Abstract
The spread of extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae (ESBL-E) is leading to increased carbapenem consumption. Alternatives to carbapenems need to be investigated. We investigated whether β-lactam/β-lactamase inhibitor (BLBLI) combinations are as effective as carbapenems in the treatment of bloodstream infections (BSI) due to ESBL-E. A multinational, retrospective cohort study was performed. Patients with monomicrobial BSI due to ESBL-E were studied; specific criteria were applied for inclusion of patients in the empirical-therapy (ET) cohort (ETC; 365 patients), targeted-therapy (TT) cohort (TTC; 601 patients), and global cohort (GC; 627 patients). The main outcome variables were cure/improvement rate at day 14 and all-cause 30-day mortality. Multivariate analysis, propensity scores (PS), and sensitivity analyses were used to control for confounding. The cure/improvement rates with BLBLIs and carbapenems were 80.0% and 78.9% in the ETC and 90.2% and 85.5% in the TTC, respectively. The 30-day mortality rates were 17.6% and 20% in the ETC and 9.8% and 13.9% in the TTC, respectively. The adjusted odds ratio (OR) (95% confidence interval [CI]) values for cure/improvement rate with ET with BLBLIs were 1.37 (0.69 to 2.76); for TT, they were 1.61 (0.58 to 4.86). Regarding 30-day mortality, the adjusted OR (95% CI) values were 0.55 (0.25 to 1.18) for ET and 0.59 (0.19 to 1.71) for TT. The results were consistent in all subgroups studied, in a stratified analysis according to quartiles of PS, in PS-matched cases, and in the GC. BLBLIs, if active in vitro, appear to be as effective as carbapenems for ET and TT of BSI due to ESLB-E regardless of the source and specific species. These data may help to avoid the overuse of carbapenems. (This study has been registered at ClinicalTrials.gov under registration no. NCT01764490.)
INTRODUCTION
The spread of extended-spectrum β-lactamases (ESBLs) in Enterobacteriaceae (ESBL-E) has become a serious public health problem worldwide (1–3). Carbapenems are considered the drugs of choice for treating severe infections caused by ESBL producers according to observational studies (1–3). This is leading to an increased consumption of carbapenems, which is particularly worrisome in a scenario where carbapenemase-producing organisms are spreading rapidly (4, 5). The search for alternatives to carbapenems for infections caused by ESBL producers is therefore a clinical priority.
ESBLs are inhibited by β-lactamase inhibitors (1). Although hyperproduction of β-lactamases or additional resistance mechanisms may hamper the activity of these compounds, β-lactam/β-lactamase inhibitor combinations (BLBLIs) such as amoxicillin-clavulanate (AMC) or piperacillin-tazobactam (PTZ) remain active against a considerable proportion of ESBL-E in many areas of the world (6–11). However, the efficacy of BLBLIs for treating serious infections caused by ESBL-E is controversial (11–13).
Designing and executing observational studies comparing the efficacy of different antimicrobials for infections caused by multidrug-resistant bacteria presents specific challenges. In fact, most studies published to date in this field have important drawbacks that seriously challenge their validity; some recommendations for improving the quality of observational studies in this field have been published (14).
We hypothesize that BLBLIs are noninferior to carbapenems for the treatment of bloodstream infections (BSI) due to ESBL-E, regardless of the source of the BSI or the Enterobacteriaceae species. Using advanced observational methods and an international effort, the objective of this study was to evaluate whether BLBLIs were as efficacious as carbapenems for the treatment of BSI due to diverse ESBL-E from different sources.
MATERIALS AND METHODS
Study design and patients.
The INCREMENT project (ClinicalTrials.gov identification no. NCT01764490) is a retrospective international cohort study including consecutive patients with clinically significant BSI due to ESBL- or carbapenemase-producing Enterobacteriaceae from January 2004 to December 2013. The overall objective of INCREMENT is to evaluate the efficacy of different antimicrobial drugs and regimens; 37 tertiary hospitals from 12 countries experienced in identifying ESBL- or carbapenemase-producing Enterobacteriaceae and collecting data from patients with BSI participated. This analysis was reported according to the STROBE recommendations (see Table S1 in the supplemental material) (15).
For this analysis and in accordance with the prespecified registered plan, patients with clinically significant monomicrobial BSI due to ESBL-E were included, provided that they received monotherapy with an active BLBLI (AMC, PTZ, or ampicillin-sulbactam [AMS]) (including those with intermediate susceptibility according to CLSI criteria [16]) or a carbapenem (including imipenem, meropenem, doripenem, and ertapenem). Data for patients were collected from charts for up to 30 days after the diagnosis of BSI; if needed, patients or relatives were contacted by phone, and mortality registers were consulted.
We constructed 3 nonmutually exclusive cohorts in order to analyze the impact of empirical and targeted therapies (ET and TT, respectively), as follows. The impact of empirical therapy was investigated in the empirical-therapy cohort (ETC), which included the patients who received monotherapy with either a BLBLI or a carbapenem that began within the first 24 h after blood cultures were taken and continued for at least 48 h (except for patients who died in ≤48 h, who were included if they received at least 1 complete day of therapy). The impact of targeted therapy was investigated in the targeted-therapy cohort (TTC), which included the patients who received a BLBLI or a carbapenem as monotherapy once the susceptibility profile was available; the targeted drug must have started in ≤5 days and been administered for at least 50% of the total duration of therapy (except for patients who died while on targeted therapy, who were included if they received at least 1 complete day of therapy). Finally, the impact of maintaining or changing the empirical therapy was investigated in the global cohort (GC), which included any patient who received either empirical or targeted monotherapy with a BLBLI or a carbapenem with the above criteria if they survived for at least 72 h (and therefore had the possibility of receiving targeted therapy). In the GC, the reference regimen for comparison was empirical and targeted therapy with a carbapenem.
The INCREMENT project was approved by the Spanish Agency of Medicines (AEMPS; code JRB-ANT-2012-01) and the Hospital Universitario Virgen Macarena Institutional Review Board (code 1921); the need to obtain written informed consent was waived. Approval was also obtained at participating centers according to local requirements.
Variables and definitions.
Data collected from all patients included demographics, nosocomial or community acquisition, underlying conditions and their severity using the McCabe classification (17), severity of acute condition at BSI presentation according to the Pitt score (18), source of BSI according to clinical and microbiological data, severe sepsis or septic shock before administration of therapy (19), antimicrobial therapy, clinical response, mortality, and length of stay after BSI. All time-dependent variables were measured with regard to the day when the blood cultures were drawn (considered day 0). Enterobacteriaceae were identified using standard microbiological techniques in each participating center. ESBL production was screened in all isolates with diminished susceptibility to cephalosporins and confirmed according to standard procedures; 2012 CLSI recommendations were used for susceptibility interpretation (16). For isolates obtained before 2012, MICs were reviewed and the susceptibility category was assigned accordingly; for 27 isolates (19 from patients treated with carbapenems and 8 from patients treated with BLBLIs), the MIC was not available or the available data showed a MIC equal to or below the older susceptibility breakpoint; these were considered susceptible if so reported by the local laboratory. Selected isolates from each center had been characterized by PCR and DNA sequencing using established methods. Nosocomial acquisition was considered when infection symptoms started >48 h after hospital admission or within 48 h of hospital discharge. Otherwise, the case was considered community onset. Times refer to the day when the blood culture that diagnosed BSI was taken. Antimicrobial therapy administered before the susceptibility results were available was considered empirical; therapy administered thereafter was considered targeted. Therapy with a BLBLI or a carbapenem was considered monotherapy if no other drug with intrinsic activity against Gram-negative organisms—including penicillins, cephalosporins, monobactams, fluoroquinolones, aminoglycosides, trimethoprim-sulfamethoxazole, fosfomycin, and colistin—was coadministered, irrespective of isolate susceptibility.
The main outcome variables were clinical response at day 14 and 30-day mortality. Clinical response was classified as cure, improvement, or failure. Clinical cure was defined as the resolution of all signs and symptoms related to the infection, with no further need for antibiotic therapy; improvement was defined as partial control or resolution of signs and symptoms related to the infection or complete resolution but continued on antibiotic therapy; and failure was defined as a clinical situation qualified as similar to or worse than the one at the bacteremia diagnosis or death due to any cause. Clinical response was dichotomized as cure/improvement versus failure.
Statistical analysis.
Separate analyses were performed for the 3 cohorts. A propensity score (PS; the probability of receiving therapy with a BLBLI) was calculated for each cohort as previously described (11); all the models obtained for propensity scores had an area under the receiver operating characteristic curve (AUROC) of ≥0.80. The PS was used in 3 ways: (i) as a covariate in multivariate analysis; (ii) to stratify the cohorts according to quartiles of the PS; and (iii) to match patients so that each patient who received treatment with a BLBLI was matched with one who received treatment with a carbapenem using calipers of a width equal to 0.2 of the standard deviation of the logit of the propensity score.
Multivariate analyses for clinical response were performed using logistic regression to control for confounding. For mortality, because proportional hazards assumptions were not fulfilled as shown by Kaplan-Meier plotting, a Cox regression analysis could not be performed, and therefore, logistic regression was also used. Variables with P values of <0.2 in the bivariate analysis were introduced into models. Interactions between therapy with BLBLIs or carbapenems and other variables were explored and were included if they caused a significant modifying effect. The Akaike information criterion (AIC) (20) was used to select the final models. Sensitivity analyses were performed by investigating the effect of BLBLI versus carbapenem therapy in specific subgroups of interest. The center effect was analyzed by including individual or grouped centers in the multivariate analysis. Additionally, a meta-regression analysis considering the geographical regions (Spain, other Mediterranean countries, and the rest of the world) was performed; the odds ratios (OR) and 95% confidence intervals (CI) for mortality in the geographical clusters and overall were calculated using a random effect model. Comparisons of matched cohorts were performed by conditional logistic regression. The analyses were performed using R software (version 3.0.1) and SPSS 15.0 software.
RESULTS
The INCREMENT database includes 1,005 patients with BSI due to ESBL-E. Two hundred seventy-eight patients did not fulfil the criteria for inclusion in this analysis; 22 of them were excluded because they died before receiving at least one complete day of therapy. Eleven of them were treated with BLBLIs (10 with PTZ and 1 with AMC), and 11 with carbapenems (10 with meropenem and 1 with imipenem). Including these patients in the analysis did not change the results. Three hundred sixty-five, 601, and 627 patients from 30 centers were included in the ETC, TTC, and GC groups, respectively (see Fig. S1 in the supplemental material). The number of cases per center in the GC ranged from 4 to 50. A total number of 207 cases from the GC were characterized by PCR amplification of blaESBL genes. The most frequent ESBLs produced by the isolates were CTX-M, in 160 cases (77.3%; 42 CTX-M-15, 27 CTX-M-1, 31 CTX-M-14, 18 CTX-M-9, 2 CTX-M-2, 1 CTX-M-1 and 39 nonspecified CTX-M enzymes), SHV-type in 22 cases (10.6%), and TEM-type in 25 cases (12.1%).
Empirical-therapy cohort.
The ETC included 365 patients: 170 received empirical therapy with a BLBLI (PTZ in 123 cases, AMC in 45, and AMS in 2), and 195 with a carbapenem (meropenem in 128 cases, imipenem in 35, and ertapenem in 32). Among the 172 patients treated empirically with PTZ, the isolate was susceptible to this antibiotic in 126 (73.2%); this was the case for 41 of 68 (60.3%) receiving AMC and for 3 of 10 (30%) receiving AMS. The isolates were susceptible to carbapenems in all patients treated with these antimicrobials.
The characteristics of patients by treatment type are shown in Table 1. The most frequent dose regimens were as follows: for PTZ, 4 g piperacillin/0.5 g tazobactam every 8 h (4/0.5 g q8h) (47% of patients) and 4/0.5 g q6h (18%); for AMC, 1/0.2 g q8h (73.6%); for imipenem, 0.5 g q6h (40%) and 0.5 g q8h (29%); for meropenem, 1 g q8h (65%) and 1 g q12h (20%); and for ertapenem, 1 g q24h (84%). The cure/improvement rates at day 14 were 78.9% for carbapenems and 80.0% for BLBLIs (absolute difference, 1.0%; 95% CI, −7.3% to 9.2%; P = 0.81). The univariate analysis of variables associated with cure/improvement is shown in Table S2 in the supplemental material.
TABLE 1.
Characteristics of patients with bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae in the empirical- and targeted-therapy cohorts
| Characteristic | No. (%) of patients (unless otherwise specified) in indicated group |
|||||
|---|---|---|---|---|---|---|
| Empirical-therapy cohort |
Targeted-therapy cohort |
|||||
| BLBLI (n = 170) | Carbapenem (n = 195) | P valuea | BLBLI (n = 92) | Carbapenem (n = 509) | P valuea | |
| Age [median (IQRb)] | 71.5 (59–79) | 66 (54.5–76) | 0.005c | 70.5 (56–80) | 68 (56–78) | 0.22c |
| Male sex | 95 (55.9) | 117 (60.0) | 0.42 | 55 (59.8) | 295 (58.0) | 0.74 |
| Enterobacteriaceae species | ||||||
| E. coli | 130 (76.3) | 136 (69.7) | 0.15 | 71 (77.2) | 368 (72.3) | 0.33 |
| K. pneumoniae | 29 (17.1) | 45 (23.1) | 0.15 | 13 (14.1) | 101 (19.8) | 0.20 |
| Other | 11 (6.5) | 14 (7.2) | 0.79 | 8 (8.7) | 40 (7.9) | 0.78 |
| Nosocomial acquisition | 75 (44.1) | 91 (46.7) | 0.63 | 38 (41.3) | 247 (48.5) | 0.2 |
| Source | ||||||
| Urinary tract | 77 (45.3) | 91 (46.7) | 0.79 | 39 (42.4) | 233 (45.8) | 0.55 |
| Biliary tract | 25 (14.7) | 24 (12.3) | 0.5 | 9 (9.8) | 62 (12.2) | 0.51 |
| Other (high-risk source) | 68d (40.0) | 80e (41.0) | 0.84 | 44f (47.8) | 214g (42.0) | 0.30 |
| ICUh admission | 13 (7.6) | 26 (13.3) | 0.071 | 4 (4.3) | 62 (12.2) | 0.02 |
| McCabe classification, nonfatal | 81 (47.6) | 95 (48.7) | 0.84 | 47 (51.1) | 263 (51.7) | 0.92 |
| Cancer | 50 (29.4) | 74 (37.9) | 0.068 | 38 (41.3) | 208 (40.9) | 0.86 |
| Pitt score [median (IQR)] | 1 (0–3) | 1 (0–3) | 0.30c | 1 (0–2) | 1 (0–2) | 0.19c |
| Severe sepsis or shock | 67 (39.4) | 72 (36.9) | 0.86 | 31 (33.7) | 164 (32.2) | 0.94 |
| Targeted therapy with: | ||||||
| Carbapenem | 80 (47.1) | 169 (86.7) | <0.0001 | |||
| BLBLI | 65 (38.2) | 8 (4.1) | <0.0001 | |||
| Other drug | 25 (14.7) | 18 (9.2) | 0.11 | |||
| Empirical therapy with: | ||||||
| Carbapenem | 4 (4.3) | 141 (27.7) | <0.0001 | |||
| BLBLI | 56 (60.9) | 140 (27.5) | <0.0001 | |||
| Other drug | 32 (34.8) | 228 (44.8) | 0.07 | |||
| Active empirical therapy | 65 (70.7) | 304 (59.7) | 0.047 | |||
| Cure/improvement | 136 (80.0) | 154 (79.0) | 0.81 | 83 (90.2) | 435 (85.5) | 0.22 |
| 30-day mortality | 30 (17.6) | 39 (20.0) | 0.60 | 9 (9.8) | 71 (13.9) | 0.28 |
P values were calculated by χ2 test, except where otherwise specified.
IQR, interquartile range.
Mann-Whitney U test.
Other sources included unknown, 21; intra-abdominal, 20; pneumonia, 12; skin, 7; vascular, 7; other, 1.
Other sources included unknown, 27; vascular, 19; intra-abdominal, 16; pneumonia, 10; skin, 5; other, 3.
Intra-abdominal, 17; unknown, 13; vascular, 6; skin, 3.
Unknown, 74; intra-abdominal, 51; vascular, 33; pneumonia, 26; skin, 15; other, 15.
ICU, intensive care unit.
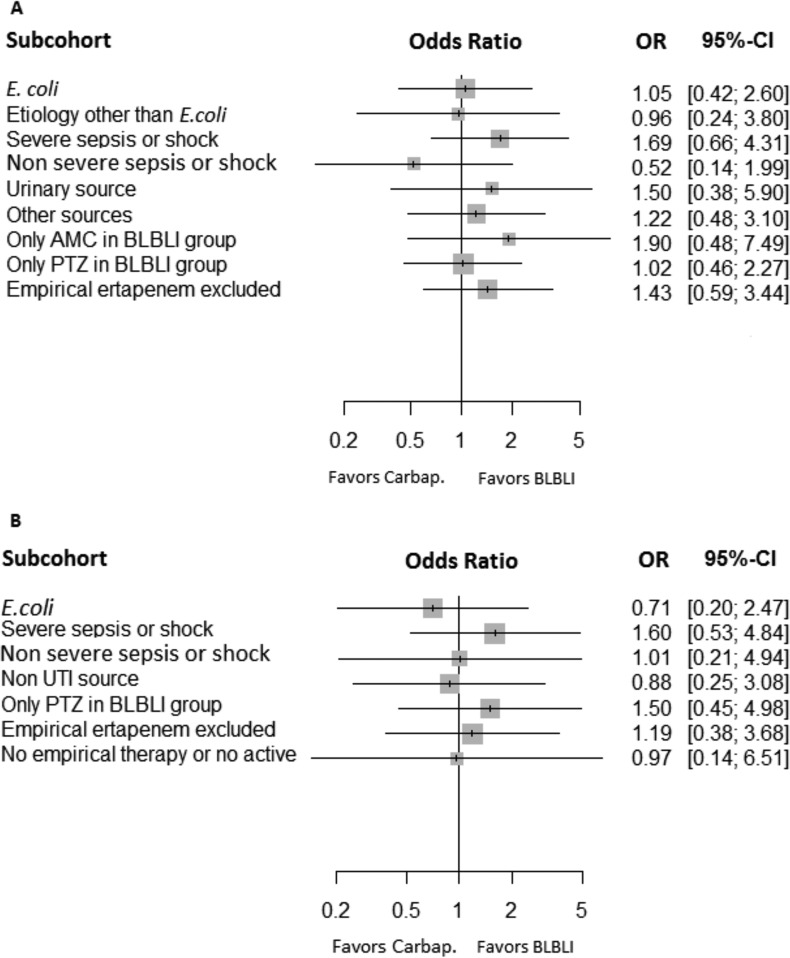
The PS-adjusted OR of empirical therapy with BLBLIs for cure/improvement was 1.03 (95% CI, 0.54 to 1.96; P = 0.9); when other potential confounders were added, including targeted therapy, the adjusted OR was 1.37 (95% CI, 0.69 to 2.76; P = 0.37) (Table 2). The addition of individual or grouped centers did not change the results. The sensitivity analysis in specific subpopulations (Fig. 1A) showed that the effects of empirical therapy with BLBLIs were similar irrespective of etiology, presentation with severe sepsis or shock, source, or specific antibiotics used.
TABLE 2.
Multivariate analysis for cure/improvement in the empirical-therapy, targeted-therapy, and global cohorts
| Variable | Adjusted OR (95% CI) | P value |
|---|---|---|
| Empirical-therapy cohort | ||
| Age (per unita) | 0.98 (0.96–1.00) | 0.07 |
| Source | ||
| Urinary | Reference for comparison | |
| Biliary tract | 0.69 (0.24–2.15) | 0.51 |
| Other (high-risk source) | 0.30 (0.15–0.59) | 0.0006 |
| McCabe classification, nonfatal | 2.64 (1.40–5.16) | 0.003 |
| Pitt score (per unit) | 0.79 (0.69–0.90) | 0.0003 |
| Severe sepsis or shock | 0.25 (0.12–0.50) | <0.0001 |
| Empirical therapy with a BLBLI | 1.37 (0.69–2.76) | 0.37 |
| Propensity score | 0.77 (0.19–3.15) | 0.71 |
| Targeted-therapy cohort | ||
| Source | ||
| Urinary tract | Reference | |
| Biliary tract | 0.88 (0.34–2.48) | 0.80 |
| Other (high-risk source) | 0.40 (0.22–0.71) | 0.002 |
| McCabe classification, nonfatal | 3.56 (2.00–6.61) | <0.0001 |
| Pitt score (per unit) | 0.80 (0.71–0.89) | <0.0001 |
| Severe sepsis or shock | 0.34 (0.19–0.61) | 0.0004 |
| Empirical therapy | ||
| Activeb | Reference | |
| Inactivec/no drugd | 0.64 (0.37–1.11) | 0.11 |
| Targeted therapy with a BLBLI | 1.61 (0.58–4.86) | 0.38 |
| Propensity score | 0.98 (0.23–4.53) | 0.98 |
| Global cohort | ||
| Source | ||
| Urinary tract | Reference | |
| Biliary tract | 0.55 (0.24–1.30) | 0.16 |
| Other (high-risk source) | 0.38 (0.20–0.70) | 0.002 |
| McCabe classification, nonfatal | 3.23 (1.83–5.93) | <0.0001 |
| Pitt score (per unit) | 0.83 (0.74–0.94) | 0.002 |
| Severe sepsis or shock | 0.32 (0.17–0.58) | 0.0002 |
| Drug used for empirical therapy-drug used for targeted therapy | ||
| Carbapenem-carbapenem | Reference | |
| BLBLI-carbapenem | 0.86 (0.28–2.53) | 0.78 |
| BLBLI-BLBLI | 1.33 (0.43–4.46) | 0.63 |
| Other drug-carbapenem | 0.83 (0.28–2.40) | 0.74 |
| Other drug-BLBLI | 1.37 (0.26–8.85) | 0.72 |
| Propensity score | 0.99 (0.23–4.21) | 0.99 |
Per unit of the score.
Carbapenems, 145; BLBLIs, 134; combined therapy, 69; other, 21.
Cephalosporin, 93; BLBLIs, 62; ciprofloxacin, 25; combined therapy, 20; other, 17.
Fifteen cases received no drug.
FIG 1.
Adjusted odds ratios and 95% confidence intervals for cure/improvement at day 14 for empirical (A) and targeted (B) therapy with BLBLIs versus carbapenems in different subgroups.
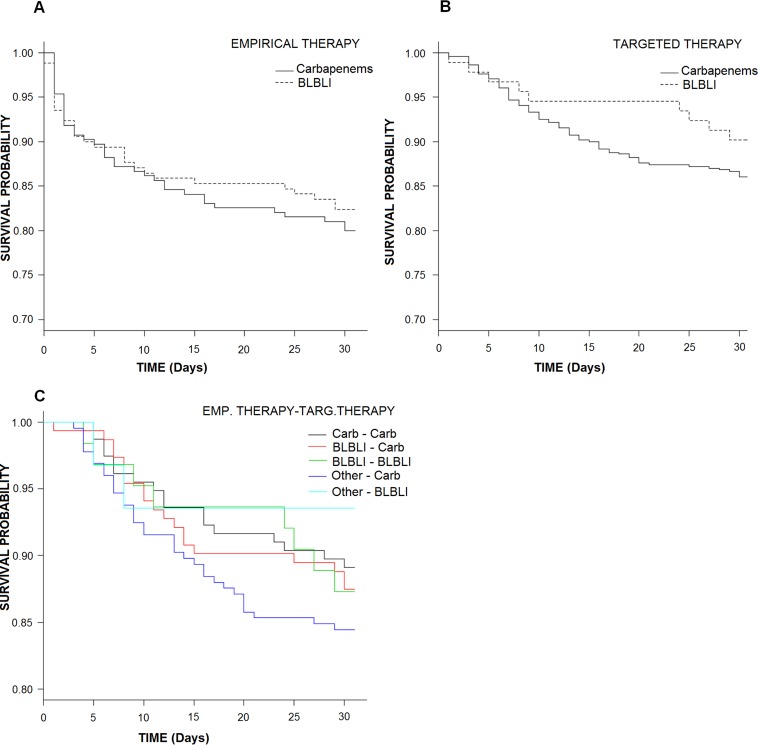
The mortality rates among patients empirically treated with carbapenems versus BLBLIs were similar (20% versus 17.6%; absolute difference at day 30, −2.4%; 95% CI, −10.2% to 5.80%; P = 0.6 by log-rank test) (Fig. 2A and Table 1). Univariate analysis of variables associated with 30-day mortality is shown in Table S3 in the supplemental material. The PS-adjusted OR of empirical therapy with BLBLIs for 30-day mortality was 0.72 (95% CI, 0.37 to 1.38; P = 0.32). When other potential confounders were added, it was 0.55 (95% CI, 0.25 to 1.18; P = 0.14) (Table 3). A meta-regression analysis considering the geographical regions (Spain, other Mediterranean countries, and the rest of the world) did not show significant differences between BLBLIs and carbapenems (see Table S4 and Fig. S2).
FIG 2.
Kaplan-Meier curves for mortality in the empirical-therapy cohort (A), the targeted-therapy cohort (B), and the global cohort (C) according to treatment regimens.
TABLE 3.
Multivariate analysis for 30-day mortality in the empirical-therapy, targeted-therapy, and global cohorts
| Variable | Adjusted OR (95% CI) | P valuea |
|---|---|---|
| Empirical-therapy cohort | ||
| Age (per unita) | 1.05 (1.02–1.07) | 0.0006 |
| Source | 4.98 (2.37–10.96) | <0.0001 |
| Urinary tract | Reference for comparison | |
| Biliary tract | 1.38 (0.37–4.64) | 0.61 |
| Other (high-risk source) | 4.50 (2.13–9.98) | 0.0001 |
| McCabe classification, nonfatal | 0.31 (0.14–0.62) | 0.002 |
| Pitt score (per unit) | 1.31 (1.14–1.51) | 0.0001 |
| Severe sepsis or shock | 6.76 (3.11–15.55) | <0.0001 |
| Empirical therapy with a BLBLI | 0.55 (0.25–1.18) | 0.14 |
| Propensity score | 1.32 (0.28–6.23) | 6.26 |
| Targeted-therapy cohort | ||
| Age (per unit) | 1.03 (1.01–1.05) | 0.003 |
| Enterobacteriaceae species | ||
| E. coli | Reference | |
| K. pneumoniae | 1.96 (0.96–3.42) | 0.04 |
| Other | 0.91 (0.30–2.51) | 0.87 |
| Source | ||
| Urinary tract | Reference | |
| Biliary tract | 0.93 (0.30–2.53) | 0.89 |
| Other (high-risk source) | 2.89 (1.55–5.55) | 0.001 |
| McCabe classification, nonfatal | 0.23 (0.12–0.43) | <0.0001 |
| Pitt score (per unit) | 1.31 (1.16–1.48) | <0.0001 |
| Severe sepsis or shock | 2.99 (1.62–5.61) | 0.0005 |
| Empirical therapy | ||
| Active | Reference | |
| No active or no empirical therapy | 1.82 (1.01–3.28) | 0.04 |
| Targeted therapy with a BLBLI | 0.59 (0.19–1.71) | 0.35 |
| Propensity score | 1.17 (0.24–5.30) | 0.84 |
| Global cohort | ||
| Age (per unit) | 1.04 (1.02–1.06) | 0.0003 |
| Enterobacteriaceae species | ||
| E. coli | Reference | |
| K. pneumoniae | 2.00 (1.01–3.88) | 0.04 |
| Other | 1.04 (0.33–2.89) | 0.94 |
| Source | ||
| Urinary tract | Reference | |
| Biliary tract | 1.17 (0.44–2.91) | 0.75 |
| Other (high-risk source) | 2.91 (1.54–5.66) | 0.001 |
| McCabe classification, nonfatal | 0.24 (0.12–0.45) | <0.0001 |
| Pitt score (per unit) | 1.25 (1.10–1.42) | 0.0004 |
| Severe sepsis or shock | 3.45 (1.82–6.70) | 0.0002 |
| Drug used for empirical therapy-drug used for targeted therapy | ||
| Carbapenem-carbapenem | Reference | |
| BLBLI-carbapenem | 0.70 (0.21–2.34) | 0.56 |
| BLBLI-BLBLI | 0.73 (0.20–2.42) | 0.61 |
| Other drug-carbapenem | 1.16 (0.37–3.76) | 0.79 |
| Other drug-BLBLI | 0.24 (0.02–1.67) | 0.18 |
| Propensity score | 0.54 (0.10–2.70) | 0.46 |
Per unit of the score.
We then performed a stratified analysis according to the quartiles of the PS; no significant differences in cure/improvement or mortality rates were shown, but it should be noted that the number of cases treated empirically with BLBLIs in the first stratum was low (Table 4). Finally, we performed a PS-based matched analysis; we could match 100 pairs of patients treated empirically with BLBLIs or carbapenems according to the PS; the matched patients did not show significant differences in exposure to variables related to empirical therapy (see Table S5 in the supplemental material); the cure/improvement and mortality rates among patients treated empirically with BLBLIs or carbapenems were 78% and 77% (P = 0.87) and 17% and 23% (P = 0.29), respectively; the Kaplan-Meier curves for survival in propensity score-matched patients are shown in Fig. S3.
TABLE 4.
Stratified analysis of outcomes of patients in the empirical- and targeted-therapy cohorts treated with β-lactam/β-lactam inhibitor combinations or carbapenems, according to quartiles of the propensity score
| Cohort, PS quartile | No. of patients with indicated outcome/no. of patients that received indicated treatment (%) |
|||||
|---|---|---|---|---|---|---|
| Cure/improvement |
Mortality |
|||||
| BLBIs | Carbapenems | P value | BLBIs | Carbapenems | P value | |
| Empirical therapy | ||||||
| n = 170 | n = 195 | n = 170 | n = 195 | |||
| 1st | 7/12 (58.3) | 59/76 (77.6) | 0.15 | 4/12 (33.3) | 18/76 (23.7) | 0.47 |
| 2nd | 29/35 (82.9) | 51/59 (86.4) | 0.76 | 5/35 (14.3) | 6/59 (10.1) | 0.78 |
| 3rd | 38/50 (76.0) | 31/41 (75.6) | 0.96 | 9/50 (18.0) | 10/41 (24.4) | 0.62 |
| 4th | 62/73 (84.9) | 13/19 (68.4) | 0.10 | 12/73 (16.4) | 5/19 (26.3) | 0.32 |
| Targeted therapy | ||||||
| n = 92 | n = 509 | n = 92 | n = 509 | |||
| 1st | 0 | 127/150 (84.7) | 0 | 21/150 (14.0) | ||
| 2nd | 2/3 (66.7) | 128/147 (87.1) | 0.30 | 1/3 (33.3) | 19/147 (12.9) | 0.30 |
| 3rd | 13/13 (100.0) | 115/137 (83.9) | 0.12 | 0/13 (0.0) | 21/137 (15.3) | 0.13 |
| 4th | 68/76 (89.5) | 65/75 (86.7) | 0.77 | 8/76 (10.5) | 10/75 (13.3) | 0.62 |
Targeted-therapy cohort.
The TTC included 601 patients: 92 received targeted therapy with a BLBLI (32 AMC and 60 PTZ), and 509 received a carbapenem (205 ertapenem, 185 meropenem, 118 imipenem, and 1 doripenem). The characteristics of patients by treatment type are shown in Table 1. The most frequent dose regimens were as follows: for PTZ, 4/0.5 g q6h (40.0%) and 4/0.5 g q8h (43.3%); for AMC, 1/0.2 g q8h (59.4%) and 2/0.5 g q8h (12.5%); for imipenem, 0.5 g q6h (49.1%) and 0.5 g q8h (20.3%); for meropenem, 1 g q8h (50.8%), 1 g q12h (15.1%) and 1 g q24h (9.2%); and for ertapenem, 1 g q24h (84.3%).
The cure/improvement rates at day 14 were 90.2% and 85.5% for carbapenems and BLBLIs, respectively (P = 0.22; absolute difference, 4.7%; 95% CI, −3.5% to 10.3%). Univariate analysis of variables associated with cure/improvement is shown in Table S6 in the supplemental material.
The PS-adjusted OR of targeted therapy with BLBLIs for cure/improvement was 1.39 (95% CI, 0.55 to 3.82; P = 0.44); when other potential confounders were added, the adjusted OR was 1.61 (95% CI, 0.58 to 4.86; P = 0.38) (Table 2). The addition of individual or grouped centers did not change the results. The results of sensitivity analysis in specific subpopulations are shown in Fig. 1B and showed no trend for different outcomes; some subpopulations, including patients with etiology other than Escherichia coli, patients with urinary source only, and AMC-treated patients only in the BLBLI group, were not considered due to very low numbers of events.
The mortality rates among patients who received targeted therapy with carbapenems or BLBLIs were similar (9.8% versus 13.9%; absolute difference at day 30, −2.4%; 95% CI, −10.2% to 5.80%; P = 0.28 by log-rank test) (Fig. 2B and Table 1). Univariate analysis of variables associated with 30-day mortality is shown in Table S7 in the supplemental material. The PS-adjusted OR of targeted therapy with BLBLIs for 30-day mortality was 0.65 (95% CI, 0.23 to 1.65; P = 0.86). When other potential confounders were added, it was 0.59 (95% CI, 0.19 to 1.71; P = 0.35) (Table 3). Meta-regression analysis considering geographical regions did not show significant differences (see Table S8 and Fig. S4).
The stratified analysis performed according to the quartiles of the PS only had a meaningful number of patients for quartiles 3 and 4; in these, no significant differences in cure/improvement or mortality rate were shown (Table 4). We could only match 55 pairs of patients receiving targeted treatment with BLBLIs or carbapenems according to the PS; the matched patients did not show significant differences in exposure to variables related to therapy (see Table S9 in the supplemental material); the cure/improvement and mortality rates among patients treated with BLBLIs or carbapenems were 89.1% and 83.6% (P = 0.40) and 7.3% and 16.4% (P = 0.14), respectively; the Kaplan-Meier curves for survival in propensity score-matched patients are shown in Fig. S5.
Global-therapy cohort.
Overall, 627 patients were included in the GTC; 156 received empirical and targeted therapy with a carbapenem, 152 received empirical therapy with a BLBLI and targeted therapy with a carbapenem, 63 received empirical and targeted therapy with a BLBLI, 225 received empirical therapy with a drug other than a carbapenem or a BLBLI and targeted therapy with a carbapenem, and 31 received empirical therapy with a drug other than a carbapenem or a BLBLI and targeted therapy with a BLBLI. The baseline features of the patients in each group are shown in Table 5. The 14-day cure/improvement rates were 87.7%, 85.5%, 87.3%, 84.9%, and 90.3%, respectively, and the 30-day mortality rates were 10.9%, 12.5%, 12.5%, 15.6%, and 6.5%, respectively.
TABLE 5.
Characteristics of patients with bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae in the global therapy cohort
| Characteristic | No. (%) of patients (unless otherwise specified) (the first drug in each pair is the empirical and the second is the targeted) |
||||
|---|---|---|---|---|---|
| Carbapenem-carbapenema (n = 156) | BLBLI-carbapenem (n = 152) | BLBLI-BLBLI (n = 63) | Other drug-carbapenem (n = 225) | Other drug-BLBLI (n = 31) | |
| Age [median (IQRb)] | 65 (50.8–75.0) | 71.5 (58.0–80.3)c | 67 (59.5–76.5) | 68 (55.0–79.0)c | 74 (56.0–83.5)c |
| Male sex | 96 (61.5) | 84 (55.3) | 37 (58.7) | 129 (57.3) | 18 (58.1) |
| Enterobacteriaceae species | |||||
| E. coli | 113 (72.4) | 106 (69.7) | 50 (79.4) | 170 (75.6) | 23 (74.2) |
| K. pneumoniae | 34 (21.8) | 34 (22.4) | 9 (14.3) | 35 (15.6) | 5 (16.1) |
| Other | 9 (5.8) | 12 (7.9) | 4 (6.3) | 20 (8.9) | 3 (9.7) |
| Nosocomial acquisition | 74 (47.4) | 67 (44.1) | 27 (42.9) | 109 (48.4) | 12 (38.7) |
| Source | |||||
| Urinary tract | 81 (51.9) | 59 (38.8) | 27 (42.9) | 103 (45.8) | 15 (48.4) |
| Biliary tract | 25 (16.0) | 40 (26.3) | 10 (15.9) | 17 (7.6) | 2 (6.5) |
| Other (high-risk source) | 50 (32.1) | 53 (34.9)c | 26 (41.3) | 105 (46.7)c | 14 (45.2) |
| ICUd admission | 19 (12.2) | 17 (11.2) | 2 (3.2)c | 27 (12.0) | 2 (6.5) |
| McCabe classification, nonfatal | 76 (48.7) | 83 (54.6) | 30 (47.6) | 108 (48.0) | 15 (48.4) |
| Pitt score [median (IQR)] | 1 (0–3) | 1 (0–2.25) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Severe sepsis or shock | 54 (34.6) | 56 (36.8) | 25 (39.7) | 70 (31.1) | 8 (25.8) |
| Cancer | 67 (42.9) | 57 (37.5) | 30 (47.6) | 97 (43.1) | 13 (41.9) |
| Active empirical therapy | 156 (100) | 96 (63.2)c | 58 (92.1) c | 78 (34.7)c | 10 (32.3)c |
| Cure/improvement | 137 (87.8) | 130 (85.5) | 55 (87.3) | 191 (84.9) | 28 (90.3) |
| 30-day mortality | 17 (10.9) | 19 (12.5) | 8 (12.7) | 35 (15.6) | 2 (6.5) |
The carbapenem-carbapenem group was considered the reference group for comparisons.
IQR, interquartile range.
The variable showed a P value of ≤0.05 in crude analysis.
ICU, intensive care unit.
Kaplan-Meier mortality curves are shown in Fig. 2C. The results of univariate analysis for cure/improvement and 30-day mortality are shown in Tables S10 and S11 in the supplemental material. In the multivariate logistic regression models, and after controlling for propensity score and other variables, empirical and targeted therapy with carbapenems showed no significant effects on cure/improvement rates at day 14 or on mortality rates compared to the rates in any of the other groups (Tables 2 and 3). Adding individual or grouped centers did not change the results.
DISCUSSION
Our results strongly support the hypothesis that active BLBLIs are not inferior to carbapenems for the treatment of BSI due to ESBL-E in different clinical scenarios. Importantly, these data not only support previous data on BSI due to E. coli with a urinary and biliary tract source (11) but also suggest that BLBLIs may, if active in vitro, be useful alternatives to carbapenems for the treatment of BSI due to any ESBL-E from any source if used at appropriate doses. Our data refer to susceptible isolates, which is clearly relevant for targeted therapy; however, it is also relevant for empirical decisions in which an evaluation of the patient's individual risk for ESBL producers and the local epidemiology data on susceptibility of ESBL producers to BLBLIs are to be taken into account.
BLBLIs have been shown to be as effective as carbapenems for the treatment of diverse severe infections in randomized trials (21). The reasons for raising doubts about the efficacy of BLBLIs in the treatment of severe infections caused by ESBL-E include the following. First, the observation that PTZ (but not AMC) is less active in vitro when tested against a high inoculum of bacteria (22). However, such an effect also occurs in non-ESBL producers. Second, the results from some animal data suggesting a lower efficacy than for carbapenems against isolates producing TEM-type ESBLs (23, 24) and some anecdotal failures (25). And third, the fact that the MICs of carbapenems (except ertapenem) are usually several dilutions below the breakpoints, while those of BLBLIs are frequently nearer the breakpoint (1, 26). In fact, a specific analysis suggested that PTZ may be less effective in patients with severe non-urinary tract bacteremic infections caused by borderline-susceptible ESBL-producing E. coli (27). Interest in BLBLIs was raised when a post hoc analysis in patients with BSI due to ESBL-producing E. coli (mostly from the urinary and biliary tracts) (11) and a recent meta-analysis of mostly small observational studies could not show that empirical or targeted therapy with BLBLIs was associated with worse outcomes than carbapenems (12). Interestingly, however, a recent retrospective study from one center showed lower survival among patients treated with BLBLIs (13).
This is an observational study, and thus, the typical limitations of this design apply, including lack of randomization, the potential effects of unmeasured variables, and residual confounding (14). Although this study has, to the best of our knowledge, the biggest sample size published to date, its statistical power is still limited and cannot exclude a potential relevant difference between carbapenems and BLBLIs. Additionally, we could not study the real exposure to the drugs used because drug levels were not measured. However, the data reflect clinical practice. Nevertheless, this study has some methodological strengths, which should be taken into consideration. The hypothesis, design, and statistical analysis plan were preregistered so as to avoid casual post hoc findings, as recommended for observational studies (14, 28, 29). Very strict criteria for the assignment of treatments were applied. Treatment changes are frequent in real life, making it difficult to assign patients appropriately to a specific antibiotic in observational studies; in fact, most observational studies lack appropriate criteria for assignment (14). We used clinical response and mortality as the main outcome measures. Previous studies mainly evaluated mortality, which has the advantage of being a “hard” outcome but may underestimate differences between drugs; we therefore also used clinical response, a “softer” but probably more sensitive outcome measure. Finally, we used advanced methods to control for confounding and center effects (14). Other strengths of our study are the inclusion of cases from diverse geographical locations, with infections caused by non-E. coli species, and with non-urinary tract sources. A randomized controlled trial would be the best way to demonstrate that BLBLIs are noninferior to carbapenems; the MERINO trial, now recruiting in Australia, Singapore, and New Zealand, will compare PTZ and meropenem in the treatment of BSI due to ESBL-E (Australian New Zealand Clinical Trials Registry no. ACTRN12613000532707; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363706).
The rates of susceptibility of ESBL-E to BLBLIs are heterogeneous, depending on the species and geographical location (6–11, 30, 31). Therefore, local susceptibility patterns of ESBL-E must be considered. However, ESBL-E are frequently susceptible to PTZ in many areas of the world. Our data among the patients treated empirically with PTZ and AMC are similar to the rates of susceptibility reported by other authors (frequently higher than 70% for PTZ [6–10] and higher than 60% for AMC [11, 30]). Finally, these results encourage the further investigation of newer BLBLI combinations that are active even against some carbapenemase-producing Enterobacteriaceae. One issue of importance is whether PTZ and AMC are equally effective. As stated above, AMC does not suffer from inoculum effect (22) and showed better activity than PTZ in an experimental murine sepsis model caused by ESBL-producing E. coli (32). Subgroup analyses did not show differences when PTZ or AMC alone was compared to carbapenems. However, more data are needed to investigate the comparative efficacies of AMC and PTZ. Of note, whether PTZ has any advantage over ertapenem as a selector for carbapenemase producers is debatable and should be studied specifically.
Klebsiella pneumoniae was independently associated with higher mortality than E. coli in adjusted analysis both in the targeted-therapy cohort and in the global-therapy cohort. Even though analyzing the impacts of specific bacteria was not an objective of this study, we think this merits further pathogenicity studies; also, this should be considered in any future outcome analysis of infections that includes different Enterobacteriaceae.
Until the results of randomized control trials are available, these data are the best evidence available to support the use of BLBLIs with in vitro activity as alternatives to carbapenems for the treatment of BSI due to ESBL-E, which may have significant implications for avoidance of the overuse of carbapenems.
Supplementary Material
ACKNOWLEDGMENTS
We thank the European Study Group of Bloodstream Infections and Sepsis (ESGBIS) from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) for endorsing the INCREMENT project. We thank Virginia Palomo for her contribution in reviewing the database and Alejandro González for his work with the online database programming.
INCREMENT project investigators: J. Gálvez, M. D. del Toro and P. Retamar (Hospital Universitario Virgen Macarena, Spain); M. Falcone and A. Russo (Policlinico Umberto I, Italy); G. Daikos (Laikon General Hospital, Greece); I. Karaiskos (Hygeia General Hospital, Greece); E. M. Trecarichi and A. R. Losito (Catholic University of the Sacred Heart, Italy); D. L. Paterson (Royal Brisbane and Women's Hospital, Australia); E. García-Vázquez and J. Gómez (Hospital Universitario Virgen de la Arrixaca, Spain); E. Roilides and E. Iosifidis (Hippokration Hospital, Thessaloniki, Greece); Y. Doi (University of Pittsburgh, USA); F. F. Tuon (Hospital da Universidade Federal do Parana, Brazil); F. Navarro and B. Mirelis (Hospital de la Santa Creu i Sant Pau, Spain); R. San Juan and M. Fernández-Ruiz (Hospital 12 de Octubre, Spain); N. Larrosa and M. Puig (Hospital Universitari Vall d'Hebrón, Spain); J. M. Cisneros and V. González (Hospital Universitario Virgen Rocío, Spain); Victoria Rucci (Hospital Español, Argentina); E. Ruiz de Gopegui and C. I. Marinescu (Hospital Universitario Son Espases, Spain); M. C. Fariñas, M. E. Cano, and M. Gozalo (Hospital Universitario Marqués de Valdecilla-IDIVAL, Spain); J. R. Paño-Pardo, and C. Navarro-San Francisco (Hospital La Paz, Spain); S. Gómez-Zorrilla, F. Tubau (Hospital Bellvitge, Spain); S. Pournaras, A. Tsakris, and O. Zarkotou (University of Athens, Greece); Ö. K. Azap (Baskent University, Turkey); A. Antoniadou and G. Poulakou (University General Hospital Attikon, Greece); D. Virmani (University of Calgary, Canada); J. Torre-Cisneros, E. Pérez-Nadales, and I. Gracia-Ahulfinger (Hospital Universitario Reina Sofía, Spain); Ö. Helvaci and A. O. Sahin (Hacettepe University, Turkey); R. Cantón and P. Ruiz (Hospital Ramón y Cajal, Spain); M. Bartoletti and M. Giannella (Teaching Hospital Policlinico S. Orsola Malpighi, Italy); F. Riemenschneider (Universitätsklinikum Tübingen, Germany); C. Badia and Mariona Xercavins (Hospital Universitari Mútua de Terrassa, Spain); and D. Fontanals and E. Jové (Hospital Parc Taulí, Spain).
J. Rodríguez-Baño has been a speaker for Merck, Astellas, AstraZeneca, Pfizer and Novartis, he has been scientific advisor for Merck, Achaogen, Roche, and AstraZeneca, and he has received unrestricted research grants from Novartis and Gilead. R. A. Bonomo received grants for research from NIH, Veteran Affairs, AstraZeneca, Merck, Melinta, and Steris. D. L. Paterson has received honoraria for advisory board participation from Merck, AstraZeneca, Cubist, Pfizer, and Novartis. Y. Carmeli received grants, honoraria, travel support, consulting fees, and other forms of financial support from Achaogen, Allecra Therapeutics, AstraZeneca, Basilea Pharmaceutica LTD, bioMérieux, Cepheid, DaVolterra, Durata Therapeutics, Intercell AG, Merck, PPD, Proteologics, Rempex Pharmaceuticals, Rib-X Pharmaceuticals, Syntezza Bioscience LTD, and Takeda Pharmaceutical. M. Tumbarello has been a scientific advisor for Gilead and Merck Sharp & Dhome, he has been a speaker for Astra Zeneca, Gilead, Merck Sharp & Dhome, Novartis, and Pfizer, and he has received research grants from Novartis and Pfizer. M. Venditti has been a speaker for Merck, Astellas, AstraZeneca, Pfizer, Gilead, and Novartis, he has been scientific advisor for Merck, Pfizer, and Astellas, and he has received unrestricted research grants from Novartis and Pfizer. B. Almirante has carried out consultancy work or received monetary payments for giving talks from Astellas, Astra Zeneca, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dhome, Novartis, and Pfizer. J. Pitout had previously received research funds from Merck and Astra Zeneca. J. Bermejo has been a speaker for Merck Sharp & Dhome, Raffo, and Elea. All other authors declare no conflict of interest.
B. Gutiérrez-Gutiérrez and J. Rodríguez-Baño had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Authors who contributed to conception or design include B. Gutiérrez-Gutiérrez, R. A. Bonomo, Y. Carmeli, D. L. Paterson, A. Pascual, and J. Rodríguez-Baño. Authors who contributed to acquisition, analysis, or interpretation of data include B. Gutiérrez-Gutiérrez, S. Pérez-Galera, E. Salamanca, M. de Cueto, E. Calbo, B. Almirante, P. Viale, A. Oliver, V. Pintado, O. Gasch, L. Martínez-Martínez, J. Pitout, M. Akova, C. Peña, J. Molina, A. Hernández, M. Venditti, N. Prim, J. Origüen, G. Bou, E. Tacconelli, M. Tumbarello, A. Hamprescht, H. Giamarellou, M. Almela, F. Pérez, M. J. Schwabe, J. Bermejo, W. Lowman, P.-R. Hsueh, M. Mora-Rillo, C. Natera, M. Souli, R. A. Bonomo, Y. Carmeli, D. L. Paterson, A. Pascual, and J. Rodríguez-Baño. The manuscript was drafted by B. Gutiérrez-Gutiérrez, S. Pérez-Galera, A. Pascual, and J. Rodríguez-Baño. Critical revision of the manuscript for important intellectual content was done by E. Salamanca, M. de Cueto, E. Calbo, B. Almirante, P. Viale, A. Oliver, V. Pintado, O. Gasch, L. Martínez-Martínez, J. Pitout, M. Akova, C. Peña, J. Molina, A. Hernández, M. Venditti, N. Prim, J. Origüen, G. Bou, E. Tacconelli, M. Tumbarello, A. Hamprescht, H. Giamarellou, M. Almela, F. Pérez, M. J. Schwabe, J. Bermejo, W. Lowman, P.-R. Hsueh, M. Mora-Rillo, C. Natera, and M. Souli. Statistical analysis was done by B. Gutiérrez-Gutiérrez, Y. Carmeli, and J. Rodríguez-Baño. Funding was obtained by R. A. Bonomo, A. Pascual, and J. Rodríguez-Baño. Supervision was provided by R. A. Bonomo, D. L. Paterson, Y. Carmeli, A. Pascual, and J. Rodríguez-Baño. Administrative, technical, or material support was provided by B. Gutiérrez-Gutiérrez, S. Pérez-Galera, E. Salamanca, M. de Cueto, E. Calbo, B. Almirante, P. Viale, A. Oliver, V. Pintado, O. Gasch, L. Martínez-Martínez, J. Pitout, M. Akova, C. Peña, J. Molina, A. Hernández, M. Venditti, N. Prim, J. Origüen, G. Bou, E. Tacconelli, M. Tumbarello, A. Hamprescht, H. Giamarellou, M. Almela, F. Pérez, M. J. Schwabe, J. Bermejo, W. Lowman, P.-R. Hsueh, M. Mora-Rillo, C. Natera, and M. Souli.
The work was funded by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, cofinanced by European Development Regional Fund titled A Way To Achieve Europe ERDF, the Spanish Network for Research in Infectious Diseases (REIPI RD12/0015), and FIS (PI10/02021). Participants in the study received support for research from the Innovative Medicines Initiative Joint Undertaking under the Combatting Bacterial Resistance in Europe—Molecule against Gram Negative Infections (COMBACTE-MAGNET) agreement 115737 resources, which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007-2013) and the European Federation of Pharmaceutical Industries and Associations (EFPIA) companies' in kind contributions (A. Pascua and J. Rodríguez-Baño), the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program, the Geriatric Research Education and Clinical Center VISN 10 (VISN 10 GRECC), and NIAID, NIH, under award numbers R01AI072219 and R01AI063517 (R. A. Bonomo). The funding organizations had no role in the design or conduct of the study, collection, management analysis, or interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00365-16.
REFERENCES
- 1.Paterson DL, Bonomo R. 2005. Extended-spectrum β-lactamase: a clinical update. Clin Microbiol Rev 18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitout JDD, Laupland KB. 2008. Extended-spectrum β-lactamase–producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Baño J, Pascual A. 2008. Clinical significance of extended-spectrum β-lactamases. Expert Rev Anti Infect Ther 6:671–683. doi: 10.1586/14787210.6.5.671. [DOI] [PubMed] [Google Scholar]
- 4.Schwaber MJ, Carmeli Y. 2008. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911–2913. doi: 10.1001/jama.2008.896. [DOI] [PubMed] [Google Scholar]
- 5.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YH, Hsueh PR, Badal RE, Hawser SP, Hoban DJ, Bouchillon SK, Ni Y, Paterson DL. 2011. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J Infect 62:280–291. doi: 10.1016/j.jinf.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Hawser S, Hoban D, Bouchillon S, Badal R, Carmeli Y, Hawkey P. 2011. Antimicrobial susceptibility of intra-abdominal gram-negative bacilli from Europe: SMART Europe 2008. Eur J Clin Microbiol Infect Dis 30:173–179. doi: 10.1007/s10096-010-1066-0. [DOI] [PubMed] [Google Scholar]
- 8.Hoban DJ, Bouchillon SK, Hawser SP, Badal RE, Labombardi VJ, DiPersio J. 2010. Susceptibility of gram-negative pathogens isolated from patients with complicated intra-abdominal infections in the United States, 2007-2008: results of the Study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob Agents Chemother 54:3031–3034. doi: 10.1128/AAC.01808-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Díaz MA, Hernández-Bello JR, Rodríguez-Baño J, Martínez-Martínez L, Calvo J, Blanco J, Pascual A, Spanish Group for Nosocomial Infections (GEIH). 2010. The diversity of Escherichia coli strains producing extended-spectrum β-lactamases in Spain: second nationwide study. J Clin Microbiol 48:2840–2845. doi: 10.1128/JCM.02147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz de Alegría C, Rodríguez-Baño J, Cano ME, Hernández-Bello JR, Calvo J, Román E, Díaz MA, Pascual A, Martínez-Martínez L, Spanish Group for Nosocomial Infections (GEIH). 2011. Klebsiella pneumoniae strains producing extended-spectrum β-lactamases in Spain: microbiological and clinical features. J Clin Microbiol 49:1134–1136. doi: 10.1128/JCM.02514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual A. 2012. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase–producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 12.Vardakas KL, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteremia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 13.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership Group. 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettiol E, Rottier WC, Del Toro MD, Harbarth S, Bonten MJ, Rodríguez-Baño J, COMBACTE consortium. 2014. Improved treatment of multidrug-resistant bacterial infections: utility of clinical studies. Future Microbiol 9:757–771. doi: 10.2217/fmb.14.35. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–537. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement CLSI M100-S22. CLSI, Wayne, PA. [Google Scholar]
- 17.McCabe WR, Jackson GG. 1962. Gram-negative bacteriemia. Etiology and ecology. Arch Intern Med 110:845–855. [Google Scholar]
- 18.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. 1989. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 87:540–546. doi: 10.1016/S0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference. Crit Care Med 31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 20.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 21.Shiber S, Yahav D, Avni T, Leibovici L, Paul M. 2015. β-Lactam/β-lactamase inhibitors versus carbapenems for the treatment of sepsis: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 70:41–47. doi: 10.1093/jac/dku351. [DOI] [PubMed] [Google Scholar]
- 22.López-Cerero L, Picón E, Morillo C, Hernández JR, Docobo F, Pachón J, Rodríguez-Baño J, Pascual A. 2010. Comparative assessment of inoculum effects on the antimicrobial activity of amoxicillin/clavulanate and piperacillin/tazobactam with ESBL-producing and non-producing Escherichia coli isolates. Clin Microbiol Infect 16:132–136. doi: 10.1111/j.1469-0691.2009.02893.x. [DOI] [PubMed] [Google Scholar]
- 23.Rice LB, Carias LL, Shlaes DM. 1994. In vivo efficacies of β-lactam-β-lactamase inhibitor combinations against a TEM-26-producing strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 38:2663–2664. doi: 10.1128/AAC.38.11.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thauvin-Eliopoulos C, Tripodi MF, Moellering RC Jr, Eliopoulos GM. 1997. Efficacies of piperacillin-tazobactam and cefepime in rats with experimental intra-abdominal abscesses due to an extended-spectrum beta-lactamase-producing strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 41:1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimhony O, Chmelnitsky I, Bardenstein R, Goland S, Hammer Muntz O, Navon Venezia S, Carmeli Y. 2006. Endocarditis caused by extended-spectrum β-lactamase–producing Klebsiella pneumoniae: emergence of resistance to ciprofloxacin and piperacillin-tazobactam during treatment despite initial susceptibility. Antimicrob Agents Chemother 50:3179–3182. doi: 10.1128/AAC.00218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Baño J, Picón E, Navarro MD, López-Cerero L, Pascual A, ESBL-REIPI Group. 2012. Impact of changes in CLSI and EUCAST breakpoints for susceptibility in bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli. Clin Microbiol Infect 18:894–900. doi: 10.1111/j.1469-0691.2011.03673.x. [DOI] [PubMed] [Google Scholar]
- 27.Retamar P, López-Cerero L, Muniain MA, Pascual Á Rodríguez-Baño J, ESBL-REIPI/GEIH Group. 2013. Impact of the MIC of piperacillin-tazobactam on the outcome of patients with bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother 57:3402–3404. doi: 10.1128/AAC.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loder E, Groves T, MacAuley D. 2010. Registration of observational studies. The next step towards research transparency. BMJ 340:c950. doi: 10.1136/bmj.c950. [DOI] [PubMed] [Google Scholar]
- 29.Thomas L, Peterson ED. 2012. The value of statistical analysis plans in observational research: defining high-quality research from the start. JAMA 308:773–774. doi: 10.1001/jama.2012.9502. [DOI] [PubMed] [Google Scholar]
- 30.Hawser SP, Badal RE, Bouchillon SK, Hoban DJ, Biedenbach DJ, Cantón R, Paterson DL. 2013. Monitoring the global in vitro activity of ertapenem against Escherichia coli from intra-abdominal infections: SMART 2002-2010. Int J Antimicrob Agents 41:224–228. doi: 10.1016/j.ijantimicag.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Qiao LD, Chen S, Yang Y, Zhang K, Zheng B, Guo HF, Yang B, Niu YJ, Wang Y, Shi BK, Yang WM, Zhao XK, Gao XF, Chen M, Tian Y. 2013. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open 3:e004152. doi: 10.1136/bmjopen-2013-004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Docobo-Pérez F, López-Cerero L, López-Rojas R, Egea P, Domínguez-Herrera J, Rodríguez-Baño J, Pascual A, Pachón J. 2013. Inoculum effect on the efficacies of amoxicillin-clavulanate, piperacillin-tazobactam, and imipenem against extended-spectrum β-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli in an experimental murine sepsis model. Antimicrob Agents Chemother 57:2109–2113. doi: 10.1128/AAC.02190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.