Abstract
We previously found the LL-37-derived peptide P60.4Ac to be effective against methicillin-resistant Staphylococcus aureus (MRSA) on human epidermal models (EMs). The goal of this study was to identify the preferred carrier for this peptide for topical application on skin and mucosal surfaces. We prepared P60.4Ac in three formulations, i.e., a water-in-oil cream with lanolin (Softisan 649), an oil-in-water cream with polyethylene glycol hexadecyl ether (Cetomacrogol), and a hydroxypropyl methylcellulose (hypromellose) 4000 gel. We tested the antimicrobial efficacy of the peptide in these formulations against mupirocin-resistant and -sensitive MRSA strains on EMs and bronchial epithelial models (BEMs). The cytotoxic effects of formulated P60.4Ac on these models were determined using histology and WST-1 and lactate dehydrogenase assays. Moreover, we assessed the stability of the peptide in these formulations with storage for up to 3 months. Killing of MRSA by P60.4Ac in the two creams was less effective than that by P60.4Ac in the hypromellose gel. In agreement with those findings, P60.4Ac in the hypromellose gel was highly effective in eradicating the two MRSA strains from EMs. We found that even 0.1% (wt/wt) P60.4Ac in the hypromellose gel killed >99% of the viable planktonic bacteria and >85% of the biofilm-associated bacteria on EMs. Hypromellose gels containing 0.1% and 0.5% (wt/wt) P60.4Ac effectively reduced the numbers of viable MRSA cells from BEMs by >90%. No cytotoxic effects of P60.4Ac in the hypromellose gel with up to 2% (wt/wt) P60.4Ac on keratinocytes in EMs and in the hypromellose gel with up to 0.5% (wt/wt) P60.4Ac on epithelial cells in BEMs were observed. High-performance liquid chromatography analysis showed that P60.4Ac was stable in the Softisan cream and the hypromellose gel but not in the Cetomacrogol cream. We conclude that P60.4Ac formulated in hypromellose gel is both stable and highly effective in eradicating MRSA from colonized EMs and BEMs.
INTRODUCTION
About 10 to 35% of humans are persistent carriers of Staphylococcus aureus, while 20 to 75% are intermediate carriers (1–3). The primary sites for colonization of humans are the nasal cavity, pharynx, perineum, and skin (3). Colonization of healthy individuals with this opportunistic bacterium causes no serious health problems; however, the risk of developing an S. aureus infection is increased in hospitalized patients or those undergoing surgery (4–6). Common infection problems include surgical site infections (7), wound and burn wound infections (5), bloodstream infections (8), catheter-related bacteremia (9, 10), and catheter (intubation)-related infections, such as pneumonia (11). Moreover, the symptoms of atopic eczema patients are often aggravated by the actions of S. aureus and S. epidermidis on their lesion sites (12, 13).
Strategies currently used to eradicate methicillin-resistant S. aureus (MRSA) from carriers involve treatment with antibiotics, such as vancomycin, mupirocin, bacitracin, rifampin, and retapamulin (14–18). It has been shown that decolonization of nasal carriers of S. aureus by treatment with mupirocin reduces the risk of surgical infection (19, 20). However, the increasing emergence of mupirocin-resistant S. aureus isolates in hospitals as well as the community underscores the need for novel antimicrobial agents with modes of action different from those of current antibiotics (21).
We consider synthetic peptides derived from naturally occurring antimicrobial peptides (AMPs), like LL-37, to be promising candidates for such agents. LL-37 is an α-helical amphipathic AMP that is cleaved from the inactive proprotein human cationic antimicrobial protein 18 (hCAP-18) and has antimicrobial activity against a wide variety of pathogens, including bacteria, fungi, and viruses, as well as immunomodulatory and wound-healing activities (for a review, see reference 22). We have previously developed the synthetic AMP P60.4Ac based on a structure-function analysis of LL-37 and have shown that it has in vitro antibacterial activity against S. aureus and Pseudomonas aeruginosa (including antibiotic-resistant strains) on wounded colonized human skin equivalents (23, 24). In addition, a polymer-lipid coating containing the peptide was found to prevent S. aureus biomaterial-associated infections in animals (25). Furthermore, local treatment with P60.4Ac was found to be beneficial in patients with therapy-resistant chronic otitis media (unpublished data).
The aim of this study was to evaluate the antimicrobial effectivity, stability, and cytotoxicity of P60.4Ac in three different formulations that may be suitable for topical application on skin and nasal mucosa. The formulations were a water-in-oil cream with lanolin (Softisan 649; referred to here as Softisan cream), an oil-in-water-type polyethylene glycol hexadecyl ether (Cetomacrogol) cream (referred to here as Cetomacrogol cream), and water-based hydroxypropyl methylcellulose (hypromellose) 4000 gel (referred to here as hypromellose gel). We found that P60.4Ac at noncytotoxic concentrations in hypromellose gel was more effective than this peptide in Softisan and Cetomacrogol creams in killing MRSA on colonized human epidermal models (EMs) and bronchial epithelial models (BEMs).
MATERIALS AND METHODS
Bacterial strains.
S. aureus strains LUH14616 and LUH15051 (23) were used in this study. Bacteria were preserved for prolonged periods in nutrient broth (Oxoid Ltd.) supplemented with 20% (vol/vol) glycerol at −80°C. Inocula from frozen cultures were grown overnight at 37°C on sheep blood agar plates (bioMérieux) before usage.
Mupirocin and Bactroban.
For comparison, 2% mupirocin calcium ointment (Bactroban nasal; GlaxoSmithKline) and a stock of mupirocin (Sigma-Aldrich) diluted to 1 mg/ml in phosphate-buffered saline (PBS) were included in this study.
Peptide.
N-terminal-acetylated and C-terminal-amidated P60.4Ac (IGKEFKRIVERIKRFLRELVRPLR; molecular mass, 3,094 Da) was synthesized by solid-phase strategies on an automated multiple-peptide synthesizer (SyroII; MultiSyntech) as described previously (24). The purity of the peptide was >95%, as determined by reverse-phase high-performance liquid chromatography (HPLC). The lyophilized peptide was stored at −20°C until use. A stock solution (100 mg/ml) of this peptide prepared in 200 mM phosphate buffer (pH 6.9) was stored at −20°C.
Compounding of P60.4Ac.
The cream base with Softisan 649 as the emulsifier was prepared by warming 28.5 g of vaselinum album (Duchefa Pharma) and 1.5 g of Softisan 649 (IMCD Benelux) to 70°C, and then the ointment was homogenized and mixed until it cooled to room temperature. The cream was added to a 50-g white aluminum tube (Spruyt Hillen BV) and stored at room temperature. The Cetomacrogol cream base was prepared as follows: 200 mg of sorbic acid was dissolved in about 30 ml of hot distilled water. Next, 15 g of cera Cetomacrogolis emulsificans, 20 g of Cetiol V oil, and 4 g of sorbitol (all from Duchefa Pharma) were together warmed to 70°C. The hot sorbic acid solution was added to the melted Cetomacrogol mixture, and it was homogenized continuously while it was cooled to room temperature. After addition of water until the final weight was 70 g, the cream was homogenized, added to 100-g white aluminum tubes, and stored at 4°C. For preparation of the hypromellose gel base, we suspended 3.75 g of hypromellose 4000 in 30 ml of propylene glycol (all from Duchefa Pharma) and added distilled water in portions until the final weight was 100 g. The gel was homogenized, kept in a refrigerator for 12 h, homogenized again, added to a 100-g white aluminum tube, and then stored at 4°C.
The creams and gels containing different amounts of P60.4Ac (range, 0.1% to 2%, wt/wt) were prepared by mixing 3.5 g of these cream/gel bases with the appropriate amount of the P60.4Ac stock solution, and then distilled water was added to a final weight of 5 g of cream or gel. The peptide-containing gels and Cetomacrogol creams were homogenized and added to sterile, 5-g eye ointment tubes (Spruyt Hillen BV). The Softisan cream containing P60.4Ac was added to a 20-ml polypropylene pot (Blockland Pack).
Analysis of the peptide content and possible breakdown products in the various ointments containing P60.4Ac using liquid chromatography.
Creams and gels containing P60.4Ac (0.5%, wt/wt) were stored for up to 3 months at 4°C and at room temperature. For analysis of the peptide content and stability in the Softisan cream, the cream was first broken by addition of water and warming to 55°C for 10 min under vigorous shaking and centrifugation as described earlier (26). An aliquot of 20 μl of the water phase was injected into the HPLC apparatus. Recovery of P60.4Ac from the Cetomacrogol cream was achieved by dissolving 0.2 g of cream in 2 ml of methanol and then adding purified water to give an emulsion, which was homogenized, and subsequently, the water phase and the fat phase were separated by centrifugation. A small sample of the water phase was used for peptide analysis. The hypromellose gel (0.15 g) was homogenized in purified water and was then ready for analysis of the peptide on the HPLC apparatus.
Measurement of the peptide content and possible breakdown products in the above-indicated fractions of the two creams and the hypromellose gel was performed by HPLC. Either sodium phenobarbital (purity, 99.7%; Bufa) dissolved in distilled water (∼0.03 mg/ml) or a solution of spironolactone (∼0.35 mg/ml; Bufa) in acetonitrile-water-trifluoroacetic acid (TFA) (500:500:1) was included as an internal standard. The HPLC apparatus was comprised of a Dionex model P680 LPG isocratic pump, a UV/visible detector (Dionex model UVD 340U), an autosampler (Dionex model ASI 100), and Chromeleon chromatography workstation (version 6.8) software. Separation of the peptide and its breakdown products in the samples was performed on a Phenomenex Nucleosil C18 analytical column (particle size, 5 μm; 150 by 4.6 mm; pore size, 300 Å), and acetonitrile-water-TFA (500:500:1) was used for isocratic elution. The detection was performed at a λ of 220 nm. The flow rate was 1 ml/min, and the pressure (ρ) was 1.14 × 107 ± 0.04 × 107 Pa. The peptide content of the creams and gel was calculated from standard curves constructed with serial dilutions of the peptide in PBS.
In vitro killing assay.
MRSA bacteria were cultured to mid-logarithmic phase at 37°C under vigorous shaking and washed once with PBS. The bacterial suspension was diluted in PBS supplemented with 1% (wt/vol) tryptic soy broth (TSB; Oxoid Ltd.) to a concentration of 1.1 × 106 CFU/ml, determined by measurement of the optical density at 600 nm. Next, 180 μl of the diluted bacterial suspension (2 × 105 CFU) was added to vials containing 20 ± 1 mg of Softisan cream, Cetomacrogol cream, or hypromellose gel or 20 ml of PBS containing increasing amounts of peptide. As a negative control, the bacteria were exposed to the placebo creams and gels or PBS without the peptide. After 1 h or 24 h at 37°C, the mixtures of bacteria and the formulations were serially diluted in PBS and plated on diagnostic sensitivity test (DST) agar plates (Oxoid Ltd.). After overnight incubation at 37°C, the number of viable bacteria was determined. Antimicrobial activity was expressed as the 99% lethal concentration (LC99), i.e., the lowest peptide concentration that killed ≥99% of bacteria after 1 h or 24 h of incubation. The lower limit of detection was 50 CFU/ml.
Ethics statement.
All primary human skin and bronchial epithelial cells used in this study were isolated from surplus tissue collected according to Article 467 of the Dutch Law on Medical Treatment Agreement and the Code for Proper Use of Human Tissue of the Dutch Federation of Biomedical Scientific Societies. None of the authors were involved in the tissue sampling. The Declaration of Helsinki principles were followed when working with human tissues.
Human EMs.
Epidermal models (EMs) were created as described before (27) by seeding 2 × 105 keratinocytes/filter insert (filter pore size, 0.4 μm; 12-well filter inserts; Corning; Costar) in Dermalife K medium including life factors (Lifeline Cell Technology) containing 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen) until the cells were confluent. Thereafter, the culture medium was replaced by keratinocyte medium (Dulbecco modified Eagle medium [DMEM; Gibco]) diluted 3:1 with Ham's F-12 medium (Gibco) supplemented with 1 μM hydrocortisone, 1 μM isoproterenol, and 0.1 μM insulin (all from Sigma-Aldrich) mixed with CNT-02-3D medium (basal medium plus supplement kit; CellnTec) supplemented with 24 μM bovine serum albumin, 25 μM palmitic acid, 15 μM linoleic acid, and 7 μM arachidonic acid (all from Sigma-Aldrich) and penicillin-streptavidin (BioWhittaker). Next, the cultures were lifted to the air-liquid interface, and after 1 day the linoleic acid concentration was increased to 30 μM. The medium was refreshed every 3 days. The EMs were cultured at the air-liquid interface for 10 days at 37°C in 7.3% CO2. These models represent the epidermal part of the human skin and contain a fully differentiated epidermis and stratum corneum (27). One day prior to exposure of the models to bacteria, the culture medium was replaced by medium without antibiotics.
Human BEMs.
Bronchial epithelial models (BEMs) were prepared as described before (28, 29). Briefly, primary bronchial epithelial cells were obtained from cancer-free lung tissue. Cells were subcultured in a 1:1 mixture of DMEM (Gibco) and bronchial epithelial growth medium (BEGM; Clonetics) supplemented with 15 ng/ml retinoic acid (Sigma-Aldrich), 1.5 μg/ml bovine serum albumin (BSA), 100 U/ml penicillin, and 100 μg/ml streptomycin. For the models, passage 2 cells were grown (40,000 cells per insert) submerged for 4 to 7 days until they reached confluence on semipermeable inserts (pore size, 0.4 μm; diameter, 12 mm; Costar; Corning) that were precoated with a mixture of 10 μg/ml BSA, 30 μg/ml bovine collagen solution (PureCol; Advanced Biomatrix), and 10 μg/ml fibronectin in PBS. Thereafter, they were cultured at the air-liquid interface for 2 weeks to induce mucociliary differentiation. To dissolve mucus threads, the BEMs were washed with 100 μl of PBS three times a week, and prior to the experiments the apical side of the BEMs was incubated with 200 μl of 0.01 M phosphate buffer (pH 7.4) for 30 min at 37°C. One day prior to exposure of the cells in the models to bacteria, the culture medium was replaced by medium without antibiotics.
Colonization and antibacterial treatment of EMs and BEMs.
For inoculation of the EMs and BEMs, bacteria were grown to log phase by culturing them for 2.5 h at 37°C in TSB at 200 rpm. This suspension was centrifuged for 10 min at 1,200 rpm, and the bacteria were resuspended in PBS. Next, the bacteria were diluted to a concentration of 3.3 × 105 CFU/ml. The models were inoculated with 300 μl of the suspension for 1 h. Thereafter, the bacterial suspension was aspirated and the models were further incubated for different time intervals. Next, 4 mg of P60.4Ac-containing Softisan cream, Cetomacrogol cream, and hypromellose gel and these ointments without peptide or Bactroban were applied on a coverslip and subsequently put on the models with minimal pressure. For comparison, a stock of P60.4Ac or mupirocin was diluted to 1 mg/ml in PBS, and 100 μl of these dilutions was applied to each model. At 4 h after application of the peptide or mupirocin, the nonadherent and loosely detachable bacteria were first collected and then the models were homogenized in 1 ml of PBS using glass potter tissue grinders (Sartorius; Fischer Scientific), serially diluted, and plated on DST plates for counting of the number of CFU. The lower limit of detection was 20 CFU.
Cryo-SEM.
Cryo-scanning electron microscopy (cryo-SEM) was used to visualize the effect of P60.4Ac on strain LUH14616 biofilms on EMs. Briefly, 24-h biofilms were exposed to P60.4Ac in PBS for 1 h. Thereafter, 2-mm biopsy specimens were quickly frozen in liquid nitrogen slush and immediately transferred to the cryo-transfer attachment (Alto2500; Gatan). The samples were sublimated at −90°C in a high vacuum for 5 min and subsequently sputter coated with a layer of 20-nm gold/palladium and examined using a JEOL JSM6700F scanning electron microscope.
Histology.
Sections of EMs were fixed in 3.7% formaldehyde and subsequently dehydrated and embedded in paraffin. Next, 5-μm sections were made and stained using hematoxylin and eosin (Klinipath) according to the manufacturer's instructions.
Cytotoxicity assays.
The effects of the formulated P60.4Ac and the cream and gel bases on cell viability in the epidermal and mucosal models were determined by the 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt (WST-1) assay for metabolic activity and the lactate dehydrogenase (LDH) release assay. Both assays were performed according to the manufacturer's instructions (Roche). Results are expressed as the percent metabolic activity and the percent LDH release relative to the values for the untreated controls.
Statistical analyses.
The Mann-Whitney U test and the Wilcoxon rank sum test were used to calculate the differences between the values for peptide-treated and control samples. Differences with P values of ≤0.05 were considered significant.
RESULTS
Stability of P60.4Ac in the different formulations.
We previously reported that 2% (wt/wt) P60.4Ac in Softisan cream remained stable for at least 6 months (26). Here, we assessed the stability of 0.5% (wt/wt) P60.4Ac in Softisan cream, Cetomacrogol cream, and hypromellose gel. All three peptide-containing formulations were stored at 4°C and ambient temperature (15 to 25°C) for up to 3 months. Using HPLC analysis, we observed that P60.4Ac in Softisan cream and hypromellose gel remained chemically stable at both temperatures with peptide yields of >90% (Table 1). Softisan cream stored at 4°C demonstrated a separation of the oil and water phases at the end of the third month and was rehomogenized before analysis. The peptide content in Cetomacrogol cream stored at ambient temperature decreased to 69.4% after 1 month and decreased further to 47.3% after 3 months (Table 1). When refrigerated, the peptide content was stable during the first month, but after 3 months the peptide content in Cetomacrogol cream dropped to 66.5% (Table 1). Within 1 month of storage at ambient temperature and 3 months in a refrigerator, the peptide-containing Cetomacrogol cream but not the cream base became light yellow. However, no degradation products of P60.4Ac could be found by HPLC analysis and mass spectrometry (data not shown). Moreover, the bactericidal effectivity of P60.4Ac in the three formulations and PBS when it was stored for 3 months at 4°C was similar to that of P60.4Ac in freshly prepared formulations (data not shown), indicating that the antibacterial activity remained intact during storage for 3 months.
TABLE 1.
Stability of P60.4Ac in the three formulations stored for 3 months at 4°C and ambient temperaturea
| Length of storage (mo) | Peptide yield (%) |
|||||
|---|---|---|---|---|---|---|
| Softisan cream |
Cetomacrogol cream |
Hypromellose gel |
||||
| 4°C | 15–25°C | 4°C | 15–25°C | 4°C | 15–25°C | |
| 0 | 90.3 | 96.6 | 95.0 | 95.5 | 99.3 | 101.6 |
| 1 | 96.7 | 91.0 | 90.6 | 69.4 | 99.3 | 102.4 |
| 3 | 95.3 | 93.8 | 66.5 | 47.3 | 93.2 | 90.0 |
Peptide yields from the Softisan cream, Cetomacrogol cream, and hypromellose gel containing 0.5% (wt/wt) P60.4Ac were measured immediately before and after a storage period of 1 and 3 months at 4°C and at ambient temperature (15 to 25°C). The peptide content of the creams and gel was calculated using a standard curve constructed with a range of concentrations of P60.4Ac in PBS. No degradation products of P60.4Ac were found. Results are means from two independent experiments.
Bactericidal effects of P60.4Ac in different formulations against MRSA LUH14616.
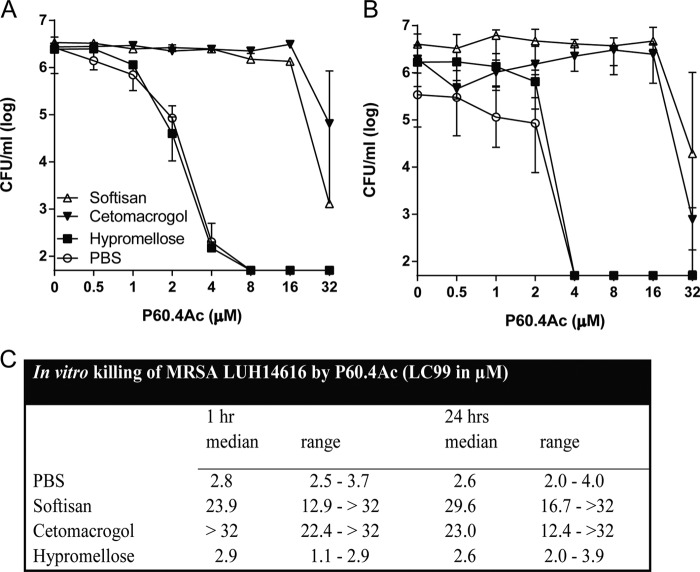
To find out if the antibacterial activity of P60.4Ac was affected by the different formulations, we exposed MRSA LUH14616 to increasing amounts of the peptide in the Softisan cream, the Cetomacrogol cream, the hypromellose gel, and PBS for 1 h and then determined the bacterial counts. Results revealed the dose-dependent killing of MRSA LUH14616 by P60.4Ac in all four formulations (Fig. 1A). Similar results were seen for 24 h of incubation of MRSA LUH14616 with P60.4Ac in the different formulations (Fig. 1B). The LC99 values for P60.4Ac in PBS and the hypromellose gel were similar, whereas the LC99 values for P60.4Ac in the Cetomacrogol and Softisan creams were considerably higher, indicating that the antibacterial activity of P60.4Ac was significantly reduced in the Cetomacrogol and Softisan creams but not in the gel (Fig. 1C).
FIG 1.
In vitro bactericidal activity of P60.4Ac in different formulations compared to that of the peptide in PBS. (A and B) The bactericidal activity of P60.4Ac in three different formulations, Softisan cream, Cetomacrogol cream, and hypromellose gel, and in PBS against MRSA strain LUH14616 after 1 h (A) and 24 h (B). The number of viable bacteria was determined microbiologically. The results are medians and interquartile ranges from 3 to 5 independent experiments. (C) LC99 (i.e., the lowest concentration of the peptide that kills 99% of the bacteria) of P60.4Ac in the different formulations. Medians and ranges from 3 to 5 independent experiments are shown.
Bactericidal effect of P60.4Ac in the different formulations on MRSA colonizing EMs.
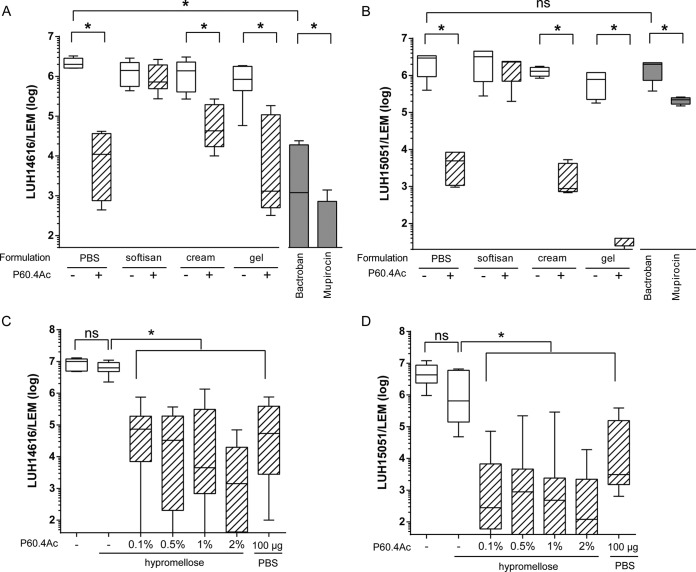
Next, we determined the bactericidal activity of P60.4Ac in the different formulations and, for comparison, the bactericidal activity of Bactroban and mupirocin on bacteria colonizing EMs. These models represent the epidermal part of the human skin and contain a fully differentiated epidermis and stratum corneum (27). EMs were inoculated with two different MRSA strains, i.e., mupirocin-sensitive strain LUH14616 and mupirocin-resistant strain LUH15051. Application of 4 mg of the Cetomacrogol cream and the hypromellose gel containing 2% (wt/wt) P60.4Ac, the equivalent of 80 μg of peptide/EM, resulted in respective 97.9% and >99.9% reductions in bacterial counts for LUH14616, which was similar to the bactericidal effect of Bactroban and mupirocin (Fig. 2A). Interestingly, P60.4Ac in the Cetomacrogol cream and hypromellose gel caused similar reductions in the bacterial counts for mupirocin-resistant strain LUH15051, whereas mupirocin and Bactroban were ineffective (Fig. 2B). P60.4Ac formulated in Softisan cream did not give a significant reduction in bacterial counts for these two strains. Since P60.4Ac in the hypromellose gel proved to be stable and highly effective against both strains of MRSA, we determined the ability of increasing amounts of P60.4Ac in the gel to eradicate LUH14616 from EMs. The results revealed that the gel containing even 0.1% (wt/wt) P60.4Ac decreased the bacterial counts for LUH14616 from 1.0 × 107 to 7.3 × 104, a reduction of 99.3% (Fig. 2C). In agreement with that finding, the 0.1% (wt/wt) P60.4Ac-containing gel led to a >99.9% reduction in bacterial counts for LUH1505, i.e., from 4.3 × 106 to 2.8 × 102 (Fig. 2D).
FIG 2.
Antibacterial effect of P60.4Ac formulated in Softisan cream, Cetomacrogol cream, and hypromellose gel. (A and B) Effect of P60.4Ac in Softisan cream, Cetomacrogol cream (cream), hypromellose gel (gel), and PBS, mupirocin in PBS, and Bactroban on the number of viable bacteria on EMs colonized with LUH14616 (A) and mupirocin-resistant S. aureus LUH15051 (B). The models were incubated with 4 mg of the ointment for 4 h. (C and D) Effect of 0.1%, 0.5%, 1%, and 2% (wt/wt) P60.4Ac in hypromellose gel on MRSA LUH14616 (C) and LUH15051 (D) colonizing EMs, determined as described in the legend to panels A and B. Medians and interquartile ranges from 3 to 5 independent experiments are presented. *, significant differences.
Effect of P60.4Ac and P60.4Ac in hypromellose gel on established biofilms.
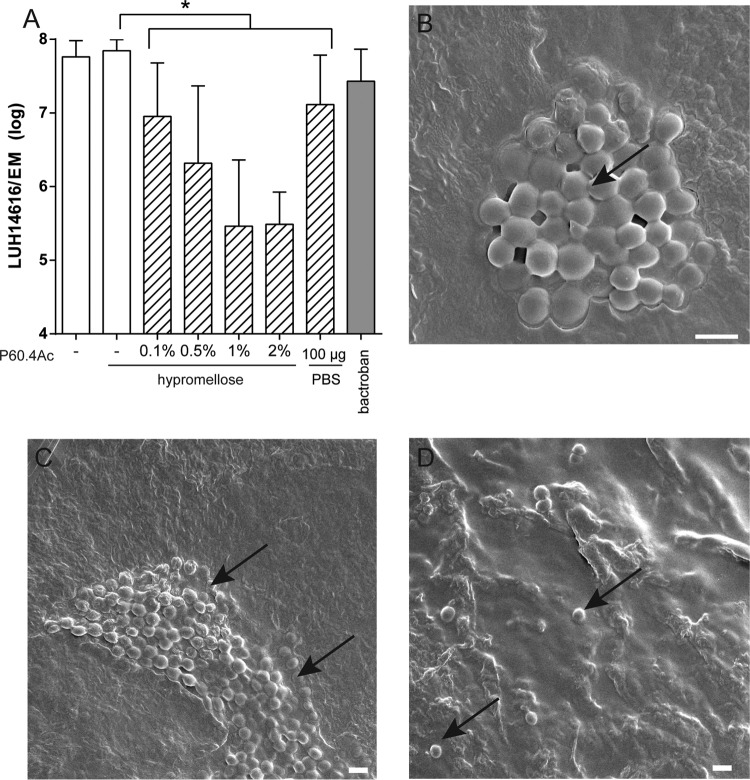
As established biofilms are a major challenge for antimicrobial agents, we determined whether P60.4Ac and P60.4Ac in the hypromellose gel could eradicate MRSA biofilms from EMs. For this purpose, MRSA LUH14616 biofilms were grown for 24 h on EMs and subsequently exposed for 4 h to 4 mg of hypromellose gel containing various amounts of P60.4Ac (range, 0% to 2% [wt/wt]). We observed a dose-dependent reduction in bacterial counts by the formulated peptide; the maximum reduction amounted to about 99% for the 1% and 2% (wt/wt) P60.4Ac formulations (Fig. 3A). In addition, we visualized the effects of P60.4Ac in PBS on MRSA LUH14616 biofilms on EMs using scanning electron microscopy (SEM). These experiments revealed mature biofilms on the stratum corneum of the EMs at 24 h after colonization by MRSA LUH14616 (Fig. 3B and C). The biofilms were completely eradicated by the peptide, with only a few single or double bacterial cells remaining on the EMs (Fig. 3D).
FIG 3.
Eradication of established biofilms from EMs by P60.4Ac. (A) Effect of exposure of LUH14616 biofilms on EMs to different concentrations of P60.4Ac in hypromellose gel for 4 h. Results are expressed as median and range for 3 different donors. (B and C) SEM microphotograph of 24-h-matured biofilms of MRSA LUH14616 on EMs. Arrows indicate biofilms. Scale bar = 1 µm. (D) SEM photograph showing occasional bacteria on EMs after 1 h of exposure of 24-h-matured biofilms to 2% (wt/wt) P60.4Ac in PBS. Arrows indicate bacterial cells. Scale bar = 1 µm.
Effects of P60.4Ac in hypromellose gel on morphology and cell viability in EMs.
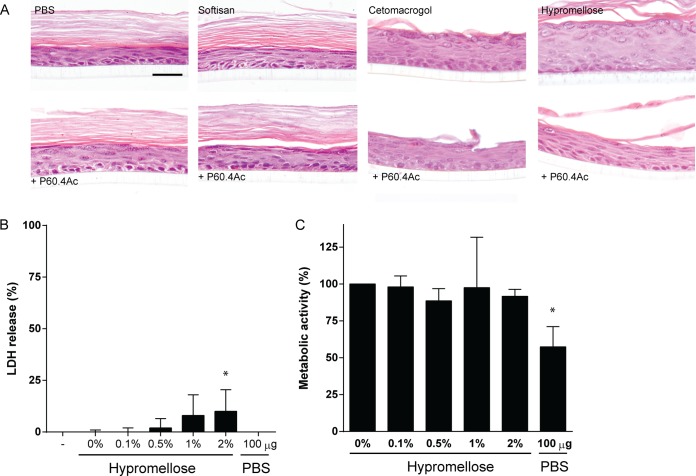
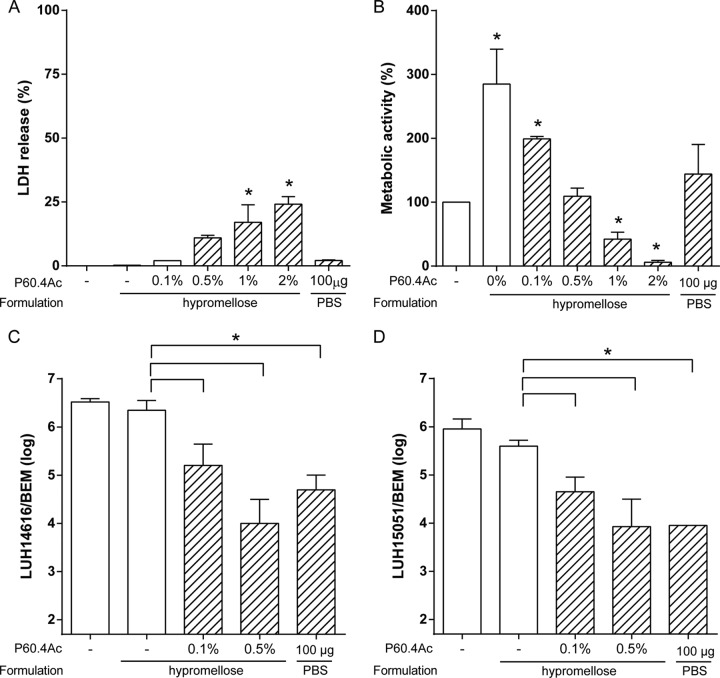
First, we determined whether the different formulations containing P60.4Ac or no peptide affected the morphology of the cells in the EMs. Staining with hematoxylin and eosin revealed no morphological changes in the cells of the EMs after exposure to the different formulations containing 2% (wt/wt) peptide or to the ointment bases alone (Fig. 4A). Since we observed that P60.4Ac formulated in the hypromellose gel was both stable and effective, we next focused on the possible cytotoxic effects of the peptide in the gel on EMs. The results revealed no significant effect of P60.4Ac at up to 2% (wt/wt) in the hypromellose gel on the metabolic activity of the cells in the EMs (Fig. 4B). In addition, we found that hypromellose gel containing 1% and 2% (wt/wt) P60.4Ac induced a small (8% to 10%) increase in the amount of LDH released by EMs compared to the amount released by treatment with the gel without peptide and no treatment (Fig. 4C).
FIG 4.
Effect of different concentrations of P60.4Ac in hypromellose gel on morphology, metabolic activity, and LDH release from EMs. (A) Hematoxylin and eosin staining of EMs after 4 h exposure to 4 mg of Softisan cream, Cetomacrogol cream, or hypromellose gel containing 2% (wt/wt) P60.4Ac or no peptide. For comparison, we included P60.4Ac dissolved in PBS (100 μg/model). LDH release (B) and metabolic activity (C) of EMs after a 4-h exposure to a concentration series of P60.4Ac in hypromellose gel. The medians and ranges from experiments with cells from 3 different donors are given. *, values significantly different from those for PBS-exposed EMs.
Effect of P60.4Ac in hypromellose gel on viability of bronchial epithelial cells and on MRSA LUH14616 colonizing BEMs.
Human bronchial epithelial models (BEMs) cultured at the air-liquid interface were selected to find out if P60.4Ac in the hypromellose gel was effective against MRSA on mucosal cell cultures (29) and to assess the nontoxic dose of the peptide. First, we assessed the cytotoxicity of P60.4Ac in the hypromellose gel on the BEMs. The metabolic activity of the cells in the BEMs was increased after application of the gel without peptide. In addition, application of gels containing 1% and 2% (wt/wt) P60.4Ac resulted in a significant reduction in metabolic activity, but application of gels containing 0.5% and 0.1% (wt/wt) peptide did not (Fig. 5A). In agreement with that finding, gels containing 1% and 2% (wt/wt) P60.4Ac resulted in increased LDH release by the cells in the BEMs, but gels containing 0.5% and 0.1% (wt/wt) peptide did not (Fig. 5B). Therefore, we assessed the effect of hypromellose gels containing 0.1% and 0.5% (wt/wt) P60.4Ac but not higher doses of the peptide on LUH14616 and LUH15051 on BEMs. The results revealed a 1- to 1.5-log reduction in bacterial counts on BEMs after application of the gel containing 0.1% (wt/wt) P60.4Ac and a >99% reduction after application of the gel containing 0.5% (wt/wt) peptide (Fig. 5C and D).
FIG 5.
Effects of P60.4Ac in hypromellose gel on epithelial cell viability and bacterial colonization of bronchial epithelial models. (A and B) BEMs were exposed for 4 h to 4 mg of hypromellose containing various amounts of P60.4Ac, and then the mitochondrial activity (A) and LDH release (B) by the models were assessed. (C and D) The effects of 0.1% and 0.5% (wt/wt) P604Ac hypromellose gels on MRSA LUH14616 (C) and LUH15051 (D) colonizing bronchial epithelial models. Results for 3 different donors are displayed as the median and interquartile range. Brackets indicate differences compared to the values for the placebo gel-exposed controls. *, a significant difference compared to the value for the untreated control, unless otherwise indicated.
DISCUSSION
Based on our earlier results (23–25; unpublished data), we considered P60.4Ac to be a potential candidate for further development as a treatment for bacterial infections resistant to current antibiotics. In this study, we evaluated the in vitro antibacterial activity, cytotoxicity, and stability of this peptide in three different formulations that may be suitable for topical application. We chose topical application of the formulated peptide because the effects of the peptide are more efficient after topical application than after systemic application, as has recently been reported for vancomycin (30), and also, systemic side effects and the effects on the normal microflora are less likely by use of topical application. These advantages may also lead to a reduced chance of bacterial resistance development (31). Based on the present results, we conclude that hypromellose gel is the preferred carrier for P60.4Ac for topical application to eliminate S. aureus, including MRSA and mupirocin-resistant MRSA, from human epidermal and mucosal surfaces. Interestingly, we found that the efficacy of P60.4Ac in hypromellose gels against MRSA on human epidermal models was higher than that of the peptide in PBS. For instance, we found 4 mg of hypromellose gels containing 0.1% (wt/wt) P60.4Ac, which is equal to 4 μg of peptide/model, to eliminate MRSA from the models, whereas the peptide in PBS was effective at 100 μg/model (23). A possible explanation could be that the peptide is gently released by the gel in sufficient amounts for longer intervals to eliminate the bacteria. The peptide formulated in the Cetomacrogol and Softisan creams was less effective, possibly because the water in which the peptide is dissolved is entrapped in the oil phase of the two creams and therefore is not efficiently released into the environment to eliminate the bacteria. A main finding from this study pertains to the action of P60.4Ac on MRSA biofilms, the normal habitat of bacteria, on epidermal models. It has been reported that up to 80% of clinical infections are biofilm infections (32). Moreover, bacteria in a biofilm can be up to 10- to 1,000-fold less sensitive to antibiotics than planktonic bacteria (33, 34). We have shown previously that bacteria in biofilms on a plastic surface are less susceptible to P60.4Ac than planktonic bacteria (23). Here, as little as 4 μg of P60.4Ac formulated in a hypromellose gel eradicated MRSA residing in a biofilm from epidermal models. In this connection, a comparison of the efficacy of the formulated P60.4Ac against bacterial biofilms with that of other novel agents, such as the acyldepsipeptide antibiotic ADEP4 (35) and bacteriolysin (36), and other antimicrobial peptides, such as lysostaphin (37), pexiganan (38), and LL-37 (39, 40), would be of interest.
In the present study, we used in vitro models that share interesting morphological and functional properties with native human skin and bronchial epithelium while presenting major advantages. These models allowed us to evaluate the antibacterial (and cytotoxic) activities of P60.4Ac while the bacteria were attached to a biological surface (residing in a biofilm or not). It is important to note the difference between this approach and testing for the antimicrobial activity of the formulated peptide in widely used assays involving bacteria in suspension, such as the commonly used MIC assay or the in vitro killing assay, also used in this study. However, it should be kept in mind that these unique tools for studying the interactions between bacteria and the human host and the effects of antimicrobial peptides on bacteria have important limitations, such as the absence of immune cells and blood flow.
Other experiments focused on the in vitro cytotoxic effects of P60.4Ac in the different formulations on cells in the epidermal and bronchial epithelial models. It was found that formulations containing up to 2% (wt/wt) P60.4Ac did not damage the keratinocytes in epidermal models, as assessed by histology and metabolic activity, but major cytotoxic effects of the peptide in hypromellose on epithelial cells in the bronchial epithelial models were observed for gels containing 1% and 2% (wt/wt) P60.4Ac. The most likely explanation for these differences is that the bronchial epithelial models are a pseudostratified layer of cells, whereas the epidermal models are multilayered with a physical barrier, i.e., the stratum corneum. We noted that application of the hypromellose gel base to epidermal and bronchial epithelial models was associated with an increase in metabolic activity. Since hypromellose gel is commonly used to rehydrate dry eyes and in in vivo studies for drug delivery, we assume that the gel is safe to use for topical purposes (41, 42).
HPLC analysis of the peptide content of these three ointments containing 0.5% (wt/wt) P60.4Ac stored for up to 3 months revealed that the peptide remained stable in the hypromellose gel and the Softisan cream, as reported earlier (26). However, analysis of P60.4Ac-containing Cetomacrogol cream showed a reduction in the peptide content of about 50% after 3 months of storage. Of note, the P60.4Ac-containing Cetomacrogol cream but not the cream without the peptide turned yellow after storage for 3 months. HPLC and mass spectrometry analysis of the P604Ac-containing Cetomacrogol creams did not reveal any peptide degradation products in the samples, indicating that the peptide is not degraded in the cream. However, we cannot exclude the possibility that the peptide is incorporated in the modified Cetomacrogol cream, preventing it from being extracted by ethanol, as we noted that the reduced peptide recovery coincided with the physical changes of the cream. In agreement with that finding, repeated extractions of the peptide from the cream yielded increased peptide amounts.
Taken together, we conclude that hypromellose gel is the preferred carrier of the synthetic peptide P60.4Ac for topical application to eliminate drug-resistant S. aureus strains from human epidermal and mucosal surfaces.
ACKNOWLEDGMENTS
We thank the Section Electron Microscopy of the Department of Molecular Cell Biology of the Leiden University Medical Center in the Netherlands for help with acquiring the electron microscopy data.
This work was supported by ZonMW (grant 95110078), Top Institute Pharma (project number T4-602), and the Dutch Burns Foundation (project number 10.106).
We have no conflicts of interest to declare.
REFERENCES
- 1.Kluytmans JA, Wertheim HF. 2005. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 33:3–8. doi: 10.1007/s15010-005-4012-9. [DOI] [PubMed] [Google Scholar]
- 2.Vandenbergh MF, Verbrugh HA. 1999. Carriage of Staphylococcus aureus: epidemiology and clinical relevance. J Lab Clin Med 133:525–534. doi: 10.1016/S0022-2143(99)90181-6. [DOI] [PubMed] [Google Scholar]
- 3.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 4.Kluytmans JA, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooistra-Smid M, Nieuwenhuis M, van Belkum A, Verbrugh H. 2009. The role of nasal carriage in Staphylococcus aureus burn wound colonization. FEMS Immunol Med Microbiol 57:1–13. doi: 10.1111/j.1574-695X.2009.00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 7.Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, Troelstra A, Box AT, Voss A, van der Tweel I, van Belkum A, Verbrugh HA, Vos MC. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 8.de Kraker ME, Wolkewitz M, Davey PG, Koller W, Berger J, Nagler J, Icket C, Kalenic S, Horvatic J, Seifert H, Kaasch AJ, Paniara O, Argyropoulou A, Bompola M, Smyth E, Skally M, Raglio A, Dumpis U, Kelmere AM, Borg M, Xuereb D, Ghita MC, Noble M, Kolman J, Grabljevec S, Turner D, Lansbury L, Grundmann H. 2011. Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 55:1598–1605. doi: 10.1128/AAC.01157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 10.Wang FD, Chen YY, Chen TL, Liu CY. 2008. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am J Infect Control 36:118–122. doi: 10.1016/j.ajic.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal VD, Maki DG, Mehta A, Alvarez-Moreno C, Leblebicioglu H, Higuera F, Cuellar LE, Madani N, Mitrev Z, Duenas L, Navoa-Ng JA, Garcell HG, Raka L, Hidalgo RF, Medeiros EA, Kanj SS, Abubakar S, Nercelles P, Pratesi RD. 2008. International Nosocomial Infection Control Consortium report, data summary for 2002-2007, issued January 2008. Am J Infect Control 36:627–637. doi: 10.1016/j.ajic.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Allen HB, Mueller JL. 2011. A novel finding in atopic dermatitis: film-producing Staphylococcus epidermidis as an etiology. Int J Dermatol 50:992–993. doi: 10.1111/j.1365-4632.2010.04648.x. [DOI] [PubMed] [Google Scholar]
- 13.Allen HB, Vaze ND, Choi C, Hailu T, Tulbert BH, Cusack CA, Joshi SG. 2014. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol 150:260–265. doi: 10.1001/jamadermatol.2013.8627. [DOI] [PubMed] [Google Scholar]
- 14.Jones RN, Fritsche TR, Sader HS, Ross JE. 2006. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob Agents Chemother 50:2583–2586. doi: 10.1128/AAC.01432-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parish LC, Parish JL. 2008. Retapamulin: a new topical antibiotic for the treatment of uncomplicated skin infections. Drugs Today (Barc) 44:91–102. doi: 10.1358/dot.2008.44.2.1153446. [DOI] [PubMed] [Google Scholar]
- 16.Rode H, Hanslo D, de Wet PM, Millar AJ, Cywes S. 1989. Efficacy of mupirocin in methicillin-resistant Staphylococcus aureus burn wound infection. Antimicrob Agents Chemother 33:1358–1361. doi: 10.1128/AAC.33.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevgi M, Toklu A, Vecchio D, Hamblin MR. 2013. Topical antimicrobials for burn infections—an update. Recent Pat Antiinfect Drug Discov 8:161–197. doi: 10.2174/15748898113089990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Hal SJ, Fowler VG Jr. 2013. Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clin Infect Dis 56:1779–1788. doi: 10.1093/cid/cit178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. 2002. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 20.van Rijen M, Bonten M, Wenzel R, Kluytmans J. 2008. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev 2008:CD006216. doi: 10.1002/14651858.CD006216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SY, Kim SM, Park SD. 2012. The prevalence, genotype and antimicrobial susceptibility of high- and low-level mupirocin resistant methicillin-resistant Staphylococcus aureus. Ann Dermatol 24:32–38. doi: 10.5021/ad.2012.24.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandamme D, Landuyt B, Luyten W, Schoofs L. 2012. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol 280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Haisma EM, de Breij A, Chan HL, van Dissel JT, Drijfhout JW, Hiemstra PS, El Ghalbzouri A, Nibbering PH. 2014. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob Agents Chemother 58:4411–4419. doi: 10.1128/AAC.02554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nell MJ, Tjabringa GS, Wafelman AR, Verrijk R, Hiemstra PS, Drijfhout JW, Grote JJ. 2006. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 27:649–660. doi: 10.1016/j.peptides.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 25.de Breij A, Riool M, Kwakman PHS, de Boer L, Cordfunke RA, Drijfhout JW, Cohen O, Emanuel N, Zaat SAJ, Nibbering PH, Moriarty T. 2016. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J Control Release 222:1–8. doi: 10.1016/j.jconrel.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Göblyös A, Schimmel KJ, Valentijn AR, Fathers LM, Cordfunke RA, Chan HL, Oostendorp J, Nibbering PH, Drijfhout JW, Hiemstra PS, den Hartigh J. 2013. Development of a nose cream containing the synthetic antimicrobial peptide P60.4Ac for eradication of methicillin-resistant Staphylococcus aureus carriage. J Pharm Sci 102:3539–3544. doi: 10.1002/jps.23695. [DOI] [PubMed] [Google Scholar]
- 27.Fischetti VA. 2008. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amatngalim GD, van Wijck Y, de Mooij-Eijk Y, Verhoosel RM, Harder J, Lekkerkerker AN, Janssen RA, Hiemstra PS. 2015. Basal cells contribute to innate immunity of the airway epithelium through production of the antimicrobial protein RNase 7. J Immunol 194:3340–3350. doi: 10.4049/jimmunol.1402169. [DOI] [PubMed] [Google Scholar]
- 29.Zuyderduyn S, Ninaber DK, Schrumpf JA, van Sterkenburg MA, Verhoosel RM, Prins FA, van Wetering S, Rabe KF, Hiemstra PS. 2011. IL-4 and IL-13 exposure during mucociliary differentiation of bronchial epithelial cells increases antimicrobial activity and expression of antimicrobial peptides. Respir Res 12:59. doi: 10.1186/1465-9921-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vingsbo Lundberg C, Frimodt-Moller N. 2013. Efficacy of topical and systemic antibiotic treatment of meticillin-resistant Staphylococcus aureus in a murine superficial skin wound infection model. Int J Antimicrob Agents 42:272–275. doi: 10.1016/j.ijantimicag.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Quirynen M, Teughels W, van Steenberghe D. 2003. Microbial shifts after subgingival debridement and formation of bacterial resistance when combined with local or systemic antimicrobials. Oral Dis 9(Suppl 1):S30–S37. [DOI] [PubMed] [Google Scholar]
- 32.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 33.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 35.Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K, Leonard SN, Smith RD, Adkins JN, Lewis K. 2013. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503:365–370. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Ghalbzouri A, Siamari R, Willemze R, Ponec M. 2008. Leiden reconstructed human epidermal model as a tool for the evaluation of the skin corrosion and irritation potential according to the ECVAM guidelines. Toxicol In Vitro 22:1311–1320. doi: 10.1016/j.tiv.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Wu JA, Kusuma C, Mond JJ, Kokai-Kun JF. 2003. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob Agents Chemother 47:3407–3414. doi: 10.1128/AAC.47.11.3407-3414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge Y, MacDonald DL, Holroyd KJ, Thornsberry C, Wexler H, Zasloff M. 1999. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob Agents Chemother 43:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamb HM, Wiseman LR. 1998. Pexiganan acetate. Drugs 56:1047–1052. doi: 10.2165/00003495-199856060-00011. [DOI] [PubMed] [Google Scholar]
- 40.Lipsky BA, Holroyd KJ, Zasloff M. 2008. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 47:1537–1545. doi: 10.1086/593185. [DOI] [PubMed] [Google Scholar]
- 41.Ticly FG, Lira RP, Zanetti FR, Machado MC, Rodrigues GB, Arieta CE. 2014. Prophylactic use of ketorolac tromethamine in cataract surgery: a randomized trial. J Ocul Pharmacol Ther 30:495–501. doi: 10.1089/jop.2013.0214. [DOI] [PubMed] [Google Scholar]
- 42.Zheng X, Goto T, Ohashi Y. 2014. Comparison of in vivo efficacy of different ocular lubricants in dry eye animal models. Invest Ophthalmol Vis Sci 55:3454–3460. doi: 10.1167/iovs.13-13730. [DOI] [PubMed] [Google Scholar]