Abstract
Antibacterial agents that kill nondividing bacteria may be of utility in treating persistent infections. Oritavancin and dalbavancin are bactericidal lipoglycopeptides that are approved for acute bacterial skin and skin structure infections in adults caused by susceptible Gram-positive pathogens. Using time-kill methodology, we demonstrate that oritavancin exerts bactericidal activity against methicillin-resistant Staphylococcus aureus (MRSA) isolates that are maintained in a nondividing state in vitro, whereas dalbavancin and the glycopeptide vancomycin do not.
TEXT
Persistent Staphylococcus aureus infections may harbor bacteria in a nondividing state in which killing by bactericidal agents is reduced relative to the killing of actively dividing bacteria (1). Hence, the use of antibacterial agents with activity against such nondividing bacteria may potentially decrease the duration of therapy that is required to treat these infections and ultimately be of benefit in their clinical management.
Oritavancin and dalbavancin are long-acting lipoglycopeptides with activity against Gram-positive bacteria (2, 3). Oritavancin has multiple mechanisms of action, and its rapid concentration-dependent bactericidal activity against S. aureus isolates in vitro results from a combination of cell wall synthesis inhibition and perturbation of membrane barrier function (4, 5). The time-dependent bactericidal activity of dalbavancin results from inhibition of cell wall synthesis via a mechanism of action that is shared with the prototypic glycopeptide vancomycin (6–8). The differences in the mechanisms of action among these agents may be important determinants of their activities against bacteria in a nondividing state. For example, oritavancin has been shown to maintain bactericidal activity in vitro against stationary-phase isolates of S. aureus in a nutrient-depleted medium, a condition in which bacterial killing by vancomycin was attenuated (a consequence of diminished cell wall synthesis) (5). In a different study, exposure of a methicillin-resistant S. aureus (MRSA) isolate to dalbavancin required 48 h to exert a ≥3-log kill against cells in a nondividing state (9). To date, no studies have directly compared the antibacterial activities of these long-acting lipoglycopeptides against either actively dividing or nondividing cells under the same test conditions in vitro. In light of this, we compare the antibacterial activities of oritavancin, dalbavancin, and vancomycin against MRSA isolates that are either actively dividing or in a nondividing state in vitro to reveal differences in the mechanisms of action of these agents that may lead to optimal therapies for infections harboring bacteria in a nondividing state.
(Part of this work was presented at the Joint 55th Interscience Conference on Antimicrobial Agents and Chemotherapy and 28th International Congress of Chemotherapy Meeting, San Diego, CA, 17 to 21 September 2015 [10].)
The four S. aureus isolates used in this study were MRSA ATCC 43300, MRSA NRS384, MRSA-heterogeneous vancomycin-intermediate S. aureus (hVISA) isolate ATCC 700698, and MRSA-hVISA NRS19. Broth microdilution MICs were determined for oritavancin (The Medicines Company, Parsippany, NJ), dalbavancin (APIChem Technology Company, Hangzhou, China), and vancomycin (Sigma-Aldrich, St. Louis, MO) following CLSI M07-A10 guidelines (11) using the S. aureus quality control (QC) isolate ATCC 29213 to confirm the appropriate assay performance and antibacterial activity of each agent. Time-kill assays of actively dividing cells were performed in cation-adjusted Mueller-Hinton broth (CAMHB) plus 0.002% polysorbate 80 using an exponentially growing inoculum at 107 CFU/ml. To assess the killing of nondividing bacteria, stationary-phase cultures (grown overnight in CAMHB at 37°C and 225 rpm) were pelleted by centrifugation (2,800 × g for 5 min) and resuspended at 107 CFU/ml in phosphate-buffered saline (PBS) containing 0.1% dextrose and 0.002% polysorbate 80. These conditions maintained cells in a viable, nondividing state for at least 48 h (9). Bacteria were exposed to an MIC-doubling dilution concentration nearest to the free peak (fCmax) for each agent, specifically, 16 μg/ml for oritavancin (fCmax of 20 μg/ml derived from a total Cmax of 138 μg/ml from a single 1,200-mg dose and 85% protein binding) and 32 μg/ml for dalbavancin (fCmax of 30 μg/ml derived from a total Cmax of 423 μg/ml from a single 1,500-mg dose and 93% protein binding) and vancomycin (fCmax of 28 μg/ml derived from a total Cmax of 63 μg/ml from a 1,000-mg dose and 55% protein binding) (2, 3, 12). For comparative purposes (and where appropriate), agents were also tested at a 16-fold increase over their respective MIC against each bacterial isolate. Bacterial viability was assessed at the indicated times by serial dilution plating using an initial dilution in 50 mg/ml of activated charcoal suspension to prevent antibiotic carryover (13). Bacteriostatic and bactericidal activities were defined as a <3-log reduction and a ≥3-log reduction in bacterial viability at 24 h (or earlier, as indicated), respectively, relative to the starting inoculum (14).
The tested S. aureus isolates were susceptible to dalbavancin (MIC ≤ 0.12 μg/ml) and vancomycin (MIC ≤ 2 μg/ml), with the sole exception that MRSA-hVISA NRS19 was nonsusceptible to dalbavancin (Table 1). MRSA isolates ATCC 43300 and NRS384 were susceptible to oritavancin (MIC ≤ 0.12 μg/ml), whereas the two MRSA-hVISA isolates were nonsusceptible to oritavancin (Table 1).
TABLE 1.
Broth microdilution modal MICs of the MRSA isolates used in this study
| Isolate | Phenotype | MIC (μg/ml)a |
||
|---|---|---|---|---|
| Dalbavancin | Oritavancin | Vancomycin | ||
| ATCC 43300 | MRSA | 0.06 (S) | 0.06 (S) | 1 (S) |
| NRS384 | MRSA | 0.06 (S) | 0.06 (S) | 0.5 (S) |
| ATCC 700698 | MRSA-hVISA | 0.06 (S) | 0.25 (NS) | 1 (S) |
| NRS19 | MRSA-hVISA | 0.25 (NS) | 0.5 (NS) | 1 (S) |
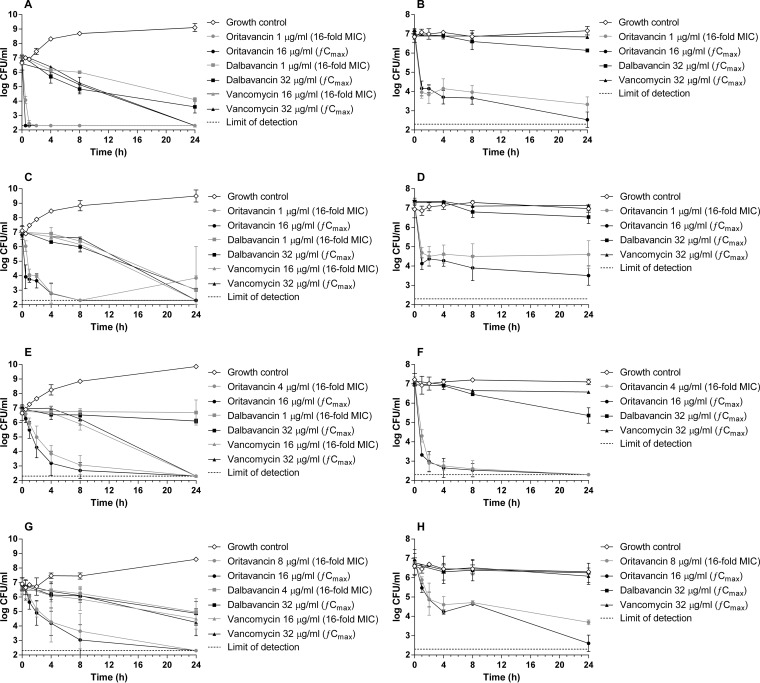
In time-kill assays using actively dividing cells, oritavancin, dalbavancin, and vancomycin exerted bactericidal activities at 24 h (≥3-log reduction in bacterial viability) against the two tested MRSA isolates (Fig. 1A and C). As expected, killing of the two MRSA isolates by oritavancin was rapid, with a >3-log reduction in CFU/ml relative to the starting inoculum occurring within 1 h at concentrations approximating 16 times the MIC of the bacterial isolates and the fCmax (16 μg/ml). In contrast, the bactericidal activities of dalbavancin and vancomycin at the tested concentrations occurred more slowly over the 24-h period. Against the two MRSA-hVISA isolates, the two tested concentrations of oritavancin exerted bactericidal activity within 8 h (reductions of 3.0- to 4.1-log CFU/ml relative to the starting inoculum) (Fig. 1E and G) despite the isolates being nonsusceptible to oritavancin. Differential killing by dalbavancin and vancomycin at the tested concentrations was observed against the MRSA-hVISA isolates (Fig. 1E and G); dalbavancin exerted bacteriostatic activity against the two MRSA-hVISA isolates (reductions of 0.1- to 1.9-log CFU/ml at 24 h relative to the starting inoculum), whereas vancomycin exerted bactericidal activity against ATCC 700698 (reductions of 4.5- to 4.7-log CFU/ml relative to the starting inoculum) and bacteriostatic activity against NRS19 (reductions of 2.2- to 2.6-log CFU/ml relative to the starting inoculum) at 24 h.
FIG 1.
Time-kill kinetics of oritavancin, dalbavancin, and vancomycin against MRSA and MRSA-hVISA isolates dividing exponentially (A, C, E, and G) or in a nondividing state (B, D, F, and H). Bacterial viability was enumerated at the indicated time points by serial dilution plating. Mean values ± standard deviations from 2 to 3 independent experiments are presented. The dashed line indicates the limit of detection (200 CFU/ml). (A and B) MRSA ATCC 43300. (C and D) MRSA NRS384. (E and F) MRSA-hVISA ATCC 700698. (G and H) MRSA-hVISA NRS19.
In time-kill assays in which the S. aureus isolates were maintained in a nondividing state in vitro, exposures to oritavancin at 16 times the MIC of the bacterial isolates resulted in bactericidal activity (reductions of 3.1- to 4.9-log CFU/ml relative to the starting inoculum) within 2 to 24 h, depending on the isolate, with the exception that the viability of MRSA NRS384 was reduced by 2.6-log CFU/ml relative to the starting inoculum at 24 h (Fig. 1B, D, F, and H). At its fCmax, oritavancin exerted bactericidal killing against the four S. aureus isolates (reductions of 3.7- to 4.8-log CFU/ml relative to the starting inoculum) within 2 to 24 h. Interestingly, the MRSA-hVISA isolate ATCC 700698 was paradoxically more readily killed by oritavancin (and dalbavancin) in a nondividing state than when the isolate was actively dividing (compare Fig. 1E and F), a sensitivity that was not shared by MRSA-hVISA NRS19 despite the same phenotype.
Exposure of the two MRSA isolates and MRSA-hVISA NRS19 in a nondividing state to the fCmax of dalbavancin (32 μg/ml) resulted in attenuated bacterial killing (killing of 0.4- to 0.9-log CFU/ml relative to the starting inoculum at 24 h) (Fig. 1B, F, and H) compared to actively dividing cells (killing of 1.9- to 4.0-log CFU/ml relative to the starting inoculum) (Fig. 1A, E, and G). MRSA-hVISA ATCC 700698 that was exposed to the fCmax of dalbavancin paradoxically showed increased killing in a nondividing state relative to actively dividing cells (killing of 1.6-log CFU/ml compared to 0.9-log CFU/ml relative to the starting inoculum at 24 h, respectively) (Fig. 1E and F). Regardless of the sensitivity of this isolate to lipoglycopeptides, exposure of all of the tested isolates to vancomycin at its fCmax (32 μg/ml) resulted in attenuated killing of nondividing cells (killing of 0.1- to 0.7-log CFU/ml relative to the starting inoculum at 24 h) compared to actively dividing cells (killing of 2.6- to 4.9-log CFU/ml relative to the starting inoculum at 24 h). Although divalent cations are not known to be required for the killing activities of dalbavancin or vancomycin, the impact, if any, of diminished ion content in the medium (PBS) that was used to assess killing of nondividing cells is unknown. However, the reduction in cell wall synthesis in nondividing cells is known to impact the activity of cell wall-active agents (5, 15, 16) and reasonably accounts for the observed marked decreases in the activities of dalbavancin and vancomycin against cells in this state.
In conclusion, the current study demonstrates the bactericidal activity of oritavancin against MRSA and MRSA-hVISA isolates in a nondividing state in vitro, conditions in which the antibacterial activities of dalbavancin and vancomycin are diminished. Despite the observed interisolate variability, oritavancin consistently exerted bactericidal activity at pharmacologically relevant exposures. The maintenance of killing by oritavancin against nondividing bacteria is likely linked to its disruption of bacterial membrane integrity, leading to depolarization, permeabilization, and cell death (5). This differential property may be of benefit for infection sites that typically harbor bacteria in the nondividing state.
REFERENCES
- 1.Hurdle JG, O'Neill AJ, Chopra I, Lee RE. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Medicines Company. 2015. Orbactiv United States prescribing information. The Medicines Company, Parsippany, NJ. [Google Scholar]
- 3.Durata Therapeutics U. S. Ltd. 2015. Dalvance United States prescribing information. Durata Therapeutics U.S. Ltd., Chicago, IL. [Google Scholar]
- 4.Belley A, McKay GA, Arhin FF, Sarmiento I, Beaulieu S, Fadhil I, Parr TR Jr, Moeck G. 2010. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother 54:5369–5371. doi: 10.1128/AAC.00760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belley A, Neesham-Grenon E, McKay G, Arhin FF, Harris R, Beveridge T, Parr TR Jr, Moeck G. 2009. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother 53:918–925. doi: 10.1128/AAC.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein BP, Draghi DC, Sheehan DJ, Hogan P, Sahm DF. 2007. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother 51:1150–1154. doi: 10.1128/AAC.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhanel GG, Calic D, Schweizer F, Zelenitsky S, Adam H, Lagace-Wiens PR, Rubinstein E, Gin AS, Hoban DJ, Karlowsky JA. 2010. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs 70:859–886. doi: 10.2165/11534440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Lopez S, Hackbarth C, Romano G, Trias J, Jabes D, Goldstein BP. 2005. In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. J Antimicrob Chemother 55(Suppl):ii21–ii24. [DOI] [PubMed] [Google Scholar]
- 9.Baldoni D, Furustrand Tafin U, Aeppli S, Angevaare E, Oliva A, Haschke M, Zimmerli W, Trampuz A. 2013. Activity of dalbavancin, alone and in combination with rifampicin, against meticillin-resistant Staphylococcus aureus in a foreign-body infection model. Int J Antimicrob Agents 42:220–225. doi: 10.1016/j.ijantimicag.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Belley A, Lalonde Seguin D, Arhin FF, Moeck G. 2015. In vitro bactericidal activity of oritavancin, dalbavancin and vancomycin against non-dividing methicillin-resistant Staphylococcus aureus, abstr A-493 Abstr Joint 55th Intersci Conf Antimicrob Agents Chemother and 28th Int Congress Chemother Meet American Society for Microbiology, Washington, DC. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Pfizer Inc. 2015. Vancomycin hydrochloride United States prescribing information. Pfizer Inc., New York, NY. [Google Scholar]
- 13.Belley A, Neesham-Grenon E, Arhin FF, McKay GA, Parr TR Jr, Moeck G. 2008. Assessment by time-kill methodology of the synergistic effects of oritavancin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother 52:3820–3822. doi: 10.1128/AAC.00361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Society for Microbiology. 2016. Antimicrobial Agents and Chemotherapy instruction to authors. American Society for Microbiology, Washington, DC: http://journalitas.asm.org/t/46519. [Google Scholar]
- 15.Mascio CT, Alder JD, Silverman JA. 2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob Agents Chemother 51:4255–4260. doi: 10.1128/AAC.00824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamp KC, Rybak MJ, Bailey EM, Kaatz GW. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob Agents Chemother 36:2709–2714. doi: 10.1128/AAC.36.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]