Abstract
Solithromycin is a new fluoroketolide. The purpose of the present study was to investigate the effect of orally administered solithromycin on the human oropharyngeal and intestinal microbiota. Thirteen healthy volunteers (median age, 27.3 years) received oral solithromycin at 800 mg on day 1 followed by 400 mg daily on days 2 to 7. Fecal and saliva samples were collected at baseline and on days 2, 5, 7, 9, 14, and 21 for pharmacokinetic and microbiological analyses. Plasma samples were collected predose on days 2, 5, and 7 as proof of exposure, and solithromycin concentration ranges were 21.9 to 258 ng/ml, 18.0 to 386 ng/ml, and 16.9 to 417 ng/ml, respectively. The solithromycin concentrations in feces were 15.8 to 65.4 mg/kg, 24.5 to 82.7 mg/kg, 21.4 to 82.7 mg/kg, 12.1 to 72.4 mg/kg, 0.2 to 25.6 mg/kg, and 0 to 0.5 mg/kg on days 2, 5, 7, 9, 14, and 21, respectively. The numbers of enterobacteria and enterococci decreased and were normalized on day 14. The numbers of lactobacilli and bifidobacteria decreased from day 2 to day 14 and were normalized on day 21. The clostridia decreased on days 2, 7, and 14 and were normalized on day 21. No Clostridium difficile strains or toxins were detected during the study period. The number of Bacteroides strains was not significantly changed. The solithromycin concentrations in saliva were 0 to 1.2 mg/liter, 0 to 0.5 mg/liter, 0 to 0.5 mg/liter, and 0 to 0.1 mg/liter on days 2, 5, 7, and 9, respectively. The numbers of streptococci decreased on day 2 and were normalized on day 5. The numbers of lactobacilli, prevotellae, fusobacteria, and leptotrichiae decreased from day 2 and were normalized on day 21.
INTRODUCTION
Solithromycin is a new fluoroketolide in the macrolide class of antibiotics. It is being developed as intravenous and oral formulations for the treatment of patients with community-acquired bacterial pneumonia (CABP) (1) and oral treatment of uncomplicated urogenital gonorrhea (2, 3). Solithromycin has activity against macrolide-resistant CABP pathogens, including Streptococcus pneumoniae, Staphylococcus aureus (including community-acquired methicillin-resistant S. aureus [MRSA]), and Mycoplasma pneumoniae and greater potency than other macrolides against Legionella pneumophila (2, 4–8).
The normal microbiota acts as a barrier against colonization by potentially pathogenic microorganisms and against overgrowth of already-present opportunistic microorganisms. Administration of antimicrobial agents, therapeutically or as prophylaxis, causes disturbances in the ecological balance between the host and the normal microbiota. Knowledge about the interaction between antimicrobial agents and the normal microbiota gives the clinician the possibility to choose agents associated with lesser degrees of ecological disturbances. Consequently, the risk of development of resistant strains and transfer of resistance elements between microorganisms is reduced. Consideration of the ecological consequences is also an important step to prevent distribution of resistant strains between persons. The extent to which disturbances occur depends on the spectrum of the agent, the dose, the route of administration, pharmacokinetic and pharmacodynamic properties, and in vivo inactivation of the agent (9).
The primary objective was to assess the impact of orally administered solithromycin on the oropharyngeal and intestinal microbiota during and after administration of 800 mg on day 1 followed by 400 mg daily on days 2 through 7 to healthy volunteers.
The secondary objectives were to explore the potential for development of resistance by measuring the susceptibility (MIC) of colonizing bacterial strains before, during, and after administration of solithromycin and to correlate oropharyngeal and intestinal microbiota patterns with solithromycin concentrations measured in saliva and feces.
MATERIALS AND METHODS
Study design.
This was a phase 1, open-label, single-center study in 12 healthy volunteers (6 males and 6 females) who completed the study to assess the impact of solithromycin on the oropharyngeal and intestinal microbiota during and after oral administration of 800 mg on day 1 followed by 400 mg daily on days 2 through 7. Subjects provided saliva and fecal samples at baseline and on days 2, 5, 7, 9, 14, and 21 for pharmacokinetic and microbiological assays. Solithromycin plasma concentrations on days 2, 5, and 7 were measured predose to provide proof of exposure.
Volunteers.
Thirteen healthy volunteers were included in the study. Volunteers were recruited through information about the study on the Clinical Pharmacology Trial Unit website of the Karolinska University Hospital (Stockholm, Sweden). A physical examination was carried out on each volunteer at the screening visit and included measurements of blood pressure, heart rate, and clinical laboratory tests as well as an interview on medical and surgical history. Inclusion criteria for men and women were age 18 to 45 years and normal findings in the medical history and physical examination. Each volunteer had a body mass index (BMI) between 19 and 30 kg/m2. The female subjects had no childbearing potential or were willing to take adequate contraceptive measures during the entire study period and for 30 days after completion of the study. The volunteers had to adhere to the visit schedule and concomitant therapy prohibitions and had to be compliant with the treatment (assessed at the screening visit). Volunteers understood and signed an informed consent form at the screening visit prior to any investigational procedure.
Exclusion criteria.
Exclusion criteria were as follows: underlying known or previous clinically significant oncologic, pulmonary, hepatic, gastrointestinal, cardiovascular, hematologic, metabolic, neurological, immunologic, nephrologic, endocrine, or psychiatric disease or current infection; women who were lactating or intended to conceive a child within the 6 months following the baseline visit; a surgical or medical condition with a history of predisposition to candidiasis overgrowth, known or suspected achlorhydria, and (within the previous 3 months) postantibiotic colitis or systemic antibiotic treatment; any condition possibly affecting drug absorption, such as gastrectomy, cholecystectomy, and surgery that bypasses or excludes the duodenum; persons with known intolerance or hypersensitivity to macrolide antibiotics (clarithromycin, azithromycin, telithromycin, or erythromycin) or who had received an investigational drug or participated in another research study within 3 months before the screening visit; a history of drug or alcohol abuse within the previous 2 years; persons who had used prescription drugs or herbal supplements within 14 days before the first dose of study drug (exceptions included hormonal contraceptives or hormone replacement therapy starting at least 3 months before the first dose of the study drug); use of any nonprescription medications, vitamins, or dietary supplements within 7 days of administration of the first dose of the study drug, unless prior approval was granted by both the investigator and sponsor (excluded from this list were intermittent use of ibuprofen, naproxen, or ≤2 g/day of acetaminophen [paracetamol]); consumption of Seville oranges or products containing Seville orange components, grapefruit, grapefruit juice, or juices containing grapefruit, within 14 days before the first dose of the study drug; presence of corrected Q-T interval (QTc) greater than 450 ms for males and 470 ms for females as corrected by the Fridericia formula or heart rate of <45 bpm; and positive urine pregnancy test at the screening visit or at baseline. Furthermore, persons with a positive test for human immunodeficiency virus (HIV types 1 and 2), hepatitis B virus surface antigen (HBsAg), or anti-hepatitis C virus (HCV) antibodies; a positive urine drug screen at the screening visit or at baseline; or a clinically significant laboratory abnormality at screening laboratory evaluation were excluded as well as those likely to require treatment during the study with drugs not permitted by the study protocol.
Approvals.
The study protocol submitted to the Regional Ethics Committee in Stockholm (Stockholm, Sweden) and to the Medical Products Agency (Uppsala, Sweden) was approved before starting the trial.
Collection of plasma samples.
Blood samples for the determination of solithromycin plasma concentrations were obtained predose on days 2, 5, and 7 as proof of exposure. The samples were collected into blood collection tubes containing lithium-heparin as an anticoagulant and were labeled appropriately. After collection, blood samples were immediately put on ice and were centrifuged within 30 min at 1,500 × g for 10 min in order to obtain plasma. Plasma samples were stored at −70°C within 30 min after centrifugation.
Collection of saliva and fecal samples.
Saliva samples were collected on days −1 (baseline), 2, 5, 7, 9, 14, and 21. Samples were collected before any intake of food or beverage, tooth brushing, or (on days 2, 5, and 7) study drug dosing. Fecal samples were collected on days −1 (baseline), 2, 5, 7, 9, 14, and 21. The first fecal specimen passed on a given day was analyzed if several were produced that day. If no fecal specimen was passed on a given day, the first subsequent specimen passed was kept. Fecal specimens passed the previous evening may have been used for samples at baseline and on days 9, 14, and 21. Saliva and fecal samples were collected in sterile tubes and containers and frozen at −70°C on site until processed.
Determination of solithromycin in plasma.
The predose plasma concentrations of solithromycin were used as proof of exposure. Plasma samples were assayed for solithromycin using a validated liquid chromatography-tandem mass spectrometry (MS) method (limit of quantification, 10 μg/liter).
Determination of solithromycin in saliva and feces.
The concentrations of solithromycin in saliva samples and feces were determined microbiologically using the agar plate diffusion method. The test agar medium was prepared with nutrient broth (BBL, Cockeysville, MD, USA) and agarose (Sigma, St. Louis, MO, USA), and the indicator strain was Micrococcus luteus ATCC 9341 for saliva and feces (American Type Culture Collection, Manassas, VA, USA). Samples were run in duplicate, and a concomitant standard series was inoculated on each agar plate. The plates were incubated aerobically for 24 h at 37°C. Concentrations of solithromycin were determined relative to the diameters of the inhibition zones caused by the corresponding concentrations from the standard series. The detection limits were 0.064 mg/liter of saliva and 0.064 mg/kg of feces (10).
Processing of specimens for microbiological analyses.
Saliva and fecal samples were suspended in prereduced peptone-yeast extract medium, diluted 10-fold, and inoculated on nonselective and selective agars (10). The aerobic agar plates were incubated for 24 h at 37°C, and anaerobic plates were incubated for 48 h at 37°C in anaerobic jars (GasPak; BBL, Cockeysville, MD, USA). Following incubation, different colony types were counted, isolated in pure culture, and identified to genus level. The lower limit of detection (LLD) for quantitative cultures was 100 CFU, i.e., log10 = 2. All isolates were identified according to Gram reaction and colony morphology, followed by biochemical tests and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Biotyper). Anaerobic microorganisms were identified by gas chromatographic analysis of metabolites from glucose or by MALDI-TOF MS. All fecal samples were analyzed for Clostridium difficile, including the predose samples, for which a real-time PCR (RT-PCR) for the toxin B gene (GenomEra Clostridium difficile assay; Abacus Diagnostica, Turku, Finland) was used.
Antibiotic susceptibility tests.
The MICs of solithromycin were determined for new colonizing strains with MICs of ≥8 mg/liter from the saliva and fecal samples by the agar dilution method (11, 12). The final inoculum for aerobic bacteria was 104 CFU per spot, and that for anaerobic bacteria was 105 CFU per spot. Inoculated plates were incubated for 24 h (aerobic bacteria) and 48 h (anaerobic bacteria). Reference strains were Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Bacteroides fragilis ATCC 25285, and Clostridium difficile ATCC 700057. The MIC was defined as the lowest concentration of the drug that inhibited growth completely.
Safety.
The following safety assessments were performed: physical examination, vital signs, electrocardiogram, adverse event (AE) monitoring, and monitoring of clinical laboratory parameters. The investigator was responsible for necessary acute medical treatment of any adverse event during the trial and ensured that appropriate medical care was maintained thereafter, if necessary.
Statistical methods.
Descriptive statistics and individual case analyses were used. For quantitation of viable counts, the numbers of CFU were determined as log10 CFU per gram of feces. A CFU value of <2 log10 was considered below the limit of detection. For calculations, a CFU number below the detection limit was set to zero. Genus-/species-specific changes in viable counts within the entire study population were analyzed to evaluate the ecological impact on the microbiota.
RESULTS
Volunteers.
Thirteen volunteers were enrolled, with 1 subject replaced and 12 completing the study. The age range of the subjects was 19 to 41 years (mean age, 27.3 years). The mean height was 174.7 cm (range, 161 to 190 cm), the mean weight was 70.2 kg (range, 61.5 to 86.9 kg), and the mean body mass index (BMI) was 23.2 kg/m2 (range, 20.7 to 26.6 kg/m2). One female subject received another antibiotic during the study, and this subject is included in safety analyses but not in microbiological analyses. Twelve subjects completed the study and are included in these microbiological results.
Effect of solithromycin on the aerobic intestinal microbiota.
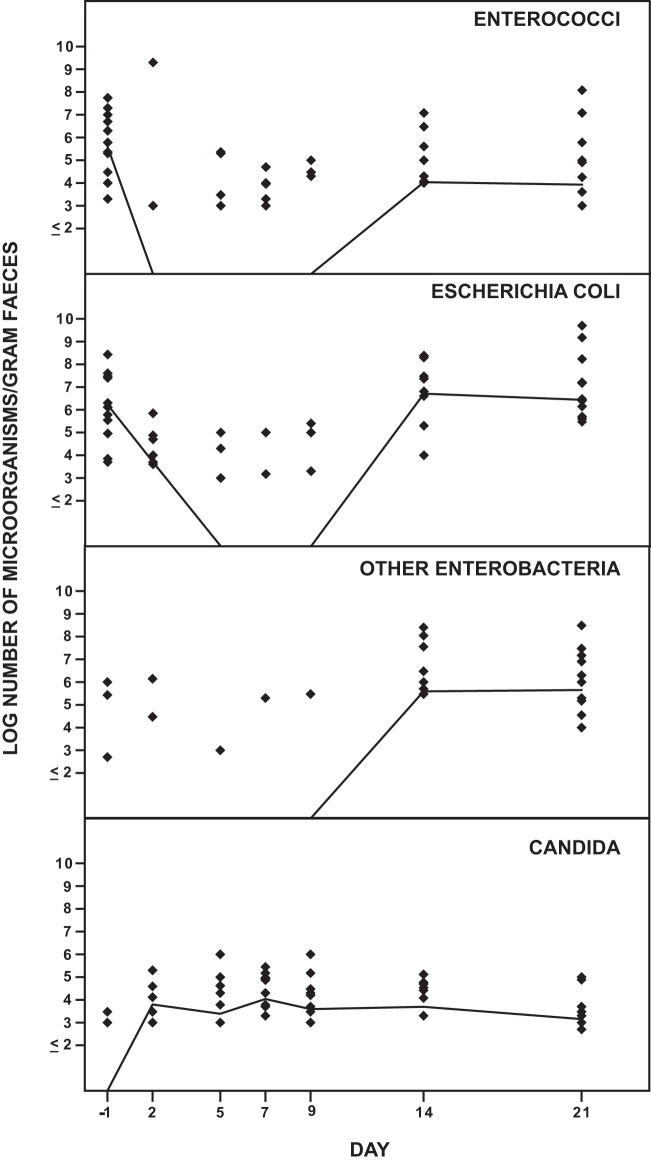
Figure 1 shows the effect of solithromycin on the aerobic intestinal microbiota. The number of E. coli strains decreased during the administration of solithromycin and was normalized on day 14. The number of other enterobacteria decreased also during the administration of solithromycin. The number of enterococci decreased from day 2 to day 9 and was normalized on day 14. The number of Candida strains was not changed during the study.
FIG 1.
Effect of solithromycin oral administration (800 mg on day 1 followed by 400 mg daily on day 2 through day 7) on the intestinal aerobic microbiota of 12 healthy volunteers. The solid line represents the median value of log CFU/gram of feces.
Effect of solithromycin on the anaerobic intestinal microbiota.
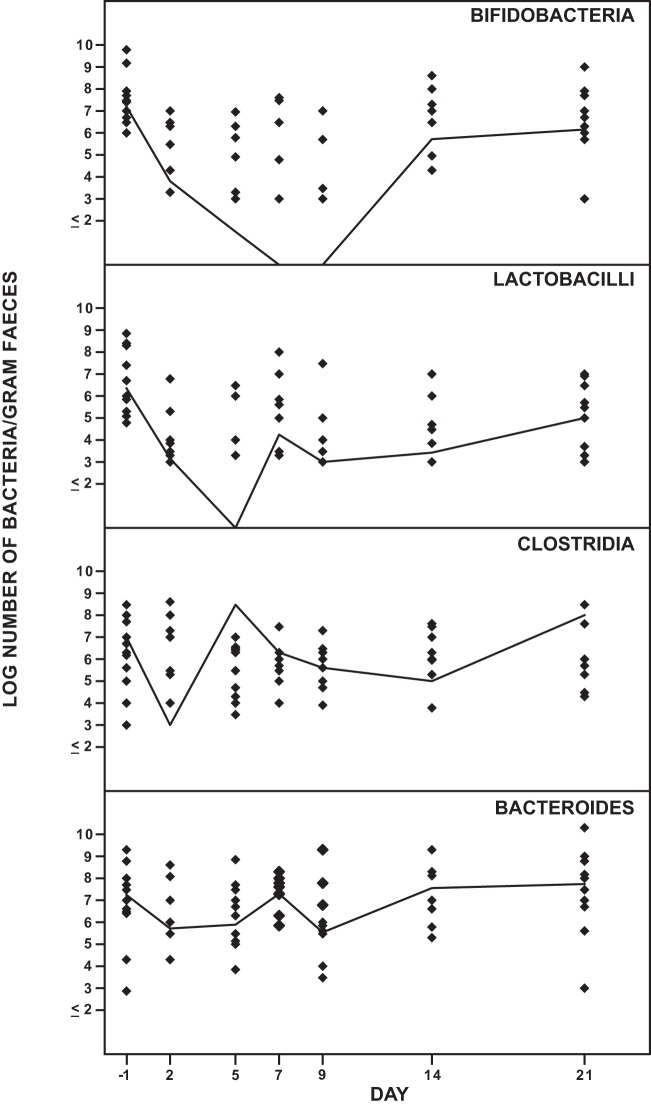
Figure 2 shows the effect of solithromycin on the anaerobic intestinal microbiota. The number of lactobacilli decreased from day 2 to day 14 and was normalized on day 21. The number of bifidobacteria decreased on day 2 and was not normalized until day 21. There was a decrease of Clostridium strains on day 2 and days 7 to 14. At day 21, clostridia were normalized. No C. difficile strains or toxins were detected. The number of Bacteroides strains was not significantly changed during the study.
FIG 2.
Effect of solithromycin oral administration (800 mg on day 1 followed by 400 mg daily on day 2 through day 7) on the intestinal anaerobic microbiota of 12 healthy volunteers. The solid line represents the median value of log CFU/gram of feces.
Effect of solithromycin on the aerobic oropharyngeal microbiota.
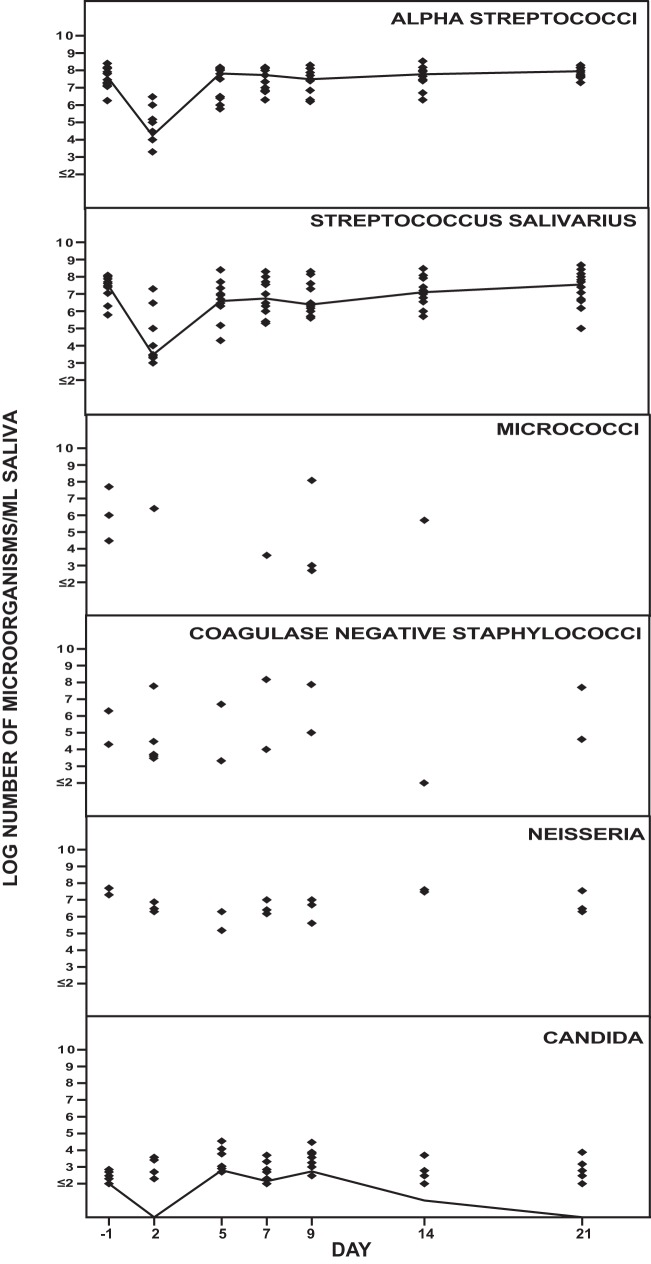
Figure 3 shows the effect of solithromycin on the aerobic oropharyngeal microbiota. The number of streptococci decreased on day 2 and was normalized on day 5. No changes in the numbers of micrococci, coagulase-negative staphylococci, or Neisseria or Candida strains were observed during the study period.
FIG 3.
Effect of solithromycin oral administration (800 mg on day 1 followed by 400 mg daily on day 2 through day 7) on the oropharyngeal aerobic microbiota of 12 healthy volunteers. The solid line represents the median value of log CFU/milliliter of saliva.
Effect of solithromycin on the anaerobic oropharyngeal microbiota.
Figure 4 shows the effect of solithromycin on the anaerobic oropharyngeal microbiota. The number of lactobacilli was partly affected during the study period. The number of Prevotella strains decreased from day 2 to day 14 and was normalized on day 21. Fusobacterium strains decreased on days 2 to 9 and were normalized by day 14. The number of Leptotrichia strains decreased on days 7 to 14 and was normalized on day 21. No changes in the numbers of Veillonella strains were observed during the study period.
FIG 4.
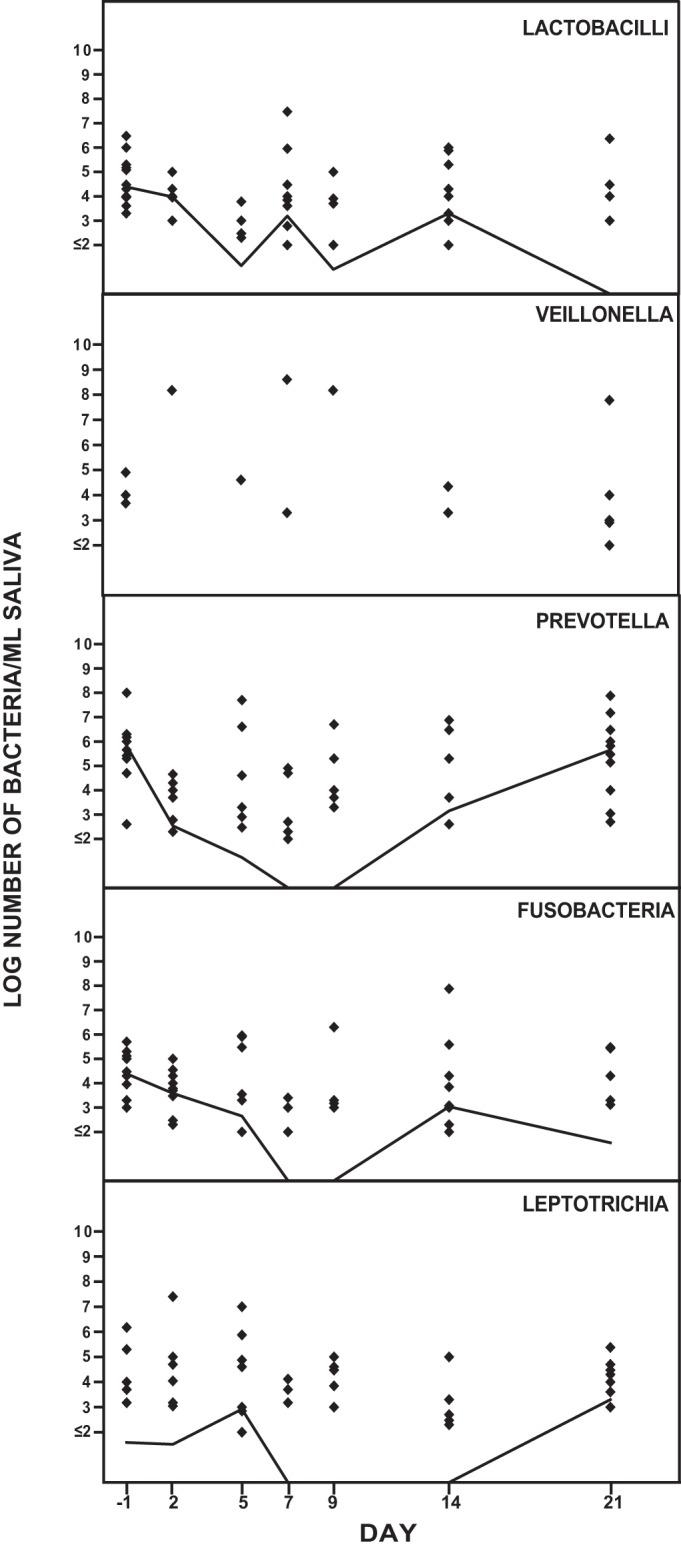
Effect of solithromycin oral administration (800 mg on day 1 followed by 400 mg daily on day 2 through day 7) on the oropharyngeal anaerobic microbiota of 12 healthy volunteers. The solid line represents the median value of log CFU/milliliter of saliva.
Plasma, saliva, and fecal concentrations of solithromycin.
The plasma concentrations of solithromycin determined by liquid chromatography-mass spectrometry are shown in Table 1. On day 2, the mean predose plasma concentration of solithromycin was 130 ng/ml and the median concentration was 85.4 ng/ml (range, 21.9 to 258 ng/ml); on day 5, the mean predose plasma concentration of solithromycin was 111 ng/ml and the median concentration was 73.3 ng/ml (range, 18.0 to 386 ng/ml); on day 7, the mean predose plasma concentration of solithromycin was 98.7 ng/ml and the median concentration was 61.6 ng/ml (range, 16.9 to 417 ng/ml).
TABLE 1.
Solithromycin plasma concentrations in 13 healthy volunteers given 800 mg solithromycin orally on day 1 followed by 400 mg solithromycin orally on day 2 through day 7
| Sample time | No. of volunteers | Concn (ng/ml) |
||
|---|---|---|---|---|
| Mean | Median | Range | ||
| Day 2 predose | 13 | 130 | 85.4 | 21.9–258 |
| Day 5 predose | 13 | 111 | 73.3 | 18.0–386 |
| Day 7 predose | 12a | 98.7 | 61.6 | 16.9–417 |
One subject missed taking the day 6 dose; therefore, the solithromycin concentration at day 7 predose for that subject was excluded.
The fecal concentrations of solithromycin as determined by bioassay are shown in Table 2. None of the volunteers had any detectable solithromycin on day −1. On day 2, the mean solithromycin concentration was 42.7 mg/kg and the median was 43.5 mg/kg (range, 15.8 to 65.4 mg/kg), and on day 5, the mean solithromycin concentration was 47.8 mg/kg and the median was 46.8 mg/kg (range, 24.5 to 82.7 mg/kg). On day 7, the mean solithromycin concentration was 39.3 mg/kg and the median was 35.4 mg/kg (range, 21.4 to 82.7 mg/kg), and on day 9, the mean solithromycin concentration was 33.1 mg/kg and the median was 24.9 mg/kg (range, 12.1 to 72.4 mg/kg). On day 14, the mean solithromycin concentration was 3.7 mg/kg and the median was 0.9 mg/kg (range, 0.2 to 25.6 mg/kg), and on day 21, the mean solithromycin concentration was 0.2 mg/kg and the median was 0.1 mg/kg (range, 0 to 0.5 mg/kg).
TABLE 2.
Fecal concentrations of solithromycin in 12 healthy volunteers receiving 800 mg solithromycin orally on day 1 followed by 400 mg solithromycin orally on day 2 through day 7
| Day | Solithromycin concn (mg/kg of feces) |
|||
|---|---|---|---|---|
| Mean | Median | Range | SD | |
| −1 | 0 | 0 | 0 | 0 |
| 2 | 42.7 | 43.5 | 15.8–65.4 | 17.7 |
| 5 | 47.8 | 46.8 | 24.5–82.7 | 17.9 |
| 7 | 39.3 | 35.4 | 21.4–82.7 | 17.1 |
| 9 | 33.1 | 24.9 | 12.1–72.4 | 20.2 |
| 14 | 3.7 | 0.9 | 0.2–25.6 | 7.2 |
| 21 | 0.2 | 0.1 | 0–0.5 | 0.2 |
The saliva concentrations of solithromycin as determined by bioassay are shown in Table 3. Peak saliva concentrations were obtained on day 2, the mean solithromycin concentration was 0.5 mg/liter, and the median was 0.5 mg/liter (range, 0 to 1.2 mg/liter).
TABLE 3.
Saliva concentrations of solithromycin in 12 healthy volunteers receiving 800 mg solithromycin orally on day 1 followed by 400 mg solithromycin orally on day 2 through day 7
| Day | Solithromycin concn (mg/liter) |
|||
|---|---|---|---|---|
| Mean | Median | Range | SD | |
| −1 | 0 | 0 | 0 | 0 |
| 2 | 0.5 | 0.5 | 0–1.2 | 0.4 |
| 5 | 0.2 | 0.1 | 0–0.5 | 0.2 |
| 7 | 0.1 | 0 | 0–0.5 | 0.2 |
| 9 | 0 | 0 | 0–0.1 | 0 |
| 14 | 0 | 0 | 0 | 0 |
| 21 | 0 | 0 | 0 | 0 |
New colonizing solithromycin-resistant bacteria in the oropharyngeal and intestinal microbiota.
All aerobic and anaerobic bacterial isolates collected from solithromycin (8-mg/liter)-containing agar plates were identified, and the MICs were determined. New colonizing bacterial isolates are shown in Table 4.
TABLE 4.
MICs of solithromycin for new colonizing bacteria in 12 healthy volunteers receiving 800 mg solithromycin orally on day 1 followed by 400 mg solithromycin orally on day 2 through day 7
| Bacterium | No. of volunteers | MIC (mg/liter) |
|---|---|---|
| Enterococcus spp. | 2 | 16 to 32 |
| Streptococcus spp. | 2 | 32 |
| Coagulase-negative staphylococci | 9 | 32 to >256 |
| Micrococcus spp. | 1 | 32 |
| Escherichia coli | 2 | 8 to >256 |
| Klebsiella spp. | 3 | 16 to 32 |
| Enterobacter spp. | 2 | 8 to 32 |
| Citrobacter spp. | 1 | 16 |
| Anaerobic cocci | 1 | 128 to >256 |
| Bifidobacterium spp. | 2 | 64 to >256 |
| Lactobacillus spp. | 6 | 8 to >256 |
| Clostridium spp. | 7 | 8 to >256 |
| Bacteroides spp. | 5 | 8 to >256 |
| Fusobacterium spp. | 1 | >256 |
| Flavonifractor spp. | 1 | >256 |
Safety and tolerability.
Thirteen subjects (6 male and 7 female) received 7 doses of solithromycin (one 800-mg dose and six 400-mg doses). One subject received 6 doses of solithromycin (one 800-mg dose and five 400-mg doses) due to missing the day 6 dose. This subject took a 400-mg dose on day 7 and was not replaced, as this missed dose on day 6 was not expected to affect the microbiological assessments. One female subject was treated with another antibiotic for cystitis prior to the day 21 assessment and was replaced with another subject.
There were no deaths, serious AEs (SAEs), or withdrawals due to AEs. Twelve (92.3%) subjects who received 800/400 mg solithromycin reported at least one AE. The majority (76.9%) of AEs were mild in intensity. The most frequently reported AEs were diarrhea (53.8%), headache (30.8%), and nasopharyngitis (30.8%).
The majority of AEs were associated with gastrointestinal disorders (69.2%). Treatment-related AEs were reported by 84.6% of subjects; most treatment-related AEs were mild, self-limiting episodes of diarrhea. One subject reported a severe AE of gastroenteritis that was considered related to solithromycin, occurring after completion of study drug dosing.
DISCUSSION
Most studies of the impact of antimicrobial agents on the normal microbiota have been carried out on the intestinal microbiota. Emergence of antimicrobial resistance frequently originates from this dense microbial population, which also is an important source of pathogens. A balanced microbiota is of importance to reduce the opportunities for pathogens to become established and to prevent colonization by resistant microbial strains. By using antimicrobial agents that do not disturb colonization resistance, the risk of emergence and spread of resistant strains between patients and dissemination of resistance determinants between microorganisms is reduced.
The factors of most importance for ecological disturbances by antibacterial agents are not only the spectrum of the agents but also their degree of absorption, route of elimination, and possible enzymatic inactivation and/or binding to human fluids and intestinal material. Knowledge of the potential of different antimicrobial agents to cause ecological disturbances in the normal microbiota is of great importance, although individual variations of pharmacokinetics, composition, and susceptibility of the normal microbiota and degree of inactivation further determine the ecological outcome of antimicrobial therapy.
There have been several investigations examining the impact of macrolides and ketolides on the intestinal and oropharyngeal microbiota (13–18).
In these investigations, erythromycin, dirithromycin, clarithromycin, roxithromycin, and telithromycin had effects on the aerobic and anaerobic intestinal microbiota, i.e., E. coli, enterococci, bifidobacteria, lactobacilli, clostridia, and bacteroides decreased during administration of the agents. New resistant strains, mainly enterobacteria, enterococci, and bacteroides, were found in most subjects. The present investigation shows the ecological effect of solithromycin on the intestinal microbiota with similar results as reported for the above-mentioned four macrolides and one ketolide. Thus, the number of E. coli strains, other enterobacteria, enterococci, bifidobacteria, lactobacilli, and clostridia decreased while the number of bacteroides and Candida strains was not significantly changed. The microbiological changes are due to the fecal concentrations of solithromycin. No C. difficile strains or toxins were detected. The protective role of Bacteroides spp. against C. difficile in the intestinal microbiota is well known and may explain this finding (19).
The impact on the oropharyngeal microbiota by solithromycin administration was not as pronounced as the impact on the intestinal microbiota. This finding is explained by the low concentration of solithromycin in saliva.
In conclusion, solithromycin has a similar ecological effect on the normal human oropharyngeal and intestinal microbiota as what has been reported for other macrolides.
ACKNOWLEDGMENT
This study was supported by a grant from Cempra, Inc.
REFERENCES
- 1.Farrell DJ, Castanheira M, Sader HS, Jones RN. 2010. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J Infect 61:476–483. doi: 10.1016/j.jinf.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Hook EW III, Golden M, Jamieson BD, Dixon PB, Harbison HS, Lowens S, Fernandes P. 2015. A phase 2 trial of oral solithromycin 1200 mg or 1000 mg as single-dose oral therapy for uncomplicated gonorrhea. Clin Infect Dis 61:1043–1048. doi: 10.1093/cid/civ478. [DOI] [PubMed] [Google Scholar]
- 3.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob Agents Chemother 56:2739–2742. doi: 10.1128/AAC.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Bambeke F, Tulkens PM. 2016. The role of solithromycin in the management of bacterial community-acquired pneumonia. Expert Rev Anti Infect Ther 14:311–324. doi: 10.1586/14787210.2016.1138857. [DOI] [PubMed] [Google Scholar]
- 5.Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, Jamieson BD, Fernandes P. 2013. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother 57:2526–2534. doi: 10.1128/AAC.00197-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallegol J, Fernandes P, Melano RG, Guyard C. 2014. Antimicrobial activity of solithromycin against clinical isolates of Legionella pneumophila serogroup 1. Antimicrob Agents Chemother 58:909–915. doi: 10.1128/AAC.01639-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen JS, Fernandes P, Unemo M. 2014. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother 58:3151–3156. doi: 10.1128/AAC.02411-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell DJ, Mendes RE, Jones RN. 2015. Antimicrobial activity of solithromycin against serotyped macrolide-resistant Streptococcus pneumoniae isolates collected from U.S. medical centers in 2012. Antimicrob Agents Chemother 59:2432–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EMEA. 2000. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. CPMP/EWP/2655/99. Committee for Proprietary Medicinal Products (CPMP), The European Agency for the Evaluation of Medicinal Products (EMEA), London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003420.pdf. [Google Scholar]
- 10.Edlund C, Beyer G, Hiemer-Bau M, Ziege S, Lode H, Nord CE. 2000. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand J Infect Dis 32:81–85. doi: 10.1080/00365540050164272. [DOI] [PubMed] [Google Scholar]
- 11.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed (M7-A9) CLSI, Wayne, PA. [Google Scholar]
- 12.CLSI. 2012b. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard, 8th ed Document M11-A8 CLSI, Wayne, PA. [Google Scholar]
- 13.Beyer G, Hiemer-Bau M, Ziege S, Edlund C, Lode H, Nord CE. 2000. Impact of moxifloxacin versus clarithromycin on normal oropharyngeal microflora. Eur J Clin Microbiol Infect Dis 19:548–550. doi: 10.1007/s100960000304. [DOI] [PubMed] [Google Scholar]
- 14.Brismar B, Edlund C, Nord CE. 1991. Comparative effects of clarithromycin and erythromycin on the normal intestinal microflora. Scand J Infect Dis 23:635–642. doi: 10.3109/00365549109105189. [DOI] [PubMed] [Google Scholar]
- 15.Eckernas SA, Grahnen A, Nord CE. 1991. Impact of dirithromycin on the normal oral and intestinal microflora. Eur J Clin Microbiol Infect Dis 10:688–692. doi: 10.1007/BF01975827. [DOI] [PubMed] [Google Scholar]
- 16.Edlund C, Alvan G, Barkholt L, Vacheron F, Nord CE. 2000. Pharmacokinetics and comparative effects of telithromycin (HMR 3647) and clarithromycin on the oropharyngeal and intestinal microflora. J Antimicrob Chemother 46:741–749. doi: 10.1093/jac/46.5.741. [DOI] [PubMed] [Google Scholar]
- 17.Heimdahl A, Nord CE. 1982. Effect of erythromycin and clindamycin on the indigenous human anaerobic flora and new colonization of the gastrointestinal tract. Eur J Clin Microbiol 1:38–48. doi: 10.1007/BF02014139. [DOI] [PubMed] [Google Scholar]
- 18.Pecquet S, Chachaty E, Tancrede C, Andremont A. 1991. Effects of roxithromycin on fecal bacteria in human volunteers and resistance to colonization in gnotobiotic mice. Antimicrob Agents Chemother 35:548–552. doi: 10.1128/AAC.35.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie TJ, Emery J, Krulicki W, Byrne B, Mah M. 2009. OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother 53:261–263. doi: 10.1128/AAC.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]