Abstract
Beta-lactams enhance the in vitro activity of daptomycin against methicillin-resistant strains of Staphylococcus aureus. Experiments were performed in a rabbit model of aortic valve endocarditis caused by methicillin-resistant daptomycin-nonsusceptible S. aureus strain CB5054 to determine if a cephalosporin, ceftriaxone, administered as a once-daily dose of 100 mg/kg of body weight, or a carbapenem, ertapenem, administered as a once-daily dose of 40 mg/kg, improved the efficacy of daptomycin, administered as a once-daily dose of 12 mg/kg. Daptomycin was ineffective alone in reducing organism densities compared to untreated controls in vegetations and spleen, but densities were 1.4 log10 CFU/g lower in kidney. The combination of daptomycin plus ceftriaxone or daptomycin plus ertapenem reduced bacterial densities in all tissues compared to single agents, with 0.6 to 1.0 log10 CFU/g fewer organisms in vegetations, 1.5 to 2.5 log10 CFU/g fewer organisms in spleen, and 1.8 to 2.5 log10 CFU/g fewer organisms in kidney, although differences were statistically significant only in spleen for daptomycin plus ceftriaxone and in kidney for daptomycin plus ertapenem. Drug exposures in rabbits were less than those achievable in humans, which may have limited the in vivo activity, particularly in vegetations.
INTRODUCTION
The combination of daptomycin and a beta-lactam enhances killing against daptomycin-susceptible and daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus (MRSA) (1–3), increases daptomycin binding to the bacterial cell membrane (2, 4, 5), and prevents the development of daptomycin resistance (3, 6, 7). Yang et al. (8) found that daptomycin plus oxacillin yielded a modest yet significant reduction (∼2 to 3 log10 CFU/g versus daptomycin alone) in MRSA tissue counts compared to the single drugs in all target organs studied (vegetations, kidneys, and spleen) in a rabbit model of endocarditis caused by a daptomycin-nonsusceptible strain. Case reports describe the successful clearance of persistent bacteremia caused by MRSA strains, including strains that are nonsusceptible to daptomycin (2, 9–11).
The majority of studies have focused on daptomycin in combination with antistaphylococcal beta-lactams (oxacillin, nafcillin, or ceftaroline); a variety of beta-lactams, including cephalosporins and carbapenems, have been shown to enhance the activity of daptomycin in vitro (3, 7), suggesting that this may be a general property of daptomycin–beta-lactam combinations. Experiments were conducted with the rabbit model of endocarditis caused by a daptomycin-nonsusceptible strain of MRSA to quantify the effect of daptomycin in combination with a once-daily cephalosporin, ceftriaxone, or a once-daily carbapenem, ertapenem, in the rabbit model of infective endocarditis (12).
MATERIALS AND METHODS
Bacterial strain.
S. aureus strain CB5054 (provided by Cubist Pharmaceuticals), a methicillin-resistant, daptomycin-nonsusceptible (Etest MICs of 24 μg/ml and 2 μg/ml, respectively) clinical isolate (8), was used to establish endocarditis. The inoculum was prepared by diluting a frozen stock in injectable 0.9% sodium chloride. The frozen stock was prepared from a culture grown overnight in tryptic soy broth. Cells were washed and resuspended in phosphate-buffered saline with 10% glycerol and stored at −80°C.
In vitro susceptibility studies.
MIC determinations were performed in triplicate using cation-adjusted Mueller-Hinton broth (CAMHB) with microdilution methods according to CLSI recommendations (13). Ca2+ was added to a final concentration of 50 μg/ml as recommended by the manufacturer for daptomycin testing.
Time-kill studies were performed in duplicate with a shaking-platform incubator at 200 rpm at 37°C in 10-ml volumes of CAMHB supplemented with 50 μg/ml Ca2+ at a starting inoculum of 106 CFU/ml. Samples (0.1 ml) were quantitatively cultured at 0, 6, and 24 h on blood agar, which was incubated for 48 h. Synergy was defined as a ≥2-log10 CFU/ml reduction for the antibiotic combination compared to the single antibiotic with the fewest CFU per milliliter at 24 h.
Animal model.
Animals were maintained in accordance with American Association for Accreditation of Laboratory Animal Care criteria. These studies were approved by the Animal Research Committee (IACUC) of the University of California, San Francisco.
Endocarditis was established in New Zealand White rabbits weighing 2 to 3 kg according to standard methods (14). Briefly, a catheter was positioned across the aortic valve and sutured in place. Forty-eight hours later, an inoculum of ∼107 CFU of the test strain in 1 ml of 0.9% saline was injected via the marginal ear vein.
Antibiotics were administered once daily for a total of 4 doses beginning 16 to 20 h after bacterial inoculation (day 1). Daptomycin was dosed at 12 mg/kg of body weight intravenously (i.v.), which corresponds to an area under the concentration-time curve (AUC) achieved by a human dose of 6 mg/ml (14); ceftriaxone was dosed at 100 mg/kg intramuscularly (i.m.); and ertapenem was dosed at 40 mg/kg i.m. A 1-ml sample of blood was obtained 1 h after dosing on day 1 or 2 and at the time of sacrifice, which was ∼24 h after the last dose of antibiotic. Samples were processed and stored as plasma for measurement of drug concentrations by high-performance liquid chromatography (HPLC). Assays were performed by Cubist Pharmaceuticals.
Untreated control rabbits were sacrificed on day 1, ∼16 to 20 h after bacterial inoculation. Antibiotic-treated rabbits were sacrificed on day 5, ∼24 h following the fourth dose. Target tissues (entire vegetation [∼0.01 to 0.05 g], kidney [0.1-g sample], and spleen [0.1-g sample]) were harvested and quantitatively cultured to determine the numbers of residual organisms. Rabbits dying before day 5 had tissues harvested and processed for culture if they received at least one dose of antibiotic and survived for at least 12 h.
Tissue samples were suspended in 1.0 ml of sterile saline and homogenized. Serial 10-fold dilutions were performed, and 0.1 ml of each dilution was plated onto blood agar. Colonies were counted after 24 to 48 h of incubation at 37°C. Tissue titers were expressed as log10 CFU per gram of tissue. The thresholds of detection were ∼1 log10 CFU/g in kidney and spleen and 1.5 log10 CFU/g in vegetations. Tissue samples showing no growth were assigned a value corresponding to the threshold of detection.
Statistical analysis.
Mean tissue titers and standard deviations (SD) were calculated for each tissue and group. Approximately 8 evaluable rabbits were required per group for 80% power and a P value of <0.05 to detect a 2-log10 CFU/g reduction in bacterial density for the more effective single agent compared to the antibiotic combination. A Mann-Whitney U test was used to compare the two groups, and differences for which P values were <0.05 were considered statistically significant.
RESULTS
In vitro susceptibility studies.
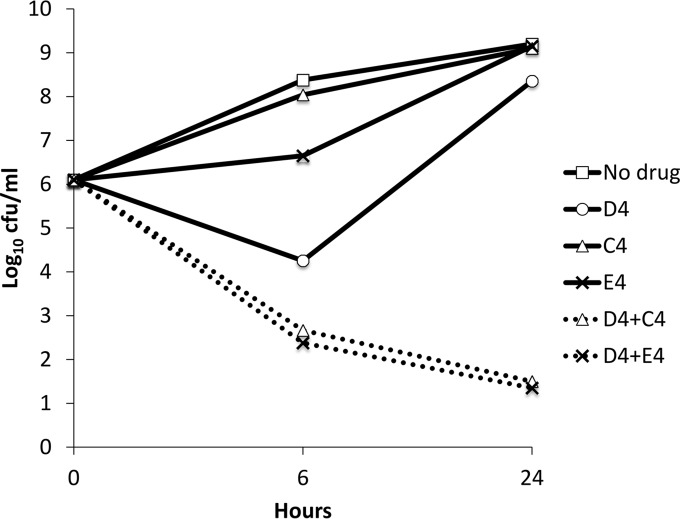
MICs were 4 μg/ml, >128 μg/ml, and 16 μg/ml for daptomycin, ceftriaxone, and ertapenem, respectively. Daptomycin at 4 μg/ml, ceftriaxone at 4 μg/ml, and ertapenem at 4 μg/ml as single agents did not inhibit the growth of CB5054 in time-kill studies. The combinations of daptomycin at 4 μg/ml plus ceftriaxone at 4 μg/ml and daptomycin at 4 μg/ml plus ertapenem at 4 μg/ml were synergistic and bactericidal, with >99.9% reductions in CFU at 24 h (Fig. 1).
FIG 1.
Time-kill studies of Staphylococcus aureus strain CB5054 exposed to daptomycin (D), ceftriaxone (C), or ertapenem (E) alone or daptomycin in combination with either ceftriaxone or ertapenem, each at a concentration of 4 μg/ml (averages of results from 2 separate experiments).
In vivo studies.
Fifty-two rabbits were inoculated with strain CB5054. Five infected rabbits died prior to being allocated to control or treatment groups. Mean tissue densities ± SD in these 5 rabbits were 7.80 ± 0.49 log10 CFU/g in vegetations, 6.08 ± 0.58 log10 CFU/g in spleen, and 6.92 ± 0.51 log10 CFU/g in kidney, values similar to those for the untreated control group (Table 1). One daptomycin-treated rabbit that was euthanized on day 5 had no catheter across the aortic valve and no organisms isolated from any tissue cultures. This rabbit was excluded from the data analysis. The 46 remaining rabbits (6 controls and 8 in each treatment group) all had bacteria isolated from one or more tissue cultures.
TABLE 1.
Numbers of rabbits per treatment group and deaths and densities of organisms in tissuea
| Treatment group | No. of rabbits (no. of deaths) | Mean density of organisms (log10 CFU/g) ± SD |
||
|---|---|---|---|---|
| Vegetationb | Spleenc | Kidneyd | ||
| Control | 6 (NA) | 8.11 ± 0.67 | 6.53 ± 0.43 | 6.91 ± 0.57 |
| Dapto | 8 (4) | 8.48 ± 1.52 | 5.95 ± 0.90 | 5.51 ± 1.40 |
| Ctrx | 8 (4) | 8.00 ± 1.42 | 6.17 ± 2.19 | 5.57 ± 2.10 |
| Erta | 8 (6) | 7.23 ± 1.64 | 4.99 ± 0.96 | 5.01 ± 1.40 |
| Dapto + Ctrx | 8 (2) | 6.94 ± 1.93 | 3.40 ± 1.87 | 3.68 ± 2.39 |
| Dapto + Erta | 8 (1) | 6.62 ± 1.16 | 3.49 ± 1.55 | 2.56 ± 1.37 |
Dapto, daptomycin; Ctrx, ceftriaxone; Erta, ertapenem; NA, not applicable.
P > 0.1 for the control group versus the daptomycin treatment group, P = 0.052 for the ceftriaxone group versus the daptomycin-ceftriaxone group, and P > 0.1 for the ertapenem group versus the daptomycin-ertapenem group.
P > 0.1 for the control group versus the daptomycin treatment group, P = 0.014 for the daptomycin group versus the daptomycin-ceftriaxone group, and P = 0.066 for the ertapenem group versus the daptomycin-ertapenem group.
P < 0.033 for the control group versus the daptomycin treatment group, P > 0.1 for the daptomycin group versus the daptomycin-ceftriaxone group, and P = 0.010 for the ertapenem group versus the daptomycin-ertapenem group.
Daptomycin alone generally was ineffective (Table 1). Although not statistically significant, daptomycin-treated rabbits had on average ∼0.5 log10 CFU/g more organisms in vegetations and 0.6 log10 CFU/g fewer organisms in spleen tissue than did controls. Daptomycin-treated rabbits had ∼1.4 log10 CFU/g fewer organisms in kidney tissue than did controls (P < 0.033).
The mean bacterial density in vegetations of rabbits treated with beta-lactams was about 1 log10 CFU/g lower than that in rabbits treated with daptomycin. Rabbits treated with the daptomycin-ceftriaxone combination had 1.1 log10 CFU/g fewer bacteria on average in vegetations than did rabbits treated with ceftriaxone alone. Rabbits treated with the daptomycin-ertapenem combination had 0.6 log10 CFU/g fewer bacteria in vegetations than did rabbits treated with ertapenem alone. Neither difference was statistically significantly (P = 0.052 and P > 0.1, respectively).
The mean bacterial density in spleen tissue was significantly lower, 2.6 log10 CFU/g, for rabbits treated with the daptomycin-ceftriaxone combination than for rabbits treated with daptomycin alone (P = 0.014), which was marginally more effective as a single agent than ceftriaxone. This combination achieved 1.8 log10 CFU/g fewer bacteria in kidney tissue than did treatment with daptomycin alone, but the difference was not statistically significant.
The bacterial density in spleen tissue was 1.5 log10 CFU/g lower for rabbits treated with the daptomycin-ertapenem combination than for those treated with ertapenem alone, but the difference between the combination and ertapenem alone was not statistically significant (P = 0.066). This combination achieved 2.4 log10 CFU/g fewer bacteria in kidney tissue than did ertapenem alone (P = 0.010).
Survival was better for rabbits treated with daptomycin combinations than for those treated with single agents. Ten of 24 rabbits treated with single agents survived the entire 4-day course of therapy, compared to 13 of 16 treated with the combination (P = 0.022).
Mean serum concentration of daptomycin, ceftriaxone, and ertapenem 1 h after dosing were 140, 300, and 214 μg/ml, respectively. Half-lives were 3.3, 3.4, and 2.5 h, respectively (Table 2).
TABLE 2.
Plasma drug concentrations and half-lives of antibiotics used for treatment
| Antibiotic | Mean concn (μg/ml) ± SD (range) (no. of samples)a |
Half-life (h) | |
|---|---|---|---|
| 1 h after dosing | Trough | ||
| Daptomycin | 140 ± 28 (103–184) (7) | 1.4 ± 0.6 (0.7–2.4) (8) | 3.3 |
| Ceftriaxone | 300 ± 76 (209–340) (5) | 2.8 ± 3.1 (0.7–8.3) (5) | 3.4 |
| Ertapenem | 214 ± 40 (176–269) (4) | 0.4 ± 0.2 (0.1–0.6) (4) | 2.5 |
One-hour samples were obtained on day 1 or 2. Trough samples were obtained at the time of sacrifice, ∼24 h after the last dose.
DISCUSSION
Daptomycin–beta-lactam combinations outperformed single-agent therapy in this model. Daptomycin generally was ineffective alone. Beta-lactams were numerically more effective agents than daptomycin in most instances, perhaps reflecting the heterogeneous phenotype of methicillin-resistant strains and the seesaw phenomenon described previously for daptomycin-nonsusceptible strains (8) such that higher daptomycin MICs are associated with greater susceptibility to beta-lactams. Survival was better and mean bacterial burdens were always lower in rabbits treated with the combination than in those treated with a single drug. The two combinations performed about the same.
Neither beta-lactam combination was particularly effective in reducing bacterial densities in vegetations compared to a beta-lactam as a single agent. Efficacy may have been limited by inadequate drug exposures. The 1-h plasma concentrations of antibiotics achieved in rabbits were similar to peak concentrations in humans given therapeutic doses, which are ∼100 μg/ml for daptomycin at 6 mg/kg once daily, 154 μg/ml for ceftriaxone at 1 g once daily, and 150 μg/ml for ertapenem at 1 g once daily (15). However, overall drug exposures were considerably lower given that drug half-lives in rabbits were approximately half or less of the half-lives in humans, which are 8 to 9 h for daptomycin, 8 h for ceftriaxone, and 4 h for ertapenem (15). It is possible that with more frequent dosing of the beta-lactams (for example, twice daily), which would more closely simulate drug exposures in humans, and with higher doses of daptomycin (the dose administered corresponded to the standard 6-mg/kg dose in humans, which is the FDA-approved dose, although doses of 8 to 12 mg/ml are often used clinically), there would have been significantly greater reductions in bacterial densities in all tissues.
The results of these experiments with a cephalosporin and a carbapenem beta-lactam antibiotic are consistent with those of the original rabbit endocarditis studies (8), conducted with the same strain as the one used here, describing enhanced activity of daptomycin in combination with oxacillin against a daptomycin-nonsusceptible strain of MRSA. The results are also consistent with a growing body of experimental and clinical data suggesting that combination therapy with daptomycin and a beta-lactam could improve outcomes of MRSA infections (1–3, 8, 9, 16–18). Given the high mortality rates associated with invasive staphylococcal infections, particularly those due to MRSA, clinical trials to test this hypothesis seem warranted.
Funding Statement
The sponsor had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The sponsor's laboratory performed assays of plasma drug concentrations.
REFERENCES
- 1.Rand KH, Houck HJ. 2004. Synergy of daptomycin with oxacillin and other beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 48:2871–2875. doi: 10.1128/AAC.48.8.2871-2875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 53:158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. 2012. β-Lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber KE, Werth BJ, Ireland CE, Stone NE, Nonejuie P, Sakoulas G, Pogliano J, Rybak MJ. 2014. Potent synergy of ceftobiprole plus daptomycin against multiple strains of Staphylococcus aureus with various resistance phenotypes. J Antimicrob Chemother 69:3006–3010. doi: 10.1093/jac/dku236. [DOI] [PubMed] [Google Scholar]
- 6.Berti AD, Wergin JE, Girdaukas GG, Hetzel SJ, Sakoulas G, Rose WE. 2012. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother 56:5046–5053. doi: 10.1128/AAC.00502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entenza JM, Giddey M, Vouillamoz J, Moreillon P. 2010. In vitro prevention of the emergence of daptomycin resistance in Staphylococcus aureus and enterococci following combination with amoxicillin/clavulanic acid or ampicillin. Int J Antimicrob Agents 35:451–456. doi: 10.1016/j.ijantimicag.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 54:3161–3169. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxi SM, Chan D, Jain V. 2015. Daptomycin non-susceptible, vancomycin-intermediate Staphylococcus aureus endocarditis treated with ceftaroline and daptomycin: case report and brief review of the literature. Infection 43:751–754. doi: 10.1007/s15010-015-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polenakovik HM, Pleiman CM. 2013. Ceftaroline for meticillin-resistant Staphylococcus aureus bacteraemia: case series and review of the literature. Int J Antimicrob Agents 42:450–455. doi: 10.1016/j.ijantimicag.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Sakoulas G, Moise PA, Casapao AM, Nonejuie P, Olson J, Okumura CY, Rybak MJ, Kullar R, Dhand A, Rose WE, Goff DA, Bressler AM, Lee Y, Pogliano J, Johns S, Kaatz GW, Ebright JR, Nizet V. 2014. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 36:1317–1333. doi: 10.1016/j.clinthera.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 12.Tattevin P, Basuino L, Bauer D, Diep BA, Chambers HF. 2010. Ceftobiprole is superior to vancomycin, daptomycin, and linezolid for treatment of experimental endocarditis in rabbits caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:610–613. doi: 10.1128/AAC.00886-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Chambers HF, Basuino L, Diep BA, Steenbergen J, Zhang S, Tattevin P, Alder J. 2009. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob Agents Chemother 53:1463–1467. doi: 10.1128/AAC.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert DN, Chambers HF, Eliopoulos GM, Saag M (ed). 2015. The Sanford guide to antimicrobial therapy 2015, 45th ed Antimicrobial Therapy, Inc, Sperryville, VA. [Google Scholar]
- 16.Werth BJ, Barber KE, Ireland CE, Rybak MJ. 2014. Evaluation of ceftaroline, vancomycin, daptomycin, or ceftaroline plus daptomycin against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model of simulated endocardial vegetations. Antimicrob Agents Chemother 58:3177–3181. doi: 10.1128/AAC.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose WE, Schulz LT, Andes D, Striker R, Berti AD, Hutson PR, Shukla SK. 2012. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 56:5296–5302. doi: 10.1128/AAC.00797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steed ME, Vidaillac C, Rybak MJ. 2010. Novel daptomycin combinations against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro model of simulated endocardial vegetations. Antimicrob Agents Chemother 54:5187–5192. doi: 10.1128/AAC.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]