Abstract
There have been concerns about an association of fluoroquinolone (FQ) use prior to tuberculosis (TB) diagnosis with adverse outcomes. However, FQ use might prevent clinical deterioration in missed TB patients, especially in those who are immunocompromised, until they receive definitive anti-TB treatment. All adult immunocompromised patients with smear-negative and culture-positive TB at a tertiary care hospital in Korea over a 2-year period were included in this study. Long-term FQ (≥7 days) use was defined as exposure to FQ for at least 7 days prior to TB diagnosis. A total of 194 patients were identified: 33 (17%) in the long-term FQ group and 161 (83%) in the comparator, including a short-term FQ group (n = 23), non-FQ group (n = 78), and a group receiving no antibiotics (n = 60). Patients in the long-term FQ group presented with atypical chest radiologic pattern more frequently than those in the comparator (77% [24/31] versus 46% [63/138]; P = 0.001). The median time from mycobacterial test to positive mycobacterial culture appeared to be longer in the long-term FQ group (8.1 weeks versus 7.7 weeks; P = 0.09), although the difference was not statistically significant. Patients in the long-term FQ group were less likely to receive empirical anti-TB treatment (55% versus 74%; P = 0.03). The median time from mycobacterial test to anti-TB therapy was longer in the long-term FQ group (4.6 weeks versus 2.2 weeks; P < 0.001), but there was no significant difference in FQ resistance (0% versus 3%; P > 0.99) or in the 30-day (6% versus 6%; P > 0.99) or 90-day (12% versus 12%; P > 0.99) mortality rate between the two groups. FQ exposure (≥7 days) prior to TB diagnosis in immunocompromised patients appears not to be associated with adverse outcomes.
INTRODUCTION
In spite of global efforts to control tuberculosis (TB), it remains a serious health concern and a major cause of death worldwide (1). As the clinical manifestations of TB are affected by the host immune response to Mycobacterium tuberculosis (2), immunocompromised patients are considered to be at particular risk of TB and are prone to disseminated disease, highlighting the importance of early diagnosis and prompt treatment in these patients (3–10). However, clinical features and radiologic findings are various and often atypical in immunocompromised patients with TB, and thus TB is occasionally misdiagnosed as other infectious diseases, which may contribute to morbidity and mortality (3–10).
Fluoroquinolones (FQs) are one of the most commonly prescribed classes of antibiotics for a broad range of bacterial infections and have excellent in vitro and in vivo activities against M. tuberculosis (11–14). They are the preferred agents for treating multidrug-resistant TB and for patients who cannot tolerate first-line drugs (1). Such a broad-spectrum antimicrobial activity of FQs has raised concerns that FQ exposure for any reason prior to TB diagnosis may be associated with adverse outcomes, such as a delay in anti-TB treatment (15–17), the emergence of FQ resistance (18–21), and increased mortality (22, 23). However, conflicting results have been obtained in several other studies (24–27), and it is theoretically possible that FQ use until definitive anti-TB treatment is given might prevent clinical deterioration in missed TB patients. We therefore evaluated the association of FQ use prior to TB diagnosis with clinical outcomes in immunocompromised patients with TB, in whom TB is quite often missed.
MATERIALS AND METHODS
Study design and patient selection.
This retrospective study was performed at the Asan Medical Center, a 2,700-bed tertiary care-affiliated teaching hospital in Seoul, Republic of Korea, a country with intermediate TB burden (annual TB incidence rate in 2012, 108 per 100,000 population). All adult patients (aged ≥18 years) with culture-confirmed TB from January 2012 through December 2013 were identified using electronic medical record databases. Microbiologic specimens for diagnosing TB were processed by standard techniques and procedures, as previously described (10). Immunocompromised patients were defined as those with underlying diseases, such as human immunodeficiency virus (HIV) infection, malignancy, liver cirrhosis, and chronic renal failure, and those receiving immunosuppressive treatment (10). Immunocompetent patients and those with smear-positive TB, who were less likely to be missed TB patients, were excluded from the analysis. The study was approved by the Asan Medical Center Institutional Review Board.
Data collection and definitions.
All medical records were retrospectively reviewed using standardized study protocols. Demographic characteristics, laboratory results, underlying diseases or conditions, patient management, and clinical outcomes were evaluated. All patients who visited our hospital were routinely required to report information about antibiotic prescription during the previous month in electronic nurse and doctor's records. In addition, we used primary care prescription records provided by referring physicians. Pulmonary and extrapulmonary TB was classified according to World Health Organization recommendations (28). Disseminated TB was defined as isolation of M. tuberculosis, positive PCR, or histologic demonstration of caseating granulomatous inflammation from blood, bone marrow, liver biopsy specimen, or at least 2 noncontiguous organs, with or without miliary lung lesions (29).
As shown in Fig. S1 in the supplemental material, empirical anti-TB therapy was classified according to the administration of the first-line anti-TB drugs, such as isoniazid (INH), rifampin (RFP), ethambutol (EMB), and pyrazinamide (PZA), before a positive mycobacterial culture or a positive M. tuberculosis PCR assay result was obtained. Definite anti-TB therapy was classified according to the administration of the first-line anti-TB drugs after a positive mycobacterial culture or a positive M. tuberculosis PCR assay result was obtained, regardless of previous antibiotic use. FQ use was classified according to the presence of FQ in the antibiotic regimen, regardless of the use of the first-line anti-TB drugs. In order to select patients who were likely to have been exposed to FQ when they had active TB, we chose the period of FQ exposure to be between 4 weeks before the date that the test that gave the first positive result of mycobacterial culture was requested and the date when anti-TB treatment was started. In addition, FQ use was further divided into short-term use (for less than 7 days) and long-term use (for 7 or more days).
Patients who had pulmonary manifestations of TB were considered to have atypical chest images if their chest images at the time of presentation did not have typical chest radiological patterns of TB as described elsewhere (30, 31). Treatment default was defined as interruption of anti-TB treatment for any reason. The primary outcomes were 30-day and 90-day mortalities.
Statistical analysis.
Statistical analyses were performed using the SPSS for Windows software package, version 21.0 (SPSS Inc., Chicago, IL). Categorical variables were analyzed using the chi-square or Fisher's exact test. Normally and non-normally distributed continuous variables were analyzed by Student's t test and the Mann-Whitney U test, respectively. Survival analysis was conducted by the Kaplan-Meier method, and 90-day cumulative survival rates were compared by the log rank test. All tests of significance were 2-tailed, and a P value of <0.05 was considered statistically significant.
RESULTS
Study population.
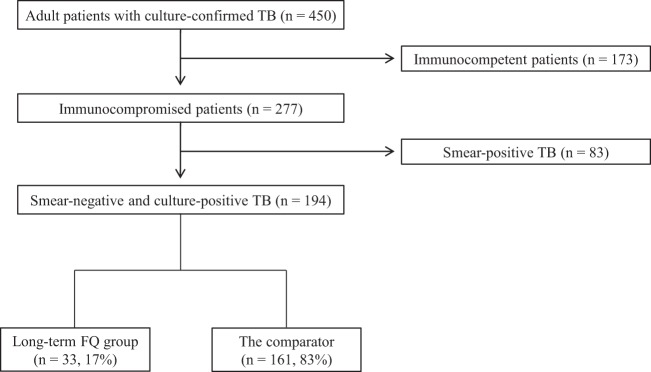
Figure 1 shows a flow diagram of the study. A total of 450 adult patients with culture-confirmed TB were identified during the study period. Of these, 173 (39%) immunocompetent patients and 83 (18%) immunocompromised patients with smear-positive TB were excluded. The remaining 194 (43%) immunocompromised patients with smear-negative and culture-positive TB were included in the final analysis. Of these 194 patients, 33 (17%) were classified in the long-term FQ group and the remaining 161 (83%) were classified in the comparator, including the short-term FQ group (n = 23), the non-FQ group (n = 78), and the group given no antibiotics (n = 60). Treatment indications for patients who received short-term (<7 days) FQ or non-FQ antibiotics are shown in Table S1 in the supplemental material.
FIG 1.
Flow diagram of the study. FQ, fluoroquinolone; TB, tuberculosis.
Demographics and clinical characteristics.
Demographics, clinical characteristics, treatments, and outcomes of the 194 immunocompromised patients with smear-negative and culture-positive TB are shown in Table 1. The mean age was 61 years (standard deviation [SD], ±15), and 141 (73%) were male. The clinical diagnoses of TB were classified as pulmonary TB (n = 160 [82%]), extrapulmonary TB (n = 23 [12%]), and disseminated TB (n = 11 [6%]). Solid tumor was the most common underlying disease or condition (64%), followed by chronic renal failure (10%), connective tissue disease (8%), hematologic malignancy (7%), transplantation (7%), liver cirrhosis (6%), and HIV infection (0.5%). Patients in the long-term FQ group were more likely to undergo transplantation than those in the comparator (15% [5/33] versus 5% [8/161]; P = 0.05). Immunosuppressive agents were more frequently used during the previous month in the long-term FQ group (49% [16/33] versus 31% [50/161]; P = 0.05). Among the patients with pulmonary manifestations of TB (87% [169/194]), patients in the long-term FQ group had atypical chest image at the time of presentation more frequently than those in the comparator (77% [24/31] versus 46% [63/138]; P = 0.001). Anti-TB drug susceptibility testing of the M. tuberculosis isolates in the first positive mycobacterial culture was available for 148 (76%) patients. Of the 33 patients in the long-term FQ group, 9 (27%) had the exposure to FQ after the anti-TB drug susceptibility test. The overall rate of multidrug resistance was 2%. Isoniazid resistance was more common in the long-term FQ group (15% [4/26] versus 4% [5/122]; P = 0.05). There was no significant difference in FQ resistance between the two groups (0% [0/26] versus 3% [3/122]; P > 0.99).
TABLE 1.
Demographics, clinical characteristics, treatments, and outcomes of the 194 immunocompromised patients with smear-negative and culture-positive tuberculosisa
| Variable | Value for: |
P value | |
|---|---|---|---|
| Long-term FQ groupb(n = 33) | Comparatorc(n = 161) | ||
| Demographics | |||
| Age, yrs (mean ± SD) | 64 ± 15 | 60 ± 15 | 0.13 |
| Male sex | 21 (64) | 120 (75) | 0.20 |
| Clinical diagnosis | |||
| Pulmonary TB | 30 (91) | 130 (81) | 0.16 |
| Extrapulmonary TB | 2 (6) | 21 (13) | 0.38 |
| Disseminated TB | 1 (3) | 10 (6) | 0.69 |
| Underlying disease | |||
| Solid tumor | 18 (55) | 107 (67) | 0.19 |
| Transplantation | 5 (15) | 8 (5) | 0.05 |
| Hematologic malignancy | 4 (12) | 10 (6) | 0.26 |
| Chronic renal failure | 4 (12) | 16 (10) | 0.75 |
| Liver cirrhosis | 3 (9) | 9 (6) | 0.43 |
| Connective tissue disease | 3 (9) | 13 (8) | 0.74 |
| HIV infection | 0 (0) | 1 (1) | >0.99 |
| Use of immunosuppressive agent (≤1 mo) | 16 (49) | 50 (31) | 0.05 |
| Atypical chest imaged | 24/31 (77) | 63/138 (46) | 0.001 |
| Anti-TB drug susceptibility test | 26 (79) | 122 (76) | 0.71 |
| Multidrug resistance | 1/26 (4) | 2/122 (2) | 0.44 |
| Resistance to isoniazid | 4/26 (15) | 5/122 (4) | 0.05 |
| Resistance to rifampin | 0/26 (0) | 2/122 (2) | >0.99 |
| Resistance to ethambutol | 1/26 (4) | 4/122 (3) | >0.99 |
| Resistance to pyrazinamide | 0/26 (0) | 1/122 (1) | >0.99 |
| Resistance to FQ | 0/26 (0) | 3/122 (3) | >0.99 |
| Time from mycobacterial test to positive mycobacterial culture, median wks (IQR) | 8.1 (5.6–9.1) | 7.7 (5.0–8.6) | 0.09 |
| Time from mycobacterial test to anti-TB therapy, median wks (IQR) | 4.6 (3.2–7.1) | 2.2 (0.6–4.3) | <0.001 |
| Treatment | |||
| Empirical anti-TB treatment | 18 (55) | 119 (74) | 0.03 |
| Definitive anti-TB treatmente | 26 (79) | 142 (88) | 0.16 |
| Defaulted from anti-TB treatmentf | 4 (12) | 7 (4) | 0.10 |
| Change of treatment regimen | 1 (3) | 12 (9) | 0.70 |
| AG exposure prior to TB diagnosisg | 1 (3) | 4 (3) | >0.99 |
| Duration of FQ use, median days (IQR) | 13 (10–18) | 2 (1–3)h | <0.001 |
| Outcome | |||
| Death before availability of positive mycobacterial culture | 3 (9) | 12 (8) | 0.72 |
| 30-day all-cause mortality | 2 (6) | 10 (6) | >0.99 |
| 90-day all-cause mortality | 4 (12) | 20 (12) | >0.99 |
Data are numbers (percentages) of patients unless indicated otherwise. AG, aminoglycoside; FQ, fluoroquinolone; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
Twenty-four (73%) and 2 (6%) were exposed to levofloxacin alone and ciprofloxacin alone, respectively. In addition, 5 (15%) were exposed to ciprofloxacin and levofloxacin, and 2 (6%) were exposed to levofloxacin and moxifloxacin.
The comparator included the short-term FQ group (n = 23), the non-FQ group (n = 78), and the group receiving no antibiotics (n = 60).
Twenty-five patients (2 in the long-term FQ group and 23 in the comparator) had extrapulmonary manifestations of TB only. Of 10 patients with disseminated TB in the non-FQ group, 8 had pulmonary plus extrapulmonary manifestations of TB and 2 had extrapulmonary manifestations of TB only.
Fifteen patients (3 in the long-term FQ group and 12 in the comparator) died before the availability of a positive mycobacterial culture, and 11 patients (4 in the long-term FQ group and 7 in the comparator) defaulted from anti-TB treatment.
Defined as treatment interruption for any reason.
No patients received AG for more than 7 days prior to TB diagnosis.
Twenty-three (14%) patients in the comparator received FQ (<7 days) prior to anti-TB treatment.
Treatments and outcomes.
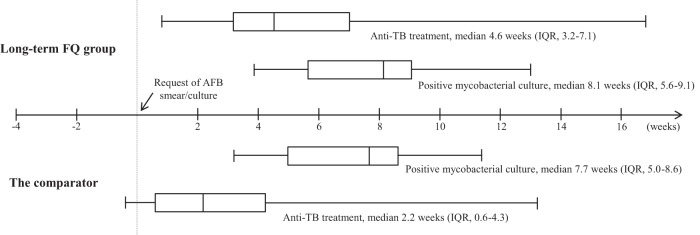
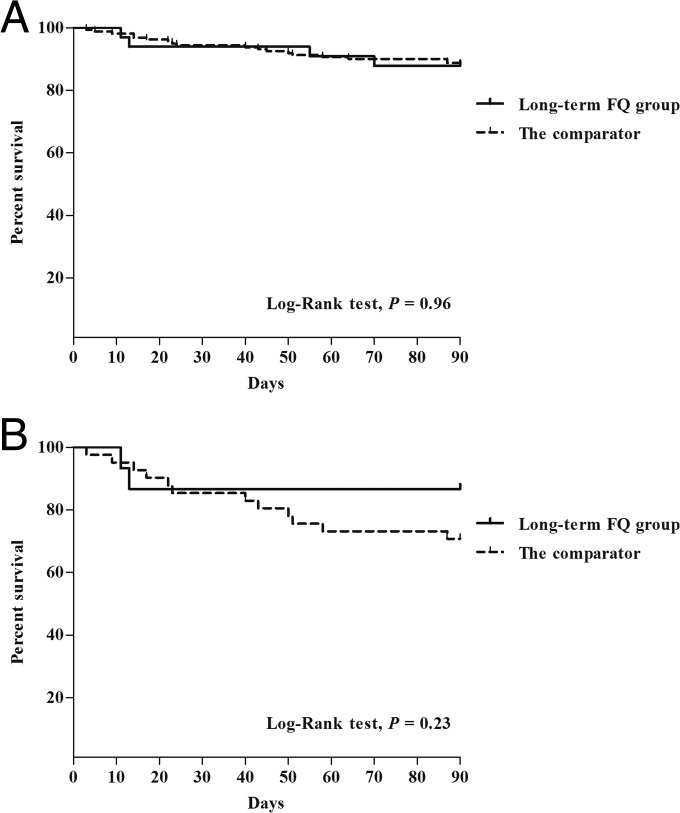
Figure 2 shows the test-to-diagnosis and test-to-treatment times in the long-term FQ group and the comparator using box-and-whisker plots. The median time from mycobacterial test to positive mycobacterial culture appeared to be longer in the long-term FQ group (8.1 weeks versus 7.7 weeks; P = 0.09), although the difference was not statistically significant. Empirical anti-TB treatment was given to 18 (55%) patients in the long-term FQ group, versus 119 (74%) in the comparator (P = 0.03). Definitive anti-TB treatment was given to 26 (79%) of the 33 patients in the long-term FQ group and to 142 (88%) of the 161 patients in the comparator (P = 0.16). The median time from mycobacterial test to anti-TB therapy was longer in the long-term FQ group (4.6 weeks) than in the comparator (2.2 weeks; P < 0.001). Five patients (1 [3%] in the long-term FQ group and 4 [3%] in the comparator; P > 0.99) were exposed to aminoglycoside (AG) antibiotics prior to TB diagnosis, and none of them received AG for more than 7 days. The median duration of long-term FQ use was 13 days (interquartile range [IQR], 10 to 18). Of the 161 patients in the comparator, 23 (14%) received FQ (<7 days) prior to anti-TB treatment, and the median duration of FQ use in those patients was 2 days (IQR, 1 to 3). The rates of death before the availability of mycobacterial culture were similar between the long-term FQ group and the comparator (9% [3/33] versus 8% [12/161]; P = 0.72). In addition, there were no significant differences in 30-day mortality (6% [2/33] versus 6% [10/161]; P > 0.99) and 90-day mortality (12% [4/33] versus 12% [20/161]; P > 0.99) between the two groups. The Kaplan-Meier survival analysis, at 90 days, showed no significant difference in survival between the two groups (P = 0.96 by log rank test) (Fig. 3A).
FIG 2.
Box-and-whisker plots showing the times between mycobacterial test to positive mycobacterial culture, and mycobacterial test to anti-TB therapy in the FQ and non-FQ groups. The boxes indicate lower and upper quartiles, the central lines indicate medians, and the ends of the whiskers indicate the minima and maxima. AFB, acid-fast bacilli.
FIG 3.
Kaplan-Meier survival curves, at 90 days, for the 194 immunocompromised patients with smear-negative and culture-positive TB (A) and subgroup analysis for the 57 patients who did not receive empirical anti-TB treatment (B).
The subgroup analysis of 57 patients who did not receive empirical anti-TB treatment, so-called “missed TB” patients (see Fig. S1 in the supplemental material), is shown in Tables S2 and S3 in the supplemental material. The 30-day mortality rates were similar between the two groups (13% [2/15] versus 17% [7/42]; P > 0.99). However, the 90-day mortality rate was lower in the long-term FQ group than in the comparator (13% [2/15] versus 31% [13/42]; P = 0.31), although this difference did not reach any statistical significance. The Kaplan-Meier survival analysis, at 90 days, showed no significant difference in survival rates between the two groups (P = 0.23 by log rank test) (Fig. 3B).
Risk factors associated with 30-day mortality.
The risk factors associated with 30-day mortality among the 194 immunocompromised patients with smear-negative and culture-positive TB are shown in Table 2. Liver cirrhosis was a significant risk factor for 30-day mortality (odds ratio [OR], 6.41; 95% confidence interval [CI], 1.48 to 27.82; P = 0.03). Atypical chest image at the time of presentation appeared to be more frequent for patients who died than those who survived (75% [9/12] versus 50% [78/157]; P = 0.09), although the difference was not statistically significant. Resistance to various anti-TB drugs, including FQ, was not associated with an increased risk of death. Patients who died were less likely to receive definitive anti-TB treatment than those who survived (25% [3/12] versus 91% [165/182]; P < 0.001). Most importantly, long-term FQ use was not a significant risk factor for 30-day mortality (OR, 0.97; 95% CI, 0.20 to 4.67; P > 0.99), while short-term FQ use (less than 7 days) was a significant risk factor for 30-day mortality (OR, 9.71; 95% CI, 2.82 to 33.43; P = 0.001). As shown in Table S1 in the supplemental material, the short-term FQ group was more likely to include sicker patients. When subgroup analysis excluding the patients (n = 5) who died rapidly within 3 days after antibiotic use was performed, the overall results were similar (see Table S4 in the supplemental material).
TABLE 2.
Univariate analysis of risk factors associated with 30-day mortality among the 194 immunocompromised patients with smear-negative and culture-positive tuberculosisa
| Variable | Value for: |
OR (95% CI) | P value | |
|---|---|---|---|---|
| Patients who died (n = 12) | Patients who survived (n = 182) | |||
| Demographics | ||||
| Age, yrs (mean ± SD) | 63 ± 12 | 61 ± 15 | 1.01 (0.97–1.05) | 0.64 |
| Male sex | 9 (75) | 132 (73) | 1.14 (0.30–4.37) | >0.99 |
| Clinical diagnosis | ||||
| Pulmonary TB | 12 (100) | 148 (81) | 1.08 (1.03–1.13) | 0.13 |
| Extrapulmonary TB | 0 (0) | 23 (13) | NA | 0.37 |
| Disseminated TB | 0 (0) | 11 (6) | NA | >0.99 |
| Underlying disease | ||||
| Solid tumor | 6 (50) | 119 (65) | 0.53 (0.16–1.71) | 0.35 |
| Transplantation | 0 (0) | 13 (7) | NA | >0.99 |
| Hematologic malignancy | 2 (17) | 12 (7) | 2.83 (0.56–14.42) | 0.21 |
| Chronic renal failure | 0 (0) | 20 (11) | NA | 0.62 |
| Liver cirrhosis | 3 (25) | 9 (5) | 6.41 (1.48–27.82) | 0.03 |
| Connective tissue disease | 1 (8) | 15 (8) | 1.01 (0.12–8.38) | >0.99 |
| HIV infection | 0 (0) | 1 (1) | NA | >0.99 |
| Immunosuppressive agent use (≤1 mo) | 6 (50) | 60 (33) | 2.03 (0.63–6.57) | 0.35 |
| Atypical chest imageb | 9/12 (75) | 78/157 (50) | 3.04 (0.79–11.65) | 0.09 |
| Anti-TB drug susceptibility test | 2 (17) | 146 (80) | 0.05 (0.01–0.24) | <0.001 |
| Multidrug resistance | 0/2 (0) | 3/146 (2) | NA | >0.99 |
| Resistance to isoniazid | 0/2 (0) | 9/146 (6) | NA | >0.99 |
| Resistance to rifampin | 0/2 (0) | 2/146 (1) | NA | >0.99 |
| Resistance to ethambutol | 0/2 (0) | 5/146 (3) | NA | >0.99 |
| Resistance to pyrazinamide | 0/2 (0) | 1/146 (1) | NA | >0.99 |
| Resistance to FQ | 0/2 (0) | 3/146 (2) | NA | >0.99 |
| Time from mycobacterial test to positive mycobacterial culture, median wks (IQR) | 5.3 (5.2–7.9) | 7.6 (5.1–8.6) | 0.81 (0.61–1.09) | 0.22 |
| Time from mycobacterial test to anti-TB therapy, median wks (IQR) | 0.6 (0.5–1.0) | 3.0 (1.0–4.6) | 0.48 (0.17–1.35) | 0.10 |
| Treatment | ||||
| Empirical anti-TB treatment | 3 (25) | 134 (74) | 0.12 (0.03–0.46) | 0.001 |
| Definitive anti-TB treatment | 3 (25) | 165 (91) | 0.03 (0.01–0.14) | <0.001 |
| Defaulted from anti-TB treatment | 0 (0) | 11 (6) | NA | >0.99 |
| Change of treatment regimen | 0 (0) | 13 (8) | NA | >0.99 |
| AG use | 0 (0) | 5 (3) | NA | >0.99 |
| FQ use | 8 (66) | 48 (26) | 5.58 (1.61–19.38) | 0.006 |
| Short-term FQ use (<7 days) | 6 (50) | 17 (9) | 9.71 (2.82–33.43) | 0.001 |
| Long-term FQ use (≥7 days) | 2 (17) | 31 (17) | 0.97 (0.20–4.67) | >0.99 |
| Duration of FQ use | NA | NA | 0.91c (0.81–1.03) | 0.14 |
| Non-FQ use | 4 (33) | 74 (41) | 0.73 (0.21–2.51) | 0.77 |
| Short-term non-FQ use (<7 days) | 1 (8) | 29 (16) | 0.48 (0.06–3.86) | 0.70 |
| Non-FQ use (≥7 days) | 3 (25) | 45 (25) | 1.02 (0.26–3.91) | >0.99 |
| No antibiotic use | 0 (0) | 60 (33) | NA | 0.02 |
Data are numbers (percentages) of patients unless indicated otherwise. AG, aminoglycoside; CI, confidence interval; NA, not available; OR, odds ratio.
Twenty-five patients among those who survived had extrapulmonary manifestations of TB only. Of 11 patients with disseminated TB in the survival group, 9 had pulmonary plus extrapulmonary manifestations of TB and 2 had extrapulmonary manifestations of TB only.
The odds ratio for the duration of FQ use indicates that for every additional 1 day there is a 0.91 times lower risk of mortality in the patients (n = 56) who received FQs.
DISCUSSION
There are several reports of the impact of FQ exposure prior to TB diagnosis on various clinical outcomes in patients with TB (15–27). However, to our knowledge, this is the first study evaluating the impact of FQ use prior to TB diagnosis on clinical outcomes in immunocompromised patients with TB. We found that long-term FQ use (≥7 days) prior to TB diagnosis was not associated with increased mortality, although the delay before initiation of anti-TB therapy was longer in the long-term FQ group. This delayed treatment appeared to be mainly caused by less empirical anti-TB treatment due to lower clinical suspicion, i.e., more so-called missed TB, in the long-term FQ group than in the comparator because of atypical presentation. It thus seems that various manifestations of TB in immunocompromised patients, especially in transplant recipients, result in more missed TB in the long-term FQ group, leading to less empirical anti-TB treatment. However, since long-term FQ exposure (≥7 days) did not significantly affect the rate of mycobacterial growth and was not associated with increased FQ resistance or increased mortality, our findings may decrease concerns about the use of FQ in immunocompromised patients in whom the probability of TB is low but cannot be completely ruled out until definitive mycobacterial culture results are available. Although we did not directly demonstrate that the FQ monotherapy prior to TB diagnosis could prevent clinical deterioration in immunocompromised patients with missed TB until they received definitive anti-TB treatment, this theoretical possibility, without any evidence of negative clinical consequences, may have important clinical implications.
Previous studies have demonstrated potential detrimental effects of FQ use in patients with undiagnosed TB, based on its strong anti-TB activity, and have warned that FQ should be avoided under conditions where a diagnosis of TB cannot be ruled out (15–23), because FQ exposure could delay the diagnosis and treatment of TB (15–17). However, in our view, the delayed diagnosis in patients given FQ may be caused by many factors; these include clinical improvement due to the FQ treatment in patients with missed TB, since this will inevitably prolong the follow-up period, reduced viability of mycobacteria due to the FQ, and simply the implications of FQ use as a proxy of lower clinical suspicion of TB. In fact, in our study, patients who received long-term FQ were more likely to have an atypical chest image at the time of presentation, suggesting that TB might be less frequently suspected in the long-term FQ group. Therefore, we conclude that FQ use after three consecutive adequate sputum mycobacterial cultures in a setting of low probability of TB, in which the toxicity of anti-TB drugs outweighs the benefit of empirical anti-TB treatment, does not necessarily delay the diagnosis of TB. A previous study performed in the United States found that the diagnostic delay was not due specifically to FQ as opposed to other antibiotic classes, suggesting that the delay was related to antibiotic use in general, or lower clinical suspicion, rather than to the anti-TB activity of FQ (24).
In addition, although there has been growing concern that the widespread use of FQ may be responsible for the increasing rate of FQ-resistant TB (18–21), no significant correlation between FQ exposure and FQ resistance was found in two studies in Korea and Taiwan, respectively (25, 26). Fortunately, previous studies have consistently reported that the exposure to FQ for less than 2 weeks appeared not to be associated with the emergence of FQ resistance (20, 21, 25), as also observed in our study, although there are case reports implying the opposite (18, 19).
The previous studies in the general population with TB found that FQ exposure prior to TB diagnosis was associated with increased mortality (22, 23). However, a recent study in Taiwan demonstrated that empirical use of FQ improved the survival rate of critically ill patients with TB that mimicked severe pneumonia, without causing treatment delay and FQ resistance (27). In that study, significantly more patients in the non-FQ group (27%) than in the FQ group (9%) died before anti-TB therapy was started. Interestingly, our data clearly showed that short-term FQ use (less than 7 days) was associated with mortality, while long-term FQ use (≥7 days) prior to TB diagnosis was not associated with mortality. These findings suggest that the presence of anti-TB activity in an empirical antibiotic regimen for a certain duration may affect the outcome in immunocompromised patients with TB. However, some biases related to treatment indication, such as short-term use of FQ (i.e., FQ use in sicker patients), should be considered. Our data together indicate that until more definite data are available, the prudent use of FQ in selected patients with missed TB who do not cross the threshold of empirical anti-TB treatment may not be harmful and could sometimes be helpful.
TB occurs with greater frequency among patients with impaired host defense, such as those with HIV infection (3, 4), malignancy (5), liver cirrhosis (6), and chronic renal failure (7) and those receiving immunosuppressive treatment (8). The clinical characteristics of TB in immunocompromised patients can include greater disease severity, more rapid progression, an increased tendency for disseminated disease, and a higher mortality rate (3–10). However, clinical suspicion of TB in these patients may be challenging because typical disease manifestations are diminished or absent (3–10), and consequently, patients are often treated empirically with multiple courses of antibiotics for presumed bacterial or fungal infections other than TB. That is, the clinical suspicion and early diagnosis of pulmonary TB rely heavily on imaging modes such as chest computed tomography (CT) because the rapid specific diagnostic tests, such as the acid-fast bacillus smear and M. tuberculosis PCR from respiratory specimens, lack sufficient sensitivity (32). Actually, our previous study has clearly demonstrated that about one-third of pulmonary TB cases in transplant recipients were very close to those of invasive pulmonary aspergillosis and about half of patients received inappropriate antifungal therapy (33). However, the first-line anti-TB drugs are difficult to liberally use in empirical therapy because of drug toxicity and interaction with other important drugs. In this context, FQ use is an attractive option in the clinical setting when an alternative diagnosis is suspected but unusual presentations of TB cannot be ruled out in immunocompromised patients.
The present study has several limitations. First, anti-TB drug susceptibility testing was performed only with the M. tuberculosis isolates from the first positive result of mycobacterial culture. Therefore, FQ susceptibilities before and after FQ exposure could not be directly compared. However, repeated anti-TB drug susceptibility testing was performed for none of the patients enrolled in this study. Indeed, anti-TB drug susceptibility tests were requested for the M. tuberculosis isolates obtained from the specimen before FQ exposure for 24 (73%) of 33 patients in the FQ group. This means that FQ resistance, if acquired, does not affect the decision on the choice of potent first-line multidrug regimen in real clinical practice. Actually, we did not find any evidence that FQ exposure in immunocompromised patients was associated with treatment failure or relapse of TB after the completion of therapy (data not shown). However, the relationship between FQ exposure and resistance should be interpreted with caution, because it is difficult to draw any confident conclusions about the relationship between FQ exposure and resistance based on our data. Second, due to the retrospective nature of our study, some information of clinical importance, such as medication history or follow-up data, may have been missed. Actually, 11 (6%) of the 194 patients defaulted from the anti-TB treatment. In addition, anti-TB drug susceptibility testing could not be performed for 46 (24%) of the patients. However, as the rates of treatment default (12% [4/33] versus 4% [7/161]; P = 0.10) and the availability of anti-TB drug susceptibility (79% [26/33] versus 76% [122/161]; P = 0.71) did not differ between the two groups, these factors are unlikely to have influenced our findings. In addition, some may argue on the potential for misclassification of antibiotic use in terms of FQ use and the validity of mortality data. However, the nurses' and doctors' records in our hospital have routinely checked the previous use of antibiotics, and a referral letter including prescription records from referring physicians is mandatory for all patients due to the national health care system. To capture mortality data, we used a unique 13-digit identification system in which all Korean residents are registered in the national administrative system. Therefore, these biases may be not so critical. Third, some may be confused by the discrepancy of the associations of short-term FQ use versus long-term FQ use with mortality. Among the patients with short-term FQ use (see Fig. S1 in the supplemental material), the mortality rate was high in those who did not receive empirical anti-TB therapy, while the mortality rate was low in those who received empirical anti-TB therapy. Therefore, we assume that the earlier anti-TB therapy is used, the better the outcome. Otherwise, patients with short-term FQ use included rapid fatal patients due to progression of pneumonia caused by TB during FQ use (see Table S1 in the supplemental material). So, the inclusion of these patients with a poor prognosis contributed to a worse outcome in those with short-term FQ use. Although any FQ use (as a whole) was associated with mortality (OR, 5.58), long-term FQ use (≥7 days) was not associated with mortality (OR, 0.97). This suggests that the high OR (9.71) of death in the short-term FQ use (<7 days) group may account for the higher mortality for any FQ group than for the non-FQ group. Fourth, this study was conducted in a country with an intermediate TB burden, and the threshold of empirical anti-TB treatment in immunocompromised patients would be different in areas with different epidemiologies of TB. Hence, the effects of FQ use in immunocompromised patients with TB, whether beneficial or harmful, may differ in countries with different TB burdens. In particular, the low pretest probability of TB in low-TB-burden countries may be more prone to lead to missed TB, so further studies are needed on the association of FQ use with clinical outcome in such countries. Finally, the main drawback of our study is that the patient number (n = 33) of the long-term FQ group is too small to draw a firm conclusion. For the same reason, we could not evaluate the impact of different kinds of FQ on clinical outcomes. Therefore, further studies with a large number of cases are needed.
In conclusion, long-term FQ exposure (≥7 days) prior to TB diagnosis in immunocompromised patients appears not to be associated with adverse outcomes. Our findings may decrease concerns about the empirical use of FQ in immunocompromised patients in the clinical setting where the diagnosis of TB cannot be ruled out but the probability of TB is not high enough to require anti-TB treatment, especially in areas where TB is endemic.
Supplementary Material
ACKNOWLEDGMENT
There are no potential conflicts of interest for any of the authors.
Funding Statement
This work was supported by grants from the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1A05004354), and by the Ministry of Education (grant NRF-2015R1D1A1A01059315) and the Asan Institute For Life Sciences (2015-1004).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01749-15.
REFERENCES
- 1.Zumla A, Raviglione M, Hafner R, von Reyn CF. 2013. Tuberculosis. N Engl J Med 368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 2.van Crevel R, Ottenhoff TH, van der Meer JW. 2002. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev 15:294–309. doi: 10.1128/CMR.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JL, Vjecha MJ, Okwera A, Hatanga E, Byekwaso F, Wolski K, Aisu T, Whalen CC, Huebner R, Mugerwa RD, Ellner JJ, Makerere. 1998. Impact of human immunodeficiency virus type-1 infection on the bacteriologic and radiographic manifestations of pulmonary tuberculosis in Uganda. Makerere University-Case Western Reserve University Research Collaboration. Int J Tuberc Lung Dis 2:397–404. [PubMed] [Google Scholar]
- 4.Lee MP, Chan JW, Ng KK, Li PC. 2000. Clinical manifestations of tuberculosis in HIV-infected patients. Respirology 5:423–426. [PubMed] [Google Scholar]
- 5.Kamboj M, Sepkowitz KA. 2006. The risk of tuberculosis in patients with cancer. Clin Infect Dis 42:1592–1595. doi: 10.1086/503917. [DOI] [PubMed] [Google Scholar]
- 6.Cho YJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. 2007. Clinical characteristics of tuberculosis in patients with liver cirrhosis. Respirology 12:401–405. doi: 10.1111/j.1440-1843.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 7.Venkata RK, Kumar S, Krishna RP, Kumar SB, Padmanabhan S, Kumar S. 2007. Tuberculosis in chronic kidney disease. Clin Nephrol 67:217–220. doi: 10.5414/CNP67217. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz P, Rodríguez C, Bouza E. 2005. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis 40:581–587. doi: 10.1086/427692. [DOI] [PubMed] [Google Scholar]
- 9.Kiyan E, Kilicaslan Z, Gurgan M, Gurgan M, Tunaci A, Yildiz A. 2003. Clinical and radiographic features of pulmonary tuberculosis in non-AIDS immunocompromised patients. Int J Tuberc Lung Dis 7:764–770. [PubMed] [Google Scholar]
- 10.Kim SH, Song KH, Choi SJ, Kim HB, Kim NJ, Oh MD, Choe KW. 2009. Diagnostic usefulness of a T-cell-based assay for extrapulmonary tuberculosis in immunocompromised patients. Am J Med 122:189–195. doi: 10.1016/j.amjmed.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Cremades R, Rodríquez JC, García-Pachón E, Galiana A, Ruiz-García M, López P, Royo G. 2011. Comparison of the bactericidal activity of various fluoroquinolones against Mycobacterium tuberculosis in an in vitro experimental model. J Antimicrob Chemother 66:2281–2283. doi: 10.1093/jac/dkr281. [DOI] [PubMed] [Google Scholar]
- 12.Ziganshina LE, Squire SB. 2008. Fluoroquinolones for treating tuberculosis. Cochrane Database Syst Rev 1:CD004795. [DOI] [PubMed] [Google Scholar]
- 13.Shim TS, Jo KW. 2013. Medical treatment of pulmonary multidrug-resistant tuberculosis. Infect Chemother 45:367–374. doi: 10.3947/ic.2013.45.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Lee CH, Kim DK, Yoon Hi Kim JY, Lee SM, Yang SC, Lee JH, Yoo CG, Lee CT, Chung HS, Kim YW, Han SK, Yim JJ. 2011. Retrospective comparison of levofloxacin and moxifloxacin on multidrug-resistant tuberculosis treatment. Korean J Intern Med 26:153–159. doi: 10.3904/kjim.2011.26.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dooley KE, Golub J, Goes FS, Merz WG, Sterling TR. 2002. Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin Infect Dis 34:1607–1612. doi: 10.1086/340618. [DOI] [PubMed] [Google Scholar]
- 16.Yoon YS, Lee HJ, Yoon HI, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. 2005. Impact of fluoroquinolones on the diagnosis of pulmonary tuberculosis initially treated as bacterial pneumonia. Int J Tuberc Lung Dis 9:1215–1219. [PubMed] [Google Scholar]
- 17.Jeon CY, Calver AD, Victor TC, Warren RM, Shin SS, Murray MB. 2011. Use of fluoroquinolone antibiotics leads to tuberculosis treatment delay in a South African gold mining community. Int J Tuberc Lung Dis 15:77–83. [PubMed] [Google Scholar]
- 18.Ginsburg AS, Grosset JH, Bishai WR. 2003. Fluoroquinolones, tuberculosis and resistance. Lancet Infect Dis 3:432–442. doi: 10.1016/S1473-3099(03)00671-6. [DOI] [PubMed] [Google Scholar]
- 19.Ginsburg AS, Hooper N, Parrish N, Dooley KE, Dorman SE, Booth J, Diener-West M, Merz WG, Bishai WR, Sterling TR. 2003. Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin Infect Dis 37:1448–1452. doi: 10.1086/379328. [DOI] [PubMed] [Google Scholar]
- 20.Devasia RA, Blackman A, Gebretsadik T, Griffin M, Shintani A, May C, Smith T, Hooper N, Maruri F, Warkentin J, Mitchel E, Sterling TR. 2009. Fluoroquinolone resistance in Mycobacterium tuberculosis: the effect of duration and timing of fluoroquinolone exposure. Am J Respir Crit Care Med 180:365–370. doi: 10.1164/rccm.200901-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long R, Chong H, Hoeppner V, Shanmuganathan H, Kowalewska-Grochowska K, Shandro C, Manfreda J, Senthilselvan A, Elzainy A, Marrie T. 2009. Empirical treatment of community-acquired pneumonia and the development of fluoroquinolone-resistant tuberculosis. Clin Infect Dis 48:1354–1360. doi: 10.1086/598196. [DOI] [PubMed] [Google Scholar]
- 22.Wang JY, Hsueh PR, Jan IS, Lee LN, Liaw YS, Yang PC, Luh KT. 2006. Empirical treatment with a fluoroquinolone delays the treatment for tuberculosis and is associated with a poor prognosis in endemic areas. Thorax 61:903–908. doi: 10.1136/thx.2005.056887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Heijden YF, Maruri F, Blackman A, Holt E, Warkentin JV, Shepherd BE, Sterling TR. 2012. Fluoroquinolone exposure prior to tuberculosis diagnosis is associated with an increased risk of death. Int J Tuberc Lung Dis 16:1162–1167. doi: 10.5588/ijtld.12.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golub JE, Bur S, Cronin WA, Gange S, Sterling TR, Oden B, Baruch N, Comstock GW, Chaisson RE. 2005. Impact of empiric antibiotics and chest radiograph on delays in the diagnosis of tuberculosis. Int J Tuberc Lung Dis 9:392–397. [PubMed] [Google Scholar]
- 25.Park IN, Hong SB, Oh YM, Lim CM, Lee SD, Lew WJ, Koh Y, Kim WS, Kim DS, Kim WD, Shim TS. 2007. Impact of short-term exposure to fluoroquinolones on ofloxacin resistance in HIV-negative patients with tuberculosis. Int J Tuberc Lung Dis 11:319–324. [PubMed] [Google Scholar]
- 26.Wang JY, Lee LN, Lai HC, Wang SK, Jan IS, Yu CJ, Hsueh PR, Yang PC. 2007. Fluoroquinolone resistance in Mycobacterium tuberculosis isolates: associated genetic mutations and relationship to antimicrobial exposure. J Antimicrob Chemother 59:860–865. doi: 10.1093/jac/dkm061. [DOI] [PubMed] [Google Scholar]
- 27.Tseng YT, Chuang YC, Shu CC, Hung CC, Hsu CF, Wang JY. 2012. Empirical use of fluoroquinolones improves the survival of critically ill patients with tuberculosis mimicking severe pneumonia. Crit Care 16:R207. doi: 10.1186/cc11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maher D, Chaulet P, Spinaci S, Harries A. 1997. Treatment of tuberculosis: guidelines for national programmes. WHO document WHO/CDS/TB/1997.220. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 29.Wang JY, Hsueh PR, Wang SK, Jan IS, Lee LN, Liaw YS, Yang PC, Luh KT. 2007. Disseminated tuberculosis: a 10-year experience in a medical center. Medicine (Baltimore) 86:39–46. doi: 10.1097/MD.0b013e318030b605. [DOI] [PubMed] [Google Scholar]
- 30.Hadlock FP, Park SK, Awe RJ, Rivera M. 1980. Unusual radiographic findings in adult pulmonary tuberculosis. Am J Roentgenol 134:1015–1018. doi: 10.2214/ajr.134.5.1015. [DOI] [PubMed] [Google Scholar]
- 31.Van Dyck P, Vanhoenacker FM, Van den Brande P, De Schepper AM. 2003. Imaging of pulmonary tuberculosis. Eur Radiol 13:1771–1785. doi: 10.1007/s00330-002-1612-y. [DOI] [PubMed] [Google Scholar]
- 32.Lee YM, Park KH, Kim SM, Park SJ, Lee SO, Choi SH, Kim YS, Woo JH, Kim SH. 2013. Diagnostic usefulness of a T-cell-based assay in patients with miliary tuberculosis compared with those with lymph node tuberculosis. Clin Infect Dis 56:e26–e29. doi: 10.1093/cid/cis872. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Kim MY, Hong SI, Jung J, Lee HJ, Yun SC, Lee SO, Choi SH, Kim YS, Woo JH. 2015. Invasive pulmonary aspergillosis-mimicking tuberculosis. Clin Infect Dis 61:9–17. doi: 10.1093/cid/civ216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.