31). The increase in the daptomycin fAUC0–24/MIC ratio in the presence of ceftolozane-tazobactam may explain the lack of resistance development in strain R8845 that occurred when daptomycin was given alone. Although resistance prevention was established in our model, ceftolozane-tazobactam was not able to restore daptomycin activity against the already daptomycin-nonsusceptible strain R8846, suggesting that whatever effect that the combination of ceftolozane and tazobactam has on daptomycin activity does not extend to daptomycin-nonsusceptible strains. The combination of daptomycin with beta-lactam/beta-lactamase inhibitors is intriguing, and further study is warranted to determine if the synergistic relationship demonstrated here is more widely applicable.
The most striking observation from our study is the enhancement of daptomycin activity demonstrated by cefazolin. Cefazolin was chosen as a comparator beta-lactam due to its previously demonstrated synergy with daptomycin in time-kill data as well as its activity against Gram-positive organisms (28). Not only did cefazolin decrease the daptomycin MIC appreciably against both MRSA strains, it also demonstrated sustained bactericidal activity at 96 h in the PK/PD models when combined with daptomycin. Of interest, the cefazolin MIC decreased as the daptomycin MIC increased between the strains, demonstrating the presence of the “seesaw effect” phenomenon, where glycopeptide and lipopeptide MIC values are inversely proportional to those of beta-lactams (32, 33). Here, the seesaw effect was not present with ceftolozane-tazobactam, perhaps suggesting that antistaphylococcal beta-lactam activity may play an important role in daptomycin synergy or, perhaps, the demonstrated PBP1 binding of cefazolin. In vitro and clinical data previously demonstrated the efficacy of daptomycin in combination with antistaphylococcal beta-lactams against MRSA (6, 10–13, 26, 34, 35). Ceftaroline, in particular, has demonstrated the ability to enhance daptomycin activity against daptomycin-nonsusceptible strains of MRSA (6, 10, 11, 13, 26). However, there have been few reports regarding the efficacy of beta-lactams without anti-MRSA activity, e.g., cefazolin, in combination with daptomycin against daptomycin-nonsusceptible MRSA. Our data suggest that enhancement of daptomycin activity is not limited to beta-lactam agents with activity against the offending organism. The ability of cefazolin to enhance the activity of daptomycin has implications for therapy, as cefazolin does not possess the anti-MRSA activity of ceftaroline and may present a viable treatment strategy for prolonged outpatient therapy. Further study on this intriguing combination against daptomycin-nonsusceptible MRSA is warranted.
This study has some limitations. Only one strain pair was studied, so it is unclear if the effects demonstrated here are applicable to a broader range of isolates. Also, our models were run over only 96 h, which is a far shorter duration than what would be used clinically for most infections. The tazobactam pharmacokinetics were also different from those that are achieved clinically, although the peak was modified in conjunction with the half-life in order to achieve an fAUC0–24 similar to that which is achieved clinically. In an effort to ensure that the extended tazobactam half-life had no effect on the models, a model was run by using the standard 1-h half-life of tazobactam in combination with daptomycin. These results were statistically similar to the values achieved in our model of daptomycin plus tazobactam with a 2.7-h half-life (see the supplemental material).
Conclusion.
Daptomycin and ceftolozane-tazobactam represent important therapeutic options against resistant bacteria that are occurring with increasing frequency. The data from this study suggest that their use in combination does not present a risk for reduced efficacy against S. aureus. The combination of ceftolozane and tazobactam, possibly due to the tazobactam component, may even enhance the activity of daptomycin. Perhaps most importantly, we have demonstrated that cefazolin is efficacious in combination with daptomycin, even when MRSA isolates are daptomycin nonsusceptible. These in vitro observations provide valuable data that can be further evaluated with clinical use.
Supplementary Material
ACKNOWLEDGMENTS
Expendable supplies for this work were supported in part by a grant from Cubist (Merck) Pharmaceuticals. M.J.R. has received grant support from, consulted for, or provided lectures for Astellas; Cubist (Merck); Forest (Allergan); Pfizer; Theravance Biopharma Antibiotics, Inc.; and Novartis. J.R.S., A.A., K.E.B., and J.H. have nothing to declare.
Funding Statement
This project did not receive any external support other than expendable supplies partially provided by Cubist (Merck) Pharmaceuticals.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01666-15.
REFERENCES
- 1.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 2.Howden BP, Peleg AY, Stinear TP. 6 April 2013. The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infect Genet Evol doi: 10.1016/j.meegid.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter CF, Chambers HF. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin Infect Dis 38:994–1000. doi: 10.1086/383472. [DOI] [PubMed] [Google Scholar]
- 4.Sader HS, Farrell DJ, Flamm RK, Jones RN. 2014. Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a worldwide surveillance programme (2005-2012). Int J Antimicrob Agents 43:465–469. doi: 10.1016/j.ijantimicag.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering RC Jr, Eliopoulos GM. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother 50:1581–1585. doi: 10.1128/AAC.50.4.1581-1585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakoulas G, Moise PA, Casapao AM, Nonejuie P, Olson J, Okumura CY, Rybak MJ, Kullar R, Dhand A, Rose WE, Goff DA, Bressler AM, Lee Y, Pogliano J, Johns S, Kaatz GW, Ebright JR, Nizet V. 2014. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 36:1317–1333. doi: 10.1016/j.clinthera.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Sakoulas G, Rose W, Rybak MJ, Pillai S, Alder J, Moellering RC Jr, Eliopoulos GM. 2008. Evaluation of endocarditis caused by methicillin-susceptible Staphylococcus aureus developing nonsusceptibility to daptomycin. J Clin Microbiol 46:220–224. doi: 10.1128/JCM.00660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steed ME, Vidaillac C, Rybak MJ. 2010. Novel daptomycin combinations against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro model of simulated endocardial vegetations. Antimicrob Agents Chemother 54:5187–5192. doi: 10.1128/AAC.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steed ME, Werth BJ, Ireland CE, Rybak MJ. 2012. Evaluation of the novel combination of high-dose daptomycin plus trimethoprim-sulfamethoxazole against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus using an in vitro pharmacokinetic/pharmacodynamic model of simulated endocardial vegetations. Antimicrob Agents Chemother 56:5709–5714. doi: 10.1128/AAC.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werth BJ, Barber KE, Ireland CE, Rybak MJ. 2014. Evaluation of ceftaroline, vancomycin, daptomycin, or ceftaroline plus daptomycin against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model of simulated endocardial vegetations. Antimicrob Agents Chemother 58:3177–3181. doi: 10.1128/AAC.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber K, Werth BJ, Rybak MJ. 2013. Evaluation of therapeutic de-escalation of the novel combination of ceftaroline (CPT) plus daptomycin (DAP) against DAP non-susceptible (DNS) methicillin-resistant Staphylococcus aureus (MRSA) in an in vitro hollow-fiber pharmacokinetic/pharmacodynamic model, poster A-467. Abstr 53rd Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 12.Barber KE, Werth BJ, Ireland CE, Stone NE, Nonejuie P, Sakoulas G, Pogliano J, Rybak MJ. 2014. Potent synergy of ceftobiprole plus daptomycin against multiple strains of Staphylococcus aureus with various resistance phenotypes. J Antimicrob Chemother 69:3006–3010. doi: 10.1093/jac/dku236. [DOI] [PubMed] [Google Scholar]
- 13.Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagace-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP III, Karlowsky JA. 2014. Ceftolozane/tazobactam: a novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 15.Solomkin J, Hershberger E, Miller B, Popejoy M, Friedland I, Steenbergen J, Yoon M, Collins S, Yuan G, Barie PS, Eckmann C. 10 February 2015. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis doi: 10.1093/cid/civ097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassetti M, Righi E. 2015. Ceftolozane/tazobactam for the treatment of complicated urinary tract and intra-abdominal infections. Future Microbiol 10:151–160. doi: 10.2217/fmb.14.112. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.American Society for Microbiology. 2015. Instructions to authors. American Society for Microbiology, Washington, DC: http://aac.asm.org/site/misc/journal-ita_abb.xhtml Accessed 20 November 2015. [Google Scholar]
- 19.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob Agents Chemother 50:3245–3249. doi: 10.1128/AAC.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas A, Udy AA, Wallis SC, Jarrett P, Stuart J, Lassig-Smith M, Deans R, Roberts MS, Taraporewalla K, Jenkins J, Medley G, Lipman J, Roberts JA. 2011. Plasma and tissue pharmacokinetics of cefazolin in patients undergoing elective and semielective abdominal aortic aneurysm open repair surgery. Antimicrob Agents Chemother 55:5238–5242. doi: 10.1128/AAC.05033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother 15(Suppl A):125–130. [DOI] [PubMed] [Google Scholar]
- 23.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother 47:1318–1323. doi: 10.1128/AAC.47.4.1318-1323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. 2012. Beta-lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rand KH, Houck HJ. 2004. Synergy of daptomycin with oxacillin and other beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 48:2871–2875. doi: 10.1128/AAC.48.8.2871-2875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose WE, Schulz LT, Andes D, Striker R, Berti AD, Hutson PR, Shukla SK. 2012. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 56:5296–5302. doi: 10.1128/AAC.00797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 54:3161–3169. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berti AD, Sakoulas G, Nizet V, Tewhey R, Rose WE. 2013. Beta-lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5005–5012. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cilli F, Aydemir S, Tunger A. 2006. In vitro activity of daptomycin alone and in combination with various antimicrobials against Gram-positive cocci. J Chemother 18:27–32. doi: 10.1179/joc.2006.18.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Smith JR, Barber KE, Raut A, Rybak MJ. 2015. Beta-lactams enhance daptomycin activity against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium in in vitro pharmacokinetic/pharmacodynamic models. Antimicrob Agents Chemother 59:2842–2848. doi: 10.1128/AAC.00053-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werth BJ, Steed ME, Ireland CE, Tran TT, Nonejuie P, Murray BE, Rose WE, Sakoulas G, Pogliano J, Arias CA, Rybak MJ. 2014. Defining daptomycin resistance prevention exposures in vancomycin-resistant Enterococcus faecium and E. faecalis. Antimicrob Agents Chemother 58:5253–5261. doi: 10.1128/AAC.00098-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber KE, Ireland CE, Bukavyn N, Rybak MJ. 2014. Observation of “seesaw effect” with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther 3:35–43. doi: 10.1007/s40121-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortwine JK, Werth BJ, Sakoulas G, Rybak MJ. 2013. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: taking advantage of the back door left open? Drug Resist Updat 16:73–79. doi: 10.1016/j.drup.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 53:158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard SN, Rolek KM. 2013. Evaluation of the combination of daptomycin and nafcillin against vancomycin-intermediate Staphylococcus aureus. J Antimicrob Chemother 68:644–647. doi: 10.1093/jac/dks453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.