Abstract
KPC-producing Klebsiella pneumoniae causes serious infections associated with high death rates worldwide. Combination therapy consisting of fosfomycin and a carbapenem is better than monotherapy to combat multidrug-resistant microorganisms, but no dosages for the combination have been defined. The MICs of meropenem and fosfomycin were evaluated against 18 clinical isolates of KPC-2-producing K. pneumoniae. The activities of combination antimicrobials were also determined by the checkerboard method. The MIC50 and MIC90 of each agent alone and in combination were challenged against short (1.5-h) or prolonged (3-h) infusion regimens of meropenem (1 g every 8 h [q8h], 1.5 g q6h, 2 g q8h) and fosfomycin (4 g q8h, 6 g q6h, 8 g q8h) by Monte Carlo simulation to evaluate the time above the MIC of the free drug concentration as a percentage of the dosing interval (fT>MIC). The monotherapy MIC50s and MIC90s were 32 and 256 mg/liter for meropenem and 64 and 512 mg/liter for fosfomycin, respectively. Antimicrobial combination increased bacterial susceptibility to 1/4 the MIC50s and to 1/8 to 1/16 the MIC90s of monotherapy. The antimicrobial combination demonstrated a synergistic effect for at least two-thirds of the isolates. In combination therapy, fosfomycin regimens of 6 g q6h and 8 g q8h as a 3-h infusion against the MIC50 and MIC90 had better chances of achieving ≥90% probability of target attainment (PTA) of 70% fT>MIC. Meropenem regimens of 1.5 g q6h and 2 g q8h in prolonged infusion can achieve close to 90% PTA of 40% fT>MIC for MIC50 but not MIC90. The significant reduction in the MIC values and the achievement of appropriate PTA demonstrated that regimens containing fosfomycin with meropenem can be effective against KPC-2-producing K. pneumoniae.
INTRODUCTION
In addition to other classes of antimicrobial agents, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae isolates are resistant to carbapenems, one of the main classes of antibiotics used against β-lactamase-producing microorganisms (1). The bacterium's high dissemination rate is a major public health problem worldwide, and the limited options of antimicrobial agents further complicate the management of infections caused by this difficult-to-treat pathogen, resulting in high morbidity and mortality rates (2, 3).
With the exception of the recently approved ceftazidime-avibactam combination, studies have shown that multidrug-resistant bacteria expressing KPC and AmpC-type β-lactamases develop secondary resistance toward β-lactamase inhibitors, including clavulanic acid, tazobactam, and sulbactam (4–6). These β-lactamase inhibitors, which were designed to combat antibiotic drug resistance, have lost their utility, exacerbating the threat to the current antimicrobial treatment option. The rediscovered “old” antibiotics, including fosfomycin and polymyxins, may offer potential treatment options against multidrug-resistant bacteria. Several clinical studies have shown that combination therapy has better success rates than monotherapy in combating multidrug-resistant infection, and a two-drug combination that included tigecycline, colistin, or meropenem was associated with lower mortality (7). However, there is no consensus on what is the best combined regimen.
Fosfomycin has broad-spectrum bactericidal activity whose mechanism of action is to prevent cell wall synthesis. It binds to the UDP-N-acetylglucosamine enolpyruvyl transferase, preventing the transpeptidation of peptidoglycan (8). Like other β-lactams, meropenem binds to penicillin-binding proteins (PBP); it exhibits high affinity for PBP 2, 3, and 4 and intermediate affinity for PBP 1a and 1b, which are also involved in cell wall synthesis (9). By impeding cell wall formation at different stages of peptidoglycan synthesis, meropenem and fosfomycin should theoretically result in synergy.
Just like many other countries combating multidrug-resistant bacteria, the health centers in Brazil are facing serious infection problems caused by KPC-producing K. pneumoniae and the Brazilian health authorities do not have defined treatment regimens for these microorganisms. Given the prohibitively high cost of developing new classes of antibiotics, the optimization of dosing regimens of existing antimicrobial agents provides a viable option to counter the immediate threat of drug-resistant microorganisms (10). In this study, we explored the checkerboard in vitro susceptibility approach and utilized a pharmacodynamic surrogate index combined with Monte Carlo simulation to evaluate treatment regimens of the meropenem-fosfomycin combination against several KPC-2-producing K. pneumoniae isolates from several Brazilian health centers.
MATERIALS AND METHODS
Microbiological organisms.
Eighteen clinical isolates of KPC-2-producing K. pneumoniae of different clones were obtained from different health centers located in seven Brazilian states from the northeast, midwest, south, and southeast regions of Brazil, representing a large part of the national territory. These isolates were selected from samples previously analyzed by Nicoletti et al. (11), which confirmed the presence of the blaKPC-2 plasmid gene by PCR (forward primer, 5′-TCGCTAAACTCGAACAGG-3′, and reverse primer, 5′-TTACTGCCCGTTGACGCCCAATCC-3′), and the resulting amplicons were sequenced in both strands (Applied Biosystems 3130 genetic analyzer). The KPC-2 amino acid sequence in each isolate was determined by a BLAST comparison of contiguous sequences against a database of known KPC-2 proteins. The genetic relationships among KPC-2-producing isolates were analyzed by multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE), using SpeI as the restriction enzyme. The specific allele sequence and sequence types were verified at the K. pneumoniae MLST website (http://bigsdb.web.pasteur.fr/klebsiella/klebsiella.html). The Escherichia coli ATCC 25922 reference strain was included as a quality control. The bacterial isolates were subcultured for 3 days on Trypticase soy agar plates containing 5% sheep blood, prior to use in the experiment.
Antimicrobial agents.
Meropenem (AstraZeneca, Cotia, São Paulo, Brazil) was generously donated by the State University of Maringa Hospital, and fosfomycin (Sigma-Aldrich, St. Louis, MO, USA) was purchased from LabCompany (Londrina, Paraná, Brazil). Fosfomycin was dissolved in water at 10 mg/ml and stored at 20°C (stock solution), and meropenem solutions were prepared at the same concentration on the day of experimentation.
MICs of single agents.
The MICs of meropenem and fosfomycin against each isolate, after two subcultures and preparation at an 0.5 McFarland standard using a nephelometer (PhoenixSpec nephelometer; BD, Sparks, MD, USA), were determined as described in the CLSI M07-A10 approved standard (12), and MICs were interpreted according to the CLSI M100-S25 guidelines (13). Considering that there were no CLSI interpretive criteria for fosfomycin susceptibility for K. pneumoniae, the CLSI methodology for E. coli strains isolated from urinary tract infections was used to evaluate susceptibility criteria for K. pneumoniae. A control group was evaluated in agar containing 25 μg/ml glucose-6-phosphate without fosfomycin. The concentration ranges of meropenem and fosfomycin tested were 0.015 to 2,048 and 0.5 to 2,048 mg/liter, respectively.
Evaluation of the antimicrobial activity of the combinations of antibiotics.
Meropenem and fosfomycin alone and in combination were evaluated by the checkerboard method in 96-well microtiter plates (Inlab, São Paulo, Brazil) (14). The inoculum of each bacterial isolate was prepared in cation-adjusted Mueller-Hinton broth at a 0.5 McFarland standard and added to the wells at a final concentration of 5 × 105 CFU/ml; the test was conducted in triplicate. After incubation at 37°C for 16 to 20 h, the modal MICs for each antibiotic and for the antibiotic combinations for each individual isolate were determined. The Loewe additivity (15) was evaluated for the combination using the following equation:
where α is the Loewe additivity index, which is used to classify the effects of the combination therapy. If α is 0, the activity of the combination is considered additive; if α is >0, the combined activity is synergistic; and if α is <0, the two drugs are antagonistic. The overall effect of the combination on the population of KPC-expressing bacterial isolates is considered synergistic if 80% of the estimated α value is >0.
A second measure for classifying the activity of the combination was the fractional inhibitory concentration index (FICI), which was calculated using the following equation: FICI = FICA + FICB, where FICA is the MIC of drug A in combination divided by the MIC of drug A alone and FICB is the MIC of drug B in combination divided by the MIC of drug B alone. The classification of the effects of combination therapy was based on the following categories: synergism, FICI ≤ 0.5; indifferent, 0.5 < FICI < 4; antagonism, FICI ≥ 4. The MIC50 and MIC90 (of monotherapy and of combinations of two antimicrobial agents) were the MICs for the median (MIC required to inhibit 50% of the isolates) and the 90th percentile of the 18 isolates.
Simulation of meropenem and fosfomycin pharmacokinetics in critically ill patients.
The population pharmacokinetic (PK) parameters of meropenem and fosfomycin in a critically ill patient population were used to simulate concentration-time profiles for estimating an individual's pharmacodynamic (PD) surrogate index. For both meropenem and fosfomycin, the PD surrogate index was best described by the time above the MIC of the free drug concentration as a percentage of the dosing interval (fT>MIC).
The demographics of 20,000 virtual patients were first simulated in a 50/50 ratio of males and females. Height was assumed to be normally distributed, with the height of males being cm (mean ± SD) and the height of females being cm (16). The weight-height relationship was based on the following equations: WTmale = exp(3.28 + 1.92 log HTmale) and WTfemale = exp(3.49 + 1.45 log HTfemale), for males and females, respectively (17), where WT refers to weight and HT refers to height. An exponential interindividual variable was incorporated into the weight-height relationship equations such that WTi = WTexp(ηi), wherein η is normally distributed with a mean of 0 and standard deviations (SD) of 0.14 and 0.17, for males and females, respectively, and i represents an individual (18).
The age of the population was uniformly distributed between 50 and 90 years of age. Serum creatinine (SCR) levels in critically ill patients with normal renal function were 0.7 ± 0.05 and 0.6 ± 0.05 mg/dl for males and females, respectively, whereas SCR levels in patients with impaired renal function were 1.5 ± 0.15 and 1.2 ± 0.15 mg/dl for males and females, respectively. Creatinine clearance (CLCR) was computed based on the modification of renal disease (MDRD) equation (19): CLCR = 186 × SCR−1.154 × age−0.203 (× 0.742 if the patient is female).
The population pharmacokinetic model for meropenem was a one-compartment model parameterized on clearance (CL) and volume of central compartment (VC). The meropenem pharmacokinetic model of Muro et al. (20) was chosen because it was shown to best predict free meropenem concentrations in critically ill patients (21). The following covariate relationship was associated with its clearance: CL (liters/h) = 11.1 × (SCR0.7)−1. The mean volume of distribution (V) was 33.6 liters. Interindividual variability for CL was assumed to be log-normally distributed with 52.1% coefficient of variation (CV). No interindividual variability was assigned to the volume of distribution. Protein binding of 2% was assumed to determine the free meropenem concentrations (21–23).
The population pharmacokinetic model for fosfomycin was a two-compartment model parameterized on CL, VC, volume of peripheral compartment (VP), and intercompartmental clearance (Q). The population pharmacokinetic model of fosfomycin in critically ill patients from the report of Parker et al. (24) was used to simulate 20,000 virtual patient profiles. Their model reported seven interoccasion CL parameters. For the purpose of simulation, the highest CL value was used. This approach veered on the conservative side to not overpredict the fosfomycin concentration in plasma. Both creatinine clearance and body weight were influential covariates. The equations for the population CL and VC incorporated these two covariates: CL (liters/h) = 5.57 × (CLCR/90), and VC (liters) = 26.5 × (WT/70)0.75. VP and Q were 22.3 liters and 19.8 liters/h, respectively. Interindividual variability was incorporated into CL and VC, assuming log-normal distribution of both parameters with CVs of 91.9% and 39%, respectively. Fosfomycin has negligible plasma protein binding (25, 26).
Pharmacodynamics.
The pharmacodynamic (PD) surrogate indices for both meropenem and fosfomycin were previously characterized by time above MIC of the free drug concentration as a percentage of the dosing interval (fT>MIC). The PD surrogate indices for meropenem and fosfomycin were 40% and 70%, respectively (22, 23, 26, 27). Pharmacodynamic analyses of antimicrobial regimens in both monotherapy and combination therapy as 0.5-h and 3-h infusions at the MIC50 or MIC90 against this isolate population were conducted to evaluate fT>MIC for each dosage regimen. The following dosage regimens were evaluated: meropenem, 1 g every 8 h (q8h) and 2 g q8h; fosfomycin, 4 g q8h and 8 g q8h. The potential importance of infusion time was evaluated for all regimens, including the short infusion of 0.5 h and the prolonged infusion of 3 h for both fosfomycin and meropenem. These regimens were chosen based on the most common regimens used in the countries in which they were registered. The dosage regimens of 1.5 g meropenem q6h and 6 g fosfomycin q6h were included considering the time-dependent action of these antimicrobials, since daily regimens including more divided doses may provide better results. These two shorter dosing intervals with more frequent dosing are less practiced in the clinic; they still provide the recommended maximum daily dosage for meropenem, 6 g a day, and for fosfomycin, 24 g a day.
Monte Carlo simulation.
Monte Carlo simulation was carried out to generate 20,000 virtual profiles with representative demographical distributions in R v.3.1.1. The plasma meropenem and fosfomycin profiles for the virtual patients were generated using NONMEM v.7.2 (ICON, Ellicott City, MD) with Advan 1 and Advan 3 subroutines, respectively. The times in both the ascending and descending phases of the time-concentration profiles in which the concentration is at the MIC were estimated by a linear interpolation algorithm in R. The duration above the MIC was determined as the difference between the two time points. The percentage of the duration above the MIC over the dosing interval was determined for each individual's profile. Probability of target attainment (PTA) for each regimen was evaluated to determine the percentage of the simulated profiles that achieved or exceeded the pharmacodynamic surrogate indices for meropenem and fosfomycin of ≥40% and ≥70% fT>MIC at increasing MICs, respectively. It was considered successful when 90% of the population reached the target values (28, 29). The results of the simulations were used to compute the cumulative fraction of response (CFR) for each dosing regimen at 40% and 70% fT>MIC of meropenem and fosfomycin, respectively. The summation of the density or percentage of bacteria at each MIC across the distribution multiplied by the PTA at the MIC is the CFR for the regimen.
Some investigators have suggested that using MIC metrics may not be sufficient in resistance suppression (30, 31). The mutant selection window (MSW) is a range of drug concentrations between the MIC for the susceptible bacteria and the mutant prevention concentration that fosters the emergence of resistant mutants (32). Firsov and colleagues have shown that time within the MSW is a suitable predictor of resistance development in Staphylococcus aureus following exposure to fluoroquinolones and that an fTMSW of >20% of the dosing interval is a useful target (33). The upper mutant prevention concentrations of the MSW were previously assumed to be 4- to 6-fold the MIC (34). Tam and colleagues, on the other hand, showed that resistance suppression in a dense Pseudomonas aeruginosa population can be achieved by maintaining trough meropenem concentrations in excess of a minimum drug concentration (Cmin)/MIC ratio of 1.7 (35). A hypothetical Cmin/MIC ratio of 2 was used to evaluate resistance suppression, and the percentage of the population that achieved or exceeded this ratio was determined for the dosage regimens of meropenem and fosfomycin described above.
RESULTS
In vitro susceptibility.
All 18 K. pneumoniae clinical isolates harbored the plasmid blaKPC-2 gene, which was confirmed by PCR and sequencing. Table 1 presents the antimicrobial susceptibility profile of 18 KPC-2-producing microorganisms to meropenem and fosfomycin as both monotherapy and combination therapy. The ranges of meropenem and fosfomycin MICs in monotherapy were 1 to 512 and 16 to 1,024 mg/liter; the MIC50s for this collection of isolates were 32 and 64 mg/liter, respectively. MIC90s were 8-fold higher. Only 2 (11%) and 10 (56%) isolates were susceptible to meropenem and fosfomycin, based on CLSI breakpoint values of ≤1 and ≤64 mg/liter, respectively.
TABLE 1.
MICs of meropenem and fosfomycin against 18 KPC-2-producing K. pneumoniae isolates in both monotherapy and combination therapy by checkerboard testa
| K. pneumoniae isolate | ST | MIC of monotherapy antimicrobial (mg/liter) |
MIC (mg/liter) of Mero-Fosfo combination | MIC fold reduction (Mero/Fosfo) | FICI | S or I based on FICI | Loewe additivity index | S or A based on Loewe index | |
|---|---|---|---|---|---|---|---|---|---|
| Mero | Fosfo | ||||||||
| Kp-3 | 70 | 4 | 16 | 4/1 | 0/16 | 1.006 | I | −0.0625 | A |
| Kp-4 | 70 | 8 | 64 | 2/8 | 4/8 | 0.375 | S | 0.625 | S |
| Kp-5 | 437 | 1 | 512 | 0.02/16 | 50/32 | 0.046 | S | 0.95 | S |
| Kp-8 | 437 | 16 | 32 | 8/4 | 2/8 | 0.625 | I | 0.375 | S |
| Kp-9 | 133 | 16 | 128 | 1/16 | 16/8 | 0.185 | S | 0.812 | S |
| Kp-10 | 437 | 64 | 128 | 2/32 | 32/4 | 0.280 | S | 0.719 | S |
| Kp-11 | 11 | 64 | 128 | 4/16 | 16/8 | 0.185 | S | 0.812 | S |
| Kp-12 | 437 | 128 | 128 | 8/16 | 16/8 | 0.185 | S | 0.812 | S |
| Kp-15 | 11 | 16 | 32 | 8/2 | 2/16 | 0.560 | I | 0.437 | S |
| Kp-19 | 437 | 64 | 64 | 4/8 | 16/8 | 0.131 | S | 0.812 | S |
| Kp-28 | 617 | 1 | 16 | 1/16 | 0/0 | 2.000 | I | −1 | A |
| Kp-38 | 437 | 32 | 64 | 2/16 | 16/4 | 0.310 | S | 0.687 | S |
| Kp-39 | 11 | 64 | 64 | 8/16 | 8/4 | 0.375 | S | 0.625 | S |
| Kp-40 | 11 | 256 | 1,024 | 64/512 | 4/2 | 0.750 | I | 0.25 | S |
| Kp-43 | 340 | 16 | 16 | 16/16 | 0/0 | 2.000 | I | −1 | A |
| Kp-44 | 11 | 512 | 256 | 32/32 | 16/8 | 0.185 | S | 0.812 | S |
| Kp-46 | 17 | 32 | 128 | 4/32 | 8/4 | 0.375 | S | 0.625 | S |
| Kp-55 | 11 | 32 | 32 | 4/2 | 8/16 | 0.131 | S | 0.812 | S |
| MIC50 | 32 | 64 | 4/16 | 8/4 | |||||
| MIC90 | 256 | 512 | 32/32 | 8/16 | |||||
ST, sequence type; Mero, meropenem; Fosfo, fosfomycin; FICI, fractional inhibitory concentration index; S, synergy; I, indifferent; A, antagonism.
For combination of the two antimicrobials, the majority of the MICs were markedly lower than the MICs of the antimicrobials in monotherapy and the resulting MIC values in the combination against these microorganisms were well within the susceptibility level of either one of the two antimicrobial agents. The MIC50s were decreased to 1/4, and for the MIC90s, there were reductions to 1/8 and 1/16 of the values in the monotherapy setting. In two-thirds of the isolates, the effect of the combination was considered synergistic by FICI scores of ≤0.5, and 83% of the isolates exhibited synergistic activities in the combination therapy based on Loewe additivity criteria. Only six isolates showed FICI values classified as indifferent (0.5 < FICI ≤ 4), and none of the isolates had FICIs of ≥4. Of the resistant isolates for which the antimicrobial monotherapy MICs were greater than the breakpoints, when they were tested against meropenem and fosfomycin in combination, the meropenem MIC for one isolate (Kp-9) and the fosfomycin MICs for seven isolates (Kp-5, Kp-9, Kp-10, Kp-11, Kp-12, Kp-44, Kp-46) were lower than the breakpoints.
Pharmacokinetic-pharmacodynamic simulations.
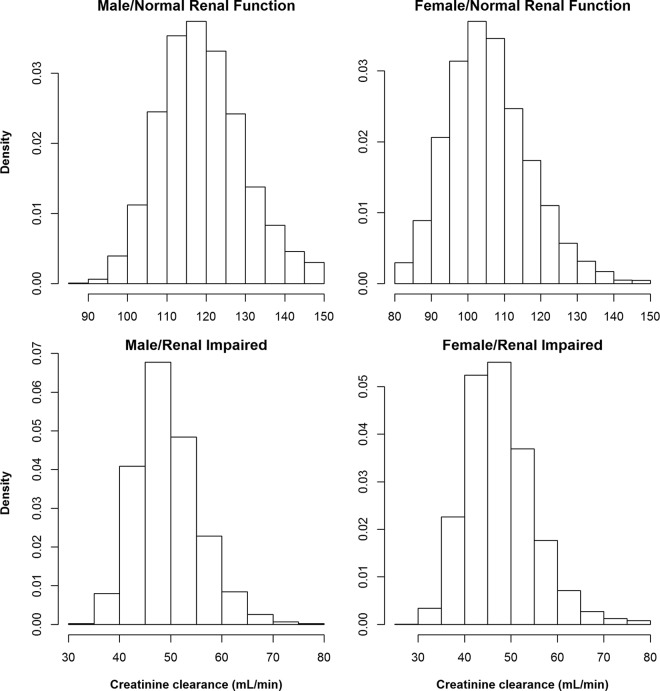
The population pharmacokinetic parameters of meropenem and fosfomycin used in the simulation were obtained from critically ill patients previously described in the literature (20, 24) and were used for simulations of pharmacodynamic surrogate indices of 20,000 virtual patients subdivided into 10,000 patients with normal renal function and 10,000 with mild to moderate renal impairment. Figure 1 shows the distribution of creatinine clearance based on the MDRD computation for male and female patients with normal renal function and mild to moderate renal impairment. For the purpose of simulation, the range of creatinine clearance for the normal renal function group is 80 to 150 ml/min and the range for the group with mild to moderate renal impairment is 30 to 80 ml/min. The upper bound for creatinine clearance is 150 ml/min, and values greater than 150 ml/min were set to 150 ml/min. The mean values and ranges for serum creatinine were selected so that the estimated creatinine clearance resulted in modal values of approximately 100 to 120 ml/min for the normal renal function group and 40 to 55 ml/min for the impaired renal function group, taking into account the weight and age distributions as well. The simulated ranges of creatinine clearance in both the normal renal function population and the impaired renal function population are well within the reported creatinine clearance range in patients treated for hospital-acquired and ventilator-associated bacterial infections (36). Creatinine clearances of <30 ml/min were not simulated, since patients with end stage renal disease (ESRD) require hemodialysis and estimations of drug clearance based on covariate relationships for ESRD patients are often inaccurate. In addition, pharmacokinetic simulations in hemodialysis patients are done differently, since hemodialysis removes drugs rapidly during the process (37).
FIG 1.
Histograms showing the distribution of creatinine clearance computed using the modification of renal disease (MDRD) equation in virtual critically ill male (left) and female (right) patients with normal renal function (top) and renal impairment (bottom).
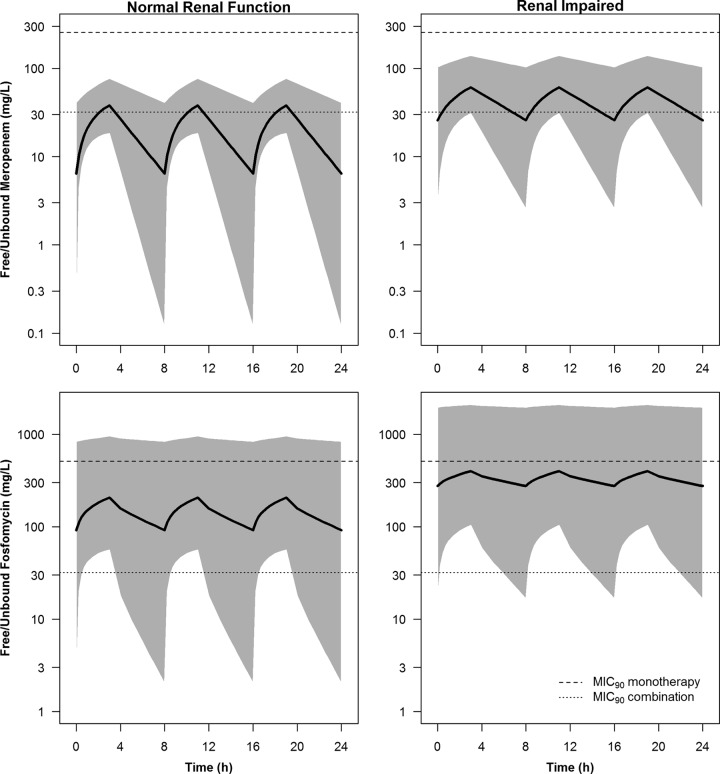
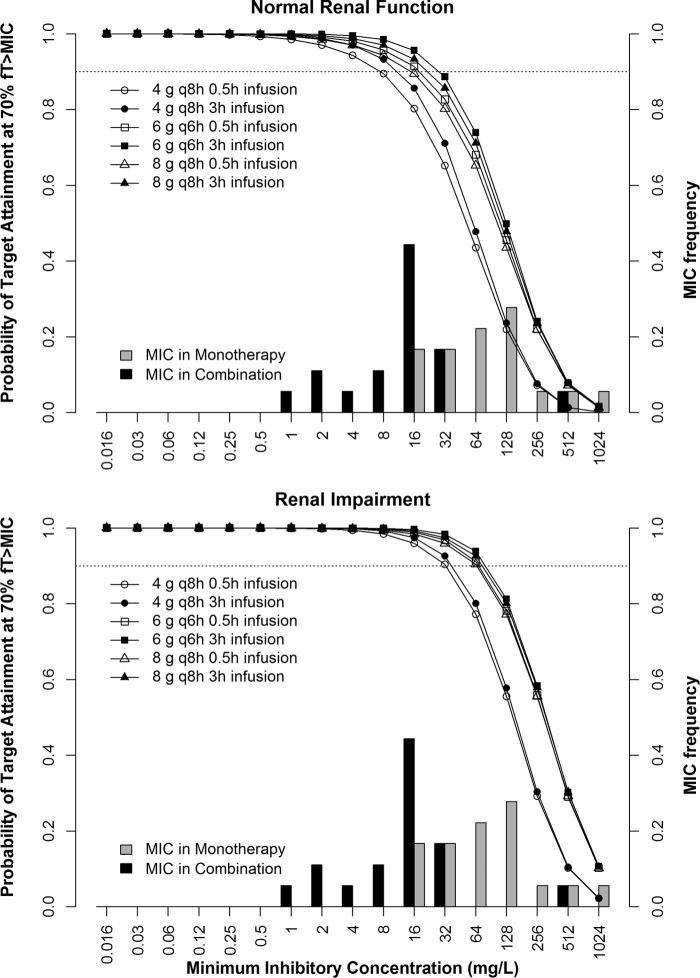
Renal function has a significant impact on the pharmacokinetics of both fosfomycin and meropenem. Figure 2 shows the simulated median and 95% prediction interval of steady-state free meropenem and fosfomycin concentrations in patients with normal renal function and those with renal impairment after the highest doses of 2 g meropenem and 8 g fosfomycin q8h were administered as a 3-h infusion. The MIC90s in monotherapy and combination therapy are also shown. The meropenem monotherapy MIC90 against KPC-producing K. pneumoniae was unattainable in all patients, while the MIC90 in the fosfomycin monotherapy situation was unattainable in the majority of the patients. The lower MIC90 for fosfomycin in the combination (32 mg/liter) was attainable in a majority of the patients, regardless of their renal function, whereas in the case of meropenem, the MIC90 was attainable in the majority of patients with renal impairment and in a lower percentage of the patients with normal renal function. Table 2 shows the probabilities of target attainment (PTA) of 40% and 70% fT>MIC for meropenem and fosfomycin, respectively, in various dosing regimens as 0.5-h and 3-h infusions. Figure 3 shows the PTA of 70% fT>MIC of several fosfomycin dosing regimens and the MIC frequency in fosfomycin monotherapy as well as in combination with meropenem. For the 0.5-h infusion, fosfomycin monotherapy regimens did not achieve 90% PTA of >70% fT>MIC against the MIC50 or MIC90 in patients with normal renal function. In patients with renal impairment, the higher doses of 6 g q6h and 8g q8h achieved 90% PTA. All fosfomycin regimens in combination with meropenem achieved ≥90% PTA against the MIC90 in patients with renal impairment but not in patients with normal renal function. Even though the prolonged infusion of 3 h improves the PTA over that of a 0.5-h infusion, fosfomycin monotherapy regimens did not reach 90% PTA against either the MIC50 or the MIC90 in patients with normal renal function. In combination with meropenem, fosfomycin in a dosing regimen of 6 g q6h as a 3-h infusion followed by 8 g q8h in an infusion of the same duration had the best chance of achieving PTA.
FIG 2.
Simulated median and 95% prediction interval of steady-state free meropenem (top) and fosfomycin (bottom) concentrations in plasma in critically ill virtual patients with normal renal function (left) and renal impairment (right). The MIC90s in monotherapy and combination therapy are shown by dashed and dotted lines.
TABLE 2.
PTAs at targeted pharmacodynamic surrogate indices (fT>MIC) for meropenem at 40% and fosfomycin at 70% for dosing regimens by infusion duration and renal function in monotherapy and combination therapy against KPC-2-producing K. pneumoniae isolatesa
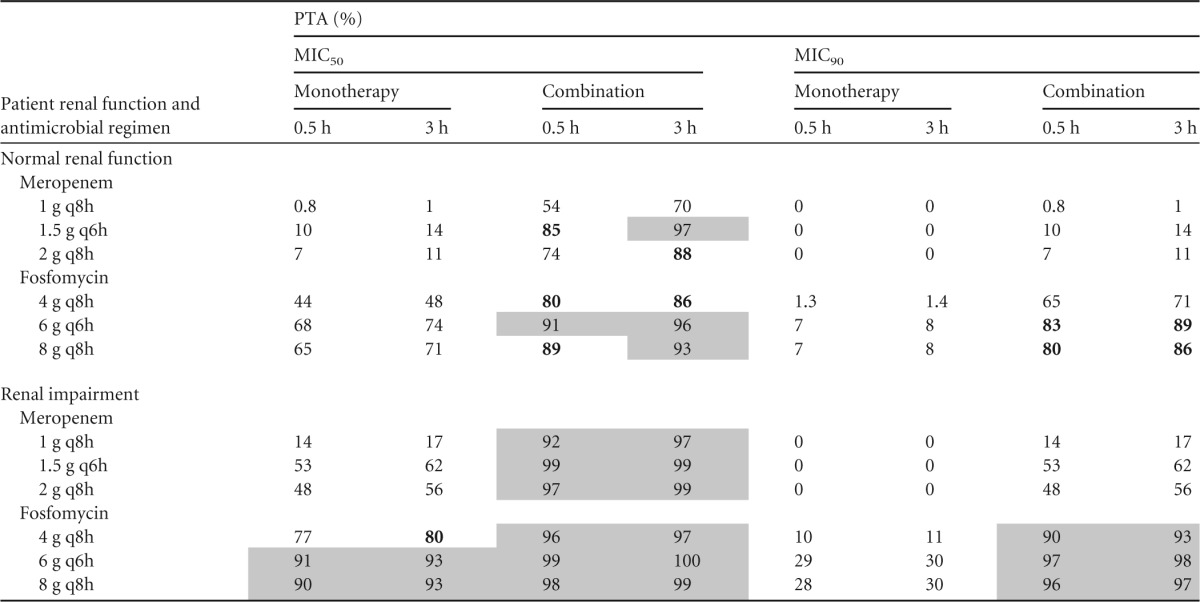
PTA, probability of target attainment; fT≥MIC, percentage of the dosing interval that free antimicrobial concentrations remain above the MIC of the bacteria. Gray shading indicates ≥90% PTA, and boldface indicates 80% to <90% PTA.
FIG 3.
MIC frequency of 18 KPC-2-producing Klebsiella pneumoniae clinical isolates that were susceptible at fosfomycin MICs in monotherapy and in combination with meropenem and probability of target attainment of 70% fT>MIC for the fosfomycin dosing regimens of 4 g q8h, 6 g q6h, and 8 g q8h in critically ill virtual patients with normal renal function (top) and renal impairment (bottom). Open symbols represent a 0.5-h infusion, and filled symbols indicate a 3-h infusion. The dotted line indicates 90% probability of target attainment.
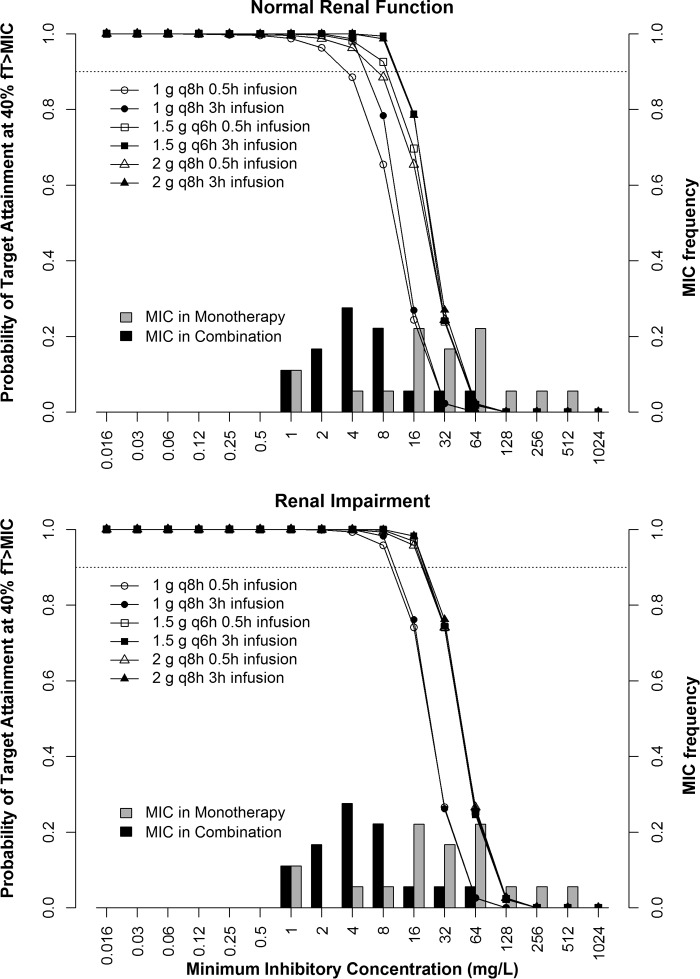
None of the meropenem dosing regimens achieved ≥90% PTA against the MIC90, regardless of renal function. Patients with renal impairment had a better chance of attaining 40% fT>MIC with meropenem at the MIC50 of the combination than patients with normal renal function. A meropenem dosing regimen of 1.5 g q6h followed by 2 g q8h as a 3-h infusion was the preferred regimen for achieving PTA. Figure 4 displays the PTA of meropenem regimens to achieve target pharmacodynamic indices in both monotherapy and combination therapy and also the MIC frequency in meropenem monotherapy and in combination therapy with fosfomycin. Against a MIC50 of 32 mg/liter in meropenem monotherapy, none of the dosing regimens achieve 90% PTA of 40% fT>MIC, whereas combination therapy resulted in ≥90% PTA of at least 40% fT>MIC for the regimens of 1.5 g q6h as a 3-h infusion. A meropenem dosing regimen of 2 g q8h as a 3-h infusion had 88% probability at the MIC50. Higher-dose combination therapy consisting of meropenem and fosfomycin and prolonged infusion demonstrated significant improvement in achieving 90% PTA against both the MIC50 and the MIC90. Fractional dosing further improves this probability.
FIG 4.
MIC frequency of 18 KPC-2-producing Klebsiella pneumoniae clinical isolates that were susceptible at meropenem MICs in monotherapy and in combination with fosfomycin and probability of target attainment of 40% fT>MIC for the meropenem dosing regimens of 1 g q8h, 1.5 g q6h, and 2 g q8h in critically ill virtual patients with normal renal function (top) and renal impairment (bottom). Open symbols represent a 0.5-h infusion, and filled symbols indicate a 3-h infusion. The dotted line indicates 90% probability of target attainment.
Table 3 summarizes the cumulative fraction of response (CFR) for each dosing regimen of meropenem and fosfomycin for the KPC-2-producing K. pneumoniae clinical isolates from various regions of Brazil. Overall, there is greater than 80% CFR for the regimens with higher doses of fosfomycin in combination with meropenem. The higher meropenem dosage regimens in combination with fosfomycin in patients with renal impairment have greater than 80% CFR, whereas this value is lower in patients with normal renal function.
TABLE 3.
Cumulative fraction of response to fosfomycin and meropenem regimens against KPC-2-producing K. pneumoniae clinical isolates from various regions of Brazil
| Patient renal function and antimicrobial regimen | CFR (%)a |
|||
|---|---|---|---|---|
| Monotherapy |
Combination |
|||
| 0.5 h | 3 h | 0.5 h | 3 h | |
| Normal renal function | ||||
| Meropenemb | ||||
| 1 g q8h | 16 | 19 | 44 | 54 |
| 1.5 g q6h | 29 | 35 | 67 | 77 |
| 2 g q8h | 25 | 31 | 59 | 69 |
| Fosfomycinc | ||||
| 4 g q8h | 40 | 44 | 78 | 82 |
| 6 g q6h | 59 | 63 | 87 | 89 |
| 8 g q8h | 57 | 61 | 86 | 89 |
| Renal impairment | ||||
| Meropenemb | ||||
| 1 g q8h | 34 | 37 | 74 | 78 |
| 1.5 g q6h | 53 | 57 | 85 | 87 |
| 2 g q8h | 50 | 54 | 83 | 86 |
| Fosfomycinc | ||||
| 4 g q8h | 66 | 68 | 91 | 93 |
| 6 g q6h | 80 | 82 | 95 | 96 |
| 8 g q8h | 79 | 81 | 95 | 95 |
Gray shading indicates ≥90% CFR, and boldface indicates 80% to <90% CFR.
CFR calculated at 40% fT>MIC for meropenem.
CFR calculated at 70% fT>MIC for fosfomycin.
In this study, we also evaluated the percentage of the virtual population for which the Cmin was 2-fold the MIC50 and MIC90 of meropenem and fosfomycin; the results are shown in Table 4. Meropenem dosing regimens in combination therapy are largely inadequate to attain a 90% probability that the Cmin/MIC ratio is ≥2 if the MIC90 is used for evaluation regardless of renal function. In patients with renal impairment, the two higher doses as a 3-h infusion were able to achieve >80% probability using the MIC50 reference. The higher fosfomycin dosage regimens in combination therapy in patients with renal impairment fared better in achieving >80% probability that the Cmin/MIC ratio was ≥2 with the MIC50 reference. Only 6 g q6h as a 3-h infusion achieved over 80% probability using the MIC90 reference.
TABLE 4.
Percentage of population whose trough drug concentrations are >2-fold the MIC against KPC-2-producing K. pneumoniae by dosing regimens, infusion duration, and renal function in monotherapy and combination therapy
| Patient renal function and antimicrobial regimen | % of population with Cmin of >2-fold the respective MICa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| MIC50 |
MIC90 |
|||||||
| Monotherapy |
Combination |
Monotherapy |
Combination |
|||||
| 0.5 h | 3 h | 0.5 h | 3 h | 0.5 h | 3 h | 0.5 h | 3 h | |
| Normal renal function | ||||||||
| Meropenem | ||||||||
| 1 g q8h | 0 | 0 | 13 | 20 | 0 | 0 | 0 | 0 |
| 1.5 g q6h | 0.34 | 0.63 | 43 | 60 | 0 | 0 | 0.34 | 0.63 |
| 2 g q8h | 0.28 | 0.47 | 31 | 42 | 0 | 0 | 0.28 | 0.47 |
| Fosfomycin | ||||||||
| 4 g q8h | 18 | 20 | 55 | 61 | 0.15 | 0.16 | 37 | 40 |
| 6 g q6h | 39 | 44 | 75 | 81 | 1.5 | 1.6 | 59 | 66 |
| 8 g q8h | 37 | 40 | 71 | 76 | 1.1 | 1.3 | 55 | 61 |
| Renal impairment | ||||||||
| Meropenem | ||||||||
| 1 g q8h | 0.78 | 1.1 | 59 | 69 | 0 | 0 | 0.78 | 1.1 |
| 1.5 g q6h | 10 | 14 | 87 | 94 | 0 | 0 | 10 | 14 |
| 2 g q8h | 8.4 | 11 | 79 | 87 | 0 | 0 | 8.4 | 11 |
| Fosfomycin | ||||||||
| 4 g q8h | 50 | 53 | 85 | 88 | 2.1 | 2.1 | 71 | 74 |
| 6 g q6h | 73 | 77 | 94 | 96 | 9.8 | 10 | 87 | 90 |
| 8 g q8h | 73 | 74 | 94 | 95 | 9.8 | 10 | 87 | 88 |
Gray shading indicates ≥90% probability that the Cmin/MIC ratio is ≥2, and boldface indicates 80% to <90% probability that the Cmin/MIC ratio is ≥2.
DISCUSSION
The dosing regimens evaluated in this study are well within the recommended daily doses used in clinical practice (23, 26). When administered as monotherapy, the meropenem and fosfomycin dosing regimens even at the maximum daily doses would not be effective against KPC-2-producing K. pneumoniae isolates, as shown by the PTA falling markedly below the 90% target for meropenem and fosfomycin PD indices of 40% and 70% fT>MIC. When administered as a single agent, neither meropenem nor fosfomycin has any utility against multidrug-resistant Gram-negative microorganisms that carry the blaKPC-2 gene. It is noted that there are other resistance mechanisms that may exist in these isolates and that were not evaluated in this study.
Combination antimicrobial therapy can potentially alleviate the global crisis of prohibitively limited antimicrobial treatment options by rescuing agents that are considered obsolete (7, 38). This study has shown that combination therapy consisting of fosfomycin and meropenem increases the susceptibility of KPC-2-producing K. pneumoniae isolates to an acceptable level for at least one of the two antimicrobial agents. A reduction to 1/4 to 1/16 the MIC50 and MIC90 of the monotherapy antimicrobials was observed in the agents used in combination therapy against this collection of clinical isolates. In a majority of the isolates tested, the actions of fosfomycin and meropenem were synergistic. In a retrospective analysis of 41 patients infected by KPC-2-producing K. pneumoniae, Qureshi et al. noted that the mortality rates of patients receiving combination regimens were markedly lower than those receiving monotherapy (13.3% versus 57.8% mortality rate), and combination therapy significantly improved patient survival (39). Tumbarello et al. demonstrated that combinations containing meropenem were associated with significantly higher survival rates when KPC-containing K. pneumoniae isolates had meropenem MICs of ≤8 mg/liter (40) and that combination therapy is effective in decreasing treatment failure (7). In 83% of the isolates in the present study, the combination with fosfomycin brought the meropenem MIC to ≤8 mg/liter, but the meropenem MIC was at or below the CLSI breakpoint of 1 mg/liter against K. pneumoniae for only 3 of the 18 isolates when the combination with fosfomycin was used. For this reason, we have seen that none of the recommended meropenem dosage regimens can achieve a 90% probability of target attainment.
Fosfomycin is an important companion drug in the combination to “rescue” meropenem's utility and is often used as an adjunct antimicrobial agent against serious infections, since fosfomycin typically demonstrates synergistic activities with other antimicrobial agents (26, 41). Fosfomycin is being utilized more frequently, particularly against multidrug-resistant bacterial infections (42), although there are reported new cases of fosfomycin resistance development (43–45). Our study shows that fosfomycin dosing regimens are more likely to achieve the PTA against the MIC90 of the combination, providing sufficient antimicrobial coverage even when the meropenem regimens at the maximum daily dosage fall short. We also evaluated a hypothetical trough/MIC ratio of ≥2 for resistance prevention and showed that the higher fosfomycin dosing regimens in a 3-h infusion in combination had >75% probability of achieving a trough/MIC ratio of ≥2 in patients with normal renal function and >90% in patients with renal impairment, with the MIC50 as a reference. With the MIC90 as a reference, the probability of achieving a trough/MIC ratio of ≥2 was smaller.
The evaluation of clinical and microbiological actions of antimicrobial regimens utilize pharmacokinetic and pharmacodynamic properties to understand the drug effects. This concept relates the characteristics of the drug, the patient, and the pathogen to derive optimized antimicrobial regimens with higher clinical and microbiological efficacies (46, 47). However, most of these evaluations are performed primarily in a monotherapy setting, and combination antimicrobial synergy studies using this concept are scarce. Recommendations for dosages of antibiotics in combination regimens should be optimized and maximized so that they can reach their respective pharmacodynamic indices and microbiological outcomes (48, 49). Our study used this concept to assess which of the combined and optimized regimens of meropenem with fosfomycin have better probabilities of therapeutic effectiveness against KPC-2-producing K. pneumoniae.
Notably, factors such as the inclusion of a second antimicrobial, prolonged infusion, increased dosage, and more divided doses show important utility in increasing the probability of target attainment, of which the inclusion of a second agent has the greatest impact. This observation corroborates the results of combination therapy against bacteria containing KPC enzymes, as demonstrated by other studies (7, 39, 40).
The antimicrobial regimens tested against the MIC50 that showed the best results were a combination of meropenem at 1.5 g q6h and fosfomycin at 6 g q6h by prolonged infusion. Due to the higher resistance to meropenem among bacterial isolates in Brazil, these isolates are still susceptible to fosfomycin but not meropenem in the fosfomycin-meropenem combination. These regimens, at the highest recommended daily dose for both agents, are administered more frequently, thus favoring the maintenance of the plasma drug concentration above the MIC for a longer period of time. Dosing regimens with more fractional doses also allowed for reduced daily doses, bringing down the cost of antimicrobial treatment, while maintaining the same efficacy in the treatment. Kotapati et al. used a meropenem regimen of 0.5 g q6h, which yielded clinical outcomes similar to those of a regimen of 1 g q8h and reduced the daily drug acquisition costs associated with antibiotic therapy (50).
Our study has two main limitations. First, the number of isolates evaluated is not very large and may bias the MIC50 and MIC90 statistics. However, these isolates demonstrated good variability in MIC ranges for both meropenem and fosfomycin; the isolates came from different regions of Brazil, thus providing a representative map of infection in the country. Second, the isolates were primarily K. pneumoniae, which is easier to treat with a β-lactam/β-lactamase inhibitor combination than P. aeruginosa (51). The outer membrane of P. aeruginosa is less permeable by antibiotics and is regulated by porins, whereas loss of porins can increase resistance to antibiotics (52–54).
The pharmacokinetic parameters of meropenem and fosfomycin used for the simulation came from one- and two-compartment models, respectively, previously developed from a critically ill population (20, 24). Other studies used a two-compartment model to characterize the total concentration of meropenem in plasma (55–58). These models were shown to underpredict free meropenem concentrations in critically ill patients, and a one-compartment model had the least bias in predicting free meropenem concentration (21). The predicted Cmax may be sensitive to the number of compartments used in the model. Cmax is also affected by the duration of infusion The distribution phase, however, is no longer apparent when the drugs are infused for more than half an hour, since the intercompartmental clearance rates reported for the two drugs are very high (24, 57). Moreover, the V for both drugs is low, indicating that the drugs are distributed extracellularly. The small volume of distribution and rapid distribution between central and peripheral compartments would result in a negligible difference in the overall probability of achieving a time-dependent pharmacodynamic index, when determining whether to use a one- or two-compartment model to predict free meropenem exposure. The predicted peak and trough plasma meropenem concentrations in this study were consistent with values reported in the literature for septic critically ill patients (59).
In conclusion, the reduction in the MICs of meropenem and fosfomycin in combination for the majority of isolates improves attainment of the target PD index, with the dosing regimens of fosfomycin with meropenem including higher daily doses, more fractionated doses, and prolonged infusion. Our study demonstrated that the antimicrobial combination consisting of meropenem and fosfomycin can be a viable alternative to combat infections caused by KPC-2-producing K. pneumoniae.
Funding Statement
Maria Cristina Bronharo Tognim received funding from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação Araucária. The funding agencies had no role in the study design, data collection and interpretation, or the decision to submit the work for publication, as these government funds are designed to encourage the training of higher education in Brazil and cover only the cost of laboratory materials.
REFERENCES
- 1.Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. 2016. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother 17:761–781. doi: 10.1517/14656566.2016.1145658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messina JA, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Scalera NM, Doi Y, Kaye KS, Evans S, Bonomo RA, Fowler VG, van Duin D. 2016. Hospital readmissions in patients with carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 37:281–288. doi: 10.1017/ice.2015.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Rosa FG, Corcione S, Cavallo R, Di Perri G, Bassetti M. 2015. Critical issues for Klebsiella pneumoniae KPC-carbapenemase producing K. pneumoniae infections: a critical agenda. Future Microbiol 10:283–294. doi: 10.2217/fmb.14.121. [DOI] [PubMed] [Google Scholar]
- 4.Smith Moland E, Hanson ND, Herrera VL, Black JA, Lockhart TJ, Hossain A, Johnson JA, Goering RV, Thomson KS. 2003. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 51:711–714. doi: 10.1093/jac/dkg124. [DOI] [PubMed] [Google Scholar]
- 5.Jacoby GA. 2009. AmpC beta-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaye KS, Gold HS, Schwaber MJ, Venkataraman L, Qi Y, De Girolami PC, Samore MH, Anderson G, Rasheed JK, Tenover FC. 2004. Variety of beta-lactamases produced by amoxicillin-clavulanate-resistant Escherichia coli isolated in the northeastern United States. Antimicrob Agents Chemother 48:1520–1525. doi: 10.1128/AAC.48.5.1520-1525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 8.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Davies TA, Shang W, Bush K, Flamm RK. 2008. Affinity of doripenem and comparators to penicillin-binding proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:1510–1512. doi: 10.1128/AAC.01529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sy SK, Derendorf H. 2014. Pharmacometrics in bacterial infections, p 229–258. In Schmidt S, Derendorf H (ed), Applied pharmacometrics, 1st ed Springer, New York, NY. [Google Scholar]
- 11.Nicoletti AG, Fehlberg LC, Picao RC, Machado Ade O, Gales AC. 2012. Clonal complex 258, the most frequently found multilocus sequence type complex in KPC-2-producing Klebsiella pneumoniae isolated in Brazilian hospitals. Antimicrob Agents Chemother 56:4563–4564. doi: 10.1128/AAC.00219-12 (Reply 56:4565, doi:.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed CLSI M07-A10. CLSI, Wayne, PA. [Google Scholar]
- 13.CLSI. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI M100-S25. CLSI, Wayne, PA. [Google Scholar]
- 14.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 15.Greco WR, Bravo G, Parsons JC. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385. [PubMed] [Google Scholar]
- 16.McDowell MA, Fryar CD, Ogden CL, Flegal KM. 2008. Anthropometric reference data for children and adults: United States, 2003-2006. Natl Health Stat Report 10:1–48. [PubMed] [Google Scholar]
- 17.Diverse Populations Collaborative Group. 2005. Weight-height relationships and body mass index: some observations from the Diverse Populations Collaboration. Am J Phys Anthropol 128:220–229. doi: 10.1002/ajpa.20107. [DOI] [PubMed] [Google Scholar]
- 18.Sy SK, Asin-Prieto E, Derendorf H, Samara E. 2014. Predicting pediatric age-matched weight and body mass index. AAPS J 16:1372–1379. doi: 10.1208/s12248-014-9657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Knight EL, Hogan ML, Singh AK. 2003. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 14:2573–2580. doi: 10.1097/01.ASN.0000088721.98173.4B. [DOI] [PubMed] [Google Scholar]
- 20.Muro T, Sasaki T, Hosaka N, Umeda Y, Takemoto S, Yamamoto H, Kamimura H, Higuchi S, Karube Y. 2011. Population pharmacokinetic analysis of meropenem in Japanese adult patients. J Clin Pharm Ther 36:230–236. doi: 10.1111/j.1365-2710.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- 21.Wong G, Farkas A, Sussman R, Daroczi G, Hope WW, Lipman J, Roberts JA. 2015. Comparison of the accuracy and precision of pharmacokinetic equations to predict free meropenem concentrations in critically ill patients. Antimicrob Agents Chemother 59:1411–1417. doi: 10.1128/AAC.04001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuti JL, Dandekar PK, Nightingale CH, Nicolau DP. 2003. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J Clin Pharmacol 43:1116–1123. doi: 10.1177/0091270003257225. [DOI] [PubMed] [Google Scholar]
- 23.Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. 2004. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther 26:1187–1198. doi: 10.1016/S0149-2918(04)80001-8. [DOI] [PubMed] [Google Scholar]
- 24.Parker SL, Frantzeskaki F, Wallis SC, Diakaki C, Giamarellou H, Koulenti D, Karaiskos I, Lipman J, Dimopoulos G, Roberts JA. 2015. Population pharmacokinetics of fosfomycin in critically ill patients. Antimicrob Agents Chemother 59:6471–6476. doi: 10.1128/AAC.01321-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goto M, Sugiyama M, Nakajima S, Yamashina H. 1981. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob Agents Chemother 20:393–397. doi: 10.1128/AAC.20.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker S, Lipman J, Koulenti D, Dimopoulos G, Roberts JA. 2013. What is the relevance of fosfomycin pharmacokinetics in the treatment of serious infections in critically ill patients? A systematic review. Int J Antimicrob Agents 42:289–293. doi: 10.1016/j.ijantimicag.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Lepe JA, Torres MJ, Smani Y, Parra-Millan R, Pachon J, Vazquez-Barba I, Aznar J. 2014. In vitro and intracellular activities of fosfomycin against clinical strains of Listeria monocytogenes. Int J Antimicrob Agents 43:135–139. doi: 10.1016/j.ijantimicag.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 28.de Kock L, Sy SK, Rosenkranz B, Diacon AH, Prescott K, Hernandez KR, Yu M, Derendorf H, Donald PR. 2014. Pharmacokinetics of para-aminosalicylic acid in HIV-uninfected and HIV-coinfected tuberculosis patients receiving antiretroviral therapy, managed for multidrug-resistant and extensively drug-resistant tuberculosis. Antimicrob Agents Chemother 58:6242–6250. doi: 10.1128/AAC.03073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sy SK, de Kock L, Diacon AH, Werely CJ, Xia H, Rosenkranz B, van der Merwe L, Donald PR. 2015. N-Acetyltransferase genotypes and the pharmacokinetics and tolerability of para-aminosalicylic acid in patients with drug-resistant pulmonary tuberculosis. Antimicrob Agents Chemother 59:4129–4138. doi: 10.1128/AAC.04049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baquero F. 1990. Resistance to quinolones in gram-negative microorganisms: mechanisms and prevention. Eur Urol 17(Suppl 1):S3–S12. [DOI] [PubMed] [Google Scholar]
- 31.Baquero F, Negri MC. 1997. Strategies to minimize the development of antibiotic resistance. J Chemother 9(Suppl 3):S29–S37. [PubMed] [Google Scholar]
- 32.Drlica K. 2003. The mutant selection window and antimicrobial resistance. J Antimicrob Chemother 52:11–17. doi: 10.1093/jac/dkg269. [DOI] [PubMed] [Google Scholar]
- 33.Firsov AA, Vostrov SN, Lubenko IY, Drlica K, Portnoy YA, Zinner SH. 2003. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob Agents Chemother 47:1604–1613. doi: 10.1128/AAC.47.5.1604-1613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khachman D, Conil JM, Georges B, Saivin S, Houin G, Toutain PL, Laffont CM. 2011. Optimizing ciprofloxacin dosing in intensive care unit patients through the use of population pharmacokinetic-pharmacodynamic analysis and Monte Carlo simulations. J Antimicrob Chemother 66:1798–1809. doi: 10.1093/jac/dkr220. [DOI] [PubMed] [Google Scholar]
- 35.Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. 2005. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:4920–4927. doi: 10.1128/AAC.49.12.4920-4927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap! Clin Infect Dis 51(Suppl 1):S103–S110. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang L, He Y, Xia H, Liu Y, Sy SK, Derendorf H. 2016. Gentamicin dosing strategy in patients with end-stage renal disease receiving haemodialysis: evaluation using a semi-mechanistic pharmacokinetic/pharmacodynamic model. J Antimicrob Chemother 71:1012–1021. doi: 10.1093/jac/dkv428. [DOI] [PubMed] [Google Scholar]
- 38.Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, Oikonomou A, Poulakou G, Roilides E, Giamarellou H. 2014. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 43:52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, Isgri S. 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 41.Albur MS, Noel A, Bowker K, MacGowan A. 2015. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. Int J Antimicrob Agents 46:560–567. doi: 10.1016/j.ijantimicag.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Sastry S, Clarke LG, Alrowais H, Querry AM, Shutt KA, Doi Y. 2015. Clinical appraisal of fosfomycin in the era of antimicrobial resistance. Antimicrob Agents Chemother 59:7355–7361. doi: 10.1128/AAC.01071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alrowais H, McElheny CL, Spychala CN, Sastry S, Guo Q, Butt AA, Doi Y. 2015. Fosfomycin resistance in Escherichia coli, Pennsylvania, USA. Emerg Infect Dis 21:2045–2047. doi: 10.3201/eid2111.150750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Zheng B, Li Y, Zhu S, Xue F, Liu J. 2015. Antimicrobial susceptibility and molecular mechanisms of fosfomycin resistance in clinical Escherichia coli isolates in mainland China. PLoS One 10:e0135269. doi: 10.1371/journal.pone.0135269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao JY, Zhu YQ, Li YN, Mu XD, You LP, Xu C, Qin P, Ma JL. 2015. Coexistence of SFO-1 and NDM-1 beta-lactamase genes and fosfomycin resistance gene fosA3 in an Escherichia coli clinical isolate. FEMS Microbiol Lett 362(1):1–7. doi: 10.1093/femsle/fnu018. [DOI] [PubMed] [Google Scholar]
- 46.Sy SK, Zhuang L, Derendorf H. 2016. Pharmacokinetics and pharmacodynamics in antibiotic dose optimization. Expert Opin Drug Metab Toxicol 12:93–114. doi: 10.1517/17425255.2016.1123250. [DOI] [PubMed] [Google Scholar]
- 47.Sy SK, Derendorf H. 2016. Pharmacokinetics I: PK-PD approach, the case of antibiotic drug development, p 185–217. In M̈uller M. (ed), Clinical pharmacology: current topics and case studies. Springer, New York, NY. [Google Scholar]
- 48.Zhuang L, Sy SK, Xia H, Singh RP, Mulder MB, Liu C, Derendorf H. 2015. Evaluation of in vitro synergy between vertilmicin and ceftazidime against Pseudomonas aeruginosa using a semi-mechanistic pharmacokinetic/pharmacodynamic model. Int J Antimicrob Agents 45:151–160. doi: 10.1016/j.ijantimicag.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Zavascki AP, Bulitta JB, Landersdorfer CB. 2013. Combination therapy for carbapenem-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther 11:1333–1353. doi: 10.1586/14787210.2013.845523. [DOI] [PubMed] [Google Scholar]
- 50.Kotapati S, Nicolau DP, Nightingale CH, Kuti JL. 2004. Clinical and economic benefits of a meropenem dosage strategy based on pharmacodynamic concepts. Am J Health Syst Pharm 61:1264–1270. [DOI] [PubMed] [Google Scholar]
- 51.Sy SK, Beaudoin ME, Zhuang L, Loblein K, Lux C, Kissel M, Tremmel R, Frank C, Strasser S, Heuberger JA, Mulder MB, Schuck VJ, Derendorf H. 21 April 2016. In vitro pharmacokinetics/pharmacodynamics of the combination of avibactam and aztreonam against MDR organisms. J Antimicrob Chemother doi: 10.1093/jac/dkw082. [DOI] [PubMed] [Google Scholar]
- 52.Hancock RE. 1998. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis 27(Suppl 1):S93–S99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 53.Hancock RE, Brinkman FS. 2002. Function of Pseudomonas porins in uptake and efflux. Annu Rev Microbiol 56:17–38. doi: 10.1146/annurev.micro.56.012302.160310. [DOI] [PubMed] [Google Scholar]
- 54.Huang H, Hancock RE. 1993. Genetic definition of the substrate selectivity of outer membrane porin protein OprD of Pseudomonas aeruginosa. J Bacteriol 175:7793–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crandon JL, Ariano RE, Zelenitsky SA, Nicasio AM, Kuti JL, Nicolau DP. 2011. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med 37:632–638. doi: 10.1007/s00134-010-2105-0. [DOI] [PubMed] [Google Scholar]
- 56.Doh K, Woo H, Hur J, Yim H, Kim J, Chae H, Han S, Yim DS. 2010. Population pharmacokinetics of meropenem in burn patients. J Antimicrob Chemother 65:2428–2435. doi: 10.1093/jac/dkq317. [DOI] [PubMed] [Google Scholar]
- 57.Li C, Kuti JL, Nightingale CH, Nicolau DP. 2006. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol 46:1171–1178. doi: 10.1177/0091270006291035. [DOI] [PubMed] [Google Scholar]
- 58.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64:142–150. doi: 10.1093/jac/dkp139. [DOI] [PubMed] [Google Scholar]
- 59.Goncalves-Pereira J, Silva NE, Mateus A, Pinho C, Povoa P. 2014. Assessment of pharmacokinetic changes of meropenem during therapy in septic critically ill patients. BMC Pharmacol Toxicol 15:21. doi: 10.1186/2050-6511-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]