Abstract
Substitution of bones is a well-established, necessary procedure to treat bone defects in trauma and orthopedic surgeries. For prevention or treatment of perioperative infection, the implantation of resorbable bone substitute materials carrying antibiotics is a necessary treatment. In this study, we investigated the newly formulated calcium-based resorbable bone substitute materials containing either gentamicin (CaSO4-G [Herafill-G]), vancomycin (CaSO4-V), or tobramycin (Osteoset). We characterized the released antibiotic concentration per unit. Bone substitute materials were implanted in bones of rabbits via a standardized surgical procedure. Clinical parameters and levels of the antibiotic-releasing materials in serum were determined. Local concentrations of antibiotics were measured using antimicrobial tests of bone tissue. Aminoglycoside release kinetics in vitro per square millimeter of bead surface showed the most prolonged release for gentamicin, followed by vancomycin and, with the fastest release, tobramycin. In vivo level in serum detected over 28 days was highest for gentamicin at 0.42 μg/ml, followed by vancomycin at 0.11 μg/ml and tobramycin at 0.04 μg/ml. The clinical parameters indicated high biocompatibility for materials used. None of the rabbits subjected to the procedure showed any adverse reaction. The highest availability of antibiotics at 14.8 μg/g on day 1 in the cortical tibia ex vivo was demonstrated for gentamicin, decreasing within 14 days. In the medulla, vancomycin showed a high level at 444 μg/g on day 1, decreasing continuously over 14 days, whereas gentamicin decreased faster within the initial 3 days. The compared antibiotic formulations varied significantly in release kinetics in serum as well as locally in medulla and cortex.
INTRODUCTION
Rapid and complete reconstruction of bone tissue through autologous or allogeneic bone or bone-related tissue is the gold standard of bone defect treatment in trauma and orthopedic surgeries. An overaging and on the other hand more and more active population raises the demand for quick and uncomplicated healing of all kinds of bone defects. At the same time, a continuous increase of hospital infections and a rising level of multiresistant bacteria are reported: osteoarticular infections increased from 2.3 to 5.8 episodes/100,000 inhabitants per year (P < 0.001) between 1985 and 2011 (1). The combination of these trends confronts active trauma and orthopedic surgeons with a rising need for effective treatment methods of bone defects as well as infections.
Resection of tumors and cystic defects, complex fractures, and the appeasement of infectious herds demand mostly replacement with allogeneic/autologous cancellous bone or bone substitute materials. Surgeons in the United States and Europe conduct roughly 500,000 surgical procedures per year using bone substitution material (2, 3); worldwide, about 2.2 million procedures are conducted in the field of orthopedic as well as trauma, neuro-, and dental surgery (4, 5).
Calcium sulfate as a resorbable slow drug release carrier is affordable, readily available, easy to sterilize, biocompatible, and visible under X ray (6–8). Calcium sulfate temporarily fills bone defects, leaving no space for bacteria to grow while at the same time providing an osteoconductive structure for ingrowing bone. While being resorbed by the body, natural bone can replace the material simultaneously. Currently used biomaterials, such as those available under the trade names Osteoset, Palacos, Palasept, Herafill, Septopal, and Genta-Coll, can release antibiotics in vivo without causing systemically toxic levels. The early use of pure calcium sulfate, so-called “plaster of Paris,” showed the disadvantage of delayed wound healing as well as osteolytic processes during resorption by pH lowering (9).
Those materials combined with potent antibiotics may be utilized to support bone healing as well as prophylactically protecting implants from infections. It is desirable to have a high local concentration of antibiotics to prevent methicillin-resistant Staphylococcus aureus (MRSA) infections without reaching toxic blood levels. Even in cases of multiresistant bacterial species, highly concentrated vancomycin can be used to treat MRSA infections by exceeding regular MICs locally (10). Previous studies have indicated that 0.8 to 1.2% of orthopedic surgical procedures (11), 3.6 to 8.1% of closed fractures, and up to 17.5 to 21.2% of open fractures in trauma surgery (12) are complicated by an infection.
The limited blood supply in the operated body region, especially the bone, and the toxicity of antibiotics reduce the effectivity of systemic antibiotic therapy. Detrimentally, any cavern caused by surgery tends to fill up with blood and as such poses an ideal breeding medium for bacteria. The implantation of antibiotic-loaded chains such as Septopal polymethylmethacrylate (PMMA) beads containing gentamicin-sulfate can bring a benefit for infected regions. Such nonresorbable materials require additional surgical intervention, constituting a burden for patient and surgeon as well as leaving another “dead space” and thus a breeding ground for further potential infections.
Adequate bone substitution material must fulfill plenty of requirements and at the same time be highly reliable to find broad acceptance and deliver positive long-term results. A high level of biochemical compatibility, as little immunologic potential as possible, and economic availability at low cost, as well as malleability and limited absorbability, influence surgeons' choice greatly.
In our study, formulations of calcium sulfate in combination with gentamicin (CaSO4-G [Herafill]) or vancomycin (CaSO4-V) and tripalmitin were tested in vitro and in vivo by implantation in rabbit tibiae. The results were compared to those obtained with commercially available tobramycin-containing Osteoset.
MATERIALS AND METHODS
Bone substitute implants.
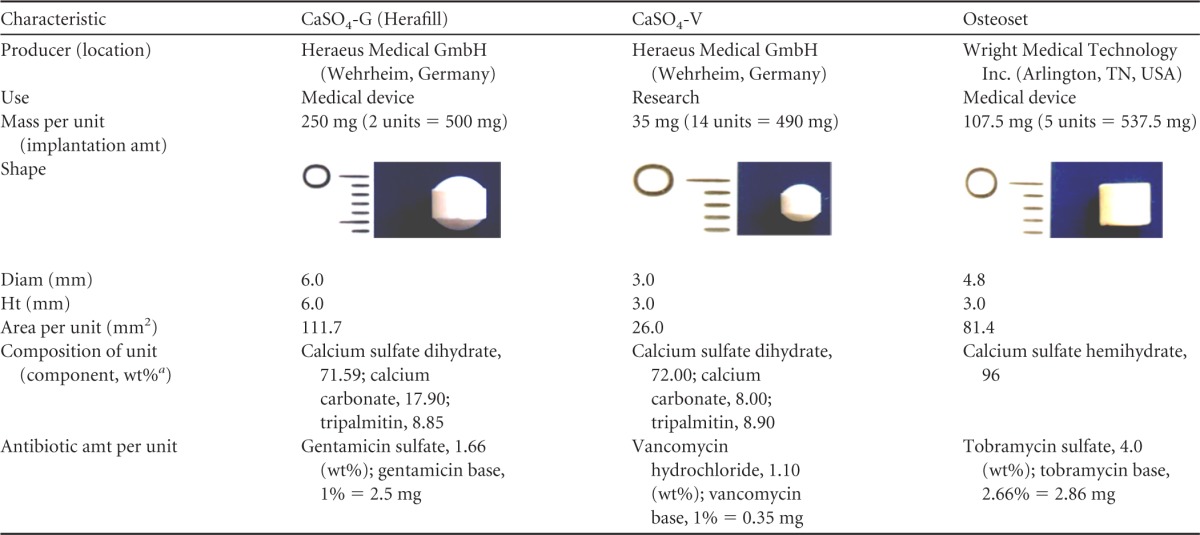
The resorbable bone substitute materials based on calcium sulfate formulations used in this study consist of calcium sulfate dehydrate, gentamicin, and tripalmitin (CaSO4-G [Herafill]), calcium sulfate dehydrate, vancomycin, and tripalmitin (CaSO4-V), and commercially available tobramycin-loaded calcium sulfate hemihydrate (Osteoset) as described in Table 1.
TABLE 1.
Comparison of the calcium sulfate-based antibiotic-loaded bone substitute beads used in this study
a wt%, percentage of weight.
Animal model.
The animal experiment was approved by the government of Upper Bavaria under the registration number 209.1/211-2531.2-22/05. Eighty-four female New Zealand White rabbits with a weight of 4.5 ± 0.5 kg were obtained and kept under standard conditions of 21 ± 2°C, relative air humidity of 55% ± 5%. Rabbits were identified via ear tattoo. Before the operation, animals were kept in the facility for 2 weeks to acclimate. Moreover, they were weighed and their blood was tested for levels of calcium and alkaline phosphatase using standard clinical chemistry laboratory techniques.
The animals were divided in 3 groups according to the test implants. Surgery was performed under general anesthesia using intramuscular injection of medetomidine, 0.25 mg/kg of body weight (Domitor; Pfizer Inc., Germany), and ketamine, 17 mg/kg (S-Ketanest; Parke-Davis GmbH). Intra- and postoperative analgesia was obtained through intravenous application of metamizole (30 mg/kg; Novaminsulfon; Ratiopharm GmbH, Germany) via a vein in the outer ear. The cornea was protected using ointment (Bepanthen; Hoffmann-LaRoche AG, Switzerland). The left hind leg undergoing surgery was shaved and the skin cleaned and disinfected (Cutasept; Bode Chemie, Germany).
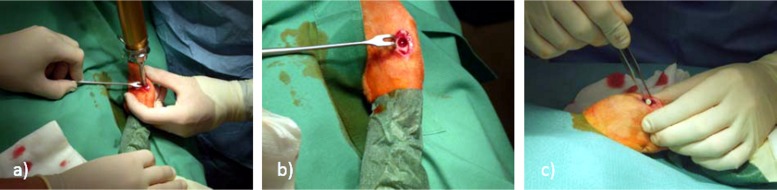
The surgical approach was conducted as displayed in Fig. 1 (lateral of the tibial tuberosity [tuberositas tibiae]): the skin was incised using a scalpel and moved to the medial side. This local skin displacement was utilized to avoid infection of the implant site through the skin wound. The incision was extended to the bone, and the periosteum was removed with a raspatory. A water-cooled surgical diamond fraise (Fig. 1a) was used to remove an 8-mm spherical bone cylinder and open the medullary cavity. Using a sterile forceps as demonstrated in Fig. 1, a matching amount of units (Herafill, 2 units; CaSO4-V, 14 units; and Osteoset, 5 units; differing numbers of units based on the various sizes of beads to match the overall implanted mass as outlined in Table 1) was inserted into the proximal medullary cavity (Fig. 1c). Following this implantation, the cavity was closed using the initially removed cylinder. Periosteum and subcutaneous tissues were readapted using resorbable suture material (3-0 Vicryl; Ethicon GmbH, Germany), and the skin was closed using nonresorbable material (3-0 Prolene; Ethicon GmbH, Germany). Thereafter, a spray bandage (Opsite; Smith & Nephew PLC, England) was applied. Anesthesia was antagonized using 0.25 mg/kg atipamezole (Antisedan; Pfizer Inc., Germany) as an intramuscular injection.
FIG 1.
(a) Fraising of the bone; (b) implant entry; (c) implant of several units of bone substitute materials.
Postoperative care.
For postoperative analgesia, buprenorphine at 0.03 mg/kg (Temgesic; Essex Pharma GmbH, Germany) was administered subcutaneously (s.c.) for 4 days every 8 h, and carprofen, 4 mg/kg s.c. (Rimadyl; Pfizer Inc., Germany), was administered for 7 days every 12 h. Daily checks included general condition, body temperature, and detailed examination of the operated leg in terms of potential inflammation signs. Blood was obtained via ear and centrifuged, and the generated serum was then stored at −20°C for further analysis of antibiotic levels. Determination of antibiotic level in serum was performed after 24, 48, and 72 h as well as after days 7, 14, 21, and 28. Weekly weight controls and clinical examinations of the animals were performed as well as testing for calcium and alkaline phosphatase using standard clinical chemistry laboratory equipment.
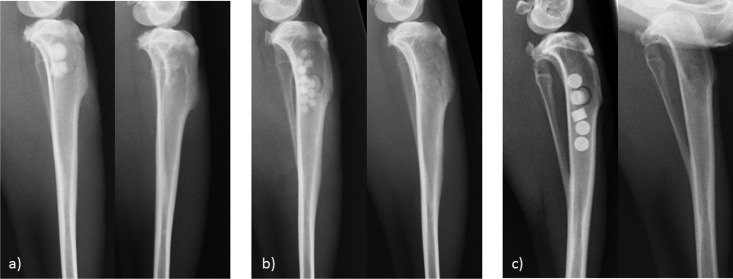
Immediately after surgery (while still under anesthesia) and at the end of observation time of 12 weeks (depending on the group), animals were X rayed in a lateral view. The X-ray images were digitally recorded and evaluated visually regarding resorption of beads and bone healing (Fig. 2). Radiologic evaluation displays a complete resorption of beads as well as good healing of bone entry. Animals were euthanized after 4, 6, 8, and 12 weeks using an overdose (50 mg/kg) of pentobarbital-sodium (Narcoren; Merial GmbH, Germany). To analyze antibiotic content, the proximal third of the tibial bone was removed and frozen at −20°C after removal of all soft tissue.
FIG 2.
X rays of tibia bones after implantation of bone substitute materials. (a) Gentamicin-containing beads (CaSO4-G [Herafill]); (b) calcium sulfate beads carrying vancomycin (CaSO4-V); (c) commercially available tobramycin-loaded calcium sulfate beads (Osteoset). In each panel, the image on the left was taken immediately after implantation and that on the right after 12 weeks in vivo.
Drug release kinetics in vitro.
Measurements of gentamicin sulfate, vancomycin, and tobramycin concentrations were conducted using a TDx Analyzer (Abbott Laboratories, Illinois, USA). In vitro release was tested in 20 ml commercially available phosphate-buffered saline (PBS). Comparable mass counts of each material were incubated at 37°C for a duration of 10 days. Release of antibiotics was analyzed daily. Every day, 20 ml supernatant was removed and replaced by 20 ml fresh PBS buffer. Antibiotic concentrations were referred to the numbers of implanted beads and normalized per square millimeter of surface area. Additionally, the amounts of antibiotic released were referred to the initially contained amount of antibiotic substance and cumulated to achieve relative release diagrams.
Biological availability.
Antibiotic levels in serum and local availability in tibia bone were consequently tested using the following procedures.
(i) Concentrations of antibiotics in serum in vivo.
The availability of antibiotic ingredients in serum was tested by drawing venous blood from the ear of the animals and centrifuging it for 10 min at 3,500 × g (Varifuge 3.0R; Heraeus Sepatech, Germany). Serum samples were taken after 24, 48, and 72 h as well as after days 7, 14, 21, and 28 and stored at −20°C until analysis at the LC Labor Consult GmbH (Germany). An ADVIA 1650 Chemistry System (Siemens Healthcare Diagnostics, Germany) was used to determine antibiotic levels in serum for gentamicin, vancomycin, and tobramycin.
(ii) Determination of local concentration of antibiotics in cortical and cancellous bone.
Standardized samples of cortical bone/medulla were weighed and incubated in 20 ml (corticalis)/10 ml (medulla) Sörensen buffer solution, and the released amounts of active ingredients were investigated. Antibiotic concentration in the tibial bone was tested on 31 samples, 11 samples with CaSO4-G (Herafill-G), 11 samples with CaSO4-V, and 9 samples with Osteoset. The sample material was the proximal third of the tibia, which was dipped in liquid nitrogen to enhance embrittlement and then sawed lengthwise. Medulla was isolated using a sharp spatula and transferred to a 50-ml Falcon tube, and weight was determined gravimetrically (Atilon ATL-224; Acculab Inc., Massachusetts, USA). Corticalis was again embrittled using liquid nitrogen and then pulverized using a bone mill for 5 min (Retsch Inc., Germany). Weight was determined again gravimetrically for the corticalis component, and antibiotic content was then determined by eluting in a Sörensen buffer at pH 7.4 and a temperature of 37°C. After 72 h of incubation, the supernatant was centrifuged and the released drug concentrations were determined indirectly via measurement of the zone of inhibition on agar plates. Determinations of active components were compared to known inhibition standards of Bacillus subtilis (ATTC 6633). The released antibiotics were then set in relation to an amount measured as per weight of bone. The clinical breakpoints of the three involved antibiotic substances for various pathogens are listed in Table 2 (13).
TABLE 2.
Selected breakpoint levels as stated by EUCASTa
| Organism(s) | Breakpoint level (μg/ml) |
||
|---|---|---|---|
| Gentamicin | Vancomycin | Tobramycin | |
| Enterobacteriaceae | 2–4 | 2–4 | |
| Pseudomonas spp. | 4 | 4 | |
| Acinetobacter spp. | 4 | 4 | |
| S. aureus and coagulase-negative staphylococci | 1 | 2–4 | 1 |
| Corynebacterium spp. | 1 | 2 | |
| Enterococcus spp. | 4 | ||
| Streptococcus groups A, B, C, and G, Streptococcus pneumoniae, and viridans group streptococci | 2 | ||
| Gram-positive anaerobes | 2 | ||
| Clostridium difficile | 2 | ||
EUCAST, European Committee on Antimicrobial Susceptibility Testing.
Statistics.
Mean values and standard deviations were calculated from at least 3 and up to 17 measurements. We used the Student t test to determine significant differences at P values of 0.05. The Gaussian error propagation law was used when appropriate.
RESULTS
Antibiotic release kinetics in vitro.
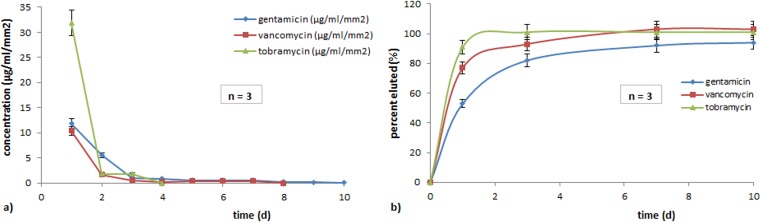
The percentage of released antibiotics was related to the content in each bone substitute unit as demonstrated in Fig. 3. The Osteoset unit had completely released tobramycin on the third day of the experiment, demonstrating the fastest drug release. Vancomycin from CaSO4-V showed 93% release on the third day and 100% release on the seventh day. Gentamicin from Herafill showed a release of 82% on the third day and 94% on the tenth day.
FIG 3.
Release of antimicrobial drugs in PBS elutions normalized per square millimeter surface of implanted beads as concentration over time (a) and as a percentage of contained substance eluted over time (b).
Figure 3a and b display detailed elution characteristics of all three substances over time, expressed as a percentage of initial value. All three drugs display an initial burst release, followed by a subsequent saturation depending on the formulation and the surface of the unit. Figure 4 describes the levels of released substances in serum over time.
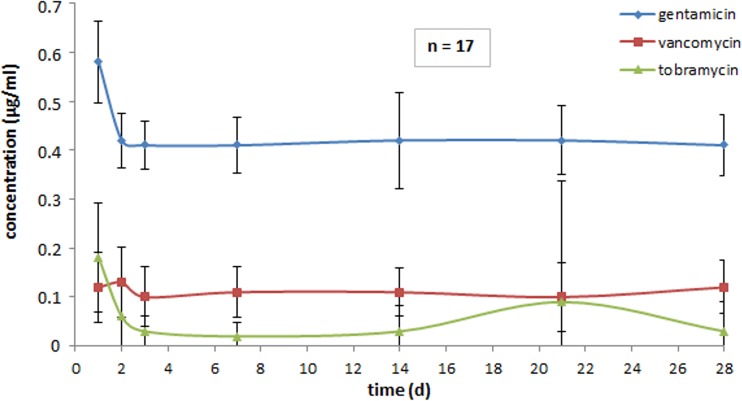
FIG 4.
Levels of antimicrobial drugs in serum.
Biological availability. (i) Levels of concentrations of antibiotics in vivo in serum.
Figure 4 displays in vivo concentration in serum following implantation of the various antibiotic CaSO4 formulations. Gentamicin (Herafill-G) showed the highest steady level with even 0.41 μg/ml at the end of the experiment. Vancomycin (CaSO4-V) levels measured were nearly homogeneous ending at the 28th day at 0.12 μg/ml. Tobramycin (Osteoset) levels in serum were heterogeneous: the peak was reached after 24 h, at 0.18 μg/ml, and levels decreased continuously to 0.02 μg/ml in the first week and then rose in the second week to 0.03 μg/ml, rising further to 0.09 μg/ml in week 3, and going down to 0.03 μg/ml in week 4. During the entire examination period of 4 weeks, all measured concentrations in serum were within the detection limit, displaying the following results: gentamicin, 0.41 to 0.58 μg/ml; vancomycin, 0.10 to 0.13 μg/ml; tobramycin, 0.03 to 0.18 μg/ml.
(ii) Antibiotic concentration in corticalis and medulla.
A preliminary examination of the beads prior to implantation showed slightly higher concentrations than declared. This may relate to a common overdosage of pharmaceutic formulations of up to 10%. Table 3 displays the mass of implanted antibiotic substances in medulla and corticalis.
TABLE 3.
Comparison of substance-specific therapeutic indices and toxic areas
| Substance therapeutic area (unit) | CaSO4-G (Herafill-G) (gentamicin) | CaSO4-V (vancomycin) | Osteoset (tobramycin) |
|---|---|---|---|
| Total substance mass implanted (mg) | 5 | 4.9 | 14.3 |
| Therapeutic index (μg/ml) | 4–12 | 5–40 | 4–8 |
| Toxic area (μg/ml) | >10–12 | >80 | >12 |
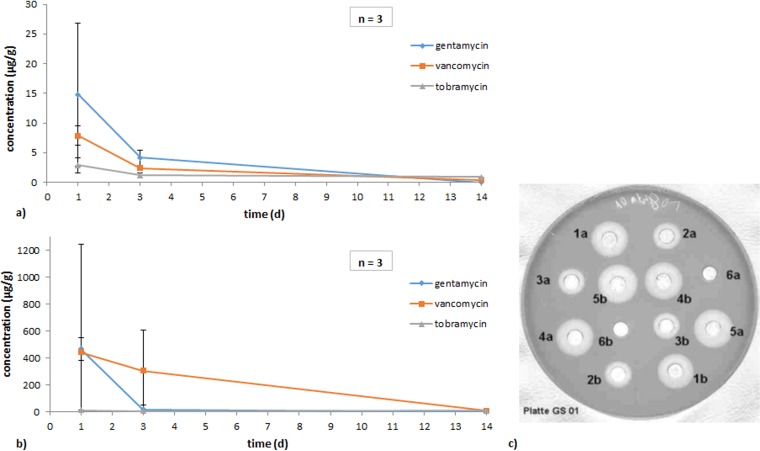
In the animal experiment, mean values of antibiotics in microgram per gram of corticalis and gram of medulla were determined (Fig. 5a and b). On day 1, 14.77 μg/g gentamicin, 7.88 μg/g vancomycin, and 2.86 μg/g tobramycin were analyzed. Three days after implantation, values had decreased significantly, and 14 days postoperation no gentamicin was found and only 0.4 and 0.88 μg/g of vancomycin and tobramycin were left.
FIG 5.
Antibiotic concentrations in 1 g of corticalis (a) and medulla (b) of rabbit tibia bones (ex vivo). (c) Exemplary plate with antibiograms for drug content determination compared to drug standards for gentamicin.
Radiologic results after implantation and 4, 6, 8, and 12 weeks later displayed various phases of unproblematic resorption. Herafill-G units were clearly visible and recognizable even after 12 weeks in the X ray. CaSO4-V units were visible until week 6, and after week 12 four of five units had completely disappeared in the X-ray image. Four weeks after surgery, all three products were visible under X ray, Osteoset rather unclearly, CaSO4-V in reduced density, and Herafill-G displaying a dense core.
Assessment of tissue compatibility of implanted antibiotic formulations was analyzed concerning clinical findings. Key aspects were appraisal of the implantation location, body weight development, and body temperature, as well as leukocyte count. Only a small corridor was left to examine biocompatibility, but no pathological processes were detected. The described reactions in combination with the histologic examination show a good biocompatibility.
DISCUSSION
Bone infections are still one of the major problems in orthopedic and trauma surgery even in the era of modern antibiotics. Bone replacement scaffolds releasing antimicrobial substances may effectively counteract infections. Earlier research concerning infection control by McConoughey et al. has proven that resorbable calcium sulfate functions as well as PMMA in the patient setting for infection control (14). In our study, CaSO4 containing antimicrobial and resorbable bone grafts differing in formulation such as composition, size, shape, and antibiotic content were compared in regard to drug release and safety. CaSO4-G (Herafill-G) showed the slowest release characteristics as well as the steadiest antibiotic levels in comparison to the applied tobramycin and vancomycin beads. To determine drug availability, released antibiotic concentrations were investigated by in vitro experiments as were levels in serum in rabbits, and the local availability in rabbit's bone was determined. None of the three bead formulations displayed any adverse reaction, while all of them were undergoing the expected partial decomposition within the tibial bone.
The use of rabbits as an animal model to investigate the pharmacological effects and safety of the newly developed calcium sulfate carrier is a common model for such investigations (15–17). Rabbits are easy to handle and to keep; their skeleton offers conditions suitable for implant of the necessary amount and size of beads. In our experiments, only female rabbits of comparable ages were used, to avoid gender- or age-specific differences. A disadvantage in the use of rabbits is the cutaneous sensitivity against injections, especially of antibiotics and their associated skin necrosis. Moreover, colonization with a rabbit-specific staphylococcus strain cannot be eliminated, which through minimal skin and mucosa lesions may enter the organism (18–23).
Three bone substitutes based on calcium sulfate were compared as carriers for antibiotics such as gentamicin, tobramycin, and vancomycin. Osteoset, as commercially available, consists only of calcium sulfate in the form of α-hemihydrate. In contrast, the examined novel preparations CaSO4-V and Herafill-G were formulated to contain, in addition, calcium sulfate dihydrates, tripalmitin, and calcium carbonate. The calcium carbonate component counteracts wound healing and osteolytic problems via buffering of the pH value in the implant region due to degradation of CaSO4 and sulfuric acid formation (9). Tripalmitin was added to slow the release of the antibiotics gentamicin and vancomycin. The use of fatty acids to influence release kinetics had previously been documented (24–26).
Following implantation, local swelling was observed in 83% of the animals after implantation of Osteoset, in 50% after CaSO4-V, and in 33% after CaSO4-G (Herafill-G). Irrespective of the implanted bone substitute, formation of hematomas was observed as well as modifications to the tissue due to resorption of the subcutaneous threads, partially resulting in indurations. This yields the assumption that swelling was caused by hematoma and surgical thread resorption rather than by the bone substitutes. Body weight decreased after surgery, probably due to indisposition of the animals and blood drawing. Temperature and leukocyte levels remained within normal physiologic range.
Literature data show that calcium sulfate is completely degraded after implantation; however, the data vary strongly in terms of the time frame as well as degrading mechanisms. Bell et al. assumed that the rapid resorption was a positive effect for bone healing (27). Petruskevicius et al., however, do not recommend Osteoset for implantation in humans, as 6 weeks is too short a time for complete resorption. In order to decrease the rate of resorption, addition of a substance, e.g., calcium phosphate, was recommended (28).
Calcium sulfate is an ideal carrier for drugs, easy to sterilize, and biodegradable and displays good release of integrated drugs. Several authors describe CaSO4 as a carrier of bone morphogenetic protein (BMP) (29–31) as well as antibiotics (32–35). Bai et al. have tested BMP-loaded calcium sulfate in a clinical trial with 16 patients and successfully induced bone healing after prior nonunion of fractures (29). Varlet has conducted research with various calcium sulfate-based antibiotic formulations to fill bone defects, resulting in successful treatment of 13 of 15 patients (36). Bouillet has successfully treated 16 of 18 patients with osteomyelitis using amoxicillin-impregnated calcium sulfate (37).
The basis of the animal experiments is the comparable mass of the bone substitutes. The number of beads implanted per animal was determined through the overall mass of the implants, resulting in various amounts of administered antibiotic as shown in Table 1. CaSO4-G (Herafill-G) and CaSO4-V are in the same range regarding their active antimicrobial content of 5.0 mg gentamicin and 4.9 mg vancomycin, while Osteoset differs strongly with 14.3 mg tobramycin. Concentrations of the released antibiotics are influenced by the content as well as by the size, surface, and composition of the carrier such as CaSO4 and palmitin.
Concerning size, CaSO4-G (Herafill-G) beads with a diameter of 6 mm are the largest beads, followed by the Osteoset beads with 4.8 mm and CaSO4-V with 3 mm. CaSO4-G (Herafill-G) thus displays the smallest surface in sum, combined with the slowest release kinetics, followed by CaSO4-V. Even though the latter has a larger surface area to interact with surrounding fluids than the Osteoset implants, the Osteoset beads have completely dissolved by day 3. This can be explained by their composition: Osteoset consists of calcium sulfate only, and the other two formulations additionally contain tripalmitate. This addition as described above results in lower drug release rates as shown by Radin et al. (38).
Release kinetics of antibiotics differ by in vitro experiment from those in vivo by intramedullary implantation. The situation is more complicated in traumatized tissue. A damaged blood supply, hematoma, or debris causes barriers for rapid diffusion in the surrounding tissue. It is crucial to maintain high local antibiotic substance concentrations for at least 48 h while not reaching systemically toxic levels in serum. A recent study from Howlin et al. has proven that an increased contact time from 24 to 72 h with the antibiotic substance results in a further 3-log reduction in the number of MRSA cells, demonstrating the importance of maintaining locally high concentrations for as long as possible (39). In our study, levels in serum varied greatly, with tobramycin at levels between 0.02 and 0.18 μg/ml, followed by vancomycin at 0.10 to 0.13 μg/ml and gentamicin at 0.41 to 0.58 μg/ml. This allows the assumption that the tested formulations did not reach systemic toxic levels of the incorporated antibiotics.
The palmitin-containing formulations show no remarkable peak or rise after implantation, while Osteoset rises to its peak 24 h after the operation. This can potentially be explained by the fact that the peak might lie prior to the drawing of the first blood sample or by the fact that the new formulation in combination with delayed resorption rates yields a continuous antibiotics release. Dahners and Funderburk found peaks 1 h after implantation with a strong decrease after 8 h (15). Furthermore, Osteoset has shown a rise in week 3 but in week 4 falls back to previous values. Taking into account the fact that mean values with high standard deviations were compared, we are careful in interpreting the peak concentration results.
In general, the levels of all three antibiotics in serum remain significantly below levels of systemic toxicity, supporting the results of Dahners and Funderburk (15).
The antibiotic content of the tibiae was ex vivo determined indirectly via the determination of inhibition zones of eluates. Within the corticalis, peak levels were measured within the first 24 h with gentamicin at 14.8 μg/g, vancomycin at 7.9 μg/g, and tobramycin at 2.9 μg/g, all decreasing within the next 3 days. Gentamicin, being at 4.1 μg/g, was thus at a therapeutic level, while the others showed minor levels, with vancomycin at 0.4 μg/g and tobramycin at 0.9 μg/g. After 12 weeks, none of the antibiotics active against coagulase-negative staphylococci could be measured in serum anymore. Interestingly, even the products already available on the market did not reach therapeutic levels. Potentially the eluates showed only a fraction of the antibiotics, especially for gentamicin still contained in the bone, as antibiotics commonly bind to the bone, forming an adsorbed inseparable unit. Gentamicin in collagen has been noted for its quick release in the initial phase, followed by a possibly constant and high level over a defined period of time, also described as “minivan-like” release model (40).
Knowledge of the bacteria found in infected bones is essential for the choice of suitable antibiotics. The most common cause of bone infection is Staphylococcus aureus, followed by Streptococcus and Pseudomonas. According to Taylor, S. aureus is the sole infectious bacterium in 33% of bone infections, and Streptococcus and Pseudomonas occur in 16% and 51%, respectively, of mixed infections and in 83% of infections with staphylococci involved (41). Gentamicin was proven successful in animal testing (15, 42) as well as in the clinical environment for local treatment (43–45), showing high effectiveness against Gram-negative microorganisms as well as S. aureus. Furthermore, gentamicin shows high thermostability at body temperature (35) and especially during both the polymerization process and the sterilization of PMMA.
In comparison with aminoglycosides, the glycopeptide vancomycin lacks this thermostability, rendering it unsuitable for formulations with PMMA due to heat development. Vancomycin currently is a reserve antibiotic against MRSA, being effective against S. aureus and Staphylococcus epidermidis as well as Enterococcus species.
In addition to the antimicrobial substance efficacy, the biocompatibility of drug-releasing beads poses an important aspect for further application. Regarding biocompatibility, the animals were regularly examined. Body temperature and leukocyte count constantly remained within the reference range. Animals were in good shape, and clinical signs of illness were unapparent. Symptoms such as pain, purulence, development of fistulas, limitation in range of motion, raised infectious parameters, or rise of body temperature (46) were not observed. According to Bencini et al., about 20% of the flora members remain within hair follicles and sebaceous glands (47).
As mentioned in earlier studies by McConoughey et al., once PMMA beads containing antibiotics release their initial burst of surface-bound antibiotics, the beads continue releasing subinhibitory concentrations of antibiotics, favoring the formation of antibiotic-resistant bacteria while providing a surface for bacterial colonization and biofilm formation (14). Neut et al. have additionally described the risk of biomaterial-associated infection on gentamicin-loaded beads, significantly attracting resistant subpopulations with extremely high MICs (48). Research by Garzoni et al. describes the special adaptability of Staphylococcus to cause varied and severe infections (49).
Overall, it was shown that pharmacokinetics vary strongly with the choice of the antibiotic substances as well as their carriers. High antibiotic concentrations were only released for the first 3 days, suggesting that local delivery of antibiotics is potentially suitable for prophylaxis, whereas for treating already-formed biofilms or intracellular bacteria it seems limited, as proposed by Howlin et al. (39).
The limitations of this study are the lack of a comparison group between local-only and systemic-only antibiotic therapy to determine the potentially different effects. Further research may focus on intracellular microbes and their treatment with effective antibiotics like rifampin. Furthermore, a bioassay was used to measure the concentration of antibiotics, while other systems like high-pressure liquid chromatography (HPLC) are more accurate.
Conclusion.
Our study showed the release characteristics of antibiotic-absorbable bone substitutes of three different formulations. Release over time as well as variations in kinetics and concentrations over time were in vitro and in vivo analyzed in animal experiments. No negative side effects were observed. It was proven that differences in the composition of bone substitute material yield differences in release characteristics.
Surgeons may consider the shown release rates and take potential advantages as well as disadvantages into account for their choice of bone substitute material. As suggested by Tan et al. in 2012, the use of antibiotic-loaded beads at the site of infection is becoming the standard of care, as the beads enable localized supra-MIC levels, which would be difficult to achieve by other means (50).
In further research, a clinical study of the vancomycin-containing beads could prove their effectiveness against methicillin-resistant bacterial stems. In a clinical daily routine with rising numbers of infections, this could reduce the medical burden and costs for many severely diseased patients.
ACKNOWLEDGMENTS
We thank the whole ZPF team of the Klinikum Rechts der Isar for their cooperation in conducting the surgical procedures as well as providing care for the animals. In addition, we thank especially M. Schnabelrauch (Innovent e.V., Jena, Germany) for his kind support in determining antimicrobial efficacy of explanted bone tissues via zones of inhibition of eluted samples.
H. Büchner and S. Vogt are employees of Heraeus Medical.
We declare that there is no conflict of interest.
Author contributions: H. Büchner and S. Vogt were involved in (i) study design and data analysis, (ii) revising the manuscript, and (iii) final approval of the manuscript.
Funding Statement
This study was funded by Heraeus Medical. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Murillo O, Grau I, Lora-Tamayo J, Gomez-Junyent J, Ribera A, Tubau F, Ariza J, Pallares R. 2015. The changing epidemiology of bacteraemic osteoarticular infections in the early 21st century. Clin Microbiol Infect 21:254.e1–e8. doi: 10.1016/j.cmi.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Bucholz RW. 2002. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 2002(395):44–52. [DOI] [PubMed] [Google Scholar]
- 3.Wirth CJ, Windhagen H. 2004. New technologies in tissue engineering. Visions of an unlimited bone generation without problems. Orthopade 33:1335–1337. (In German.) [DOI] [PubMed] [Google Scholar]
- 4.Lewandrowski KU, Gresser JD, Wise DL, Trantol DJ. 2000. Bioresorbable bone graft substitutes of different osteoconductivities: a histologic evaluation of osteointegration of poly(propylene glycol-co-fumaric acid)-based cement implants in rats. Biomaterials 21:757–764. doi: 10.1016/S0142-9612(99)00179-9. [DOI] [PubMed] [Google Scholar]
- 5.Muschler GF, Negami S, Hyodo A, Gaisser D, Easley K, Kambic H. 1996. Evaluation of collagen ceramic composite graft materials in a spinal fusion model. Clin Orthop Relat Res 1996(328):250–260. [DOI] [PubMed] [Google Scholar]
- 6.Mackey D, Varlet A, Debeaumont D. 1982. Antibiotic loaded plaster of Paris pellets: an in vitro study of a possible method of local antibiotic therapy in bone infection. Clin Orthop Relat Res 1982(167):263–268. [PubMed] [Google Scholar]
- 7.Peltier LF. 1961. The use of plaster of Paris to fill defects in bone. Clin Orthop 21:1–31. [PubMed] [Google Scholar]
- 8.Peltier LF, Jones RH. 1978. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J Bone Joint Surg 60:820–822. [PubMed] [Google Scholar]
- 9.Coetzee AS. 1980. Regeneration of bone in the presence of calcium sulfate. Arch Otolaryngol 106:405–409. doi: 10.1001/archotol.1980.00790310029007. [DOI] [PubMed] [Google Scholar]
- 10.Stemberger A, Grimm H, Bader F, Rahn HD, Ascherl R. 1997. Local treatment of bone and soft tissue infections with the collagen-gentamicin sponge. Eur J Surg Suppl 1997(578):17–26. [PubMed] [Google Scholar]
- 11.Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. 1997. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg 79:590–595. [DOI] [PubMed] [Google Scholar]
- 12.Boxma H, Broekhuizen T, Patka P, Oosting H. 1996. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch Trauma Trial. Lancet 347:1133–1137. doi: 10.1016/S0140-6736(96)90606-6. [DOI] [PubMed] [Google Scholar]
- 13.European Committee on Antimicrobial Susceptibility Testing. 2015. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0, 2015. http://www.eucast.org.
- 14.McConoughey SJ, Howlin RP, Wiseman J, Stoodley P, Calhoun JH. 2015. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res B Appl Biomater 103:870–877. doi: 10.1002/jbm.b.33247. [DOI] [PubMed] [Google Scholar]
- 15.Dahners LE, Funderburk CH. 1987. Gentamicin-loaded plaster of Paris as a treatment of experimental osteomyelitis in rabbits. Clin Orthop Relat Res 1987(219):278–282. [PubMed] [Google Scholar]
- 16.Ljubovic E, Nikulin A. 1956. Plastic plombage in experimental bone regeneration. Acta Med Iugosl 10:1–36. (In German.) [PubMed] [Google Scholar]
- 17.Orsini G, Ricci J, Scarano A, Pecora G, Petrone G, Iezzi G, Piattelli A. 2004. Bone-defect healing with calcium-sulfate particles and cement: an experimental study in rabbit. J Biomed Mater Res B Appl Biomater 68:199–208. [DOI] [PubMed] [Google Scholar]
- 18.Hermans K, De Herdt P, Devriese LA, Godard C, Haesebrouck F. 2000. Colonisation of rabbits with Staphylococcus aureus after experimental infection with high and low virulence strains. Vet Microbiol 72:277–284. doi: 10.1016/S0378-1135(99)00179-0. [DOI] [PubMed] [Google Scholar]
- 19.Hermans K, Devriese LA, Haesebrouck F. 2003. Rabbit staphylococcosis: difficult solutions for serious problems. Vet Microbiol 91:57–64. doi: 10.1016/S0378-1135(02)00260-2. [DOI] [PubMed] [Google Scholar]
- 20.Meulemans L, Hermans K, Duchateau L, Haesebrouck F. 2007. High and low virulence Staphylococcus aureus strains in a rabbit skin infection model. Vet Microbiol 125:333–340. doi: 10.1016/j.vetmic.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Vancraeynest D, Hermans K, Haesebrouck F. 2004. Genotypic and phenotypic screening of high and low virulence Staphylococcus aureus isolates from rabbits for biofilm formation and MSCRAMMs. Vet Microbiol 103:241–247. doi: 10.1016/j.vetmic.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Vancraeynest D, Hermans K, Martel A, Vaneechoutte M, Devriese LA, Haesebrouck F. 2004. Antimicrobial resistance and resistance genes in Staphylococcus aureus strains from rabbits. Vet Microbiol 101:245–251. doi: 10.1016/j.vetmic.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Vershigora AE, Ovod VV, Vikhot NE, Mikhal'skii LA. 1986. Staphylococcal adherence to rabbit epithelial cells. Zh Mikrobiol Epidemiol Immunobiol 1986(5):37–41. (In Russian.) [PubMed] [Google Scholar]
- 24.Coraca-Huber DC, Putzer D, Fille M, Hausdorfer J, Nogler M, Kuhn KD. 2014. Gentamicin palmitate as a new antibiotic formulation for mixing with bone tissue and local release. Cell Tissue Bank 15:139–144. doi: 10.1007/s10561-013-9384-y. [DOI] [PubMed] [Google Scholar]
- 25.Obermeier A, Matl FD, Schwabe J, Zimmermann A, Kuhn KD, Lakemeier S, von Eisenhart-Rothe R, Stemberger A, Burgkart R. 2012. Novel fatty acid gentamicin salts as slow-release drug carrier systems for anti-infective protection of vascular biomaterials. J Mater Sci Mater Med 23:1675–1683. doi: 10.1007/s10856-012-4631-5. [DOI] [PubMed] [Google Scholar]
- 26.Obermeier A, Schneider J, Wehner S, Matl FD, Schieker M, von Eisenhart-Rothe R, Stemberger A, Burgkart R. 2014. Novel high efficient coatings for anti-microbial surgical sutures using chlorhexidine in fatty acid slow-release carrier systems. PLoS One 9:e101426. doi: 10.1371/journal.pone.0101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell WH. 1964. Resorption characteristics of bone and bone substitutes. Oral Surg Oral Med Oral Pathol 17:650–657. doi: 10.1016/0030-4220(64)90372-X. [DOI] [PubMed] [Google Scholar]
- 28.Petruskevicius J, Nielsen S, Kaalund S, Knudsen PR, Overgaard S. 2002. No effect of Osteoset, a bone graft substitute, on bone healing in humans: a prospective randomized double-blind study. Acta Orthop Scand 73:575–578. doi: 10.1080/000164702321022875. [DOI] [PubMed] [Google Scholar]
- 29.Bai MH, Liu XY, Ge BF, Yallg C, Chen DA. 1996. An implant of a composite of bovine bone morphogenetic protein and plaster of Paris for treatment of femoral shaft nonunions. Int Surg 81:390–392. [PubMed] [Google Scholar]
- 30.Rosenblum SF, Frenkel S, Ricci JR, Alexander H. 1993. Diffusion of fibroblast growth factor from a plaster of Paris carrier. J Appl Biomater 4:67–72. doi: 10.1002/jab.770040109. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki Y, Oida S, Akimoto Y, Shioda S. 1988. Response of the mouse femoral muscle to an implant of a composite of bone morphogenetic protein and plaster of Paris. Clin Orthop Relat Res 1988(234):240–249. [PubMed] [Google Scholar]
- 32.Benoit MA, Mousset B, Delloye C, Bouillet R, Gillard J. 1997. Antibiotic-loaded plaster of Paris implants coated with poly lactide-co-glycolide as a controlled release delivery system for the treatment of bone infections. Int Orthop 21:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowyer GW, Cumberland N. 1994. Antibiotic release from impregnated pellets and beads. J Trauma 36:331–335. doi: 10.1097/00005373-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Miclau T, Dahners LE, Lindsey RW. 1993. In vitro pharmacokinetics of antibiotic release from locally implantable materials. J Orthop Res 11:627–632. doi: 10.1002/jor.1100110503. [DOI] [PubMed] [Google Scholar]
- 35.Mousset B, Benoit MA, Delloye C, Bouillet R, Gillard J. 1995. Biodegradable implants for potential use in bone infection. An in vitro study of antibiotic-loaded calcium sulphate. Int Orthop 19:157–161. [DOI] [PubMed] [Google Scholar]
- 36.Varlet A, Dauchy P. 1983. Plaster of Paris pellets containing antibiotics in the treatment of bone infection. New combinations of plaster with antibiotics. Rev Chir Orthop Reparatrice Appar Mot 69:239–244. (In French.) [PubMed] [Google Scholar]
- 37.Bouillet R, Bouillet B, Kadima N, Gillard J. 1989. Treatment of chronic osteomyelitis in Africa with plaster implants impregnated with antibiotics. Acta Orthop Belg 55:1–11. (In French.) [PubMed] [Google Scholar]
- 38.Radin S, Campbell JT, Ducheyne P, Cuckler JM. 1997. Calcium phosphate ceramic coatings as carriers of vancomycin. Biomaterials 18:777–782. doi: 10.1016/S0142-9612(96)00190-1. [DOI] [PubMed] [Google Scholar]
- 39.Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS, Stoodley P. 2015. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother 59:111–120. doi: 10.1128/AAC.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruszczak Z, Friess W. 2003. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Delivery Rev 55:1679–1698. doi: 10.1016/j.addr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Taylor GJ, Bannister GC, Calder S. 1990. Perioperative wound infection in elective orthopaedic surgery. J Hosp Infect 16:241–247. doi: 10.1016/0195-6701(90)90112-2. [DOI] [PubMed] [Google Scholar]
- 42.Cornell CN, Tyndall D, Waller S, Lane JM, Brause BD. 1993. Treatment of experimental osteomyelitis with antibiotic-impregnated bone graft substitute. J Orthop Res 11:619–626. doi: 10.1002/jor.1100110502. [DOI] [PubMed] [Google Scholar]
- 43.Ascherl R, Stemberg A, Lechner F, Blumel G. 1990. Local treatment of infection with collagen gentamicin. Aktuelle Probl Chir Orthop 34:85–93. (In German.) [PubMed] [Google Scholar]
- 44.Gollwitzer H, Thomas P, Diehl P, Steinhauser E, Summer B, Barnstorf S, Gerdesmeyer L, Mittelmeier W, Stemberger A. 2005. Biomechanical and allergological characteristics of a biodegradable poly(D, L-lactic acid) coating for orthopaedic implants. J Orthop Res 23:802–809. doi: 10.1016/j.orthres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Klemm K. 1979. Gentamicin-PMMA-beads in treating bone and soft tissue infections (author's transl). Zentralbl Chir 104:934–942. (In German.) [PubMed] [Google Scholar]
- 46.Schwameis E, Abdolvahab F, Wurnig C. 1996. Osteomyelitis. Clinical aspects, diagnosis and therapy. Radiologe 36:823–833. (In German.) [DOI] [PubMed] [Google Scholar]
- 47.Bencini PL, Galimberti M, Signorini M, Crosti C. 1991. Antibiotic prophylaxis of wound infections in skin surgery. Arch Dermatol 127:1357–1360. [PubMed] [Google Scholar]
- 48.Neut D, van de Belt H, Stokroos I, van Horn JR, van der Mei HC, Busscher HJ. 2001. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother 47:885–891. doi: 10.1093/jac/47.6.885. [DOI] [PubMed] [Google Scholar]
- 49.Garzoni C, Kelley WL. 2009. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol 17:59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Tan HL, Lin WT, Tang TT. 2012. The use of antimicrobial-impregnated PMMA to manage periprosthetic infections: controversial issues and the latest developments. Int J Artif Organs 35:832–839. doi: 10.5301/ijao.5000163. [DOI] [PubMed] [Google Scholar]