Abstract
Polymyxins have emerged as a last-resort treatment of extensively drug-resistant (XDR) Gram-negative Bacillus (GNB) infections, which present a growing threat. Individualized polymyxin-based antibiotic combinations selected on the basis of the results of in vitro combination testing may be required to optimize therapy. A retrospective cohort study of hospitalized patients receiving polymyxins for XDR GNB infections from 2009 to 2014 was conducted to compare the treatment outcomes between patients receiving polymyxin monotherapy (MT), nonvalidated polymyxin combination therapy (NVCT), and in vitro combination testing-validated polymyxin combination therapy (VCT). The primary and secondary outcomes were infection-related mortality and microbiological eradication, respectively. Adverse drug reactions (ADRs) between treatment groups were assessed. A total of 291 patients (patients receiving MT, n = 58; patients receiving NVCT, n = 203; patients receiving VCT, n = 30) were included. The overall infection-related mortality rate was 23.0% (67 patients). In the multivariable analysis, treatment of XDR GNB infections with MT (adjusted odds ratio [aOR], 8.49; 95% confidence interval [CI], 1.56 to 46.05) and NVCT (aOR, 5.75; 95% CI, 1.25 to 25.73) was associated with an increased risk of infection-related mortality compared to that with treatment with VCT. A higher Acute Physiological and Chronic Health Evaluation II (APACHE II) score (aOR, 1.14; 95% CI 1.07 to 1.21) and a higher Charlson comorbidity index (aOR, 1.28; 95% CI, 1.11 to 1.47) were also independently associated with an increased risk of infection-related mortality. No increase in the incidence of ADRs was observed in the VCT group. The use of an individualized antibiotic combination which was selected on the basis of the results of in vitro combination testing was associated with significantly lower rates of infection-related mortality in patients with XDR GNB infections. Future prospective randomized studies will be required to validate these findings.
INTRODUCTION
Infections caused by extensively drug-resistant (XDR) bacteria are emerging as a serious threat worldwide, including Singapore (1, 2). These resistant bacteria display nonsusceptibility to at least one antibiotic in all but two antimicrobial categories or less (i.e., the bacteria are susceptible to antibiotics in only one or two antimicrobial categories) (3). XDR infections are often associated with nosocomial infections (2), posing a severe hazard to public health care in terms of increased cost, morbidity, and mortality (1, 4). XDR Gram-negative Bacillus (GNB) infections are of particular concern due to the paucity of existing antibiotics available for efficacious treatment, and this concern is compounded by the drying pipeline of new antibiotics for the treatment of GNB infections (5).
Polymyxins, an old class of antibiotics consisting of polymyxin B and colistin (polymyxin E) in the form of the prodrug colistimethate sodium (CMS), have experienced a resurgence as last-resort options for the treatment of XDR GNB infections (6). Inevitably, in recent years, increasing numbers of reports of polymyxin heteroresistance have surfaced from in vitro studies (7–13). Some studies have indicated that synergistic effects can be achieved by combining polymyxins with other antibiotics, in turn leading to enhanced bactericidal activity and increased suppression of heteroresistance (7–11). For this reason, the use of polymyxin-based combination therapy instead of polymyxin monotherapy (MT) has been advocated. To date, the theoretical advantage of polymyxin-based combination therapy for the treatment of XDR GNB infections has not yet been realized in the majority of the published clinical studies (6, 14–19). In several of these studies, the reported outcomes reveal inconclusive results as to whether monotherapy or combination therapy is the superior option (14–19). It should be noted that the polymyxin-based antibiotic combinations that are employed in most of these studies are based on expert opinion and are not guided by any in vitro combination testing methods. Hence, any added therapeutic efficacy that the combination therapy possesses over monotherapy may have been potentially negated by the presence of strain-specific resistance of the XDR GNB to the polymyxin-based combinations chosen in these studies.
At Singapore General Hospital (SGH), a modified time-kill method known as multiple-combination bactericidal testing (MCBT) has been developed and used since 2009 to provide the in vitro combination testing-guided selection of antibiotic combinations effective against individual strains of XDR GNB in individual patients (20, 21). To the best of our knowledge, no other institution has used in vitro methods to prospectively guide polymyxin-based combination therapy for the clinical management of XDR GNB infections in individual patients. MCBT is carried out immediately after an infectious disease (ID) physician requests that a certain XDR GNB isolate be tested. The MCBT result is reported to the ID physician by an ID pharmacist within 48 h of the request.
In this study, we aimed to compare and evaluate the clinical efficacy of polymyxin combination therapy validated/guided by MCBT with nonvalidated/unguided polymyxin combination therapy and polymyxin monotherapy in the treatment of XDR GNB infections.
MATERIALS AND METHODS
Study design.
A multicenter retrospective cohort study of patients who were treated with polymyxins for XDR GNB infections during the period from 2009 to 2014 was conducted. The medical institutions involved were SGH, a 1,700-bed public acute tertiary care hospital, and Mount Elizabeth Orchard Hospital, a 345-bed private acute tertiary care hospital. The study was approved by the institutional review board of each hospital.
Study population.
All patients with suspected XDR GNB infections and prescribed polymyxins from 2009 to 2014 were identified from each hospital's electronic pharmacy database. Patients were included in the study if (i) an XDR GNB infection (evidenced by positive cultures and clinically diagnosed by an ID physician) was present, (ii) the XDR-GNB infection was treated with polymyxins for at least 48 h, (iii) the patients were adequately treated for any other concomitant non-XDR GNB infections, and (iv) the XDR GNB isolate was polymyxin sensitive. Polymyxin use was defined as the use of intravenous (i.v.) polymyxin B, i.v. CMS, or nebulized CMS administered alone or in combination with an i.v. polymyxin(s). Patients with XDR GNB cultures assessed to be colonizers or contaminants or who had incomplete patient records were excluded.
The patients included in the study were classified into one of the three treatment groups based on the type of treatment received. To be classified into the individual treatment groups, each patient had to receive the treatment defined for the group for at least 48 h. For antibiotics administered at dosing frequencies of more than 24 h between doses, e.g., in renal function-adjusted dosing regimens, at least two doses had to be administered. No patient was classified into more than one group. Polymyxin monotherapy (MT) was defined as treatment with a polymyxin(s) not overlapping with any other antibiotic for the treatment of GNB infections for the entire course of polymyxin therapy. Nonvalidated polymyxin combination therapy (NVCT) was defined as treatment with a polymyxin(s) overlapping with another antibiotic(s) for the treatment of GNB infection, regardless of whether the antibiotic was meant to be used for the treatment of the XDR infection or for the expansion of the antimicrobial spectrum, at any time during the course of polymyxin therapy. Validated polymyxin combination therapy (VCT) was defined as treatment with a polymyxin-based antibiotic combination selected on the basis of MCBT results at any time during the course of polymyxin therapy.
Microbiology.
GNB isolates were identified. Susceptibility testing to, and MICs of, various antibiotics were determined using the Kirby-Bauer disk diffusion method (22) and the Etest method (23), respectively, and the results were interpreted according to the latest Clinical and Laboratory Standards Institute criteria (13). A XDR GNB was defined as a GNB with nonsusceptibility to at least one agent in all but two antimicrobial categories or less (i.e., the bacterial isolates remained susceptible to antibiotics in only one or two categories). Upon request for MCBT by the attending clinicians, the implicated GNB cultures were sent to the SGH pharmacy anti-infective laboratory. Antibiotic combinations active in vitro were identified using the MCBT method, and appropriate individualized pharmacokinetics/pharmacodynamics-based regimens were recommended by an ID pharmacist.
Data collection and definitions.
Patient demographics, concomitant comorbidities, characteristics of the infection, treatment details, and patient outcomes were extracted from the medical records.
The Charlson comorbidity index (CCI) (24) and Acute Physiological and Chronic Health Evaluation II (APACHE II) (25) score were calculated on the day of initiation of polymyxin treatment as a gauge of the patient's risk of death on the basis of concomitant comorbidities and disease severity, respectively. The specific site of infection was determined on the basis of the attending clinician's diagnosis and was redesignated into the broad categories of sites of infection defined by the Centers for Disease Control and Prevention (CDC) (26).
An adequate i.v. polymyxin dose was defined as i.v. polymyxin B doses of at least 20,000 IU/kg of body weight/day administered in two divided doses (27) and/or i.v. CMS doses consisting of a loading dose equivalent to 9 × 106 IU and minimum maintenance doses equivalent to 9 × 106 IU/day administered in two or more doses (28). It was assumed that both polymyxins exhibited the same efficacy and toxicity if they were given at adequate doses. An adequate nebulized CMS dose was defined as a minimum of 3 × 106 IU/day administered in three separate doses (29).
Infection-related mortality and microbiological eradication were the primary and secondary outcomes of the study, respectively. Infection-related mortality was defined as in-hospital mortality secondary to the XDR infection(s), as evaluated by the attending ID physician. In-hospital patient mortality that occurred after the documented clinical resolution of the infection was not considered to be infection related. Microbiological eradication was defined as two consecutive XDR-GNB-negative cultures of clinical samples obtained from the site of infection. If samples for follow-up cultures were unavailable, microbiological eradication was assumed when clinical resolution of the infection was documented and the patients were discharged well with no readmission in the next 30 days for the same XDR GNB infection. In addition, microbiological noneradication was assumed if the patient experienced infection-related mortality and samples for follow-up cultures were unavailable.
Reported adverse drug reactions (ADRs) and resolution of these incidents were also recorded. Incidences of nephrotoxicity documented in the patient's case notes were recorded, and the severities were classified according to the RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) criteria (30). Other ADRs, such as hepatotoxicity and neurotoxicity, were also recorded. ADRs were considered to be unresolved if the patients experienced mortality before complete resolution of the ADRs.
Statistical analyses.
All statistical analyses were performed using Stata 13 software. Continuous variables were compared using the Mann-Whitney U or Kruskal-Wallis test, and categorical variables were compared using either Pearson's chi-square test or Fisher's exact test, as appropriate. Continuous and ordinal data were expressed as medians and ranges; categorical data were expressed as the number and percentage of incidences. All tests were two-tailed, and a P value of ≤0.05 was considered significant.
To identify independent predictors of infection-related mortality, covariates with clinical plausibility and P values of ≤0.2 in the univariate mortality analysis were entered into the logistic regression multivariable model in a forward stepwise manner. Variables between each treatment arm were also compared in order to identify potential confounders that may have contributed to differences in infection-related mortality. Covariates with P values of ≤0.2 in the univariate treatment arm analysis were additionally included in the model to adjust for confounding due to differences in treatment groups. The results of the logistic regression were expressed as the adjusted odds ratio (aOR) and 95% confidence interval (CI).
RESULTS
Study population.
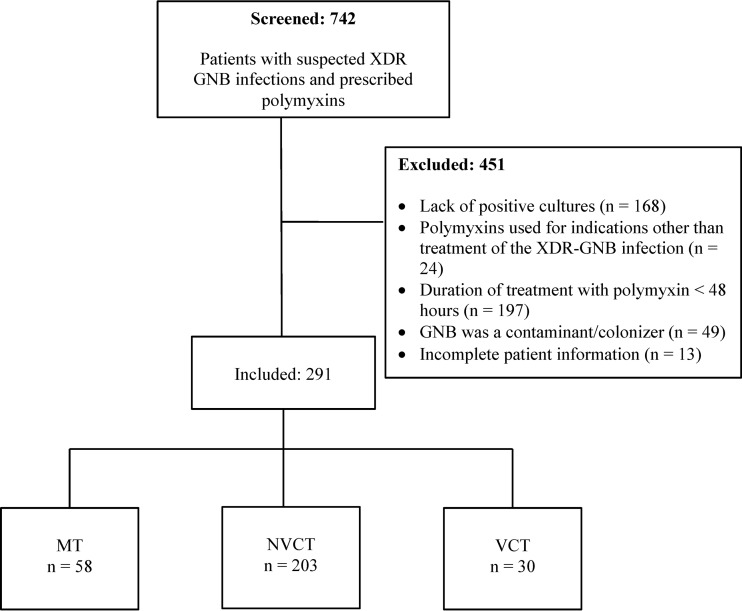
From 2009 to 2014, there were 742 patients who had a suspected XDR GNB infection and were prescribed i.v. polymyxins. Four hundred fifty-one patients did not meet the study inclusion criteria and were excluded from the study; 291 patients fulfilled the criteria for study inclusion and were included in the analysis (Fig. 1). Of these, 58 patients received MT, 203 patients received NVCT, and 30 patients received VCT.
FIG 1.
Flowchart of number of patients screened and included in each treatment group. Abbreviations: GNB, Gram-negative Bacillus; MT, polymyxin monotherapy; NVCT, nonvalidated polymyxin combination therapy; VCT, validated polymyxin combination therapy; XDR, extensively drug resistant.
Patient characteristics stratified by treatment groups.
We describe the patient baseline characteristics, stratified by treatment group, in Table 1. Upon comparison of the baseline characteristics, it was observed that the VCT group had a significantly higher CCI (median, 4.5; range, 0 to 14; P < 0.001) and APACHE II score (median, 16; range, 5 to 31; P = 0.023) than the other groups. The proportions of patients with skin and soft tissue infections (3.3%, P < 0.001) and urinary tract infections (0%, P = 0.038) were significantly lower in the VCT group. The XDR GNB involved was also significantly different between the three groups: Acinetobacter baumannii was more prevalent in the MT and NVCT groups (P < 0.001), while Klebsiella pneumoniae (P = 0.006) and Pseudomonas aeruginosa (P < 0.001) were more prevalent in the VCT group.
TABLE 1.
Baseline and infection characteristics of patients receiving MT, NVCT, and VCT
| Characteristic | Result for the following treatment arm: |
P value | ||
|---|---|---|---|---|
| MT (n = 58) | NVCT (n = 203) | VCT (n = 30) | ||
| Demographics | ||||
| Median (range) age (yr) | 63 (16–93) | 58 (16–87) | 59 (21–92) | 0.389 |
| No. (%) of male patients | 37 (63.8) | 137 (67.5) | 19 (63.3) | 0.815 |
| No. (%) of patients with the following comorbidities: | ||||
| Ischemic heart disease | 14 (24.1) | 45 (22.2) | 5 (16.7) | 0.721 |
| Congestive heart failure | 6 (10.3) | 15 (7.4) | 6 (20.0) | 0.081 |
| Cerebrovascular disease | 6 (10.3) | 18 (8.9) | 7 (23.3) | 0.056 |
| Diabetes mellitus | 29 (50.0) | 72 (35.5) | 11 (36.7) | 0.131 |
| Chronic kidney disease | 19 (32.8) | 52 (25.6) | 6 (20.0) | 0.387 |
| Hepatic disease | 4 (6.9) | 17 (8.4) | 4 (13.3) | 0.582 |
| Malignancy disease | 9 (15.5) | 42 (20.7) | 12 (40.0) | 0.025 |
| Autoimmune disease | 1 (1.7) | 4 (2.0) | 3 (10.0) | 0.037 |
| Immunocompromised | 5 (8.6) | 13 (6.4) | 10 (33.3) | 0.037 |
| Median (range) CCI | 2 (0–8) | 2 (0–10) | 4.5 (0–14) | <0.001 |
| Median (range) APACHE II score | 12 (0–23) | 14 (0–29) | 16 (5–31) | 0.023 |
| No. (%) of patients with the following primary infection or site of infectiona: | ||||
| Central nervous system | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0.805 |
| Nosocomial pneumonia | 18 (31.0) | 89 (43.8) | 11 (36.7) | 0.194 |
| Tracheobronchitis | 1 (1.7) | 3 (1.5) | 0 (0.0) | 0.784 |
| Skin and soft tissue | 21 (36.2) | 84 (41.4) | 1 (3.3) | <0.001 |
| Bone and joint | 4 (6.9) | 15 (7.4) | 4 (13.3) | 0.504 |
| Gastrointestinal system | 1 (1.7) | 11 (5.4) | 2 (6.7) | 0.450 |
| Urinary tract | 11 (19.0) | 25 (12.3) | 0 (0.0) | 0.038 |
| Blood | 13 (22.4) | 62 (30.5) | 13 (43.3) | 0.127 |
| Secondary bacteremia | 4 (6.9) | 39 (19.2) | 11 (36.7) | 0.003 |
| No. (%) of patients in whom the following XDR GNB was responsible for infectionb: | ||||
| A. baumannii | 47 (81.0) | 170 (83.7) | 11 (36.7) | <0.001 |
| P. aeruginosa | 9 (15.5) | 38 (18.7) | 14 (46.7) | <0.001 |
| K. pneumoniae | 2 (3.4) | 7 (3.4) | 5 (16.7) | 0.006 |
| Other XDR-GNBc | 2 (3.4) | 5 (2.5) | 0 (0.0) | 0.289 |
| Details of polymyxin used | ||||
| Median (range) duration between XDR culture and treatment (days) | 3 (0–55) | 3 (0–79) | 3 (1–39) | 0.046 |
| No. (%) of patients who received polymyxin B | 44 (21.7) | 162 (79.8) | 30 (100) | 0.016 |
| Median (range) avg no. of IU of polymyxin B/kg/day administered to each patient | 18,006 (2,315–33,846) | 21,467 (3,163–45,977) | 25,000 (25,000–25,000) | <0.001 |
| No. (%) of patients who received i.v. CMS | 7 (12.1) | 39 (19.2) | 1 (3.3) | 0.056 |
| Median (range) avg no. of IU of i.v. CMS/day administered to each patient | 2,500,000 (1,000,000–3,500,000) | 4,090,000 (1,530,000–9,000,000) | 9,000,000 | 0.009 |
| No. (%) of patients who received nebulized CMS | 16 (27.6) | 81 (39.9) | 11 (36.7) | 0.231 |
| Median (range) avg no. of IU of nebulized CMS/day administered to each patient | 6,000,000 (3,000,000–6,000,000) | 6,000,000 (2,860,000–9,000,000) | 6,000,000 (6,000,000–6,000,000) | 0.122 |
| Median (range) proportion of treatment days with adequate i.v. polymyxin dosese | 0.00 (0.00–1.00) | 0.52 (0.00–1.00) | 1.00 (1.00–1.00) | <0.001 |
| Median (range) proportion of treatment days with adequate nebulized CMS dosese | 1.00 (1.00–1.00) | 1.00 (0.86–1.00) | 1.00 (1.00–1.00) | 0.845 |
| Median (range) duration (days) of polymyxin treatment | 9.5 (3–27) | 15 (3–139) | 16.5 (6–126) | <0.001 |
There may be multiple primary sites of XDR GNB infection per patient.
There may be multiple XDR GNB cultured at each site of infection.
Other GNB included Stenotrophomonas maltophilia (n = 1), Enterobacter cloacae (n = 2), Pseudomonas putida (n = 2), a Citrobacter species (n = 1), and an Enterobacter species (n = 1).
The patient may have received ≥1 polymyxin in the course of treatment.
Defined as the proportion of i.v. polymyxin B or i.v. CMS treatment days on which each patient received adequate doses of the respective polymyxin.
Overall, the median time from the time of the culture isolation to the time of receipt of polymyxin therapy for the treatment of the XDR-GNB infections was 3 days (range, 0 to 79 days). Two-hundred thirty-six patients received i.v. polymyxin B, while 47 patients received i.v. CMS. In addition, 108 patients received nebulized CMS with or without i.v. polymyxins. The median i.v. polymyxin B and i.v. CMS doses administered to the patients across all treatment groups were 21,949 IU/kg/day (range, 2,315 to 45,977 IU/kg/day) and 3,800,000 IU/day (range,1,000,000 to 9,000,000 IU/day), respectively. All patients in the VCT group were maintained on adequate i.v. polymyxin B or i.v. CMS doses for the entire treatment duration; in contrast, patients in the NVCT group were maintained on adequate i.v. polymyxin B or i.v. CMS doses for only half of the treatment duration (median proportion of polymyxin treatment days on adequate i.v. polymyxins in the NVCT group, 0.52 [range, 0.00 to 1.00]).
Patient outcomes.
The overall infection-related mortality rate was 23.0% (67 patients). Upon a crude comparison of the 3 groups, 13 (22.4%), 50 (24.6%), and 4 (13.3%) patients in the MT, NVCT, and VCT groups, respectively, died (P = 0.387). A high rate of bacteriological eradication was observed (222/291, 76.3%), with bacteriological eradication being achieved in 77.6%, 74.4%, and 86.7% of the patients in the MT, NVCT, and VCT groups, respectively, (P = 0.325). All patients who did not have infection-related mortality experienced microbiological eradication, except for two patients from the NVCT arm (98.2%): one was discharged against medical advice and lost to follow-up, and the other patient was terminally discharged due to medical futility (worsening pneumonia despite prolonged antibiotic therapy). Two patients died due to the XDR-GNB infection, despite having two consecutive negative cultures of samples from the site of infection. We further described the types of antibiotics used in combination with polymyxins in the NVCT and VCT groups and the respective infection-related mortalities observed with each type of combination therapy in Table 2. As shown, polymyxins in combination with carbapenems were most commonly employed in both the NVCT group (61.1%) and the VCT group (66.7%). For each individual polymyxin combination, lower rates of infection-related mortality were observed in the VCT group than the NVCT group.
TABLE 2.
Antibiotics used in combination with polymyxins in patients receiving NVCT and VCT
| Antibiotic used in combination with polymyxinsa | NVCT group (n = 203) |
VCT group (n = 30) |
||
|---|---|---|---|---|
| No. (%) of patients | No. (%) of patients with infection-related mortality | No. (%) of patients | No. (%) of patients with infection-related mortality | |
| Carbapenemsb | 124 (61.1) | 37 (29.8) | 20 (66.7) | 4 (20.0) |
| Fluoroquinolonesc | 52 (25.6) | 16 (30.8) | 7 (23.3) | 1 (14.3) |
| Beta-lactam–beta-lactamase inhibitord | 51 (25.1) | 8 (15.7) | 1 (3.3) | 0 (0.0) |
| Aminoglycosidese | 14 (6.9) | 4 (28.6) | 6 (20.0) | 1 (16.7) |
| Cephalosporinsf | 37 (18.3) | 6 (16.2) | 0 (0.0) | |
| Aztreonam | 13 (6.4) | 2 (15.4) | 4 (13.3) | 0 (0.0) |
| Rifampin | 28 (13.8) | 11 (39.3) | 4 (13.3) | 1 (25.0) |
| Tigecycline | 46 (22.7) | 12 (26.1) | 1 (3.3) | 0 (0.0) |
Each patient could receive more than 1 type of polymyxin combination throughout the treatment course.
The carbapenems used included meropenem, ertapenem, doripenem, and imipenem-cilastin.
The fluoroquinolones used included levofloxacin and ciprofloxacin.
The beta-lactam–beta-lactamase inhibitors used included piperacillin-tazobactam, ampicillin-sulbactam, and amoxicillin-clavulanate.
The aminoglycosides used included amikacin and gentamicin.
The cephalosporins used included ceftazidime, ceftriaxone, and cefepime.
Observed incidences of possible toxicities attributed to antibiotic use are summarized in Table 3. Overall, one or more ADRs occurred in 119 (40.9%) patients. Nephrotoxicity was the most common ADR, occurring in 86.6% of all patients who experienced ADRs. VCT was not associated with a higher incidence of ADRs than NVCT and MT (P = 0.215). Overall, ADRs were well managed, and the majority was resolved upon discharge (73.1%), with no differences between treatment arms being observed (P = 0.545). Thirty-two (26.9%) patients did not experience resolution of their ADRs; of these, 26 patients died before resolution of the ADRs. For the remaining 6 patients, 4 patients experienced nephrotoxicity but had trends of improving serum creatinine levels at the point of discharge. The other 2 patients displayed no signs of improvement in the ADRs: one patient experienced potentially irreversible hyperpigmentation attributed to polymyxin B, and the other experienced end-stage kidney disease and had to undergo dialysis.
TABLE 3.
Possible ADRs due to antibiotic use between treatment arms
| Group or ADR | No. (%) of patients in the following polymyxin treatment arm: |
P value | ||
|---|---|---|---|---|
| MT (n = 58) | NVCT (n = 203) | VCT (n = 30) | ||
| All patients with an ADR | 18 (31.0) | 89 (43.8) | 12 (40.0) | 0.215 |
| Patients in which the ADR resolveda | 14 (77.8) | 63 (70.8) | 10 (83.3) | 0.545 |
| Nephrotoxicity based on RIFLE criteria | 16 (27.6) | 74 (36.5) | 8 (26.7) | 0.313 |
| Risk of renal dysfunction | 8 (13.8) | 20 (9.9) | 4 (13.3) | 0.637 |
| Injury to the kidney | 5 (8.6) | 31 (15.3) | 0 (0.0) | 0.038 |
| Failure of kidney function | 3 (5.2) | 22 (10.8) | 3 (10.0) | 0.434 |
| Loss of kidney function | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0.013 |
| End-stage kidney disease | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0.805 |
| Nephrotoxicity not included in the RIFLE criteriab | 0 (0.0) | 4 (2.0) | 1 (3.3) | 0.460 |
| Total incidence of nephrotoxicityc | 16 (27.6) | 78 (38.4) | 9 (30.0) | 0.254 |
| Neurotoxicity | 1 (1.7) | 10 (4.9) | 1 (3.3) | 0.543 |
| Hepatotoxicity | 2 (3.4) | 8 (3.9) | 0 (0.0) | 0.542 |
| Othersd | 2 (3.4) | 13 (6.4) | 3 (10.0) | 0.468 |
The percentages in parentheses refer to the percentage of patients who experienced a resolution of the adverse drug reactions among all patients who experienced adverse drug reactions.
These patients experienced electrolyte imbalances due to nephropathy without a concomitant rise in the serum creatinine level of ≥1.5 times the baseline level (a requisite for the least severe grade of nephrotoxicity under the RIFLE criteria).
Includes incidences of nephrotoxicity classified under the RIFLE criteria and those that do not meet its requirements.
Others included hyperpigmentation (n = 2), drug-related exanthem (n = 5), drug-related fever (n = 1), bronchospasm (n = 1), phlebitis (n = 1), nausea and vomiting (n = 3), diarrhea (n = 3), abdominal pain (n = 1), thrombocytopenia (n = 1), leukopenia (n = 1), and pancytopenia (n = 1).
Risk factors associated with in-hospital mortality.
The results of bivariate analysis, stratified by the presence or absence of infection-related mortality, are shown in Table 4. We observed that the group with infection-related mortality was significantly younger (median age, 58 years; P = 0.047) and had a higher median CCI (median CCI, 4; P < 0.001) and a higher median APACHE II score (median APACHE II score, 18; P < 0.001). The group with infection-related mortality also had a significantly higher proportion of cases of nosocomial pneumonia (67.1%, P < 0.001) and secondary bacteremia (28.4%, P = 0.019). In the multivariable analysis, treatment of XDR GNB infections with MT (aOR, 8.49; 95% CI, 1.56 to 46.05) and NVCT (aOR, 5.75; 95% CI, 1.25 to 25.73) was associated with a risk of infection-related mortality approximately 8 times and 6 times, respectively, compared to that from treatment with VCT after adjusting for potential confounders (i.e., VCT was associated with a significantly lower risk of infection-related mortality than MT and NVCT) (Table 5). A higher APACHE II score (aOR, 1.14; 95% CI, 1.07 to 1.21) and a higher CCI (aOR, 1.28; 95% CI, 1.11 to 1.47) were also independently associated with an increased risk of infection-related mortality.
TABLE 4.
Potential risk factors for infection-related mortality in patients with XDR GNB infections and treated with polymyxin MT or guided and nonguided combination therapy
| Characteristic | Result for patients with: |
P value | |
|---|---|---|---|
| No infection-related mortality (n = 224) | Infection-related mortality (n = 67) | ||
| Demographics | |||
| Median (range) age (yr) | 65 (16–92) | 58 (16–93) | 0.047 |
| No. (%) of male patients | 147 (65.6) | 46 (68.7) | 0.645 |
| No. (%) of patients with the following comorbidities: | |||
| Ischemic heart disease | 42 (18.8) | 22 (32.9) | 0.015 |
| Congestive heart failure | 18 (8.0) | 9 (13.4) | 0.182 |
| Cerebrovascular disease | 19 (8.5) | 12 (17.9) | 0.028 |
| Diabetes mellitus | 83 (37.1) | 29 (43.3) | 0.358 |
| Chronic kidney disease | 53 (23.7) | 24 (35.8) | 0.048 |
| Hepatic disease | 19 (8.5) | 6 (9.0) | 0.904 |
| Malignancy disease | 45 (20.1) | 18 (26.9) | 0.237 |
| Autoimmune disease | 6 (2.7) | 2 (3.0) | 0.893 |
| Immunocompromised | 17 (7.6) | 11 (16.4) | 0.032 |
| Median (range) CCI | 2 (0–14) | 4 (0–10) | <0.001 |
| Median (range) APACHE II score | 12 (0–28) | 18 (9–31) | <0.001 |
| No. (%) of patients with the following primary infection or site of infectiona: | |||
| Central nervous system | 1 (0.4) | 0 (0.0) | 0.584 |
| Nosocomial pneumonia | 73 (32.6) | 45 (67.1) | <0.001 |
| Tracheobronchitis | 4 (1.8) | 0 (0.0) | 0.271 |
| Skin and soft tissue | 85 (37.9) | 21 (31.3) | 0.324 |
| Bone and joint | 21 (9.4) | 2 (3.0) | 0.089 |
| Gastrointestinal system | 11 (4.9) | 3 (4.5) | 0.884 |
| Urinary tract | 29 (12.9) | 7 (10.4) | 0.586 |
| Blood | 62 (27.7) | 26 (38.8) | 0.082 |
| Secondary bacteremia | 35 (15.6) | 19 (28.4) | 0.019 |
| No. (%) of patients in whom the following XDR GNB was responsible for infectionb: | |||
| Acinetobacter baumannii | 171 (76.3) | 57 (85.1) | 0.128 |
| Pseudomonas aeruginosa | 52 (23.2) | 9 (13.4) | 0.084 |
| Klebsiella pneumoniae | 13 (5.8) | 1 (1.5) | 0.148 |
| Othersc | 3 (1.3) | 1 (1.5) | 0.925 |
| Treatment detailsd | |||
| No. (%) of patients who received polymyxin B | 185 (82.6) | 51 (76.1) | 0.235 |
| Median (range) avg no. of IU of polymyxin B/kg/day administered to each patient | 21,919 (2,315–45,977) | 22,189 (3,163–34,231) | 0.971 |
| No. (%) of patients who received i.v. CMS | 34 (15.2) | 13 (19.4) | 0.410 |
| Median (range) avg no. of IU of i.v. CMS/day administered to each patient | 4,000,000 (1,000,000–9,000,000) | 3,050,000 (1,530,000–9,000,000) | 0.410 |
| Median (range) proportion of treatment days with adequate i.v. polymyxin B or i.v. CMS dosese | 0.70 (0.00–1.00) | 0.38 (0.00–1.00) | 0.169 |
| No. (%) of patients who received nebulized CMS | 69 (30.8) | 39 (58.2) | <0.001 |
| Median (range) avg no. of IU of nebulized CMS/day administered to each patient | 6,000,000 (3,000,000–9,000,000) | 6,000,000 (2,860,000–6,000,000) | 0.405 |
| Median (range) proportion of treatment days with adequate nebulized CMS dosese | 1.00 (1.00–1.00) | 1.00 (0.86–1.00) | 0.178 |
| Median (range) duration (days) between XDR culture and treatment | 3 (0–45) | 2 (1–4) | 0.137 |
| No. (%) of patients receiving the following treatment type: | |||
| MT | 45 (20.1) | 13 (19.4) | 0.902 |
| NVCT | 153 (68.3) | 50 (74.6) | 0.323 |
| VCT | 26 (11.6) | 4 (6.0) | 0.183 |
There may be multiple primary sites of XDR GNB infection per patient.
There may be multiple XDR GNB cultured at each site of infection.
Other GNB included Stenotrophomonas maltophilia (n = 1), Enterobacter cloacae (n = 2), Pseudomonas putida (n = 2), a Citrobacter species (n = 1), and an Enterobacter species (n = 1).
Patients may have received ≥1 polymyxin in the course of treatment.
Proportion of adequate i.v. polymyxin/i.v. CMS/nebulized CMS doses is defined as the proportion of polymyxin treatment days in which each patient received adequate doses of the respective polymyxin.
TABLE 5.
Multivariable logistic regression model for significant predictors of infection-related mortality
| Covariatea | aOR | 95% CI |
|---|---|---|
| APACHE II score | 1.14 | 1.07–1.21 |
| CCI | 1.28 | 1.11–1.47 |
| MT | 8.49 | 1.56–46.05 |
| NVCT | 5.75 | 1.25–25.73 |
Other factors that were included in the multivariable regression model but that were not significant included nosocomial pneumonia, bone and joint infection, secondary bacteremia, A. baumannii as the infecting organism, P. aeruginosa as the infecting organism, proportion of treatment days with adequate i.v. polymyxin doses, and receipt of nebulized CMS. Factors that were evaluated but that did not remain in the stepwise regression model included K. pneumoniae as the infecting organism, bloodstream infection, average number of doses of polymyxin B administered, and duration (in days) between XDR culture and treatment.
DISCUSSION
This is the first study to evaluate the clinical efficacy of MCBT-guided polymyxin-based treatments of XDR GNB infections and compare it with that of unguided polymyxin-based antibiotic combinations and polymyxin monotherapy. We showed that the use of MCBT-guided polymyxin-based combination therapy for the treatment of XDR GNB infections was independently associated with a decreased risk of infection-related mortality.
Use of a combination of polymyxins with another antibiotic for the treatment of polymyxin-susceptible GNB was first proposed to overcome some shortcomings of polymyxin monotherapy, such as the potential for therapeutic failure due to the presence of polymyxin heteroresistance (7, 8, 10, 11). Against isolates with high polymyxin MICs (≥2 mg/liter), polymyxins may achieve only limited bacterial killing, considering the unbound polymyxin concentrations that are achievable in patients (31, 32). Hence, while monotherapy may be appropriate for patients with less severe infections caused by isolates which are highly susceptible to polymyxins, critically ill patients with deep-seated infections or infections caused by isolates with high polymyxin MICs would likely benefit the most from rationally optimized polymyxin combination therapy. In addition, a number of recent studies have suggested that polymyxin monotherapy may be inferior to therapy with other drugs for the treatment of GNB, further corroborating the idea that combination therapy is necessary (33, 34).
Like previous nonrandomized studies, we observed large variability in the baseline and treatment characteristics between the treatment groups in our study (35, 36). It is noteworthy that patients in the VCT group had significantly more comorbidities, higher APACHE II scores, and higher incidences of secondary bacteremia (bacteremia complicating a primary infection site). Despite this, patients in the VCT group still had lower mortality rates (13%) than patients in the other two groups (for patients receiving MT, the mortality rate was 22%; for patients receiving NVCT, the mortality rate was 25%), although these differences in mortality rates were not statistically significant in the unadjusted comparison of the treatment groups. Unlike patients in the MT or NVCT group, all patients in the VCT group received adequate i.v. polymyxin doses for the entire treatment duration. This can be attributed to the fact that when an MCBT result is reported, the corresponding antimicrobial doses selected to maximize the probability of achieving the target values for the pharmacokinetic/pharmacodynamic parameters in the specific patient are also simultaneously recommended to the ID physician by the ID pharmacist. This is in contrast to the situation for patients in the MT and NCVT groups, whereby antimicrobial doses were selected on the basis of the individual physician's discretion and may be reduced out of a fear of nephrotoxicity. Furthermore, some physicians also opted to reduce the doses of i.v. polymyxins for patients in the NVCT group, based on the belief that lower doses of each antibiotic were needed due to the presence of synergism when antibiotics are employed in combination.
To account for potential confounding due to differences in baseline and treatment characteristics, we performed a multivariable regression analysis to elucidate factors that were independently associated with infection-related mortality in our study cohort. In addition to the stepwise inclusion of variables that were found to be different (P < 0.2) between patients who died and those who did not die, we also included variables that were different between the treatment groups to account for potential selection bias. Of note, our final model accounted for the severity and type of illness in the patient, the proportion of patients receiving appropriate i.v. polymyxin doses, as well as the differences in the causative GNB organisms. After adjusting for potential confounders in baseline characteristics, we found that treatment of XDR GNB infections with MT and NVCT was associated with approximately 8 times and 6 times the risk of infection-related mortality, respectively, compared to that with treatment with VCT. In other words, VCT was associated with a significantly lower rate of infection-related mortality than MT and NVCT in our adjusted analysis. A higher APACHE II score and a higher CCI were also found to independently increase the risk of infection-related mortality, which may explain the lack of a treatment effect of VCT in the unadjusted analysis, given the significantly higher APACHE II score and CCI in the VCT group. The incidences of nephrotoxicity were similar across all treatment groups, despite the fact that the polymyxin doses in the VCT arm were higher than those in the NVCT or MT arm, The rates of nephrotoxicity observed in our study were comparable to the rates reported in previous studies, which ranged from 23 to 46% (14, 19, 37).
The findings of our study were similar to those of a previous study (35). Shields et al. compared the use of colistin-based antibiotic combinations guided by in vitro testing to those unguided by in vitro testing for the treatment of XDR A. baumannii infections and observed improved outcomes with in vitro testing-guided combinations (35). The authors postulated that the poor outcomes observed in patients treated with previously reported antibiotic combinations were due to different mechanisms of resistance in the strains of XDR A. baumannii present in their patient population. This concept appears to be highly applicable in our study, particularly in view of the varied resistance genotypes harbored by local XDR GNB isolates (21). Our study findings lend further credence to the concept that polymyxin combinations selected on the basis of information previously published in the literature or anecdotal experience will likely be inadequate due to strain-specific resistance and that the selection of individualized polymyxin-based combinations through individualized prospective testing is necessary for the treatment of infections caused by unique strains of XDR GNB in individual patients.
Our study is not without limitations. First, like most retrospective and nonrandomized studies, we observed significant differences in baseline characteristics between the treatment groups, which may result in confounding. In an attempt to address the most important confounders, we conducted a multivariable regression analysis, ensuring that statistically significant confounders with clinical plausibility were maintained in our final multivariable model. However, we acknowledge that such analyses cannot control for unknown confounders. Furthermore, the nonrandomized nature of the study may have led to potential bias (e.g., selection bias), which cannot be fully addressed using a multivariable regression model. Second, patients with infection-related mortality received a significantly lower proportion of appropriate i.v. polymyxin doses over the treatment duration. This highlights the importance of appropriate polymyxin doses in ensuring good patient outcomes, as suggested by multiple recently published pharmacokinetic/pharmacodynamic and clinical studies (32, 38, 39). Unfortunately, we were unable to conduct a subgroup analysis to determine if the outcomes for patients in the MT and NVCT groups with fully adequate i.v. polymyxins (i.e., adequate i.v. polymyxin doses for 100% of the entire treatment duration) were comparable to those for patients in the VCT group with fully adequate i.v. polymyxins. This is because the i.v. polymyxin doses administered to each patient were often changed during the treatment duration, particularly in the MT and NVCT groups. Hence, too few patients received the entire adequate course of i.v. polymyxins to allow any sound statistical analysis. Moving forward, future studies, preferably controlled and randomized trials, with larger numbers of patients will be required to validate our results.
Conclusions.
This is the first study to review and compare the clinical efficacy of MCBT-guided polymyxin-based antibiotic combination therapy with polymyxin monotherapy and unguided polymyxin combination therapy for the treatment of XDR GNB infections. The use of MCBT-guided polymyxin therapy was associated with rates of infection-related mortality significantly lower than those that occurred after polymyxin monotherapy and unguided polymyxin combination therapy and potentially has a critical role in guiding the future use of polymyxin combinations in patients with XDR GNB infections. The majority of toxicities associated with antibiotic treatment were generally well controlled and resolved upon the discontinuation of treatment. VCT is not associated with an increased risk of ADRs compared to the risk with NVCT and MT. Further prospective studies, preferably randomized trials, with larger patient populations are necessary to validate the efficacy and safety of MCBT-guided polymyxin combination therapy.
ACKNOWLEDGMENTS
We declare that there are no conflicts of interest.
This work was supported in part by a National Medical Research Council exploratory and developmental grant (EDG11May068) and Singapore General Hospital research grants (SRG#09/2011, SRG#15/2011, and SRG/C4/04/2014).
Funding Statement
This work was supported and funded in part by the National Medical Research Council (NMRC) (EDG11May068). This work, including the efforts of Andrea Lay-Hoon Kwa, was funded by Singapore General Hospital (SGH) (SRG#9/2011, SRG#15/2011, and SRG/C4/04/2014). This work was supported in part by a National Medical Research Council Centre Grant (NMRC/CG/016/2013).
The funders had no role in study design, data collection and analysis, decision to publish, preparation of the manuscript, or the decision to submit the work for publication.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Hsu L-Y, Tan T-Y, Jureen R, Koh T-H, Krishnan P, Tzer-Pin Lin R, Wen-Sin Tee N, Tambyah PA. 2007. Antimicrobial drug resistance in Singapore hospitals. Emerg Infect Dis 13:1944–1947. doi: 10.3201/eid1312.070299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 4.Livermore DM. 2007. Introduction: the challenge of multiresistance. Int J Antimicrob Agents 29:S1–S7. doi: 10.1016/S0924-8579(07)00158-6. [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. 2013. 10 × ′20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Y, Lee W, Kwa AL. 2015. Polymyxin B versus colistin: an update. Expert Rev Anti Infect Ther 13:1481–1497. doi: 10.1586/14787210.2015.1093933. [DOI] [PubMed] [Google Scholar]
- 7.O'Hara JA, Ambe LA, Casella LG, Townsend BM, Pelletier MR, Ernst RK, Shanks RM, Doi Y. 2013. Activities of vancomycin-containing regimens against colistin-resistant Acinetobacter baumannii clinical strains. Antimicrob Agents Chemother 57:2103–2108. doi: 10.1128/AAC.02501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim TP, Tan TY, Lee W, Sasikala S, Tan TT, Hsu LY, Kwa AL. 2011. In-vitro activity of polymyxin B, rifampicin, tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii in Singapore. PLoS One 6:e18485. doi: 10.1371/journal.pone.0018485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim TP, Lee W, Tan TY, Sasikala S, Teo J, Hsu LY, Tan TT, Syahidah N, Kwa AL. 2011. Effective antibiotics in combination against extreme drug-resistant Pseudomonas aeruginosa with decreased susceptibility to polymyxin B. PLoS One 6:e28177. doi: 10.1371/journal.pone.0028177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon NC, Png K, Wareham DW. 2010. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 54:5316–5322. doi: 10.1128/AAC.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Bergen PJ, Bulitta JB, Tsuji B, Forrest A, Nation RL, Li J. 2013. Synergistic activity of colistin and rifampin combination against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:3738–3745. doi: 10.1128/AAC.00703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yau W, Owen RJ, Poudyal A, Bell JM, Turnidge JD, Yu HH, Nation RL, Li J. 2009. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infect 58:138–144. doi: 10.1016/j.jinf.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Barin J, Martins AF, Heineck BL, Barth AL, Zavascki AP. 2013. Hetero- and adaptive resistance to polymyxin B in OXA-23-producing carbapenem-resistant Acinetobacter baumannii isolates. Ann Clin Microbiol Antimicrob 12:15. doi: 10.1186/1476-0711-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A, Viscoli C, Zarrilli R, Gallo C, Utili R. 2013. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 15.Falagas ME, Rafailidis PI, Kasiakou SK, Hatzopoulou P, Michalopoulos A. 2006. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin-meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin Microbiol Infect 12:1227–1230. doi: 10.1111/j.1469-0691.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 16.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108-2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 19.Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, Kokturk F, Ornek T, Celebi G. 2013. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect 141:1214–1222. doi: 10.1017/S095026881200194X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai YY, Lim TP, Sasikala S, Rafida N, Tan TY, Lee W, Hsu LY, Teo J, Tan TT, Kwa AL. 2012. Comparison of a rapid multiple combination bactericidal testing method versus conventional time-kill studies for patients infected with extreme drug-resistant Acinetobacter baumannii with decreasing susceptibilities to polymyxin B. Abstr 22nd Eur Congr Clin Microbiol Infect Dis (ECCMID), London, United Kingdom. [Google Scholar]
- 21.Teo J, Cai Y, Lim TP, Tan TT, Kwa AL. 2016. Carbapenem resistance in gram-negative bacteria: the not-so-little problem in the little red dot. Microorganisms 4:13. doi: 10.3390/microorganisms4010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer AW, Kirby WM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496. [PubMed] [Google Scholar]
- 23.Baker CN, Stocker SA, Culver DH, Thornsberry C. 1991. Comparison of the E Test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J Clin Microbiol 29:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 26.CDC/NHSN. 2014. CDC/NHSN surveillance definitions for specific types of infections. CDC, Atlanta, GA: http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf. [Google Scholar]
- 27.Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin Microbiol Rev 21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumbarello M, De Pascale G, Trecarichi EM, De Martino S, Bello G, Maviglia R, Spanu T, Antonelli M. 2013. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest 144:1768–1775. doi: 10.1378/chest.13-1018. [DOI] [PubMed] [Google Scholar]
- 30.Bellomo R, Ronco C, Kellum J, Mehta R, Palevsky P, Acute Dialysis Quality Initiative workgroup. 2004. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergen PJ, Landersdorfer CB, Zhang J, Zhao M, Lee HJ, Nation RL, Li J. 2012. Pharmacokinetics and pharmacodynamics of ‘old' polymyxins: what is new? Diagn Microbiol Infect Dis 74:213–223. doi: 10.1016/j.diagmicrobio.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 33.Rigatto MH, Ribeiro VB, Konzen D, Zavascki AP. 2013. Comparison of polymyxin B with other antimicrobials in the treatment of ventilator-associated pneumonia and tracheobronchitis caused by Pseudomonas aeruginosa or Acinetobacter baumannii. Infection 41:321–328. doi: 10.1007/s15010-012-0349-z. [DOI] [PubMed] [Google Scholar]
- 34.Kvitko CH, Rigatto MH, Moro AL, Zavascki AP. 2011. Polymyxin B versus other antimicrobials for the treatment of Pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother 66:175–179. doi: 10.1093/jac/dkq390. [DOI] [PubMed] [Google Scholar]
- 35.Shields RK, Kwak EJ, Potoski BA, Doi Y, Adams-Haduch JM, Silviera FP, Toyoda Y, Pilewski JM, Crespo M, Pasculle AW, Clancy CJ, Nguyen MH. 2011. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis 70:246-252. doi: 10.1016/j.diagmicrobio.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Rigatto MH, Vieira FJ, Antochevis LC, Behle TF, Lopes NT, Zavascki AP. 2015. Polymyxin B in combination with antimicrobials lacking in vitro activity versus polymyxin B in monotherapy in critically ill patients with Acinetobacter baumannii or Pseudomonas aeruginosa infections. Antimicrob Agents Chemother 59:6575–6580. doi: 10.1128/AAC.00494-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, Costa LW, Zavascki AP. 2015. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemother 70:1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 38.Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. 2012. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 54:1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]