Abstract
Synergy between colistin and the signal peptidase inhibitor MD3 was tested against isogenic mutants and clinical pairs of Acinetobacter baumannii isolates. Checkerboard assays and growth curves showed synergy against both colistin-susceptible strains (fractional inhibitory concentration index [FICindex] = 0.13 to 0.24) and colistin-resistant strains with mutations in pmrB and phosphoethanolamine modification of lipid A (FICindex = 0.14 to 0.25) but not against colistin-resistant Δlpx strains with loss of lipopolysaccharide (FICindex = 0.75 to 1). A colistin/MD3 combination would need to be targeted to strains with specific colistin resistance mechanisms.
TEXT
Acinetobacter baumannii is an important nosocomial pathogen that is able to acquire or develop resistance to multiple antibiotics (1). The frequency of multidrug-resistant (MDR) clinical isolates has increased in recent years. One of “agents of last resort” with activity against A. baumannii MDR strains is colistin (COL) (polymyxin E) (2), a polycationic antimicrobial peptide which targets the polyanionic bacterial lipopolysaccharide (LPS) (3). Two mechanisms of resistance to colistin are the most studied in A. baumannii (4, 5). One involves the total loss of LPS by means of inactivation of the lipid A biosynthetic pathway. Mutations in any of the first three genes involved in lipid A biosynthesis (lpxA, lpxC, and lpxD) prevent interaction with colistin and result in high-level resistance (6). A second mechanism, previously studied by our group, involves mutations and increased expression of pmrAB genes. These result in the addition of phosphoethanolamine residues to lipid A, which decreases the negative charge displayed on the LPS (7, 8). Colistin resistance is concerning; with no new commercial antimicrobials available for use against MDR isolates, there is an urgent need to develop compounds to address this clinical issue (9, 10).
Recently, enhanced antimicrobial activity of colistin in combination with a synthetic β-aminoketone, MD3 [1-(2,5-dichlorophenyl)-3-(dimethylamino)propan-1-one], an inhibitor of bacterial type I signal peptidases (SPases), was described. It was demonstrated that SPase inhibition by MD3 in combination with outer membrane permeabilizing agents (colistin or sodium hexametaphosphate [NaHMP]) was increased in A. baumannii and other species (11). Bacterial SPases are involved in the maturation of proteins through cleavage of the amino-terminal signal peptides of translocated proteins (12). Permeabilization of the outer membrane should enable more-efficient access of MD3 to cytoplasm-located SPases. The aim of the present study was therefore to evaluate the synergistic effects of the combination of MD3 and colistin against strains of A. baumannii harboring well-characterized mechanisms of colistin resistance.
The combination of MD3 and colistin (COL) was tested against A. baumannii strains with deficits in LPS biosynthesis (inactivating mutations in lpx genes) and strains with lipid A modifications (mutations in pmrB). The bacterial strains used were the antibiotic-susceptible A. baumannii ATCC 19606 type strain and isogenically derived colistin-resistant (Colr) mutants ATCC 19606ΔlpxA, ATCC 19606ΔlpxC, ATCC 19606ΔlpxD (6), and ATCC 19606pmrB (7). Also tested were the MDR but colistin-susceptible (Cols) clinical isolate A. baumannii ABRIM (13) and its derived Colr ABRIMpmrB mutant (7). Finally, two pairs of Cols/Colr A. baumannii isolates, AB248/AB249pmrB and AB299/AB347pmrB, recovered consecutively from two intensive-care units (ICU) patients before and after colistin therapy, were studied (14). Molecular mechanisms of colistin resistance in each of these strains have been extensively characterized previously (Table 1). The MICs of COL and MD3 were determined by broth microdilution following CLSI criteria (15). Colistin MICs were also confirmed by Etest (bioMérieux, France) in the presence of 1 to 4 mg/liter of MD3. The activity of COL in combination with MD3 was assessed in checkerboard assays (16, 17). After 20 h of incubation, 10-μl volumes of alamarBlue reagent (Thermo-Scientific, USA) were added to all wells to identify those containing viable bacteria. The fractional inhibitory concentration index (FICindex) was calculated as follows: FICindex = FICCOL + FICMD3 = MIC [COLMD3]/[COL] + MIC [MD3COL]/[MD3]. The FIC data were interpreted as follows: FICindex = ≤0.5, synergy; FICindex = >0.5 to 4, no interaction (17). The MD3 compound was obtained from the Infectious Disease Research Institute of Seattle (USA).
TABLE 1.
Descriptions of bacterial strains and MICs of colistin and MD3 alone and in combinationa
| A. baumannii strain | MIC (mg/liter) |
FICindex | Description | Source or reference | |||
|---|---|---|---|---|---|---|---|
| Colistin | MD3 | COLMD3 | MD3COL | ||||
| ATCC 19606 | 0.5 | 32 | 0.06 | 2 | Synergy | A. baumannii type strain | ATCC |
| ATCC 19606pmrB | 64 | 16 | 0.5 | 4 | Synergy | Isogenic derivative mutant of ATCC 19606; single amino acid substitution (Ala227Val) in PmrB | 7 |
| ATCC 19606ΔlpxA | 64 | 2 | 16 | 1 | No interaction | Isogenic derivative mutant of ATCC 19606; 445-bp deletion at nucleotide 364 within the lpxA gene and frameshift after H121 | 6 |
| ATCC 19606ΔlpxC | 128 | 2 | 64 | 1 | No interaction | Isogenic derivative mutant of ATCC 19606; 84-bp deletion within the lpxC gene | 6 |
| ATCC 19606ΔlpxD | 2,056 | 2 | 1,028 | 1 | No interaction | Isogenic derivative mutant of ATCC 19606; single-base deletion at nucleotide 364 of the lpxD gene and frameshift after K317 | 6 |
| ABRIM | 0.5 | 32 | 0.06 | 4 | Synergy | A. baumannii clinical isolate | 13 |
| ABRIMpmrB | 32 | 8 | 0.5 | 1 | Synergy | Isogenic derivative mutant of ABRIM; single amino acid substitution (Asn353Tyr) in PmrB | 7 |
| AB248 | 0.25 | 64 | 0.03 | 1 | Synergy | A. baumannii clinical isolate | 14 |
| AB249pmrB | 256 | 32 | 0.25 | 8 | Synergy | Isogenic clinical isolate derivative of AB248; single amino acid substitution (Pro233Ser) in PmrB | 14 |
| AB299 | 0.25 | 64 | 0.03 | 4 | Synergy | A. baumannii clinical isolate | 14 |
| AB347pmrB | 64 | 16 | 0.25 | 4 | Synergy | Isogenic clinical isolate derivative of AB299; single amino acid substitution (Pro170Leu) in PmrB | 14 |
COLMD3, colistin MICs in the presence of MD3; MD3COL, MD3 MICs in the presence of colistin; ATCC, American Type Culture Collection.
Growth curve analyses were performed using COL and MD3 at the fixed concentrations that resulted in synergy when COL and MD3 were combined in checkerboards. A. baumannii cultures were grown overnight, and 5 × 105 CFU/ml was used to inoculate 100 μl of Mueller-Hinton II broth in 96-well plates. Optical density was monitored using an Epoch-2 Microplate Spectrophotometer for 18 h (BioTek, USA).
Synergy observed in Cols and Colr strains with amino acid modifications in PmrB.
The MICs of colistin and MD3 for the studied strains are shown in Table 1. The MICs of colistin were concordant with those previously published (14, 18). MD3 alone had little activity against any of the A. baumannii strains tested. However, there was a clear inverse relationship between susceptibility to MD3 and the MIC of COL, which could have been due in part to easier transport of MD3 through a modified outer membrane. All the Colr mutants showed a MD3 MIC 2-fold to 16-fold lower than that seen with the wild-type parents (Table 1).
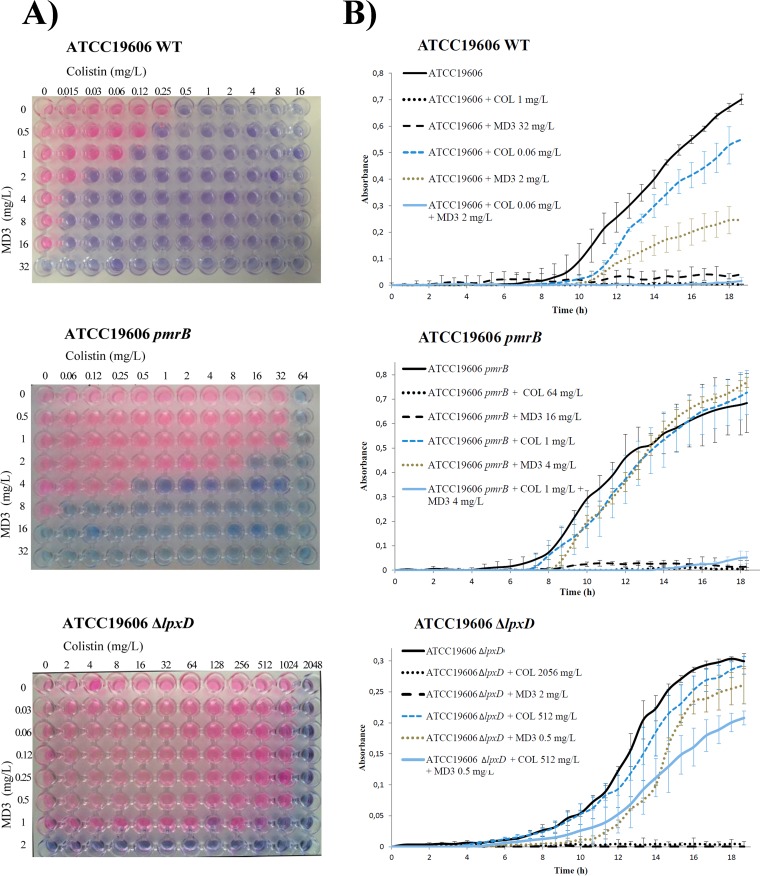
Clear synergy was seen against the Cols ATCC 19606 parental strain (FICindex = 0.18), the Cols clinical strains (FICindex = 0.13 to 0.24), and all the Colr strains with modifications in pmrB (including both the ATCC 19606pmrB strain [FICindex = 0.26] and the clinical isolates [FICindex = 0.14 to 0.25]). However, no synergy (interpreted as no interaction) was observed against the Δlpx strains (FICindex = 0.75 to 1) (Table 1 and Fig. 1A). In Colr Δlpx mutants, the use of MD3 resulted in a reduction in the MIC of COL of only 2-fold to 4-fold. This was in contrast to 128-fold and 1,028-fold reductions at 4 mg/liter and 8 mg/liter of MD3 for the ATCC 19606pmrB mutant (from 64 mg/liter to 0.5 and 0.06 mg/liter, respectively). Similarly, assays performed using the ABRIMpmrB, AB249pmrB, and AB347pmrB Colr clinical strains showed reductions in the COL MIC from 32 to 0.5 mg/liter, 256 to 0.25 mg/liter, and 64 to 0.25 mg/liter, respectively (64-fold to 256-fold), with MD3 added at 1 mg/liter. A decrease in the COL MIC of 8-fold to 16-fold was also observed with the Cols clinical strains. These results confirm a potent synergistic effect of MD3 in combination with COL against Colr strains with pmrB gene mutations promoting phosphoethanolamine modifications but not against Colr strains with complete loss of the LPS.
FIG 1.
Assessment of synergy. (A) Checkerboard assays using colistin and MD3 performed on cultures of ATCC 19606, ATCC 19606pmrB, and ATCC 19606ΔlpxD A. baumannii strains. Pink wells indicate growth. Blue wells indicate growth inhibition. (B) Growth curves of the same strains in the absence of antimicrobials (black line) and in the presence of colistin at their MIC (black dotted line), of MD3 at their MIC (black dashed line), of colistin at their COLMD3 MIC (blue dashed line), of MD3 at their MD3COL MIC (green dotted line), and of colistin and MD3 at the COLMD3 andMD3COL MICs (blue line). COLMD3, MICs of colistin in the presence of MD3; MD3COL, MICs of MD3 in the presence of colistin. Because of the different methodologies used in the two assays, for the growth curve assays, strains ATCC 19606 and ATCC 19606pmrB were grown in 1 mg/liter of colistin instead 0.5 mg/liter as MIC controls. Independent assays were performed at least three times.
The effects of MD3 and COL on the growth and fitness of A. baumannii ATCC 19606, ATCC 19606pmrB, and ATCC 19606ΔlpxD are shown in Fig. 1B. When A. baumannii ATCC 19606 was grown in the presence of concentrations of COL and MD3 that were below the MICs of the compounds (0.06 mg/liter and 2 mg/liter, respectively), the strain was able to grow, although its fitness was affected. However, when the two compounds were combined at those concentrations, synergy and complete inhibition of growth were observed. Similarly, the ATCC 19606pmrB mutant was able to grow in the presence of 1 mg/liter of COL or 4 mg/liter of MD3 (64-fold below the MIC for COL and 4-fold below the MIC for MD3) but growth was totally inhibited in the presence of COL and MD3 in combination. In contrast, in the case of ATCC 19606ΔlpxD (COL and MD3 MICs of 2,056 mg/liter and 2 mg/liter, respectively), no synergistic inhibition of growth was seen even with COL at 512 mg/liter. Against the clinical pairs ABRIM/ABRIMpmrB, AB248/AB249pmrB, and AB299/AB347pmrB, the combination of the two compounds at subinhibitory concentrations inhibited growth, while with the ΔlpxA and ΔlpxC Colr mutants, the growth was not affected, as seen with the ΔlpxD mutant (data not shown). These assays confirmed the results obtained using the checkerboard method.
This study confirmed that combining MD3 and COL increases the susceptibility of Cols A. baumannii strains, as previously reported (11). Moreover, we have demonstrated that there is also a significant synergistic effect against Colr isolates that is dependent on the mechanism of colistin resistance. In Colr A. baumannii strains harboring phosphoethanolamine modifications in lipid A, potent synergy between MD3 and COL was observed, but no interaction was seen when colistin resistance was mediated by lipid A deficiency. We also observed that the acquisition of colistin resistance in A. baumannii by means of a loss of LPS paradoxically increases the susceptibility to MD3.
In most published reports from studies of Colr A. baumannii clinical isolates, resistance has been found to be mediated by modifications of the pmrAB genes (14, 19–22), as in those included in this study. Although clinical isolates with loss of LPS have been described (6, 23), complete loss of LPS promotes drastic changes in bacterial cellular architecture and significant loss of fitness (24), whereas colistin resistance due to lipid A modifications leads a low or null fitness cost in the presence of antimicrobial selective pressure (18, 25). The relevant fitness burdens may affect the ability of Colr A. baumannii to persist and spread in the clinical environment; therefore, it is most likely that Colr clinical isolates of A. baumannii carry mutations in pmrAB genes rather than in lpx genes.
Colistin resistance due to phosphoethanolamine modification mediated by a plasmid-encoded enzyme, MCR-1, has recently been described in Enterobacteriaceae (26). This highlights the need for compounds, alone or in combination, able to target Colr bacteria. We conclude that the MD3 compound could be used in the future for development of therapy against infections caused by Colr A. baumannii and other MDR Gram-negative pathogens.
ACKNOWLEDGMENTS
We thank J. D. Boyce for the kind gift of the A. baumannii ATCC 19606ΔlpxA (AL1851), ATCC 19606ΔlpxC (AL1842), and ATCC 19606ΔlpxD (AL1852) strains. We also thank S. Pournaras for the kind gift of the A. baumannii AB248, AB249, AB299, and AB347 strains.
We declare that we have no conflicts of interest.
Funding Statement
This work was supported by the Spanish National Plans for Scientific Research, Development and Technological Innovation 2008-2011 and 2013-2016 and funded by the ISCIII-General Subdirection of Assessment and Promotion of the Research European Regional Development Fund (ERDF) “A way of making Europe” and also by the Spanish Network for Research in Infectious Diseases (REIPI RD12/0015).
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huttner B, Jones M, Rubin MA, Neuhauser MM, Gundlapalli A, Samore M. 2012. Drugs of last resort? The use of polymyxins and tigecycline at US Veterans Affairs medical centers, 2005–2010. PLoS One 7:e36649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock RE. 1997. Peptide antibiotics. Lancet 349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 5.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother 53:3628–3634. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempf M, Rolain JM. 2012. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents 39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Durante-Mangoni E, Utili R, Zarrilli R. 2014. Combination therapy in severe Acinetobacter baumannii infections: an update on the evidence to date. Future Microbiol 9:773–789. doi: 10.2217/fmb.14.34. [DOI] [PubMed] [Google Scholar]
- 11.Personne Y, Curtis MA, Wareham DW, Waite RD. 2014. Activity of the type I signal peptidase inhibitor MD3 against multidrug-resistant Gram-negative bacteria alone and in combination with colistin. J Antimicrob Chemother 69:3236–3243. doi: 10.1093/jac/dku309. [DOI] [PubMed] [Google Scholar]
- 12.Paetzel M, Karla A, Strynadka NC, Dalbey RE. 2002. Signal peptidases. Chem Rev 102:4549–4580. doi: 10.1021/cr010166y. [DOI] [PubMed] [Google Scholar]
- 13.Bou G, Oliver A, Martínez-Beltrán J. 2000. OXA-24, a novel class D beta-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob Agents Chemother 44:1556–1561. doi: 10.1128/AAC.44.6.1556-1561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pournaras S, Poulou A, Dafopoulou K, Chabane YN, Kristo I, Makris D, Hardouin J, Cosette P, Tsakris A, Dé E. 2014. Growth retardation, reduced invasiveness, and impaired colistin-mediated cell death associated with colistin resistance development in Acinetobacter baumannii. Antimicrob Agents Chemother 58:828–832. doi: 10.1128/AAC.01439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing: 17th informational supplement M07-A9. CLSI, Wayne, PA, USA. [Google Scholar]
- 16.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. A critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 17.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 18.Beceiro A, Moreno A, Fernández N, Vallejo JA, Aranda J, Adler B, Harper M, Boyce JD, Bou G. 2014. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob Agents Chemother 58:518–526. doi: 10.1128/AAC.01597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Bae IK, Jeong SH, Yong D, Lee K. 2015. In vivo selection of pan-drug resistant Acinetobacter baumannii during antibiotic treatment. Yonsei Med J 56:928–934. doi: 10.3349/ymj.2015.56.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesho E, Yoon EJ, McGann P, Snesrud E, Kwak Y, Milillo M, Onmus-Leone F, Preston L, St Clair K, Nikolich M, Viscount H, Wortmann G, Zapor M, Grillot-Courvalin C, Courvalin P, Clifford R, Waterman PE. 2013. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis 208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- 21.Mavroidi A, Likousi S, Palla E, Katsiari M, Roussou Z, Maguina A, Platsouka ED. 2015. Molecular identification of tigecycline- and colistin-resistant carbapenemase-producing Acinetobacter baumannii from a Greek hospital from 2011 to 2013. J Med Microbiol 64:993–997. doi: 10.1099/jmm.0.000127. [DOI] [PubMed] [Google Scholar]
- 22.Durante-Mangoni E, Del Franco M, Andini R, Bernardo M, Giannouli M, Zarrilli R. 2015. Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis 82:222–226. doi: 10.1016/j.diagmicrobio.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Selasi GN, Nicholas A, Jeon H, Lee YC, Yoo JR, Heo ST, Lee JC. 2015. Genetic basis of antimicrobial resistance and clonal dynamics of carbapenem-resistant Acinetobacter baumannii sequence type 191 in a Korean hospital. Infect Genet Evol 36:1–7. doi: 10.1016/j.meegid.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Soon RL, Nation RL, Cockram S, Moffatt JH, Harper M, Adler B, Boyce JD, Larson I, Li J. 2011. Different surface charge of colistin-susceptible and -resistant Acinetobacter baumannii cells measured with zeta potential as a function of growth phase and colistin treatment. J Antimicrob Chemother 66:126–133. doi: 10.1093/jac/dkq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wand ME, Bock LJ, Bonney LC, Sutton JM. 2015. Retention of virulence following adaptation to colistin in Acinetobacter baumannii reflects the mechanism of resistance. J Antimicrob Chemother 70:2209–2216. doi: 10.1093/jac/dkv097. [DOI] [PubMed] [Google Scholar]
- 26.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]