Abstract
Gepotidacin, a novel triazaacenaphthylene antibacterial agent, is the first in a new class of type IIA topoisomerase inhibitors with activity against many biothreat and conventional pathogens, including Neisseria gonorrhoeae. To assist ongoing clinical studies of gepotidacin to treat gonorrhea, a multilaboratory quality assurance investigation determined the reference organism (N. gonorrhoeae ATCC 49226) quality control MIC range to be 0.25 to 1 μg/ml (88.8% of gepotidacin MIC results at the 0.5 μg/ml mode).
TEXT
Antimicrobial resistance (AMR) among Neisseria gonorrhoeae isolates continues to be reported worldwide; most recently, reports included high-level AMR to the fluoroquinolones, extended-spectrum β-lactams, and azithromycin (1–4). To address this public health concern, new antimicrobial agents, drug combinations, and compounds having novel modes of action are being studied (5–11).
Gepotidacin (GSK2140944) is a novel triazaacenaphthylene antibacterial agent, the first in a new class of bacterial type IIA topoisomerase inhibitors. This novel agent does not share cross-resistance with the fluoroquinolones due to a different binding mode and exhibits in vitro activity against levofloxacin-resistant Staphylococcus aureus (MIC90, 0.5 μg/ml) and Streptococcus pneumoniae (MIC90, 0.5 μg/ml) (12, 13). One potential focus of clinical use would be the treatment of gonorrhea, especially in geographic areas where AMR has become highly prevalent (2). To facilitate gepotidacin development and to expand the standardized susceptibility testing to this agent for sexually transmitted diseases, a Clinical and Laboratory Standards Institute (CLSI) M23-style quality control (QC) study (14) was conducted to establish the agar dilution MIC QC range for testing N. gonorrhoeae ATCC 49226 by the CLSI M07-A10 method (15). These results could be used during ongoing clinical investigations along with previously published QC investigations by our group (16).
The study protocol, with eight participating laboratories, was organized and monitored by JMI Laboratories (North Liberty, IA, USA). The participant laboratories (and site director) were the Infectious Diseases Research Laboratory, Detroit, MI (M. Zervos); ThermoFisher Scientific, Cleveland, OH (C. Knapp); University of Rochester, Rochester, NY (D. Hardy); University of Alberta, Edmonton, Canada (R. Rennie); Cleveland Clinic Foundation, Cleveland, OH (G. Procop); JMI Laboratories, North Liberty, IA (R. Jones); Methodist Hospital, Indianapolis, IN (G. Denys); and Tufts University Medical Center, Boston, MA (D. Snydman).
The site investigators applied the CLSI M07-A10 agar dilution method (15) for testing the N. gonorrhoeae ATCC 49226 QC organism. Each site was provided with 3 lots of GC agar base deeps (BD, Difco, and Oxoid) with 1% defined growth supplement to test gepotidacin (GlaxoSmithKline; lot 132377141) and the control agent ciprofloxacin (Sigma; lot BCBM7969V) across 7 doubling dilution steps (0.06 to 4 and 0.0005 to 0.03 μg/ml, respectively). Ten replicates of each drug and medium lot (only one lot for ciprofloxacin) were performed over at least 2 testing days with a maximum of 5 replicates per day. This design generated 30 MIC results at each laboratory site, or 240 total gepotidacin MIC values (80 for ciprofloxacin). Colony counts to monitor inoculum density were determined at each location. The average inoculum count across all sites and experiments was 1.6 × 105 CFU/spot (range, 7.4 × 104 to 5.0 × 105 CFU/spot).
Gepotidacin MIC results for N. gonorrhoeae ATCC 49226 were very reproducible at each laboratory. All 8 participant sites had a modal value of 0.5 μg/ml, and the majority of gepotidacin MIC results (57 to 79 of 80 replicate MICs) were at the mode with each GC agar base lot. The geometric mean MIC results only varied from 0.40 to 0.50 μg/ml among the 8 participant sites and ranged from 0.41 to 0.51 μg/ml for the three tested medium lots.
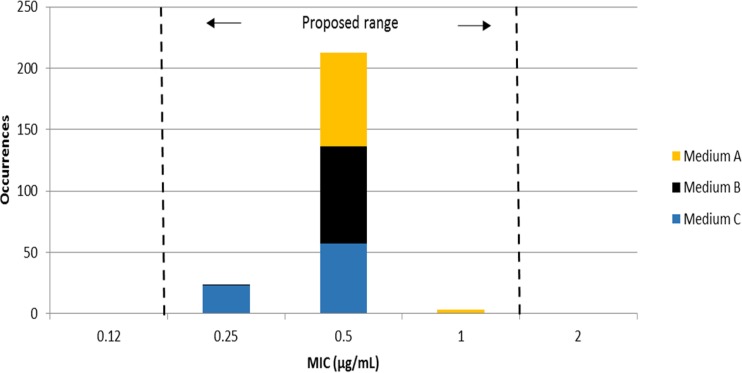
Figure 1 shows the gepotidacin MIC distribution for N. gonorrhoeae ATCC 49226 (240 values). A total of 213 MIC results (88.8%) were at the 0.5 μg/ml mode, and all MIC results were within the proposed 3-log2 dilution QC range of 0.25 to 1 μg/ml, as calculated from the Gavan method (14). The Range Finder statistical method (17) also suggested an identical MIC QC range. All ciprofloxacin control MIC values were within the CLSI-published range of 0.001 to 0.008 μg/ml (18). The control QC strain MIC for gepotidacin was consistent with reported clinical N. gonorrhoeae strains (23), having MIC50 and MIC90 results of 0.12 and 0.25 μg/ml, respectively.
FIG 1.
Gepotidacin (GSK2140944) reference agar dilution MIC results from an 8-laboratory investigation to determine QC ranges for N. gonorrhoeae ATCC 49226. All results are within the proposed MIC range (0.25 to 1 μg/ml).
Gepotidacin, with a unique mode of action, provides an opportunity to further study a novel agent for the treatment of uncomplicated gonorrhea, thus potentially limiting the probability of cross-resistance or coresistance (12, 19). The in vitro activity of gepotidacin (MIC mode, 0.5 μg/ml) against the N. gonorrhoeae QC strain (ATCC 49226), having nonsusceptible characteristics to penicillin and tetracycline, appears promising when combined with favorable findings from safety and pharmacokinetic/pharmacodynamic studies (20, 21). As AMR among N. gonorrhoeae continues to evolve in numerous countries, gepotidacin warrants further study (as listed at ClinicalTrials.gov) (22) supported by reference-quality susceptibility tests (15, 18) and guided by the MIC QC guidance published here.
ACKNOWLEDGMENTS
This study was supported by GlaxoSmithKline (GSK; Collegeville, PA, USA) and has been funded in whole or in part with federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority under contract HHSO100201300011C. R.N.J., K.A.F., J.E.R., and R.K.F. are employees of JMI Laboratories who received grant funds to study gepotidacin and were paid consultants to GSK in connection with the development of the manuscript. N.E.S.-O. is an employee of GSK.
We thank the contributing laboratories (personnel and directors) for their excellent support of this protocol.
JMI Laboratories, Inc., has also received research and educational grants in 2014 and 2015 from Achaogen, Actavis, Actelion, Allergan, American Proficiency Institute (API), AmpliPhi, Anacor, Astellas, AstraZeneca, Basilea, Bayer, BD, Cardeas, Cellceutix, CEM-102 Pharmaceuticals, Cempra, Cerexa, Cidara, Cormedix, Cubist, Debiopharm, Dipexium, Dong Wha, Durata, Enteris, Exela, Forest Research Institute, Furiex, Genentech, GSK, Helperby, ICPD, Janssen, Lannett, Longitude, Medpace, Meiji Seika Kasha, Melinta, Merck, Motif, Nabriva, Novartis, Paratek, Pfizer, Pocared, PTC Therapeutics, Rempex, Roche, Salvat, Scynexis, Seachaid, Shionogi, Tetraphase, The Medicines Co., Theravance, ThermoFisher, VenatoRX, Vertex, Wockhardt, Zavante, and some other corporations. Some JMI employees are advisors/consultants for Allergan, Astellas, Cubist, Pfizer, Cempra, and Theravance. We have no speaker bureaus or stock options to declare.
Funding Statement
This study was supported by GlaxoSmithKline (GSK; Collegeville, PA, USA) and has been funded in whole or in part with federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority under contract HHSO100201300011C. R.N.J., K.A.F., J.E.R., and R.K.F. are employees of JMI Laboratories who received grant funds to study gepotidacin and were paid consultants of GSK in connection with the development of the manuscript. N.E.S.-O. is an employee of GSK.
REFERENCES
- 1.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buono SA, Watson TD, Borenstein LA, Klausner JD, Pandori MW, Godwin HA. 2015. Stemming the tide of drug-resistant Neisseria gonorrhoeae: the need for an individualized approach to treatment. J Antimicrob Chemother 70:374–381. doi: 10.1093/jac/dku396. [DOI] [PubMed] [Google Scholar]
- 3.Kirkcaldy RD, Soge O, Papp JR, Hook EW III, del Rio C, Kubin G, Weinstock HS. 2015. Analysis of Neisseria gonorrhoeae azithromycin susceptibility in the United States by the Gonococcal Isolate Surveillance Project, 2005 to 2013. Antimicrob Agents Chemother 59:998–1003. doi: 10.1128/AAC.04337-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen SC, Yin YP, Dai XQ, Unemo M, Chen XS. 2016. First nationwide study regarding ceftriaxone resistance and molecular epidemiology of Neisseria gonorrhoeae in China. J Antimicrob Chemother 71:92–99. doi: 10.1093/jac/dkv321. [DOI] [PubMed] [Google Scholar]
- 5.Biedenbach DJ, Turner LL, Jones RN, Farrell DJ. 2012. Activity of JNJ-Q2, a novel fluoroquinolone, tested against Neisseria gonorrhoeae, including ciprofloxacin-resistant strains. Diagn Microbiol Infect Dis 74:204–206. doi: 10.1016/j.diagmicrobio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Barbee LA, Soge OO, Holmes KK, Golden MR. 2014. In vitro synergy testing of novel antimicrobial combination therapies against Neisseria gonorrhoeae. J Antimicrob Chemother 69:1572–1578. doi: 10.1093/jac/dkt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauser C, Hirzberger L, Unemo M, Furrer H, Endimiani A. 2015. In vitro activity of fosfomycin alone and in combination with ceftriaxone or azithromycin against clinical Neisseria gonorrhoeae isolates. Antimicrob Agents Chemother 59:1605–1611. doi: 10.1128/AAC.04536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob Agents Chemother 56:2739–2742. doi: 10.1128/AAC.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hook EW III, Golden M, Jamieson BD, Dixon PB, Harbison HS, Lowens S, Fernandes P. 2015. A phase 2 trial of oral solithromycin 1,200 mg or 1,000 mg as single-dose oral therapy for uncomplicated gonorrhea. Clin Infect Dis 61:1043–1048. doi: 10.1093/cid/civ478. [DOI] [PubMed] [Google Scholar]
- 10.Unemo M, Ringlander J, Wiggins C, Fredlund H, Jacobsson S, Cole M. 2015. High in vitro susceptibility to the novel spiropyrimidinetrione ETX0914 (AZD0914) among 873 contemporary clinical Neisseria gonorrhoeae isolates from 21 European countries from 2012 to 2014. Antimicrob Agents Chemother 59:5220–5225. doi: 10.1128/AAC.00786-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkcaldy RD, Weinstock HS, Moore PC, Philip SS, Wiesenfeld HC, Papp JR, Kerndt PR, Johnson S, Ghanem KG, Hook EW III. 2014. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 59:1083–1091. doi: 10.1093/cid/ciu521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, Huang J, Jones E, Jones J, Brown KK, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Shen C, Shillings A, Theobald AJ, Wohlkonig A, Pearson ND, Gwynn MN. 2010. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 13.Bouchillon S, Hackel M, Miller LA, Scangarella-Oman NE. 2013. In vitro activity of GSK2140944, a novel topoisomerase inhibitor, against isolates associated with lower respiratory tract and skin infections, abstr F-1216. 53rd Intersci Conf Antimicrob Agents Chemother, 10 to 13 September, 2013, Denver, CO. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2008. M23-A3. Development of in vitro susceptibility testing criteria and quality control parameters—3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2015. M07-A10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th edition. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Ross JE, Scangarella-Oman NE, Flamm RK, Jones RN. 2014. Determination of disk diffusion and MIC quality control guidelines for GSK2140944, a novel bacterial type II topoisomerase inhibitor antimicrobial agent. J Clin Microbiol 52:2629–2632. doi: 10.1128/JCM.00656-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnidge J, Bordash G. 2007. Statistical methods for establishing quality control ranges for antibacterial agents in Clinical and Laboratory Standards Institute susceptibility testing. Antimicrob Agents Chemother 51:2483–2488. doi: 10.1128/AAC.01457-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; 26th informational supplement. CLSI M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Ehmann DE, Lahiri SD. 2014. Novel compounds targeting bacterial DNA topoisomerase/DNA gyrase. Curr Opin Pharmacol 18:76–83. doi: 10.1016/j.coph.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Tiffany CA, Hossain M, McDonald M, Patel A, Lerman S, Patel P, Widdowson K, Dumont E. 2013. Safety and pharmacokinetics of single escalating oral doses of GSK2140944, a novel bacterial topoisomerase inhibitor, abstr F-1218. 53rd Intersci Conf Antimicrob Agents Chemother, 10 to 13 September, 2013, Denver, CO. [Google Scholar]
- 21.So W, Crandon JL, Nicolau DP. 2015. Pharmacodynamic profile of GSK2140944 against methicillin-resistant Staphylococcus aureus in a murine lung infection model. Antimicrob Agents Chemother 59:4956–4961. doi: 10.1128/AAC.00625-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinicaltrials.gov. 2016. A dose-ranging study evaluating the efficacy, safety, and tolerability of GSK2140944 in the treatment of uncomplicated urogenital gonorrhea caused by Neisseria gonorrhoeae. https://clinicaltrials.gov/ct2/show/NCT02294682?term=GSK2140944&rank=8.
- 23.Farrell DJ, Sader HS, Rhomberg PR, Scangarella-Oman NE, Flamm RK. 2016. Gepotidacin (GSK2140944) in vitro activity against Neisseria gonorrhoeae (MIC/MBC, kill kinetics, checkerboard, PAE/SME tests), poster 461. ASM Microbe 2016, Boston, Massachusetts, 16 to 20 June 2016. [Google Scholar]