Abstract
Thanatin (THA) displays potent antibiotic activity, especially against extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli both in vitro and in vivo, with minimal hemolytic toxicity and satisfactory stability in plasma. However, the high cost of thanatin significantly limits its development and clinical application. To reduce the cost of peptide synthesis, a formulation of cyclic thanatin (C-thanatin) called linear thanatin (L-thanatin) was synthesized and its activity was evaluated in vivo and in vitro. Results showed that C-thanatin and L-thanatin MICs did not differ against eight Gram-negative and two Gram-positive bacterial strains. Furthermore, the survival rates of ESBL-producing-E. coli-infected mice were consistent after C-thanatin or L-thanatin treatment at 5 or 10 mg/kg of body weight. Neither C-thanatin nor L-thanatin showed toxicity for human red blood cells (hRBCs) and human umbilical vein endothelial cells (HUVECs) at a concentration as high as 256 μg/ml. Results of circular dichroism spectroscopy indicated that the secondary structure of L-thanatin is extremely similar to that of C-thanatin. Membrane permeabilization and depolarization assays showed that C-thanatin and L-thanatin have similar abilities to permeabilize the outer and inner membranes and to induce membrane depolarization in ESBL-producing E. coli. However, neither of them caused significant HUVEC membrane permeability. These findings indicate that the two peptides have similar effects on bacterial cell membranes and that the disulfide bond in thanatin is not essential for its antimicrobial activities in vivo and in vitro. L-thanatin is thus a promising low-cost peptide candidate for treating ESBL-producing E. coli infections.
INTRODUCTION
Thanatin (THA) is a cationic antimicrobial peptide that was first discovered in the hemipteran insect Podisus maculiventris. This peptide is composed of 21 amino acids (GSKKPVPIIYCNRRTGKCQRM), with an intramolecular disulfide bridge between the two cysteines at positions 11 and 18. Our previous studies revealed that thanatin exhibits potent antibiotic activity, especially against extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli, both in vitro and in vivo, with a low inherent ability to induce microbial resistance, minimal hemolytic toxicity, and high stability in plasma (1, 2).
Despite their promise for clinical benefit, the high cost of producing peptide drugs such as thanatin significantly limits their development and clinical application. Compared with conventional antibiotics, the costs of cationic antimicrobial peptides are always prohibitively high for large-scale production (3). Intrachain disulfide bonds exist in many naturally occurring peptides and play important roles in biological activities. However, synthesis of peptide drugs with one or more disulfide bridges is complicated and expensive (4).
Previous studies found that the disulfide bonds of protegrin-1 are not essential for its antimicrobial and hemolytic activities in vitro (5). To reduce the cost of thanatin synthesis, native cyclized thanatin (C-thanatin) and its analogue linear thanatin (L-thanatin) were synthesized in the present study. Their antimicrobial activities were evaluated in vitro and in vivo against eight Gram-negative and two Gram-positive bacterial strains with septicemic BALB/c mice.
MATERIALS AND METHODS
Chemicals.
All antibiotics used in this study were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All other chemicals and reagents used were of analytical grade.
Organisms.
The multidrug-resistant (MDR) clinical isolates methicillin-resistant Staphylococcus epidermidis (MRSE), Pseudomonas aeruginosa XJ75315, ESBL-producing Klebsiella pneumoniae XJ75297, and enterohemorrhagic E. coli (EHEC) were obtained from the clinical laboratory of Xijing Hospital (Xi'an, China). E. coli ATCC 25922, S. aureus ATCC 29213, P. aeruginosa ATCC 27853, ESBL-producing E. coli ATCC 35218, Salmonella enterica serovar Typhimurium SL1344, and Shigella flexneri ATCC 12022 from the Chinese National Center for Surveillance of Antimicrobial Resistance were used as reference strains.
Peptide synthesis.
L-thanatin (GSKKPVPIIYCNRRTGKCQRM) was synthesized by classic Fmoc methodology. Then, L-thanatin in reduced form was taken up in the oxidation buffer (1 mg per 10 ml) and C-thanatin was formed (6). The crude compound was purified to over 95% chromatographic homogeneity by reverse-phase high-performance liquid chromatography (RP-HPLC) (data not shown), and the purified compound was identified by mass spectrometry (MS). The molecular masses of the detected L-thanatin and C-thanatin were 2,435.95 and 2,433.95 Da, respectively, which were in good agreement with their calculated masses (2,435.97 and 2,433.97 Da, respectively).
Bacterial susceptibility testing.
The MIC values of C-thanatin and L-thanatin were determined by a microdilution method according to the CLSI guideline. Mueller-Hinton (MH) broth (100 μl) containing ESBL-producing E. coli ATCC 35218 (106 CFU/ml) was added to 100 μl of the culture medium containing the test compound (0.25 to 256 μg/ml in serial 2-fold dilutions). The plates were incubated at 37°C for 20 h in an incubator.
Compounds were added to cell cultures to achieve final concentrations of 1 μg/ml C-thanatin, 1 μg/ml L-thanatin, and 24 μg/ml ampicillin, with the addition of an equal volume of diluent (0.2% bovine serum albumin and 0.01% acetic acid) as a control. The growth curves for ESBL-producing E. coli ATCC 35218 were obtained using a Bioscreen C growth curve analyzer (Labsystems Diagnostics Oy, Helsinki, Finland) at 37°C and 600 nm at 1-h intervals for 24 h. The working volume in the wells of the Bioscreen plate was 300 μl and was comprised of 150 μl of bacterium suspension (106 CFU/ml) and 150 μl of drug solution.
In vitro toxicity.
Hemolysis was evaluated by determining the amount of hemoglobin released in 2% suspensions of fresh human erythrocytes (7, 8). Suspensions of human red blood cells (hRBCs) (100 μl) were added to a 96-well microtiter plate and incubated individually with Cap11-1-18m2, C-thanatin, or L-thanatin at 8, 16, 32, 64, 128, or 256 μg/ml for 6 h at 37°C. Hemolysis values of 0% and 100% were determined with a spectrophotometer in a phosphate-buffered saline (PBS) solution and 1% Triton X-100, respectively.
The cytotoxicity of C-thanatin or L-thanatin for the human umbilical vein endothelial cells (HUVECs) was determined by a standard MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay (9, 10). Briefly, cells (5 × 103 cells/well) were seeded in a 96-well microtiter plate with 100 μl Dulbecco's modified Eagle medium (DMEM) and 10% fetal bovine serum (FBS) in every well for 12 h and were then exposed with or without C-thanatin and L-thanatin at various concentrations (16 to 256 μg/ml) for 48 h. After drug treatment, an MTT solution (final concentration, 0.5%) was added, and cells were incubated for another 4 h at 37°C. Dimethyl sulfoxide (DMSO) (150 μl) was added to each well after removal of the supernatant, and the absorbance at 490 nm was measured using a microplate reader.
In vivo antibacterial activity.
To test whether C-thanatin and L-thanatin could provide the same survival benefit in vivo, male BALB/c mice 6 to 8 weeks of age and weighing 18 to 22 g were used in this study. The experimental and animal care procedures were approved by the animal care and use committee of Fourth Military Medical University. Sepsis was induced by intraperitoneal (i.p.) administration of an inoculum of 4 × 107 CFU ESBL-producing E. coli ATCC 35218 in 0.4 ml of Mueller-Hinton broth. The mice received a dose of C-thanatin and L-thanatin (5 or 10 mg/kg of body weight) intraperitoneally at 1, 6, 20, and 28 h after bacterial challenge, with the addition of an equal volume of diluent (0.2% bovine serum albumin and 0.01% acetic acid) as a control. The survival of 12 mice in each group was monitored for 7 days after infection, and the cumulative percentages of survival were determined.
Acute toxicity (11).
The acute toxicity experiment was performed by i.p. injection of the test C-thanatin and L-thanatin compounds into groups of 10 BALB/c mice. The dose of peptide administered per mouse was 60 mg/kg. Animals were directly inspected for adverse effects for 4 h, and mortality was monitored for 15 days thereafter.
CD spectra.
Circular dichroism (CD) spectra of C-thanatin and L-thanatin were measured by the use of a BioLogic MOS 450 CD spectrophotometer (BioLogic, France) equipped with nitrogen-purging capabilities at room temperature. The CD spectra were recorded in the range of 190 to 260 nm with a response rate of 0.5 s at 1.0-nm bandwidth and corrected by subtraction of the background scan with water. The concentration of both C-thanatin and L-thanatin was 0.5 mg/ml, and a 2-mm-path-length cuvette was used. The scan was repeated three times. The mean residue ellipticity (MRE) ([θ]; given in degrees per square centimeter per decimole) was calculated as [θ] = [θ]obs × (MRW/10lc), where [θ]obs is the observed ellipticity (in millidegrees), MRW is the mean residue molecular weight of the peptide, l is the path length of the cell (in centimeters), and c is the peptide concentration (in milligrams per milliliter) (12). After 72 h at −20°C, the experiment was repeated as described above.
Membrane permeabilization assay.
Mid-log-phase E. coli ATCC 35218 was diluted to 108 CFU/ml. The peptides were then added to cell cultures to achieve a final concentration of 6 μg/ml C-thanatin or L-thanatin with the addition of an equal volume of diluent as a control. A 1-ml volume of E. coli ATCC 35218 was collected at different time points. The outer membrane permeabilization activity of the peptides was determined as previously described (13). The collected cells were washed and resuspended in 5 mM HEPES and 5 mM glucose buffer (pH 7.2) and then incubated with NPN (1-N-phenylnaphthylamine) (10 μl from a 500 μM stock in acetone) for 30 min at 25°C. The samples were then transferred to cuvettes, and fluorescence was measured using an F-2500 fluorescence spectrophotometer (Hitachi, Japan) at 350 nm (excitation wavelength) and 420 nm (emission wavelength). The inner membrane permeabilization activity of the peptides was measured by the uptake of propidium iodide (PI) (14, 15). The collected cells were washed and resuspended as described above, and then 10 μM PI was added to the cells and the reaction mixture was incubated for 30 min at 25°C. The fluorescence of the dye was monitored using a fluorescence spectrophotometer at 535 nm (excitation wavelength) and 617 nm (emission wavelength).
HUVECs were maintained in DMEM containing 10% fetal bovine serum (FBS) at 37°C with 5% CO2. When the cells reached confluence, the media with FBS was removed, and the cells were washed with 1× PBS, trypsinized, centrifuged (800 rpm for 5 min), and counted in a hemocytometer. Thereafter, cells (1 × 105/well) were plated into 24-well plates and the stock solution of peptides was added to cell cultures at a final concentration of 6 μg/ml C-thanatin or L-thanatin, with the addition of an equal volume of diluent as a negative control and 0.1% Triton X-100 as a positive control. Subsequently, 1 ml HUVECs was harvested at 0, 30, 60, 90, 120, 150, 180, 210, and 240 min, washed, and resuspended in PBS. Then, 10 μM PI was added to the cells, after which they were incubated and monitored as mentioned above.
Membrane depolarization assay.
The cytoplasmic membrane depolarization activities of the peptides were determined using the membrane potential-sensitive dye disC3(5) (3,3′-dipropylthiadicarbocyanine iodide) (14). The harvested E. coli ATCC 35218 cells collected at different time points were washed with 5 mM HEPES and 5 mM glucose and were resuspended in 5 mM glucose–5 mM HEPES buffer–100 mM KCl solution in a 1:1:1 ratio. The cells were first treated with 0.2 mM EDTA (pH 8.0) in order to permeabilize the outer membrane to allow dye uptake. Then, a stock solution of disC3(5) was added to achieve a final concentration of 120 nM and quenching was allowed to occur at room temperature for 30 min. The fluorescence of the dye was monitored using a fluorescence spectrophotometer at 622 nm (excitation wavelength) and 670 nm (emission wavelength).
Statistical analyses.
Data are expressed as means ± standard deviations (SD) in Fig. 1, 2, and 3B (see also Fig. 5A to C). Data are shown as means ± standard errors of the means (SEM) (see Fig. 5D). Two-way analysis of variance (ANOVA) was used for the data presented in Fig. 1 and 2A (see also Fig. 5). Comparisons of three or more groups were performed using one-way ANOVA (Fig. 2B and 3B). Survival curves were calculated by the Kaplan-Meier method (Fig. 3A). Statistical analyses were done using Prism software (version 6; GraphPad, CA). P values of ≤0.05 was considered statistically significant.
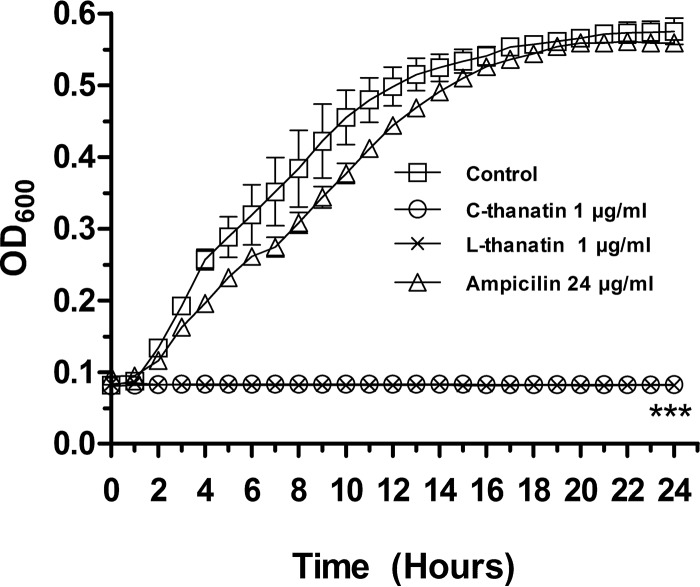
FIG 1.
Growth curves of C-thanatin, L-thanatin, and ampicillin. C-thanatin, L-thanatin, and ampicillin were added to cell cultures containing ESBL-producing E. coli ATCC 35218 to achieve a final concentration of 1 μg/ml C-thanatin or L-thanatin and 24 μg/ml ampicillin with the addition of an equal volume of diluent as a control. The optical density at 600 nm (OD600) of the cell suspensions was measured automatically with an automated Bioscreen C system in regular intervals of 1 h for 24 h. ***, P < 0.001 (versus control group).
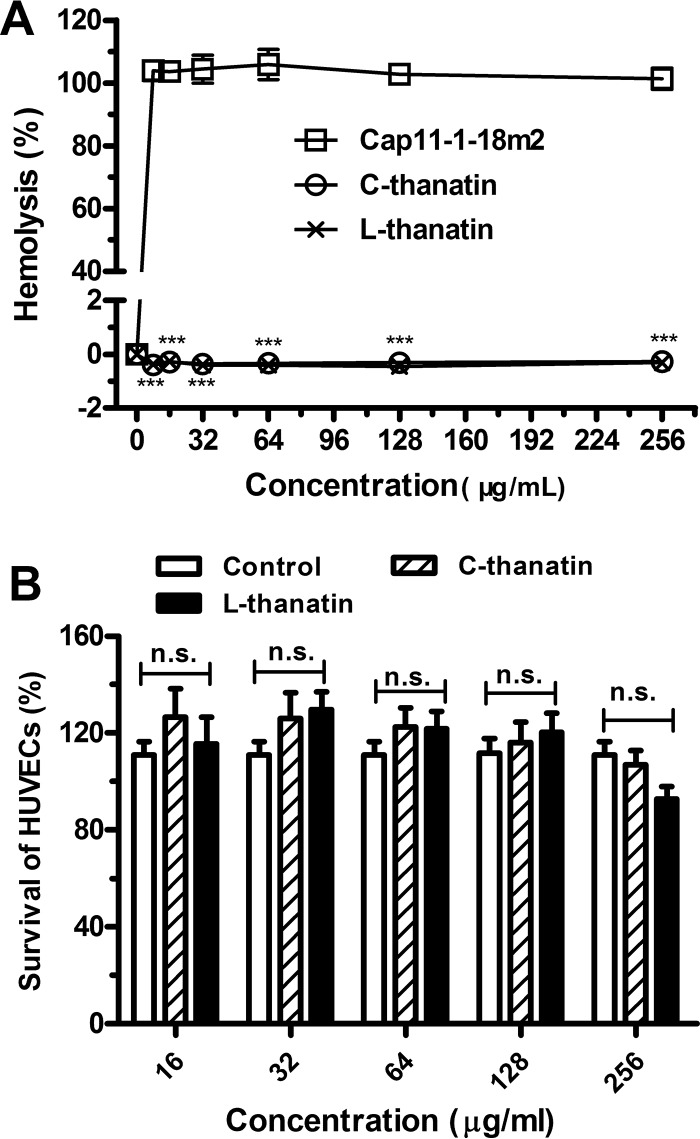
FIG 2.
Toxicity of C-thanatin and L-thanatin in vitro. (A) Hemolysis of C-thanatin and L-thanatin was determined on human red blood cells (2% hematocrit) after 6 h of incubation. The data were shown as means ± SD of results determined for 4 samples. ***, P < 0.001 (versus the Cap11-1-18m2-treated group). (B) The absorbance at 490 nm of HUVECs after incubation without or with various concentrations of C-thanatin and L-thanatin for 48 h. Each plot was obtained from a representative experiment, and the data points are the means of the results from four replicates ± SD. The data were shown as means ± SD of results determined for 8 samples. n.s., no significant difference from control group results.
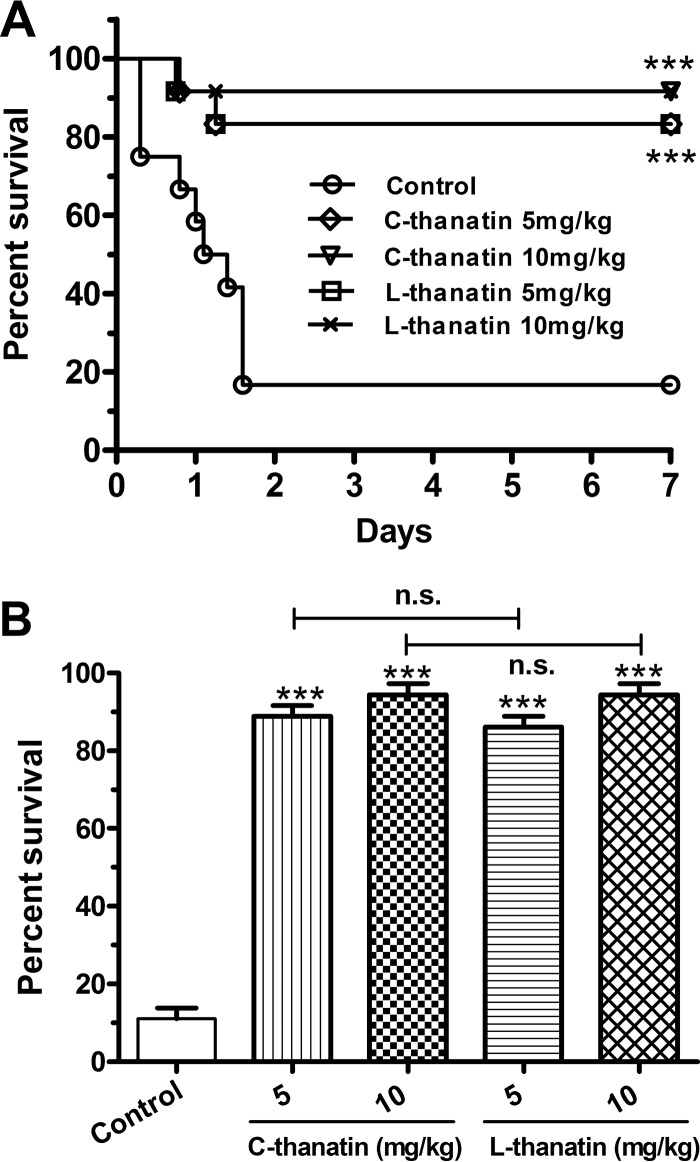
FIG 3.
Both C-thanatin and L-thanatin rescued BALB/c sepsis in mice caused by ESBL-producing E. coli ATCC 35218. Data represent survival rates of BALB/c mice (n = 12 mice/group) inoculated with ESBL-producing E. coli ATCC 35218 and treated with a 5 or 10 mg/kg dose of C-thanatin and L-thanatin and the same amount of sterile water by i.p. injection at 1, 6, 20, and 28 h after bacterial challenge. ***, P < 0.001 (versus control group), n.s., no significant difference.
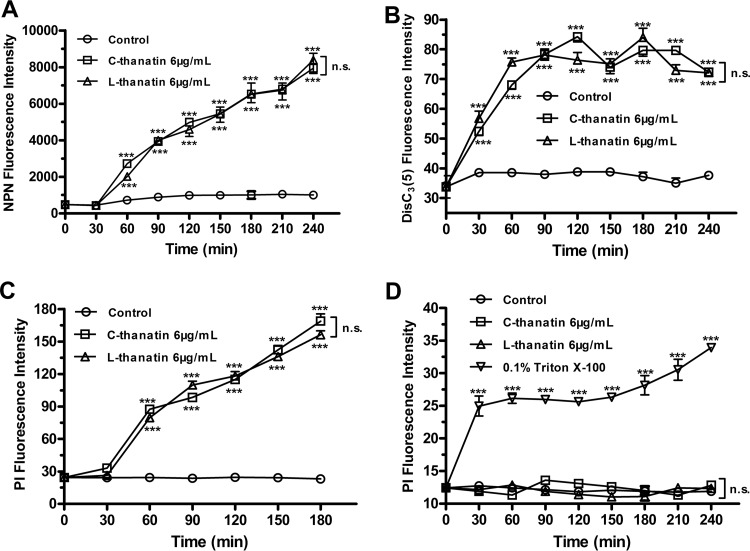
FIG 5.
Membrane permeabilization and depolarization assay. (A) Outer membrane permeabilization of E. coli ATCC 35218 with NPN dye incubation after C-thanatin or L-thanatin treatment. (B) Cytoplasmic membrane depolarization of E. coli ATCC 35218 treated with diSC3(5) dye incubation after C-thanatin or L-thanatin treatment. (C) Cytoplasmic membrane permeabilization of E. coli ATCC 35218 treated with PI dye incubation after C-thanatin or L-thanatin treatment. (D) Cytoplasmic membrane permeabilization of HUVECs treated with PI dye incubation after C-thanatin or L-thanatin treatment. ***, P < 0.001 (versus control results).
RESULTS
Bacterial susceptibility testing and growth assay.
No differences between the C-thanatin and L-thanatin MICs were observed. Both C-thanatin and L-thanatin exerted potent antibacterial effects against almost all Gram-negative bacteria tested, including clinically prevalent and challenging species such as ESBL-producing E. coli ATCC 35218, with most MIC values found to be ≤1 μg/ml (Table 1). Meanwhile, the MIC values of C-thanatin and L-thanatin against Gram-positive bacteria, S. aureus ATCC 29213, and MRSE were similar (Table 1). Growth of ESBL-producing E. coli ATCC 35218 was totally inhibited in the group treated with 1 μg/ml C-thanatin or L-thanatin. However, it was not affected by treatment with 24 μg/ml ampicillin (Fig. 1).
TABLE 1.
MICs of C-thanatin and L-thanatin in Mueller-Hinton broth culture
| Strain | MIC (μg/ml) |
|||
|---|---|---|---|---|
| C-thanatin | L-thanatin | Ceftazidime | Amikacin | |
| S. aureus ATCC 29213 | 256 | 256 | 8 | 1 |
| MRSE | 16 | 16 | 256 | 1 |
| P. aeruginosa ATCC 27853 | 16 | 16 | 1 | 1 |
| MDR P. aeruginosa XJ75315 | 1 | 1 | 64 | 4 |
| E. coli ATCC 25922 | 1 | 1 | 0.5 | 1 |
| ESBL-producing E. coli ATCC 35218 | 1 | 1 | 0.5 | 4 |
| EHEC | 1 | 1 | 0.5 | 4 |
| ESBL-producing K. pneumoniae XJ75297 | 1 | 1 | >256 | >256 |
| S. Typhimurium SL1344 | 1 | 1 | 0.5 | 4 |
| S. flexneri ATCC 12022 | 0.25 | 0.25 | 4 | 4 |
Toxicity.
Neither C-thanatin nor L-thanatin showed hemolytic toxicity in 2% hRBC suspensions at 256 μg/ml, a concentration 250 times higher than the MIC values for ESBL-producing E. coli ATCC 35218 (Fig. 2A). Moreover, the levels of viability of HUVECs were not different between the control group and the C-thanatin or L-thanatin-treated groups at the concentration of 256 μg/ml (Fig. 2B). These results suggested that neither C-thanatin nor L-thanatin exhibited toxicity for mammalian cells.
No adverse reaction was observed in BABL/c mice, and all mice survived for 15 days after i.p. injection of 60 mg/kg C-thanatin and L-thanatin.
In vivo antibacterial activity of C-thanatin and L-thanatin.
To study the bactericidal effects of C-thanatin and L-thanatin in vivo, we monitored their effects on the survival of mice infected with ESBL-producing E. coli ATCC 35218. Ten mice died after bacterial challenge within 2 days in the ESBL-producing-E. coli-infected group (twelve mice per group). After bacterial challenge, the survival rates were 16.7%, 83.3%, 83.3%, 91.7%, and 91.7% for mice treated with diluent, 5 mg/kg C-thanatin, 5 mg/kg L-thanatin, 10 mg/kg C-thanatin, and 10 mg/kg L-thanatin, respectively (Fig. 3). These data indicated that the in vivo antibacterial properties of C-thanatin and L-thanatin were similar.
Secondary structures of the peptides.
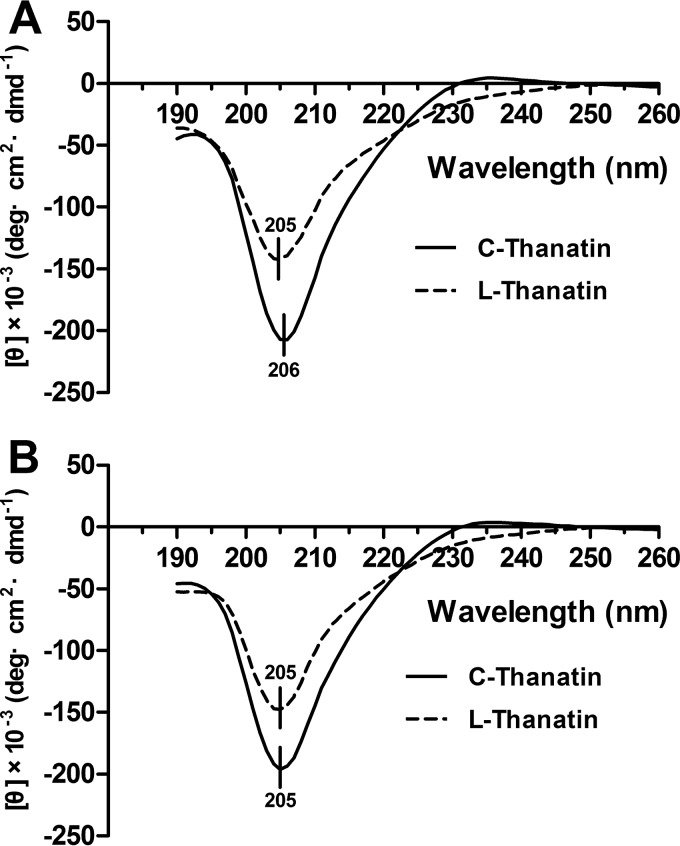
In this study, the C-thanatin spectrum displayed one negative spike at 206 nm and L-thanatin displayed a consistent pattern with one negative spike at 205 nm (Fig. 4A). After being kept at −20°C for 3 days, C-thanatin and L-thanatin both had a negative spike at 205 nm (Fig. 4B). These results showed that C-thanatin and L-thanatin had highly similar secondary structures.
FIG 4.
CD spectra of C-thanatin and T-thanatin used in this study. (A) The concentration of both C-thanatin and T-thanatin was 0.5 mg/ml. (B) After being kept at −20°C for 3 days, the sample data were determined as described in the text. The scan was repeated three times.
Bacterial outer membrane permeabilization assay.
NPN is a small hydrophobic molecule that cannot cross the intact bacterial outer membrane. However, when the outer cell membrane is damaged, NPN goes into the phospholipid layer and exhibits increased fluorescence. Therefore, NPN can be used to identify the kinetics of outer membrane permeabilization. Similar time-dependent rises in fluorescence intensity were observed after both C-thanatin treatment and L-thanatin treatment, and no significant differences were detected in fluorescence levels between samples treated with these two peptides (Fig. 5A).
Bacterial inner membrane depolarization assay.
C-thanatin or L-thanatin treatment caused an increase in disC3(5) fluorescence intensity in a time-dependent manner (Fig. 5B), indicating that both peptides can cause destabilization of the cytoplasmic membrane. There was no significant difference in disC3(5) release between samples treated with C-thanatin and those treated with L-thanatin (Fig. 5B), which indicated that the two peptides have similar abilities to cause E. coli inner membrane depolarization.
Bacterial inner membrane permeabilization assay.
Bacterial membrane permeability was investigated by using red propidium iodide (PI), which enters cells only when the cell membrane is compromised and fluoresces upon binding to nucleic acids. PI fluorescence intensity increased in an obvious and time-dependent manner after C-thanatin or L-thanatin treatment, and no difference was observed in the effects of these two peptides (Fig. 5C). These results indicate that the two peptides cause similar increases in bacterial cell membrane permeability.
Cell membrane permeabilization assay.
In contrast to bacterial cytoplasmic membranes, the HUVEC membrane was not affected by C-thanatin and L-thanatin. No increase in PI fluorescence intensity was observed after C-thanatin or L-thanatin treatment for 4 h, which indicated that neither of the peptides caused significant cell membrane permeability (Fig. 5D).
DISCUSSION
Thanatin is the only insect peptide with a β-hairpin motif (16), which resembles a hairpin with two adjacent beta strands in the primary structure. A cysteine containing a sulfur atom is present in each of the beta strands. These sulfur atoms form a covalent disulfide bond that stabilizes the tertiary structure in several proteins (17). Lee et al. (18) found that the linear derivative of arenicin-1 was less active against bacterial cells than native arenicin-1. Its disulfide bridge plays important roles in its biological activities. Similarly, Haag et al. (19) found that the nodule-specific cysteine-rich peptide (NCR247) had decreased activity against Sinorhizobium meliloti when cysteines were substituted for serines or the S-S bridges were changed from cysteines 1-2 and 3-4 to cysteines 1-3 and 2-4.
However, other data differ from the above-mentioned results. Wu et al. (20) found that human β-defensin-3 (hBD-3) remained unaffected by bactericidal activity in the absence of a disulfide bridge. Mangoni et al. (21) and Dawson and Liu (5) reported that linear PG-1 was at least as active as cyclic PG-1 against E. coli and that their hemolytic activities were also similar (5). Unexpectedly, Schroeder et al. found that hBD1 with reduced disulfide bridges became a potent antimicrobial peptide against the opportunistic pathogenic fungus Candida albicans as well as against the anaerobic and Gram-positive commensals of Bifidobacterium and Lactobacillus species (22). These data showed that disulfide bonds of antimicrobial peptides are not essential for their antimicrobial activities.
In the current study, we investigated whether thanatin molecules without disulfide bridges retain potent antimicrobial activity. Previous studies showed that, after its two cysteine residues were modified with tert-butyl groups, thanatin exhibited improved antimicrobial activity toward Micrococcus luteus but lower activity against E. coli (23). These data suggested that the disulfide bridge in thanatin is closely related to its antimicrobial activity and specificity (23). Orikasa et al. (24) found that chemically modified thanatin with a normal octyl group (C8H17) in the side chain of cysteine residues exhibited 8-fold-higher antimicrobial activity against M. luteus than wild-type thanatin.
To investigate the role of the disulfide bond in thanatin, we synthesized linear L-thanatin and assessed the antimicrobial activities of both types of thanatin in vivo and in vitro. Our data showed that both C-thanatin and L-thanatin displayed potent antibiotic activities, especially against Gram-negative bacteria, including susceptible and resistant strains. The C-thanatin and L-thanatin MICs were not significantly different for all the tested bacteria (eight Gram-negative and two Gram-positive species). The in vivo results showed that thanatin significantly improved the survival rate of ESBL-producing-E. coli-infected mice from 16.7% for the control group to 83.3% and 91.7% for the groups treated with 5 and 10 mg/kg C-thanatin or L-thanatin, respectively. As expected, the same survival rates were observed in ESBL-producing-E. coli-infected mice after L-thanatin treatment. Furthermore, neither C-thanatin nor L-thanatin showed toxicity for hRBCs and HUVECs at concentrations as high as 256 μg/ml, which was 250 times higher than the MIC values for ESBL-producing E. coli ATCC 35218. Highly similar CD detection results were also found for C-thanatin and L-thanatin. Overall, these results indicate that the disulfide bond of thanatin is dispensible for its secondary structure, antimicrobial activity, and cell toxicity.
Experiments assessing membrane permeabilization and depolarization showed that C-thanatin and L-thanatin have similar abilities to permeabilize the outer and inner membranes and to induce membrane depolarization in ESBL-producing E. coli. This indicates that the two peptides have very similar actions with respect to bacterial cell membranes. However, neither causes significant membrane permeability in HUVECs, suggesting that they have high selectivity with respect to Gram-negative bacterial cell membranes.
Membrane disruption is the main mechanism of action of many antimicrobial peptides, but a single peptide can have multiple cellular targets that simultaneously contribute to the death of the microorganism (25). For example, indolicidin appears to promote significant membrane depolarization and DNA synthesis inhibition (26, 27). Nisin not only binds to lipid II-forming membrane pores (28) and lipids III and IV interfering with teichoic and lipoteichoic acid biosynthesis (29) but also stimulates autolysin activity. PR-39 can block bacterial DNA and protein synthesis (30) and can inhibit the degradation of inhibitor of nuclear factor κB (31), thereby attenuating inflammation. However, the intracellular targets of thanatin have not been reported to date.
In conclusion, our data demonstrated that the disulfide bond in thanatin is not necessary for its antimicrobial activity. Therefore, due to its simpler synthesis process and lower cost of production, L-thanatin can be used as a low-cost alternative to native C-thanatin for antimicrobial treatment.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (no. 81001460, 81471997, and 81473252).
We thank Xiuli Xu (Xijing Hospital, Shaanxi, China) for clinical strains.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Hou Z, Da F, Liu B, Xue X, Xu X, Zhou Y, Li M, Li Z, Ma X, Meng J, Jia M, Wang Y, Luo X. 2013. R-thanatin inhibits growth and biofilm formation of methicillin-resistant Staphylococcus epidermidis in vivo and in vitro. Antimicrob Agents Chemother 57:5045–5052. doi: 10.1128/AAC.00504-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou Z, Lu J, Fang C, Zhou Y, Bai H, Zhang X, Xue X, Chen Y, Luo X. 2011. Underlying mechanism of in vivo and in vitro activity of C-terminal-amidated thanatin against clinical isolates of extended-spectrum beta-lactamase-producing Escherichia coli. J Infect Dis 203:273–282. doi: 10.1093/infdis/jiq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marr AK, Gooderham WJ, Hancock RE. 2006. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KM, Kumari YB, Mallikharjunasarma D, Bulliraju K, Sreelatha V, Ananda K. 2012. Large scale solid phase synthesis of peptide drugs: use of commercial anion exchange resin as quenching agent for removal of iodine during disulphide bond formation. Int J Pept 2012:323907. doi: 10.1155/2012/323907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson RM, Liu CQ. 2010. Disulphide bonds of the peptide protegrin-1 are not essential for antimicrobial activity and haemolytic activity. Int J Antimicrob Agents 36:579–580. doi: 10.1016/j.ijantimicag.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Fehlbaum P, Bulet P, Chernysh S, Briand JP, Roussel JP, Letellier L, Hetru C, Hoffmann JA. 1996. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc Natl Acad Sci U S A 93:1221–1225. doi: 10.1073/pnas.93.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kustanovich I, Shalev DE, Mikhlin M, Gaidukov L, Mor A. 2002. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J Biol Chem 277:16941–16951. doi: 10.1074/jbc.M111071200. [DOI] [PubMed] [Google Scholar]
- 8.Tossi A, Scocchi M, Zanetti M, Gennaro R, Storici P, Romeo D. 1997. An approach combining rapid cDNA amplification and chemical synthesis for the identification of novel, cathelicidin-derived, antimicrobial peptides. Methods Mol Biol 78:133–150. [DOI] [PubMed] [Google Scholar]
- 9.Xue X, Chen X, Mao X, Hou Z, Zhou Y, Bai H, Meng J, Da F, Sang G, Wang Y, Luo X. 2013. Amino-terminated generation 2 poly(amidoamine) dendrimer as a potential broad-spectrum, nonresistance-inducing antibacterial agent. AAPS J 15:132–142. doi: 10.1208/s12248-012-9416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loreto ES, Mario DA, Denardi LB, Alves SH, Santurio JM. 2011. In vitro susceptibility of Pythium insidiosum to macrolides and tetracycline antibiotics. Antimicrob Agents Chemother 55:3588–3590. doi: 10.1128/AAC.01586-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navon-Venezia S, Feder R, Gaidukov L, Carmeli Y, Mor A. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob Agents Chemother 46:689–694. doi: 10.1128/AAC.46.3.689-694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MK, Cha L, Lee SH, Hahm KS. 2002. Role of amino acid residues within the disulfide loop of thanatin, a potent antibiotic peptide. J Biochem Mol Biol 35:291–296. doi: 10.5483/BMBRep.2002.35.3.291. [DOI] [PubMed] [Google Scholar]
- 13.Hancock RE, Farmer SW, Li ZS, Poole K. 1991. Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and OmpF porin of Escherichia coli. Antimicrob Agents Chemother 35:1309–1314. doi: 10.1128/AAC.35.7.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uppu DS, Akkapeddi P, Manjunath GB, Yarlagadda V, Hoque J, Haldar J. 2013. Polymers with tunable side-chain amphiphilicity as non-hemolytic antibacterial agents. Chem Commun 49:9389–9391. doi: 10.1039/c3cc43751e. [DOI] [PubMed] [Google Scholar]
- 15.Yarlagadda V, Akkapeddi P, Manjunath GB, Haldar J. 2014. Membrane active vancomycin analogues: a strategy to combat bacterial resistance. J Med Chem 57:4558–4568. doi: 10.1021/jm500270w. [DOI] [PubMed] [Google Scholar]
- 16.Panteleev PV, Bolosov IA, Balandin SV, Ovchinnikova TV. 2015. Structure and biological functions of beta-hairpin antimicrobial peptides. Acta Naturae 7:37–47. [PMC free article] [PubMed] [Google Scholar]
- 17.Ladenstein R, Ren B. 2006. Protein disulfides and protein disulfide oxidoreductases in hyperthermophiles. FEBS J 273:4170–4185. doi: 10.1111/j.1742-4658.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JU, Kang DI, Zhu WL, Shin SY, Hahm KS, Kim Y. 2007. Solution structures and biological functions of the antimicrobial peptide, arenicin-1, and its linear derivative. Biopolymers 88:208–216. doi: 10.1002/bip.20700. [DOI] [PubMed] [Google Scholar]
- 19.Haag AF, Kerscher B, Dall'Angelo S, Sani M, Longhi R, Baloban M, Wilson HM, Mergaert P, Zanda M, Ferguson GP. 2012. Role of cysteine residues and disulfide bonds in the activity of a legume root nodule-specific, cysteine-rich peptide. J Biol Chem 287:10791–10798. doi: 10.1074/jbc.M111.311316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W. 2003. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci U S A 100:8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangoni ME, Aumelas A, Charnet P, Roumestand C, Chiche L, Despaux E, Grassy G, Calas B, Chavanieu A. 1996. Change in membrane permeability induced by protegrin 1: implication of disulphide bridges for pore formation. FEBS Lett 383:93–98. doi: 10.1016/0014-5793(96)00236-0. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. 2011. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature 469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T, Yamamoto N, Tamura A, Murabayashi S, Hashimoto S, Shimada H, Taguchi S. 2008. NMR based structure-activity relationship analysis of an antimicrobial peptide, thanatin, engineered by site-specific chemical modification: activity improvement and spectrum alteration. Biochem Biophys Res Commun 369:609–615. doi: 10.1016/j.bbrc.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 24.Orikasa Y, Ichinohe K, Saito J, Hashimoto S, Matsumoto K, Ooi T, Taguchi S. 2009. The hydrophobicity in a chemically modified side-chain of cysteine residues of thanatin is related to antimicrobial activity against Micrococcus luteus. Biosci Biotechnol Biochem 73:1683–1684. doi: 10.1271/bbb.90183. [DOI] [PubMed] [Google Scholar]
- 25.Guilhelmelli F, Vilela N, Albuquerque P, Derengowski LDS, Silva-Pereira I, Kyaw CM. 2013. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front Microbiol 4:353. doi: 10.3389/fmicb.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbalakshmi C, Sitaram N. 1998. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett 160:91–96. doi: 10.1111/j.1574-6968.1998.tb12896.x. [DOI] [PubMed] [Google Scholar]
- 27.Nan YH, Park KH, Park Y, Jeon YJ, Kim Y, Park IS, Hahm KS, Shin SY. 2009. Investigating the effects of positive charge and hydrophobicity on the cell selectivity, mechanism of action and anti-inflammatory activity of a Trp-rich antimicrobial peptide indolicidin. FEMS Microbiol Lett 292:134–140. doi: 10.1111/j.1574-6968.2008.01484.x. [DOI] [PubMed] [Google Scholar]
- 28.Yount NY, Yeaman MR. 2013. Peptide antimicrobials: cell wall as a bacterial target. Ann N Y Acad Sci 1277:127–138. doi: 10.1111/nyas.12005. [DOI] [PubMed] [Google Scholar]
- 29.Müller A, Ulm H, Reder-Christ K, Sahl HG, Schneider T. 2012. Interaction of type A lantibiotics with undecaprenol-bound cell envelope precursors. Microb Drug Resist 18:261–270. doi: 10.1089/mdr.2011.0242. [DOI] [PubMed] [Google Scholar]
- 30.Bals R, Wilson JM. 2003. Cathelicidins—a family of multifunctional antimicrobial peptides. Cell Mol Life Sci 60:711–720. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anbanandam A, Albarado DC, Tirziu DC, Simons M, Veeraraghavan S. 2008. Molecular basis for proline- and arginine-rich peptide inhibition of proteasome. J Mol Biol 384:219–227. doi: 10.1016/j.jmb.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]