Abstract
Efflux pumps of the resistance nodulation cell division (RND) transporter family, such as AcrB of Escherichia coli, play an important role in the development of multidrug resistance, but the molecular basis for their substrate promiscuity is not yet completely understood. From a collection of highly clarithromycin-resistant AcrB periplasmic domain mutants derived from in vitro random mutagenesis, we identified variants with an unusually altered drug resistance pattern characterized by increased susceptibility to many drugs of lower molecular weight, including fluoroquinolones, tetracyclines, and oxazolidinones, but unchanged or increased resistance to drugs of higher molecular weight, including macrolides. Sequencing of 14 such “divergent resistance” phenotype mutants and 15 control mutants showed that this unusual phenotype was associated with mutations at residues I38 and I671 predominantly to phenylalanine and threonine, respectively, both conferring a similar susceptibility pattern. Reconstructed I38F and I671T single mutants as well as an engineered I38F I671T double mutant with proved efflux competence revealed an equivalent phenotype with enhanced or unchanged resistance to many large AcrB substrates but increased susceptibility to several lower-molecular-weight drugs known to bind within the distal binding pocket. The two isoleucines located in close vicinity to each other in the lower porter domain of AcrB beneath the bottom of the proximal binding pocket may be part of a preferential small-drug entrance pathway that is compromised by the mutations. This finding supports recent indications of distinct entrance channels used by compounds with different physicochemical properties, of which molecular size appears to play a prominent role.
INTRODUCTION
Bacterial multidrug resistance (MDR) is one of the great challenges in view of increasing resistance rates, particularly among Gram-negative pathogens and limited development of new therapeutic compounds (1). In Gram-negative bacteria, the outer membrane influx barrier and MDR efflux contribute to poor susceptibility to various antibiotics (2, 3). Several classes of bacterial drug exporters are known (4, 5), among them the resistance nodulation cell division (RND)-type transporters, which are considered the most efficient MDR efflux systems of Gram-negative organisms. RND pumps cooperate with an outer membrane channel and membrane fusion proteins, thereby bridging the inner membrane, the periplasm, and the outer membrane. Some of the RND pumps have a narrow substrate range (e.g., the aminoglycoside exporter AcrD from Escherichia coli and MexY from Pseudomonas aeruginosa), while many of them confer resistance to a wide variety of chemically unrelated compounds. The latter group includes the constitutively expressed trimeric exporter AcrB, the major MDR efflux pump of E. coli which works together with the outer membrane channel TolC and the AcrA membrane fusion proteins.
AcrB serves as a model RND transporter and has been extensively investigated. Anchored in the inner membrane by 12 transmembrane helices (TM1 to -12), its periplasmic part is built from two large loops between TM1 and TM2 and TM7 and TM8 (6, 7) comprising the TolC docking domain and the porter domain (with subdomains PN1, PN2, PC1, and PC2). The latter harbors a proximal and a distal substrate binding pocket (PBP and DBP, respectively), also designated “access” and “deep binding pocket,” respectively (8, 9). The PBP is located between subdomains PC1 and PC2 and is separated from the DPB (between PC1 and PN2) by a flexible loop, the so-called “switch-loop” (or “G-loop,” since it is glycine rich) (8, 10). The two binding pockets are known to play a major role in recognizing and accommodating substrates during a cyclic transport process enabled by proton-motive force that is generated by proton translocation from the TM domain of the efflux pump (11). Each protomer of the trimeric AcrB complex is thought to pass through a conformational change from an access, via binding, to an extrusion state, while the substrate is thought to be forced from the PBP to the DBP and finally to the gate directed to the outer membrane channel TolC (12–14).
A strikingly wide variety of compounds with different sizes and chemical properties are known to be extruded by AcrB from E. coli (3, 15) and similarly from the close homolog MexB from P. aeruginosa (10, 16). While substrate specificity-determining sites have been demonstrated within the DBP and the PBP (9, 15, 17–19), less information is available about putative entrance channels. Data predominantly from crystallization and computer modeling studies have suggested a vestibule and a cleft entrance. The vestibule channel appears to be opened to the so-called “groove” between TM8 and TM9 and is thought to trap substrates from the outer leaflet of the inner membrane, whereas the cleft pathway is thought to allow the entrance of substrates from the periplasm between subdomains PC1 and PC2 further apart from the upper ending of the TM domain (9, 12–14, 20–22). A third option, an entrance on the back side of AcrB open to the central cavity built from the intermolecular space between the protomers is considered to be less likely (13, 21).
In addition to crystallization, computational, and site-directed mutagenesis studies, in vitro random mutagenesis is known to be a powerful tool to get insights into the structure-function relationship of proteins, even of membrane transporters (23–26). This approach may help to locate besides targets of potential efflux pump inhibitors (EPIs) (27, 28) binding sites and the translocation pathways of drugs and other substrates.
In the present study, we screened a library of highly clarithromycin-resistant AcrB variants derived from chromosome-based in vitro random mutagenesis of the periplasmic porter domain and identified a number of AcrB mutations with a divergent impact on the susceptibility to different drugs and chemicals. We here report that mutations at residues I38 and I671, which appear to constitute a narrow site in the lower part of the AcrB periplasmic porter domain, lead to this “divergent resistance” (DR) phenotype with unchanged or even enhanced AcrB-associated resistance to many drugs of higher molecular weight but considerably increased susceptibility to drugs with lower molecular weights. These findings demonstrate new pump substrate specificity determinants within the lower porter domain in addition to those previously described at the proximal and distal AcrB binding pockets.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and chemical compounds.
An overview of the bacterial strains and plasmids used in this study is given in Table 1. Bacteria were cultivated on Luria-Bertani (LB) broth agar (1.5%) at 37°C, supplemented with drug if needed and as indicated, or in LB broth for MIC, accumulation, and efflux assays.
TABLE 1.
Strains and plasmids used in this study
| Straina or plasmid | Origin | Source or reference |
|---|---|---|
| Strains | ||
| 3-AG100 | E. coli K-12 AG100 derivative, AcrAB-TolC-overexpressing mar mutant | Kern et al. (37) |
| ΔAcrB mutant | acrB-deficient 3-AG100 strain, derived by total deletion of acrB | Schuster et al. (27) |
| I38F mutant | Chromosomal AcrB variant of 3-AG100 derived from in vitro random mutagenesis | This study |
| I671T mutant | Chromosomal AcrB variant of 3-AG100 derived from in vitro random mutagenesis | This study |
| I671T ΔAcrB mutant | acrB (::rpsL neo) knockout strain derived from AcrB variant I671T | This study |
| I38F I671T mutant | Chromosomal AcrB variant of 3-AG100 derived by site-directed mutagenesis | This study |
| Plasmids | ||
| pAcrBwt | pET24a-acrBwt | Kindly provided by Klaas Martinus Posb |
| pAcrB-I38F | From plasmid-based site-directed mutagenesis of acrB using pAcrBwt | This study |
| pAcrB-I671T | From plasmid-based site-directed mutagenesis of acrB using pAcrBwt | This study |
| pRed/ET | Curable plasmid, expression of λ phage-derived proteins (assistance of homologous recombination) | Counter-selection BAC modification kitc |
Additional chromosomal AcrB mutants derived from in vitro random mutagenesis are listed in Table 3.
Institute of Biochemistry, Goethe University, Frankfurt, Germany.
Gene Bridges, Heidelberg, Germany.
Chemicals were obtained from Sigma (Taufkirchen, Germany) with the following exceptions: linezolid (Zyvoxid; 2-mg/ml solution) was obtained from Pfizer (Berlin, Germany), tedizolid from Gentaur (Kampenhout, Belgium), phosphate-buffered saline (PBS) from Lonza (Verviers, Belgium), and 1,2′-dinaphthylamine (1,2′-DINA) from TCI-Europe (Zwijndrecht, Belgium).
In vitro random mutagenesis and library construction.
Chromosomal acrB mutants from the acrB-overexpressing E. coli strain 3-AG100 were generated by an in vitro random mutagenesis method (error-prone PCR combined with homologous recombination) by targeting the gene regions corresponding to the two large periplasmic loops LP1 (amino acids 29 to 330) and LP2 (amino acids 561 to 862) separately as described recently (27). The resultant AcrB mutants were selected in a single step on LB agar plates containing 16 μg/ml clarithromycin (CLR) (as the pump substrate) and 25 μg/ml phenylalanine-arginine-β-naphthylamide (PAβN) (as the putative efflux pump inhibitor [29]). (The intrinsic MIC of PAβN determined with the parental E. coli strain 3-AG100 is >512 μg/ml, that of CLR is 256 μg/ml, and that of CLR in the presence of 25 μg/ml PAβN is 8 μg/ml).
Plasmid-based site-directed mutagenesis.
Mutations of interest were reconstructed within plasmid pET24a-acrBwt using the site-directed mutagenesis kit Q5 (New England BioLabs, Frankfurt, Germany) according to the manufacturer's instructions. Introduction of the desired mutations was confirmed by sequencing, and the resulting plasmids were expressed from the ΔAcrB E. coli strain.
Chromosome-based site-directed mutagenesis.
The I38F I671T double mutant was constructed from the AcrB mutant harboring the I671T single mutation using a two-step homologous recombination procedure with the Gene Bridges counter-selection BAC modification kit according to the manufacturer's protocol (Gene Bridges, Heidelberg, Germany). Briefly, the gene region corresponding to periplasmic loop 1 was replaced with an rpsL-neo cassette, which was exchanged in a second step with a PCR product amplified from genomic DNA of the I38F single mutant as the template (using proofreading enzyme Q5 polymerase from New England BioLabs, Frankfurt, Germany): the forward primer was 5′-AAACAGGAGCCGTTAAGACA-3′, and the reverse primer was 5′-CGGAAGTTCTGCAGGAACAG-3′. The counterselected mutants with replaced rpsL-neo cassette were confirmed by sequencing. The oligonucleotides used for amplification of the rpsL-neo cassette with homology arms adjacent to the acrB gene region corresponding to periplasmic loop 1 were reported previously (27).
Molecular standard techniques.
PCR amplification, DNA agarose gel electrophoresis, DNA extraction, and DNA purification were carried out following standard procedures. Sanger sequencing of PCR products amplified from acrB was performed by Seqlab (Göttingen, Germany).
Susceptibility testing.
The MICs of 16 drugs were determined by a standard 2-fold microdilution assay with inocula of 1.5 × 105 CFU/ml LB prepared from fresh overnight cultures on LB agar plates using 96-well custom plates (Merlin, Bornheim-Hersel, Germany). Ninety-six-well format 2-fold serial dilutions were also applied to determine the MICs of additional test drugs, including tedizolid, oleandomycin, tylosin, and josamycin, as well as those of efflux pump inhibitors (EPIs) and dyes. In order to evaluate EPI efficiency, MIC testing was additionally performed in the presence of 25 μg/ml PAβN. The inoculated microdilution assays were incubated for 20 h at 37°C. They were done at least in triplicate (from different overnight cultures), and the median was determined. Gentamicin was used throughout as control (no AcrB substrate).
Dye accumulation and real-time efflux assays.
Bacterial cells freshly grown overnight on LB agar plates were resuspended in PBS supplemented with 0.4% d-glucose to an optical density at 600 nm (OD600) of 1. Dye was added, and fluorescence was measured at 37°C over time (30 min). Ethidium bromide was used at a final concentration of 2.5 μM (excitation wavelength, 518 nm; emission, 605 nm), Hoechst 33342 at 2.5 μM (excitation, 350 nm; emission, 461 nm), berberine at 30 μg/ml (excitation, 355 nm; emission, 517 nm), and pyronin Y at 2.5 μM (excitation, 545 nm; emission, 570 nm).
The 1,2′-DINA real-time efflux assay was performed according to a protocol published previously (30) using a final 1,2′-DINA concentration of 32 μM, with fluorescence measurements at excitation and emission wavelengths of 370 and 420 nm, respectively, with automated injection of glucose. The Nile red real-time efflux assays were carried out as described earlier using a final Nile red concentration of 5 μM (31, 32). Dye assays were done in triplicate, and fluorescence measurements were carried out with the Tecan Infinite M200PRO fluorescence plate reader (Tecan, Männedorf, Switzerland).
Visualization of I38 and I671 in the three-dimensional structure of AcrB.
The residues identified were visualized, and distances of side chains were measured within the access, binding, and extrusion state of the AcrB protomer (PDB accession no. 2HRT; chains A, B, and C) using the molecular visualization program PyMOL, version 1.3, 2010, Schrodinger, LLC (www.pymol.org).
RESULTS
Selection and screening of AcrB periplasmic domain variants.
In a recent in vitro random mutagenesis study with the aim to discover targets of EPIs within the E. coli efflux pump AcrB (27), we were unable to detect acrB mutations conferring resistance to the pump-inhibitory action of PAβN. However, from error-prone PCR-based random mutagenesis targeting the periplasmic domain of AcrB from E. coli, we had obtained mutants at rates of 0.065% (from 8 × 104 plated cells) and 0.15% (from 3 × 105 cells) for the large periplasmic loops 1 (LP1) and 2 (LP2), respectively, growing in the presence of a CLR-PAβN combination that was growth inhibitory for the parental strain. The MICs of several pump substrates in the absence and presence of PAβN revealed no changes in the drug efflux-inhibitory action of PAβN for these mutants, which instead showed an up to 4-fold increased resistance to CLR itself. With further analysis, it became apparent that from these highly CLR-resistant LP1 and LP2 mutants, 15% (from 52 tested) and 20% (from 182 tested), respectively, displayed an unusual resistance pattern characterized by consistent ≥4-fold-increased susceptibility to several drugs (including linezolid, chloramphenicol, cefuroxime, oxacillin, tetracycline, and levofloxacin) but no or smaller changes in the MICs of other drugs, including macrolides and novobiocin (Table 2; see Table S1 in the supplemental material), here called the “divergent resistance” (DR) phenotypic profile.
TABLE 2.
Resistance profile of DR and non-DR AcrB mutants in comparison with the parental E. coli strain 3-AG100 overexpressing wild-type AcrBa
| Strain | MIC (μg/ml)b |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LZD | CHL | CXM | OXA | CLI | TET | TGC | LVX | NOV | RIF | CLR | ERYc | AZM | |
| 3-AG100 (parental strain) | 1,024 | 16 | 16 | 512 | 512 | 4 | 0.5 | 2 | 1,024 | 16 | 256 | >1,024 | 256 |
| DR AcrB mutants | 64 | 1 | 1 | 64 | 128 | 1 | 0.13 | 0.25 | 512 | 16 | 1,024 | >1,024 | 256 |
| Non-DR AcrB mutants | 512 | 8 | 16 | 512 | 512 | 4 | 0.5 | 2 | 512 | 16 | 1,024 | >1,024 | 256 |
DR mutants have an unusually altered pattern of resistance to AcrB substrates that we call the “divergent resistance” (DR) pattern, and non-DR phenotype mutants have significantly changed susceptibility only to CLR, in comparison with the parental E. coli strain 3-AG100 overexpressing wild-type AcrB.
MICs were determined for several DR and non-DR phenotype mutants (Table 3; see Tables S1 and S2 in the supplemental material) and did not differ within the two groups by more than one dilution step from those listed here (with the exception of the CXM MIC of the non-DR mutant CLP-I-13 [see Table S2]). LZD, linezolid; CHL, chloramphenicol; CXM, cefuroxime; OXA, oxacillin; CLI, clindamycin; TET, tetracycline; TGC, tigecycline; LVX, levofloxacin; NOV, novobiocin; RIF, rifampin; CLR, clarithromycin; ERY, erythromycin; AZM, azithromycin. MIC changes of ≥4-fold compared to the MIC for parental strain 3-AG100 are depicted in boldface.
The limit of ERY solubility in broth is 1,024 μg/ml.
Mutations within DR and non-DR periplasmic domain variants.
Due to the mutagenesis method that was used here, alterations of the periplasmic domain of AcrB were expected. We sequenced the acrB genes of LP1 and LP2 mutants (seven each) of the DR phenotype and of seven LP1 and eight LP2 variants without DR as a control. (For the phenotype of non-DR mutants, see Table 2 and Table S2 in the supplemental material.) All sequenced DR variants derived from LP1 harbored an amino acid substitution at I38, while all those from LP2 showed amino acid substitutions at residue I671 (Table 3; see Table S1 in the supplemental material). The two most frequently detected alterations, I38F and I671T, occurred even as single mutations and were not found in the non-DR strains. However, as shown in Table 3 and Table S2, some non-DR mutants contained single mutations in close vicinity to I38 or I671 (Y35N, A39T, V672M, and E673G).
TABLE 3.
Periplasmic domain mutants achieved from in vitro random mutagenesis and their genotypes and associated resistance phenotypesa
| AcrB region targeted by in vitro random mutagenesis | AcrB mutation(s) | Total no. of mutants with the indicated genotype | Resistance phenotype |
|---|---|---|---|
| LP1 | I38N G147D | 1 | DR |
| I38F A103T A160T | 1 | DR | |
| I38F R259S | 1 | DR | |
| I38F N144S A209E | 1 | DR | |
| I38F | 1 | DR | |
| I38S P116L A294V | 1 | DR | |
| I38F P50L T196 M | 1 | DR | |
| A22P T85A | 1 | Non-DR | |
| Y35N | 1 | Non-DR | |
| A39T | 2 | Non-DR | |
| P224L F316S | 1 | Non-DR | |
| P224T | 1 | Non-DR | |
| T85A | 1 | Non-DR | |
| LP2 | T600I I671T A752V A831T | 1 | DR |
| I671T S813P A840T | 1 | DR | |
| I671T | 3 | DR | |
| N613S I671T E693D A831V | 1 | DR | |
| I671T R699L S807P | 1 | DR | |
| V672M | 1 | Non-DR | |
| E673G | 1 | Non-DR | |
| G621S K632R Q846H | 1 | Non-DR | |
| L828F | 1 | Non-DR | |
| L828S K835N | 1 | Non-DR | |
| R586P G675A | 1 | Non-DR | |
| R717S | 1 | Non-DR | |
| K603R G621V M649V R717L F801L | 1 | Non-DR |
See also Tables S1 and S2 in the supplemental material. Mutations exclusively occurring within DR phenotype mutants are depicted in boldface. For definitions of the DR (boldface) and non-DR phenotypes, see Table 2.
I38 and I671 in the three-dimensional structure of AcrB.
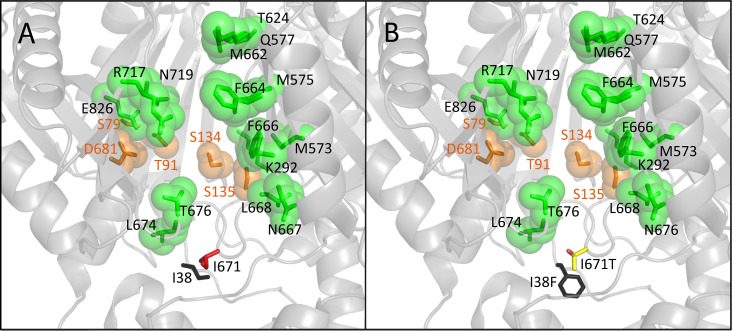
When using the 2HRT structure of AcrB, I38 and I671 mapped in close proximity, with their hydrophobic side chains facing each other and apparently constituting a narrow site (Fig. 1). The two residues belong to two loops—one connecting TM1 and PN1 (residues 34 to 40) and the other connecting PC1 and PC2 (residues 664 to 679). PyMOL measurement of distances between the C5 atom of I38 and C4 of I671 in the access, binding, and extrusion states (2HRT chains A, B, and C) revealed changes from 5.5 Å over 3.8 Å to 5.9 Å, respectively.
FIG 1.
(A) Partial view of the binding state monomer of AcrB shown as a light-colored cartoon (2HRT, chain B, side view from the external periplasmic cleft). Phenylalanine residues of the distal binding pocket (DBP) and residues of the proximal binding pocket (PBP) are depicted as blue and green spheres, respectively, according to a 2012 publication by Vargiu and Nikaido (18). The G-loop (switch-loop) separating the DBP and the PBP is partially hidden. I38 and I671 side chains are shown as sticks, with dashed lines indicating the site of distance measurement. The magenta oval illustrates the cleft entrance with magenta-labeled residues. The bulky and the thin black arrows indicate putative passage routes of high- and low-molecular-weight drugs, respectively. PN1, PN2, PC1, and PC2, subdomains of the porter domain; TD, TolC docking domain; TM, transmembrane domain. (B and C) Enlarged view of the I38-I671 “neighborhood” within the binding state (B) and access monomers (C) showing additional residues mutated in non-DR variants. Residues from the loop between TM1 and PN1 and those from the loop between PC1 and PC2 are shown as black and red sticks, respectively. Images were created using PyMOL (www.pymol.org).
Plasmid-based reconstruction of I38F and I671T.
In order to confirm the impact of the two I38F and I671T substitutions on the altered substrate specificity of AcrB and to exclude other mutations relevant for the observed DR phenomenon, we reconstructed the single mutations I38F and I671T within the wild-type (wt) acrB gene-harboring plasmid pAcrBwt by site-directed mutagenesis. Expression of the resulting single-mutation-containing plasmids in an E. coli acrB deletion (ΔAcrB) strain demonstrated the ability of I38F as well as I671T to confer the observed DR resistance phenotype. As shown in Table 4, the two plasmid-borne AcrB single mutations each increased the susceptibility to linezolid and several other pump substrates but were associated with unchanged susceptibility to others and increased resistance to clarithromycin.
TABLE 4.
Resistance profiles of site-directed mutagenesis-constructed E. coli AcrB mutants and wild-type acrB-overexpressing 3-AG100 reference strain
| Strain | MIC (μg/ml)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LZD | CHL | CXM | OXA | CLI | TET | TGC | LVX | NOV | RIF | CLR | ERYb | AZM | |
| ΔAcrB mutant (derived from 3-AG100) | 16 | 1 | 0.25 | 0.5 | 4 | 0.5 | 0.06 | 0.06 | 2 | 8 | 4 | 16 | 2 |
| Plasmid-based acrB strains | |||||||||||||
| ΔAcrB × pAcrBwt strain | 256 | 2 | 2 | 64 | 256 | 2 | 0.25 | 0.25 | 32 | 8 | 64 | 512 | 32 |
| ΔAcrB × pAcrB-I38F strain | 32 | 1 | 0.25 | 16 | 32 | 0.5 | 0.13 | 0.13 | 32 | 8 | 128 | 512 | 32 |
| ΔAcrB × pAcrB-I671T strain | 32 | 1 | 0.25 | 16 | 32 | 0.5 | 0.13 | 0.13 | 32 | 8 | 256 | 512 | 32 |
| Chromosome-based acrB mutants and parental strain | |||||||||||||
| 3-AG100 (parental reference strain) | 1,024 | 16 | 16 | 512 | 512 | 4 | 0.5 | 2 | 1,024 | 16 | 256 | >1,024 | 256 |
| I671T ΔAcrB mutant | 16 | 2 | 0.25 | 0.5 | 4 | 0.5 | 0.13 | 0.06 | 2 | 8 | 4 | 8 | 2 |
| Constructed I38F I671T mutant | 16 | 1 | 0.25 | 8 | 16 | 0.5 | 0.13 | 0.06 | 256 | 16 | 512 | 256 | 32 |
LZD, linezolid; CHL, chloramphenicol; CXM, cefuroxime; OXA, oxacillin; CLI, clindamycin; TET, tetracycline; TGC, tigecycline; LVX, levofloxacin; NOV, novobiocin; RIF, rifampin; CLR, clarithromycin; ERY, erythromycin; AZM, azithromycin. MIC changes of ≥4-fold detected from plasmid-based and chromosome-based AcrB mutants with point mutations compared to the ΔAcrB × pAcrBwt and 3-AG100 wild-type AcrB-expressing strains, respectively, are depicted in boldface.
The limit of ERY solubility in broth is 1,024 μg/ml.
acrB gene knockout of the I671T mutant and chromosome-based I38F I671T double mutant.
In order to substantiate the importance of the putative I38-I671 narrow site for substrate specificity and to explore whether the coexistence of I38F and I671T might potentiate rather than mitigate the DR pattern, we engineered a chromosome-based I38F I671T double mutant. We used site-directed mutagenesis to introduce mutation I38F within a single mutation I671T-containing strain. The procedure required an intermediate acrB knockout (I671T ΔAcrB) strain that notably revealed no differences in the MIC levels compared to those detected from the ΔAcrB strain derived from the parental wild-type AcrB-expressing E. coli strain 3-AG100 (Table 4). This finding indicates that, in fact, there are no other mutations outside from acrB responsible for the observed DR phenotype of the I671T mutant.
As shown in Tables 4 and 5, the constructed I38F I671T double mutation yielded a strain with obviously preferential efflux not only of CLR but also of other macrolides, including oleandomycin, tylosin, and josamycin. The MIC levels of erythromycin and azithromycin were increased compared with AcrB inactivation but did not reach the wild-type level. Testing of other additional drugs, including a new oxazolidinone (tedizolid), another tetracycline (minocycline), and two further fluoroquinolones (ciprofloxacin and moxifloxacin), confirmed a DR pattern with improved efflux of selected macrolides and less efficient pumping of many other compounds. In contrast to the single mutations, the I38F I671T double mutation additionally increased, to a certain extent, the susceptibility to rifaximine (unlike rifampin) and novobiocin.
TABLE 5.
Susceptibility to additional low- and high-molecular-weight drugs of the site-directed constructed (chromosome-based) AcrB I38F I671T double mutant strain compared to the ΔAcrB mutant and wild-type AcrB-overexpressing strain 3-AG100
| Strain | MIC (μg/ml)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| TEDb | MIN | MXF | CIP | RIX | OLMb | TYL | JOS | |
| ΔAcrB mutant | 1 | 0.125 | 0.06 | 0.06 | 4 | 4 | 16 | 4 |
| 3-AG100 | >512 | 4 | 4 | 2 | 64 | 512 | 1,024 | 1,024 |
| I38F I671T mutant | 4 | 0.25 | 0.25 | 0.13 | 16 | >512 | 1,024 | 1,024 |
TED, tedizolid; MIN, minocycline; MXF, moxifloxacin; CIP, ciprofloxacin; RIX, rifaximin. Macrolides: OLM, oleandomycin; TYL, tylosin; JOS, josamycin. MIC changes of ≥4-fold detected from the double mutant compared to the parental wild-type AcrB-overexpressing strain 3-AG100 are in boldface.
With TED and OLM, the solubility of the drugs in broth was limited to the MICs detected with strain 3-AG100 and the I38F I671T double mutant, respectively.
Dye accumulation and real-time efflux.
For the functional evaluation of the mutated efflux transporter, we used dye accumulation and real-time efflux assays and compared single I38F and I671T mutants as well as the I38F I671T double mutant with parental E. coli strain 3-AG100 and its corresponding ΔAcrB derivative. As shown in Table 6, the AcrB-overexpressing parental strain 3-AG100 showed increased resistance to the four different dyes used in accumulation assays compared to the AcrB-inactivated strain, and this correlated with decreased dye accumulation. The changes in dye MICs and accumulation for the DR phenotype-associated AcrB mutants was variable. With the exception of pyronin Y, which in the case of the double mutant revealed values only marginally lower than those of the ΔAcrB strain, the dye accumulation levels relative to that of the ΔAcrB strain were significantly lower for all mutants, consistent with functioning efflux. Accumulation levels for all dyes but ethidium were highest for the double mutant, and this correlated well with lower dye MICs indicating less efficient efflux compared to that in the single mutants, particularly for berberine and pyronin Y. However, as shown in Fig. 2, differences in accumulation of Hoechst 33342 with the single mutants and the double mutant were only marginal. Very low dye accumulation, similar to that seen in wild-type cells, was observed for berberine in both single mutants (Table 6), suggesting unimpaired efflux of this compound.
TABLE 6.
Accumulation and MICs of dyes by wild-type acrB-overexpressing strain 3-AG100, the corresponding acrB deletion strain, and DR AcrB mutants
| Strain | % dye accumulationa |
MIC (μg/ml)b |
||||||
|---|---|---|---|---|---|---|---|---|
| Ethidium | Hoechst 33342 | Berberine | Pyronin Y | Ethidiumc | Hoechst 33342 | Berberinec | Pyronin Y | |
| ΔAcrB mutant | 100 | 100 | 100 | 100 | 8 | 1 | 128 | 2 |
| 3-AG100 | 35.3 ± 7 | 39.4 ± 5 | 28.1 ± 3 | 62.5 ± 6 | >512 | 4 | >1,024 | 64 |
| I38F mutant | 66.8 ± 20 | 56.5 ± 7 | 21.4 ± 5 | 59.6 ± 5 | 512 | 4 | ND | 16 |
| I671T mutant | 44.6 ± 8 | 55.7 ± 7 | 27.2 ± 7 | 73.1 ± 5 | >512 | 4 | ND | 16 |
| I38F I671T mutant | 40.4 ± 4 | 64.6 ± 10 | 53.0 ± 6 | 98.5 ± 1 | 128 | 2 | 256 | 4 |
The values shown represent the mean percentage ± standard deviation of accumulation at 30 min in comparison with values for the ΔAcrB mutant set at 100% (Fig. 2). Pyronin Y accumulation was calculated from the quenching effect (decrease of fluorescence) caused by intercellular accumulation at 10 min (the time of the quenching maximum reached from the ΔAcrB mutant).
MIC changes of ≥4-fold detected from DR AcrB mutants compared to parental strain 3-AG100 are depicted in boldface. ND, not determined.
With ethidium and berberine, the solubility of the dyes in broth was limited to the MICs detected with parental strain 3-AG100.
FIG 2.

Accumulation of Hoechst 33342 from wild-type acrB-overexpressing strain 3-AG100, the acrB deletion (ΔAcrB) strain, and AcrB mutants determined by fluorescent measurement. RFU, relative fluorescence units.
Since accumulation study results can be confounded by altered influx capacities, we further measured real-time efflux using suitable dyes (1,2′-DINA and Nile red). After dye loading of the deenergized cells and subsequent energization by adding glucose, the levels of 1,2′-DINA and Nile red efflux of the single and double mutants were similar and did not differ significantly from wild-type cell dye efflux efficiency (Fig. 3) (data from single mutants not shown), again confirming that the mutants were efflux competent.
FIG 3.
Real-time efflux assays with Nile red (A) and 1,2′-DINA (B) with wild-type acrB-overexpressing strain 3-AG100, the acrB deletion (ΔAcrB) strain, and the I38F I671T double mutant. The arrows indicate the addition of glucose for reenergization of the cells. RFU, relative fluorescence units.
DISCUSSION
The findings from our in vitro random mutagenesis studies demonstrate that amino acid substitutions at a specific site within the lower AcrB porter domain of the MDR efflux transporter AcrB not known to be part of the proximal or distal binding pockets dramatically alter the pump substrate selectivity. Replacement of one or both of the two I38 or I671 isoleucine residues by aromatic or more polar and bulkier amino acids remarkably increased the susceptibility to several drugs that typically have low molecular weight by impaired efflux of these compounds. In contrast, such replacement did not change or, in fact, even increased resistance to selected high-molecular-weight compounds (molecular weight of >600).
Based on the three-dimensional 2HRT protein structure of AcrB, the two isoleucines appear to build a narrow site with their aliphatic side chains facing each other. The bottleneck is situated above the upper ending of the TM domain close to the bottom of the PBP (Fig. 1), which might be affected by the mutations too. Residue I38 (belonging to a loop connecting TM1 and PN1) is located more to the back side of the ArcB protomer in orientation to the central cavity built from the three protomers, whereas residue I671 (belonging to a loop connecting PC1 and PC2) is located more to the periplasmic front side. The PC1-PC2 loop was predicted by free energy calculation to undergo conformational changes during the translocation of doxorubicin (22), and its movement was also reported in studies of AcrB cocrystallized with linezolid (33), one of the low-molecular-weight compounds substantially affected by I38 and I671 mutations. Consistent with these observations is that the distance between the isoleucine side chains as measured within the asymmetric 2HRT protein structure suggests a widening during the access and extrusion but a narrowing within the binding state monomer.
It may appear somehow surprising in this context that nearby single mutations V672M, E673G, G675A, Y35N, and A39T, found within some of our control mutants, were not able to confer the DR phenotype with decreased resistance to lower-molecular-weight drugs seen with mutations at I38 or I671. Visual examination of the three-dimensional protein structure model gives a possible explanation since the side chains of V672, E673, Y35, A39, and G675 are not tangent to the I38-I671 interspace (Fig. 1B and C). The unique importance of the latter regarding the translocation of small drugs is further highlighted by the inability of V672M, E673G, G675A, A39T, and Y35N to confer the DR phenotype.
Significantly increased resistance to CLR—a property shared by all mutants, whether of the DR phenotypes or not—has obviously been mediated through I38F and I671T, as well as through V672M, E673G, A39T, and Y35N with similar efficiency. We speculate that all these mutations affect the PBP, which has been suggested to preferentially bind high-molecular-weight drugs like erythromycin (Fig. 4) and rifampin during the translocation process (9, 32). Evidence of an important role of residues in this particular area for substrate specificity has been derived from studies demonstrating effects of mutations at E673, adjacent residues T676 and Q569, and at Q34 (34–36). Notably, all of the investigated non-DR mutants (Table 3; see Table S2 in the supplemental material) harbor at least one mutation that might affect the binding capacity of the PBP (with two exceptions—both containing a substitution at P224 located at the intermonomer connecting loop), and those mutations, thus, are likely to be the reason for enhanced CLR resistance. For instance, mutations L828F, L828S, R717S, and R717L might influence the macrolide binding of the PBP via the periplasmic cleft side (18), G675A, V672M, E673G, A39T, and Y35N via the bottom side, and T85A, G621V, and G621S, presumably via the switch-loop (G-loop) at the upper end of the PBP. This flexible loop separating the PBP and the DBP (8) (Fig. 1) has been shown to impair macrolide susceptibility when mutated (10, 19).
FIG 4.
Residues I38 and I671 shown as sticks within a section of the AcrB access state monomer (2HRT, chain A). Residues of the proximal binding pocket are shown as transparent green spheres, and those reported by Nakashima et al. (9) to be involved in erythromycin binding are shown as transparent orange spheres. (A) Nonmutated residues I38 and I671; (B) mutated residues detected from DR mutants.
Even though the mutagenesis method used minimizes the occurrence of mutations outside the addressed gene region (23), spontaneous additional mutations (drug target mutations or mutations leading to altered cell wall components or expression of another efflux pump) cannot be completely excluded. However, we were able to prove the essential role for the DR phenotype of I38F and I671T by plasmid-borne reconstruction. Also, the impact of I671T was clearly demonstrated by constructing an acrB deletion strain (from the chromosomal I671T AcrB variant) that revealed identical behavior to the acrB-deficient strain derived from the parental strain, E. coli 3-AG100. The engineered double mutant gave additional proof of the correlation between the DR resistance pattern and mutations at I38 and I671, although on drugs of higher molecular weight, there were somewhat opposing effects to the findings with the single mutants. The two voluminous and polar residues introduced in close proximity to each other might cause a more serious disturbance of the structural surrounding by an expected repulsion effect. This could particularly affect the binding capacity of the adjacent proximal binding pocket (Fig. 4) and thereby the resistance to drugs of higher molecular weight.
Proper functioning of the mutated AcrB transporter has been demonstrated by real-time efflux assays with two different dyes, 1,2′-DINA and Nile red, showing unimpaired efflux competence of the mutants, including the double mutant. In contrast to many low-molecular-weight drugs, the entry into the translocation pathway for these small highly lipophilic compounds does not appear to be compromised, an observation supporting the hypothesis that size is not the only discriminating property for the route of transport used. Furthermore, a third entrance in addition to the vestibule and the cleft cannot be totally excluded. The differently affected transport of further dyes might reflect their various physicochemical properties and reveal the substrate discriminating impact of mutations I38F and I671T. Some discrepancy in values from dye MIC and accumulation might arise from the different time spans of the assays, meaning that the cells are exposed to the chemicals for many hours or only minutes.
Since an impact of mutations at I38 and I671 on the binding capacity of the adjacent PBP is conceivable, it might also explain the compromised efflux of low-molecular-weight drugs. However, it is believed that these substrates pass through the PBP without specific binding to reach their binding site within the DBP (21, 22). Several mutations compromising the resistance to drugs of lower molecular weight were described within the DBP (17), and minocycline has been found trapped there in the binding state protomer of cocrystallized AcrB (12). We now show that substitutions at I38 and I671, an area far from this cavity at the opposite side of the switch-loop below the PBP, can dramatically increase the susceptibility to smaller drug compounds. We believe that the I38-I671 site belongs to one of the putative entrance channels discovered from the three-dimensional crystallization structures (12–14) and a few computer simulations. Besides the above-mentioned free energy calculation study of the doxorubicin transport in AcrB (22), a molecular simulation of drug uptake pathway options has been published previously and describes distinct entrance channels for drugs of different molecular sizes. Low-molecular-weight drugs have been suggested to use the vestibule entrance that opens close to the inner membrane, whereas larger drugs have been predicted to enter through the periplasmic cleft entrance about 15 Å above, between PC1 and PC2 (Fig. 1A). The study proposes two different possible pathways for the translocation of lower-molecular-weight drugs, both using the vestibule entrance channel. I671 is shown as a residue belonging to the front and the back vestibule and I38 as a residue of the back vestibule (21). From the same study, as well as those from other authors, it is supposed that not only the molecular size but also further properties (e.g., lipophilicity and the polar surface area) might be critical for the transport of substrates and the translocation pathways used in RND efflux transporters (16).
With the discovery of a highly substrate-selective narrow site supposedly belonging to the vestibule entrance channel of AcrB by in vitro random mutagenesis, we believe we provide further evidence for the existence of previously suggested distinct entrance pathways for drugs of different molecular sizes, even though other physicochemical properties of substrates may play additional roles. I38 and I671 AcrB variants should be subjected to crystallization and computational analyses to elucidate the function of this narrow site in the translocation pathway in more detail. It might be of similar importance as the switch-loop at the opposite end of the PBP. The identification of residues lining the route of transport—particularly those conferring substrate selectivity—offers new perspectives regarding the design of efflux-incompatible or -inhibitory drugs, for which plugging a bottleneck might be an option.
Supplementary Material
ACKNOWLEDGMENT
We thank Klaas Martinus Pos, Goethe University, Frankfurt, Germany, for kindly providing plasmid pET24a-acrBwt.
Funding Statement
This work was supported in part by grants (to W.V.K.) from the Innovative Medicines Initiative (IMI) Joint Undertaking (project ND4BB translocation no. 115524, including contributions from the European Union's seventh framework program and EFPIA companies) and from the German Federal Ministry of Education and Research (BMBF 01KI9951).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00314-16.
REFERENCES
- 1.Bassetti M, Merelli M, Temperoni C, Astilean A. 2013. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob 12:22. doi: 10.1186/1476-0711-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li XZ, Plesiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikaido H, Pagès JM. 2012. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36:340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delmar JA, Su CC, Yu EW. 2014. Bacterial multidrug efflux transporters. Annu Rev Biophys 43:93–117. doi: 10.1146/annurev-biophys-051013-022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole K. 2005. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother 56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 6.Elkins CA, Nikaido H. 2002. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J Bacteriol 184:6490–6498. doi: 10.1128/JB.184.23.6490-6499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujihira E, Tamura N, Yamaguchi A. 2002. Membrane topology of a multidrug efflux transporter, AcrB, in Escherichia coli. J Biochem 131:145–151. doi: 10.1093/oxfordjournals.jbchem.a003069. [DOI] [PubMed] [Google Scholar]
- 8.Eicher T, Cha HJ, Seeger MA, Brändstatter L, El-Delik J, Bohnert JA, Kern WV, Verrey F, Grütter MG, Diederichs K, Pos KM. 2012. Transport of drugs by the multidrug transporter AcrB involves an access and a deep binding pocket that are separated by a switch-loop. Proc Natl Acad Sci U S A 109:5687–5692. doi: 10.1073/pnas.1114944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakashima R, Sakurai K, Yamasaki S, Nishino K, Yamaguchi A. 2011. Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket. Nature 480:565–569. doi: 10.1038/nature10641. [DOI] [PubMed] [Google Scholar]
- 10.Cha HJ, Müller RT, Pos KM. 2014. Switch-loop flexibility affects transport of large drugs by the promiscuous AcrB multidrug efflux transporter. Antimicrob Agents Chemother 58:4767–4772. doi: 10.1128/AAC.02733-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeger MA, von Ballmoos C, Verrey F, Pos KM. 2009. Crucial role of Asp408 in the proton translocation pathway of multidrug transporter AcrB: evidence from site-directed mutagenesis and carbodiimide labeling. Biochemistry 48:5801–5812. doi: 10.1021/bi900446j. [DOI] [PubMed] [Google Scholar]
- 12.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. 2006. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443:173–179. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 13.Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. 2006. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 313:1295–1298. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 14.Sennhauser G, Amstutz P, Briand C, Storchenegger O, Grütter MG. 2007. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol 5:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takatsuka Y, Chen C, Nikaido H. 2010. Mechanism of recognition of compounds of diverse structures by the multidrug efflux pump AcrB of Escherichia coli. Proc Natl Acad Sci U S A 107:6559–6565. doi: 10.1073/pnas.1001460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreier J, Ruggerone P. 2015. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front Microbiol 6:660. doi: 10.3389/fmicb.2015.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohnert JA, Schuster S, Seeger MA, Fähnrich E, Pos KM, Kern WV. 2008. Site-directed mutagenesis reveals putative substrate binding residues in the Escherichia coli RND efflux pump AcrB. J Bacteriol 190:8225–8229. doi: 10.1128/JB.00912-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargiu AV, Nikaido H. 2012. Multidrug binding properties of the AcrB efflux pump characterized by molecular dynamics simulations. Proc Natl Acad Sci U S A 109:20637–20642. doi: 10.1073/pnas.1218348109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehmeier C, Schuster S, Fähnrich E, Kern WV, Bohnert JA. 2009. Site-directed mutagenesis reveals amino acid residues in the Escherichia coli RND efflux pump AcrB that confer macrolide resistance. Antimicrob Agents Chemother 53:329–330. doi: 10.1128/AAC.00921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eicher T, Brandstätter L, Pos KM. 2009. Structural and functional aspects of the multidrug efflux pump AcrB. Biol Chem 390:693–699. doi: 10.1515/BC.2009.090. [DOI] [PubMed] [Google Scholar]
- 21.Yao XQ, Kimura N, Murakami S, Takada S. 2013. Drug uptake pathways of multidrug transporter AcrB studied by molecular simulations and site-directed mutagenesis experiments. J Am Chem Soc 135:7474–7485. doi: 10.1021/ja310548h. [DOI] [PubMed] [Google Scholar]
- 22.Zuo Z, Wang B, Weng J, Wang W. 2015. Stepwise substrate translocation mechanism revealed by free energy calculations of doxorubicin in the multidrug transporter AcrB. Sci Rep 5:13905. doi: 10.1038/srep13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lluis MW, Godfroy JI III, Yin H. 2013. Protein engineering methods applied to membrane protein targets. Protein Eng Des Sel 26:91–100. doi: 10.1093/protein/gzs079. [DOI] [PubMed] [Google Scholar]
- 24.Malle E, Zhou H, Neuhold J, Spitzenberger B, Klepsch F, Pollak T, Bergner O, Ecker GF, Stolt-Bergner PC. 2011. Random mutagenesis of the prokaryotic peptide transporter YdgR identifies potential periplasmic gating residues. J Biol Chem 286:23121–23131. doi: 10.1074/jbc.M111.239657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. 2011. Random mutagenesis of the proton-coupled folate transporter (SLC46A1), clustering of mutations, and the bases for associated losses of function. J Biol Chem 286:24150–24158. doi: 10.1074/jbc.M111.236539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gros Y, Schuldiner S. 2010. Directed evolution reveals hidden properties of VMAT, a neurotransmitter transporter. J Biol Chem 285:5076–5084. doi: 10.1074/jbc.M109.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuster S, Kohler S, Buck A, Dambacher C, König A, Bohnert JA, Kern WV. 2014. Random mutagenesis of the multidrug transporter AcrB from Escherichia coli for identification of putative target residues of efflux pump inhibitors. Antimicrob Agents Chemother 58:6870–6878. doi: 10.1128/AAC.03775-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opperman TJ, Nguyen ST. 2015. Recent advances toward a molecular mechanism of efflux pump inhibition. Front Microbiol 6:421. doi: 10.3389/fmicb.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee VJ. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother 45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohnert JA, Schuster S, Szymaniak-Vits M, Kern WV. 2011. Determination of real-time efflux phenotypes in Escherichia coli AcrB binding pocket phenylalanine mutants using a 1,2′-dinaphthylamine efflux assay. PLoS One 6:e21196. doi: 10.1371/journal.pone.0021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohnert JA, Karamian B, Nikaido H. 2010. Optimized Nile red efflux assay of AcrAB-TolC multidrug efflux system shows competition between substrates. Antimicrob Agents Chemother 54:3770–3775. doi: 10.1128/AAC.00620-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyer R, Ferrari A, Rijnbrand R, Erwin AL. 2015. A fluorescent microplate assay quantifies bacterial efflux and demonstrates two distinct compound binding sites in AcrB. Antimicrob Agents Chemother 59:2388–2397. doi: 10.1128/AAC.05112-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung LW, Kim HB, Murakami S, Gupta G, Kim CY, Terwilliger TC. 2013. Crystal structure of AcrB complexed with linezolid at 3.5 A resolution. J Struct Funct Genomics 14:71–75. doi: 10.1007/s10969-013-9154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husain F, Nikaido H. 2010. Substrate path in the AcrB multidrug efflux pump of Escherichia coli. Mol Microbiol 78:320–330. doi: 10.1111/j.1365-2958.2010.07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi N, Tamura N, van Veen HW, Yamaguchi A, Murakami S. 2014. β-Lactam selectivity of multidrug transporters AcrB and AcrD resides in the proximal binding pocket. J Biol Chem 289:10680–10690. doi: 10.1074/jbc.M114.547794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao W, Warren MS, Black DS, Satou T, Murata T, Nishino T, Gotoh N, Lomovskaya O. 2002. On the mechanism of substrate specificity by resistance nodulation division (RND)-type multidrug resistance pumps: the large periplasmic loops of MexD from Pseudomonas aeruginosa are involved in substrate recognition. Mol Microbiol 46:889–901. doi: 10.1046/j.1365-2958.2002.03223.x. [DOI] [PubMed] [Google Scholar]
- 37.Kern WV, Oethinger M, Jellen-Ritter AS, Levy SB. 2000. Non-target gene mutations in the development of fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother 44:814–820. doi: 10.1128/AAC.44.4.814-820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.