Abstract
This study aimed to evaluate the trypanocidal activity of mycophenolic acid (MPA) and its derivatives for Trypanosoma congolense. The proliferation of T. congolense was completely inhibited by adding <1 μM MPA and its derivatives. In addition, the IMP dehydrogenase in T. congolense was molecularly characterized as the target of these compounds. The results suggest that MPA and its derivatives have the potential to be new candidates as novel trypanocidal drugs.
TEXT
Trypanosoma congolense causes animal African trypanosomiasis (AAT) in livestock. The lack of effective vaccines makes the use of chemotherapeutic agents the most effective measure for controlling AAT. Limited numbers of commercial drugs have long been used to treat AAT. The emergence of drug-resistant trypanosomes and cases of drug-refractory trypanosomiasis have been reported (1–4), underscoring the need for development of new drugs.
A candidate target for drug development is IMP dehydrogenase (IMPDH). This enzyme is very important in the Trypanosoma spp. because it lacks a de novo purine synthesis pathway, which makes the purine nucleotide synthesis in these parasites solely dependent on a salvage pathway in the glycosomes (5–7). IMPDH converts IMP into XMP through this pathway, which is a rate-limiting step in the metabolism of guanine nucleotides (8). Mycophenolic acid (MPA), compound 1, is a well-known IMPDH inhibitor (Fig. 1). Its enzymatic activity has already been proven in many protozoan parasites (9–14). The antiprotozoan activities of MPA against Babesia spp. were reported in in vivo and in vitro studies (9, 15). Thus, the activity of MPA against IMPDH is expected to lead to a novel strategy for the development of trypanocides.
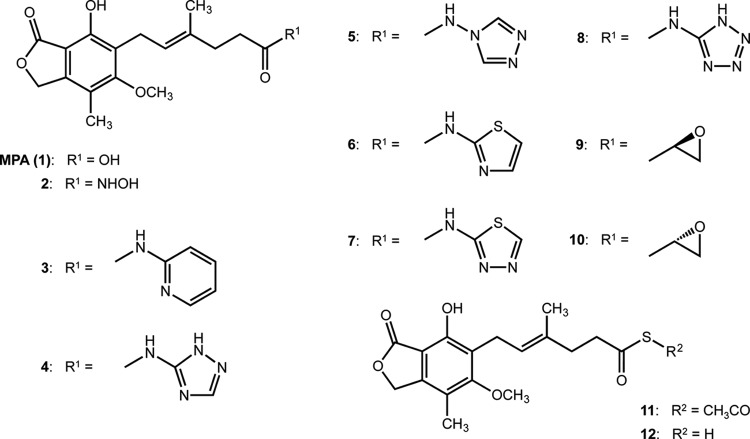
FIG 1.
The structures of mycophenolic acid (MPA) and its derivatives.
The novel IMPDH orthologue of T. congolense (TcIMPDH) (accession no. LC094350) was identified from T. congolense resequencing data (unpublished data). The recombinant TcIMPDH showed IMPDH activity in vitro (see Fig. S1A and B in the supplemental material). The nanomolar levels of MPA clearly inhibited NADH production by TcIMPDH in a dose-dependent manner (50% inhibitory concentration [IC50] = 26.2 nM) (see Fig. S1C in the supplemental material). The expression profile and cellular localization of TcIMPDH were analyzed by Western blotting and immunofluorescence microscopy. TcIMPDH was expressed in glycosomes as granulated forms throughout the life cycle stages of T. congolense (see Fig. S2 in the supplemental material). TcIMPDH was expressed at similar levels in bloodstream form (BSF), procyclic form (PCF), and epimastigote form (EMF). In contrast, TcIMPDH expression in the metacyclic form (MCF) was significantly lower than in the other stages (P < 0.05, Tukey's multiple-comparison test). This result suggests that purine synthesis is highly important in the proliferative stages of the parasite but not in the nonproliferative MCF stage.
The aim of this study was to reveal the trypanocidal activities of MPA derivatives for developing effective trypanocidal drugs. Various inhibitory activities and the cell-differentiation activity of MPA derivatives against mammalian cells have been reported in vitro. Some MPA derivatives (compounds 2, 4, 9, and 10) have shown particularly significant inhibitory activities against human IMPDH and were observed to induce erythroid differentiation in K562 cells (16, 17). The earlier reports suggested that some MPA derivatives might be specific inhibitors for Trypanosoma. The chemical structures of the MPA derivatives in this study are shown in Fig. 1. We evaluated the trypanocidal activity against T. congolense, T. b. brucei, and T. evansi using an ATP-based luciferase viability system (18). To evaluate the trypanocidal activity of MPA (compound 1) and its derivatives in vitro, BSFs were cultivated with 1 μM of each compound. At 1 μM, nine derivatives showed <10% anti-T. congolense activity (Table 1). In contrast, only three compounds, 1, 2, and 4, inhibited T. congolense growth by 99.60 ± 0.38%, 94.46 ± 3.89%, and 98.87 ± 0.78% at 1 μM, respectively (Table 1). Although compound 1 showed high trypanocidal activity against T. b. brucei and T. evansi, compounds 2 and 4 showed lower inhibitory activities at 1 μM against T. b. brucei and T. evansi than against T. congolense (Table 1). The low plasma membrane permeability of compounds 3, 5, 6, 7, 8, 11, and 12 might account for their low trypanocidal activity, while the low trypanocidal activity of compounds 9 and 10 against all of the tested trypanosome species and of compound 2 against T. b. brucei and T. evansi suggests their low affinity with these trypanosome IMPDHs or the deactivation of these compounds by other species-specific enzymes in cytosol. The IC50s of compounds 1, 2, and 4 to T. congolense were 0.10 ± 0.04, 0.56 ± 0.21, and 0.16 ± 0.04 μM, respectively (Table 2). The IC50s of these three compounds to MDBK cells were 0.52 ± 0.12, 1.40 ± 0.18, and 0.84 ± 0.21 μM, respectively. The selectivity indices of MPA and the two derivatives in T. congolense were 5.14, 2.62, and 5.10, respectively (Table 2). However, the higher IC50s and lower selectivity indices of these three compounds were shown in T. b. brucei and T. evansi (Table 2). The cytotoxicity of these compounds was higher than that of commercial drugs (19). However, the IC50s of compounds 1 and 4 for T. congolense BSF were comparable to those of two commercially available trypanocides (pentamidine [0.17 μM] and diminazene [0.11 μM]) against T. congolense (18). These results suggest that compounds 1, 2, and 4 might be potential lead compounds in the development of trypanocides, especially against T. congolense.
TABLE 1.
Trypanocidal activity of MPA and its derivatives
| Compound | Inhibition rate (%)a |
||
|---|---|---|---|
| T. congolense | T. b. brucei | T. evansi | |
| 1 (MPA) | 99.60 ± 0.38 | 82.99 ± 2.82 | 90.53 ± 1.22 |
| 2 | 94.46 ± 3.89 | 5.24 ± 13.12 | 14.21 ± 8.64 |
| 3 | 2.36 ± 8.64 | 7.83 ± 10.35 | 16.66 ± 5.55 |
| 4 | 98.87 ± 0.78 | 46.13 ± 5.21 | 42.79 ± 4.58 |
| 5 | 4.65 ± 15.29 | 14.29 ± 34.17 | 32.43 ± 4.88 |
| 6 | 1.45 ± 10.94 | 22.27 ± 4.81 | 17.11 ± 6.14 |
| 7 | 4.59 ± 15.12 | 14.50 ± 13.76 | 29.44 ± 10.03 |
| 8 | 3.59 ± 14.06 | 22.99 ± 12.90 | 19.94 ± 8.44 |
| 9 | 0.06 ± 8.66 | 9.28 ± 5.15 | 11.99 ± 1.59 |
| 10 | 3.15 ± 8.43 | 9.03 ± 7.91 | 9.49 ± 6.13 |
| 11 | 6.51 ± 14.38 | 16.47 ± 6.97 | 12.79 ± 4.49 |
| 12 | 3.03 ± 12.91 | 11.56 ± 4.17 | 13.61 ± 8.67 |
| Pentamidineb | 99.93 ± 0.07 | 99.96 ± 0.06 | 99.94 ± 0.07 |
| Controlc | 0.00 ± 1.74 | 0.48 ± 1.58 | −0.24 ± 2.25 |
Trypanocidal activity of MPA (compound 1) and 11 MPA derivatives (see Fig. 1) at a concentration of 1 μM was evaluated for T. congolense IL3000 strain, T. b. brucei GUTat 3.1 strain, and T. evansi Tansui strain. The inhibition rate was calculated from 3 independent experiments and expressed as the mean inhibition rate ± SD.
Pentamidine 500 ng/ml was used as a 100% inhibition control.
HMI-9 medium with 0.25% dimethyl sulfoxide (DMSO) was used as a 0% inhibition control.
TABLE 2.
IC50 and selectivity index of MPA and MPA derivatives 2 and 4 against T. b. brucei and T. evansi
| Compound | IC50 (μM)a for: |
Selectivity indexa,b for: |
|||||
|---|---|---|---|---|---|---|---|
| T. congolense | T. b. brucei | T. evansi | MDBK cell | T. congolense | T. b. brucei | T. evansi | |
| 1 (MPA) | 0.10 ± 0.04 | 0.62 ± 0.05 | 0.61 ± 0.002 | 0.52 ± 0.12 | 5.14 | 0.84 | 0.85 |
| 2 | 0.56 ± 0.21 | >2.5 | >2.5 | 1.4 ± 0.18 | 2.62 | ND | ND |
| 4 | 0.16 ± 0.04 | 1.26 ± 0.009 | 1.38 ± 0.10 | 0.84 ± 0.21 | 5.10 | 0.67 | 0.61 |
All values were calculated from 3 independent experiments and expressed as means ± SD.
Mean IC50 of MDBK cells/mean IC50 of trypanosomes. ND, not determined.
To clarify the mode of action of compounds 1 and 4 in trypanosomes, the effects of guanosine and xanthine supplementation on the trypanocidal effects of these compounds were examined. The IC50s of compounds 1 and 4 were increased by guanosine in a dose-dependent manner (Table 3), while xanthine supplementation did not alter the IC50s of either compound 1 or compound 4 in T. congolense BSF (Table 3). These results suggest that guanosine was transported into the T. congolense BSF and converted into GMP as a purine nucleotide source, while no xanthine was transported or converted into XMP by hypoxanthine-guanine phosphoribosyltransferase in T. congolense. We therefore concluded that the proliferation inhibitory effects of MPA against T. congolense BSF were caused by the inhibition of intracellular TcIMPDH.
TABLE 3.
Effects of guanosine and xanthine on parasite proliferation under IMPDH inhibition by MPA and N-(2,3,5-triazolyl) mycophenolic amide (compound 4)
| Guanosine or xanthine supplementation (μM) | IC50 (μM)a with: |
|||
|---|---|---|---|---|
| Guanosine for compound: |
Xanthine for compound: |
|||
| 1 (MPA) | 4 | 1 (MPA) | 4 | |
| 250 | >5.0 | >5.0 | 0.09 ± 0.001 | 0.21 ± 0.01 |
| 50 | 0.29 ± 0.19 | 0.50 ± 0.31 | 0.09 ± 0.003 | 0.22 ± 0.01 |
| 0 | 0.07 ± 0.006 | 0.13 ± 0.02 | 0.09 ± 0.004 | 0.22 ± 0.02 |
All values were calculated from 3 independent experiments and are shown as means ± SD.
Hypoxanthine and inosine were predicted to be the main purine sources in T. brucei (20). Hypoxanthine and inosine have also been shown to be present in the blood at higher concentrations than other purines (21), suggesting their roles as the main purine sources in trypanosomes and that they are supplied via the salvage pathway. The concentration of purine bases and nucleosides in the extracellular environment is lower than that in the intracellular environment (21). T. brucei spp. proliferate in blood circulation and then invade the central nervous system through the blood-brain barrier (22, 23), while T. congolense only proliferates in blood circulation by adhesion to the vascular endothelium (24). In conclusion, MPA and its derivatives might therefore also inhibit trypanosome proliferation in vivo, particularly in T. congolense.
Nucleotide sequence accession number.
The sequence for the novel IMPDH orthologue of T. congolense (TcIMPDH) can be found in the GenBank database under accession no. LC094350.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yoko Matsushita for her excellent technical assistance.
This study was financially supported by the Japan Society for the Promotion of Science (JSPS), Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and the AMED/JICA SATREPS.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02816-15.
REFERENCES
- 1.Baker N, de Koning HP, Maser P, Horn D. 2013. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol 29:110–118. doi: 10.1016/j.pt.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delespaux V, Geysen D, Van den Bossche P, Geerts S. 2008. Molecular tools for the rapid detection of drug resistance in animal trypanosomes. Trends Parasitol 24:236–242. doi: 10.1016/j.pt.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Delespaux V, Dinka H, Masumu J, Van den Bossche P, Geerts S. 2008. Five-fold increase in Trypanosoma congolense isolates resistant to diminazene aceturate over a seven-year period in Eastern Zambia. Drug Resist Updat 11:205–209. doi: 10.1016/j.drup.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Pinder M, Authie E. 1984. The appearance of isometamidium resistant Trypanosoma congolense in West Africa. Acta Trop 41:247–252. [PubMed] [Google Scholar]
- 5.Hammond DJ, Gutteridge WE. 1984. Purine and pyrimidine metabolism in the Trypanosomatidae. Mol Biochem Parasitol 13:243–261. doi: 10.1016/0166-6851(84)90117-8. [DOI] [PubMed] [Google Scholar]
- 6.Boitz JM, Ullman B, Jardim A, Carter NS. 2012. Purine salvage in Leishmania: complex or simple by design? Trends Parasitol 28:345–352. doi: 10.1016/j.pt.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vertommen D, Van Roy J, Szikora JP, Rider MH, Michels PA, Opperdoes FR. 2008. Differential expression of glycosomal and mitochondrial proteins in the two major life-cycle stages of Trypanosoma brucei. Mol Biochem Parasitol 158:189–201. doi: 10.1016/j.molbiopara.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Shu Q, Nair V. 2008. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med Res Rev 28:219–232. doi: 10.1002/med.20104. [DOI] [PubMed] [Google Scholar]
- 9.Cao S, Aboge GO, Terkawi MA, Zhou M, Luo Y, Yu L, Li Y, Goo Y, Kamyingkird K, Masatani T, Suzuki H, Igarashi I, Nishikawa Y, Xuan X. 2013. Cloning, characterization and validation of inosine 5′-monophosphate dehydrogenase of Babesia gibsoni as molecular drug target. Parasitol Int 62:87–94. doi: 10.1016/j.parint.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Umejiego NN, Li C, Riera T, Hedstrom L, Striepen B. 2004. Cryptosporidium parvum IMP dehydrogenase: identification of functional, structural, and dynamic properties that can be exploited for drug design. J Biol Chem 279:40320–40327. doi: 10.1074/jbc.M407121200. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan WJ Jr, Dixon SE, Li C, Striepen B, Queener SF. 2005. IMP dehydrogenase from the protozoan parasite Toxoplasma gondii. Antimicrob Agents Chemother 49:2172–2179. doi: 10.1128/AAC.49.6.2172-2179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobie F, Berg A, Boitz JM, Jardim A. 2007. Kinetic characterization of inosine monophosphate dehydrogenase of Leishmania donovani. Mol Biochem Parasitol 152:11–21. doi: 10.1016/j.molbiopara.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Bessho T, Morii S, Kusumoto T, Shinohara T, Noda M, Uchiyama S, Shuto S, Nishimura S, Djikeng A, Duszenko M, Martin SK, Inui T, Kubata KB. 2013. Characterization of the novel Trypanosoma brucei inosine 5′-monophosphate dehydrogenase. Parasitology 140:735–745. doi: 10.1017/S0031182012002090. [DOI] [PubMed] [Google Scholar]
- 14.Digits JA, Hedstrom L. 1999. Kinetic mechanism of Tritrichomonas foetus inosine 5′-monophosphate dehydrogenase. Biochemistry 38:2295–2306. doi: 10.1021/bi982305k. [DOI] [PubMed] [Google Scholar]
- 15.Cao S, Aboge GO, Terkawi MA, Zhou M, Kamyingkird K, Adjou Moumouni PF, Masatani T, Igarashi I, Nishikawa Y, Xuan X. 2014. Mycophenolic acid, mycophenolate mofetil, mizoribine, ribavirin, and 7-nitroindole inhibit propagation of Babesia parasites by targeting inosine 5′-monophosphate dehydrogenase. J Parasitol 100:522–526. doi: 10.1645/13-278.1. [DOI] [PubMed] [Google Scholar]
- 16.Sunohara K, Mitsuhashi S, Shigetomi K, Ubukata M. 2013. Discovery of N-(2,3,5-triazoyl)mycophenolic amide and mycophenolic epoxyketone as novel inhibitors of human IMPDH. Bioorg Med Chem Lett 23:5140–5144. doi: 10.1016/j.bmcl.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Mitsuhashi S, Takenaka J, Iwamori K, Nakajima N, Ubukata M. 2010. Structure-activity relationships for inhibition of inosine monophosphate dehydrogenase and differentiation induction of K562 cells among the mycophenolic acid derivatives. Bioorg Med Chem 18:8106–8111. doi: 10.1016/j.bmc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Suganuma K, Allamanda P, Hakimi H, Zhou M, Angeles JM, Kawazu S, Inoue N. 2014. Establishment of ATP-based luciferase viability assay in 96-well plate for Trypanosoma congolense. J Vet Med Sci 76:1437–1441. doi: 10.1292/jvms.14-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sykes ML, Baell JB, Kaiser M, Chatelain E, Moawad SR, Ganame D, Ioset JR, Avery VM. 2012. Identification of compounds with anti-proliferative activity against Trypanosoma brucei brucei strain 427 by a whole cell viability based HTS campaign. PLoS Negl Trop Dis 6:e1896. doi: 10.1371/journal.pntd.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Koning HP, Bridges DJ, Burchmore RJ. 2005. Purine and pyrimidine transport in pathogenic protozoa: from biology to therapy. FEMS Microbiol Rev 29:987–1020. doi: 10.1016/j.femsre.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Traut TW. 1994. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 22.Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, Krishna S. 2003. The trypanosomiases. Lancet 362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 23.Mulenga C, Mhlanga JD, Kristensson K, Robertson B. 2001. Trypanosoma brucei brucei crosses the blood-brain barrier while tight junction proteins are preserved in a rat chronic disease model. Neuropathol Appl Neurobiol 27:77–85. doi: 10.1046/j.0305-1846.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 24.Shakibaei M, Milaninezhad M, Risse HJ. 1994. Immunoelectron microscopic studies on the specific adhesion of Trypanosoma congolense to cultured vascular endothelial cells. J Struct Biol 112:125–135. doi: 10.1006/jsbi.1994.1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.