Abstract
A series of colistin-resistant Escherichia coli clinical isolates was recovered from hospitalized and community patients in South Africa. Seven clonally unrelated isolates harbored the mcr-1 gene located on different plasmid backbones. Two distinct plasmids were fully sequenced, and identical 2,600-bp-long DNA sequences defining a mcr-1 cassette were identified. Promoter sequences responsible for the expression of mcr-1, deduced from the precise identification of the +1 transcription start site for mcr-1, were characterized.
TEXT
The recent identification of a plasmid-encoded polymyxin resistance mechanism (MCR-1) among human and animal enterobacterial isolates is a source of concern (1). Actually, polymyxins (colistin and polymyxin B) are the last-resort antibiotics for treating infections caused by carbapenemase producers. MCR-1 is a phosphoethanolamine transferase that modifies the lipopolysaccharide by adding phosphoethanolamine to lipid A, leading to resistance to polymyxins (1). This resistance trait is transferable and has been reported so far mostly in Enterobacteriaceae from animal isolates but also in those from human isolates and from food products (2–8). First identified in China as published in November 2015 (1), MCR-1-producing isolates are mostly Escherichia coli strains that have been reported in many different countries scattered throughout Europe, Asia, and North America. In Africa, PCR and in silico analysis identified a few MCR-1-positive E. coli isolates from chicken from Algeria and from a single human E. coli isolate from Nigeria (9).
Our study was initiated by the isolation of seven colistin-resistant enterobacterial E. coli isolates from patients hospitalized in different hospitals in Johannesburg and Pretoria, South Africa, and also from community patients in Johannesburg from March 2014 to June 2015. The clonal relationships of the isolates were first evaluated by pulsed-field gel electrophoresis analysis as described previously (10), and the results showed that the seven isolates belonged to six distinct clones (data not shown) (Table 1). Only isolates Af31 and Af48, both from Johannesburg, were indistinguishable. However, isolate Af31 was from a community patient whereas isolate Af48 was from a hospitalized patient. Multilocus sequence typing performed as described previously (10) confirmed that all isolates were distinct, with the exception of isolates Af31 and Af48 (Table 1). Among those isolates, Af31 and Af45 remained susceptible to all ß-lactams, Af23 and Af24 exhibited a penicillinase phenotype related to TEM-1 production, and isolates Af40 and Af49 exhibited an extended-spectrum-β-lactamase (ESBL) phenotype related to CTX-M-55 according to molecular analyses (11). Isolate Af48 exhibited an AmpC-type cephalosporinase phenotype related to CMY-2. Interestingly, isolate Af31 was resistant to florfenicol and possessed the floR gene that we previously identified in another MCR-1-positive E. coli isolate from Switzerland (12). It was noteworthy that all isolates were resistant to sulfonamides, tetracyclines, and fluoroquinolones (Table 1), which are antibiotics that are extensively prescribed in veterinary medicine (13).
TABLE 1.
Features of the MCR-1-producing colistin-resistant E. coli isolates and patient characteristicsa
| Strain no. | Date of isolation | Site of isolation | Gender | Age range of the patient (yrs) | Origin (city) | MIC of colistin (μg/ml) | β-lactam resistance phenotype | PFGE profile | Sequence type | mcr-1-positive plasmid type | mcr-1-positive plasmid size (ca. kb) | Coresistance markers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Af23 | March 2014 | Blood | M | 50–59 | Pretoria | 4 | Penicillinase (TEM-1) | B | ST10 | IncI2 | 70 | CIP, TET, SXT |
| Af24 | March 2014 | Pus | F | 40–49 | Johannesburg | 4 | Penicillinase (TEM-1) | C | ST1007 | IncI2 | 65 | CIP, TET, SXT |
| Af31 | October 2014 | Urine | F | 60–69 | Johannesburg | 8 | Susceptible | A | ST624 | IncHI2 | 150 | CIP, TET, SXT, CHL, FLO, KAN |
| Af40 | March 2015 | Wound | F | 60–69 | Johannesburg | 4 | ESBL (CTX-M-55) | D | ST57 | IncI2 | ND | CIP, TET, CHL, FOS, KAN, TOB, SXT |
| Af45 | May 2015 | Urine | F | 60–69 | Johannesburg | 8 | Susceptible | E | ST101 | IncI2 | 70 | TET, SXT |
| Af48 | May 2015 | Urine | F | 60–69 | Johannesburg | 4 | AmpC (CMY-2) | A | ST624 | IncX4 | 30 | CIP, TET, SXT, CHL |
| Af49 | May 2015 | Urine | F | 30–39 | Pretoria | 4 | ESBL (CTX-M-55) | F | ST226 | ND | ND | CIP, TET, SXT, CHL, FOS, KAN, TOB |
PFGE, pulsed-field gel electrophoresis; M, male; F, female; ND, not determined; CIP, ciprofloxacin; TET, tetracycline; SXT, trimethoprim plus sulfamethoxazole; CHL, chloramphenicol; FLO, florfenicol; KAN, kanamycin; TOB, tobramycin; FOS, fosfomycin; CHL, chloramphenicol.
Mating-out assays were performed with all mcr-1-positive isolates as donors and E. coli J53 (azide resistant) as the recipient as described previously (14). Selection was performed on Trypticase-soy agar plates supplemented with colistin (2 μg/ml) and sodium azide (100 μg/ml). Transconjugants were obtained for all donors except for Af49. They exhibited colistin MIC values of 4 to 8 μg/ml (resistance cutoff being at 2 μg/ml) compared to 0.25 μg/ml for the E. coli J53 recipient strain. No additional resistance marker was cotransferred along with the mcr-1 gene in any of the E. coli transconjugants. PCR-based replicon typing (15) as well as primers specific for plasmid types IncX4 (X4-Fw [5′-AGCAAACAGGGAAAGGAGAAGACT-3′] and X4-Rv [5′-TACCCAAATCGTAACCTG-3′]) and IncI2 (I2-Fw [5′-TGCAGCTTGCTGTGATTAGC-3′] and I2-Rv [5′-TTCGCTGTTCATCATACGGC-3′]) identified different mcr-1-bearing plasmid backbones, including IncI2, IncHI2, and IncX4, differing in sizes and structures (Table 1). Surprisingly, the two clonally related Af31 and Af48 isolates harbored two different mcr-1-positive plasmid types corresponding to the IncHI2- and IncX4-type scaffolds, respectively.
PCR mapping was performed by referring to the sequence of the reference plasmid identified in China (pHNSHP45; GenBank accession no. KP347127) (1) in order to characterize the genetic environment of the mcr-1 gene among the different E. coli isolates. Insertion sequence ISApl1 was identified upstream of mcr-1 in all but one isolate (Af48). Downstream of mcr-1, the closely related genetic environment previously identified for plasmid pHNSHP45 was found in all isolates.
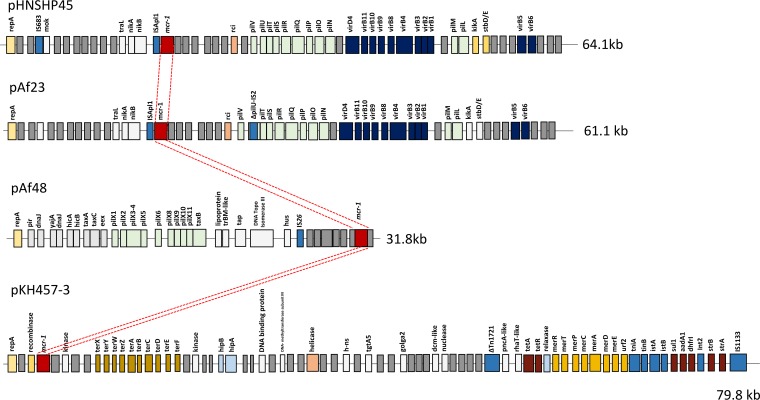
Considering that several plasmid types were identified, two of them were entirely sequenced. Sequencing was performed by using Illumina technology (Fasteris, Plan-les-Ouates, Switzerland). The mcr-1-positive pAf23 plasmid from isolate Af23 was retained since it was an IncI2-type plasmid, as was plasmid pHNSHP45 from the pioneer study (1). The two plasmids were almost completely identical (99% identical at the nucleotide level), with pAf23 being 61.1 kb in size and pHNSHP45 being 64.1 kb, with an additional IS683 element (Fig. 1). No other resistance gene was identified on pAf23 (Table 1). Therefore, the occurrence of the mcr-1 gene in Af23 was related to the acquisition of a plasmid that may be considered structurally related to the Chinese index plasmid.
FIG 1.
Schematic map of the mcr-1-bearing plasmids. pHNSHP45 is the reference plasmid from China (GenBank accession no. KP0347127) (1), the IncP-type pKH457-3 is from Belgium (17), pAf23 is the IncI2-type plasmid recovered from isolate Af23 (GenBank accession no. KX032519) (pAf23), and pAf48 is the IncX4-type plasmid from isolate Af48 (GenBank accession no. KX032520) (pAf48). Open reading frames are indicated by small and vertical rectangles. Colors correspond to loci combining genes acting for the same function (e.g., pil-type genes for the pilus apparatus). The mcr-1 cassette is represented by a red box, and the conserved mcr-1 cassette identified on the different plasmids is highlighted by red dashed lines. The sizes of the respective plasmids are indicated.
The second sequenced mcr-1 plasmid, plasmid pAf48 from isolate Af48, was 31.8 kb in size and exhibited an IncX4-type scaffold (Fig. 1). Again, no resistance gene other than the mcr-1 gene was identified on that plasmid (Table 1). A similar (92.3% overall identity at the nucleotide level) but mcr-1-negative plasmid backbone was identified in the United Kingdom, which was plasmid pSAM7 from E. coli recovered from cattle and harboring the blaCTX-M-14 ESBL gene (16).
In order to understand the process of acquisition of the mcr-1 gene in different plasmid backbones, a detailed comparison of the different mcr-1-positive plasmid sequences was established. Identical 2,600-bp sequences that might be defined as representative of a mcr-1 cassette were identified in plasmids pAf23 and pAf48 but also in the pHNSHP45 IncI2-type plasmid from China (1) and the pKH457-3 IncP-type plasmid from Belgium (17) through our in silico analysis. The 5′ extremity of that cassette started with CAAAT, and the 3′ extremity ended with AAGTT (Fig. 2). At those extremities, no putative inverted repeat sequence was identified that might have corresponded to features usually identified at insertion sequence extremities or at the extremities of mobile insertion cassette (mic) elements such as that containing the qnrS2 quinolone resistance gene (18). In addition, no putative target site duplication was identified on both extremities of that cassette, likely ruling out an in trans transposition process.
FIG 2.
Sequence of the mcr-1 cassette. The 2,600-bp-long sequence is shown, with the start and stop codons of mcr-1 being in bold. The sense of transcription of mcr-1 is indicated by an arrow. Amino acids of the MCR-1 sequence are indicated below the nucleotide sequence. The P1-mcr-1 promoter sequences are indicated, with the corresponding −35 and −10 boxes being underlined, as well as the +1 transcription start.
A detailed analysis of the mcr-1 cassette showed that the mcr-1 start codon was identified at position 79 and that 791 bp separated the stop codon of mcr-1 gene from the right end of the cassette (Fig. 2). A putative promoter region (called P1-mcr-1) was identified within the first 79 bp of the cassette, corresponding to sequences −35 (TGGATT) and −10 (TATAAT) being separated by a 16-bp sequence. Therefore, expression of mcr-1 may be driven by a promoter which is part of the mobile cassette, thus making this element autonomous in term of transcription. In addition, by analysis of the sequences located upstream of the mcr-1 cassette in plasmids pAf23 and pHNSHP45, the ISApl1 insertion sequence element was identified, being located 9 bp upstream of the mcr-1 cassette. In order to confirm that P1-mcr-1 was indeed the correct promoter leading to mcr-1 expression, and also to verify whether the occurrence of ISApl1 could modify the +1 transcription start of mcr-1, mapping of this transcription start site was performed by 5′ rapid amplification of cDNA ends (5′-RACE), as described previously (19). Total RNA was isolated from the different strains studied using an RNeasy Midi kit (Qiagen, Courtaboeuf, France) and the manufacturer's recommendations. 5′-RACE reactions were performed using 5 μg of total RNA of each strain (Af23 and Af48) and a 5′/3′ RACE kit (2nd generation; Roche Diagnostics, Rotkreuz, Switzerland) following the manufacturer's recommendations and using primers SP1 (5′-AAAATAACTGGTCACCGCGC-3′), SP2 (5′-ACAGGCTTTAGCACATAGCG-3′), and SP3 (5′-AAAGAGCACGACAGCGATCG-3′). Considering the +1 transcription site identified, results confirmed that P1-mcr-1 was indeed the promoter of mcr-1 expression (Fig. 2). Also, the same site was identified in both isolate Af23 and isolate Af48, thus indicating that the occurrence of ISApl1 in Af23 did not modify the promoter sequence of mcr-1 (data not shown).
Our report describes the first MCR-1-producing E. coli isolates from South Africa, recovered from community and distantly located hospitalized patients. We identified the same clone (ST624) in both hospital- and community-acquired isolates, that clone being identified as an avian pathogenic E. coli isolate causing colibacillosis in poultry in Spain and China (20, 21), but also in isolates from patients in France and Japan (22). This feature, together with the identification of the floR gene associated with resistance to florfenicol (that antibiotic being given only in veterinary medicine), further suggests the animal origin of that resistance trait (23).
Overall, this study further showed the wide spread of the mcr-1 gene on different plasmid backbones and in different E. coli clonal backgrounds. A detailed genetic analysis identified an mcr-1-containing cassette that could have been mobilized from its original (and still unknown) reservoir to its host and that could be a source of transferable polymyxin resistance. The mechanism of mobilization of this mcr-1 cassette remains to be determined, since it might correspond to an unknown genetic process.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this work have been deposited in the GenBank nucleotide database under accession no. KX032519 (pAf23) and KX032520 (pAf48).
ACKNOWLEDGMENTS
This work has been funded by the University of Fribourg, Fribourg, Switzerland, and by the ANIHWA–ERA-NET project PRAHAD funded by the Federal Food Safety and Veterinary Office, Bern, Switzerland.
We thank Surbhi Malhotra-Kumar for providing us with the DNA sequence of plasmid pKH457-3 for comparison.
REFERENCES
- 1.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Figueiredo R, Henriques A, Sereno R, Mendonça N, da Silva GJ. 2015. Antimicrobial resistance and extended-spectrum β-lactamases of Salmonella enterica serotypes isolated from livestock and processed food in Portugal: an update. Foodborne Pathog Dis 12:110–117. doi: 10.1089/fpd.2014.1836. [DOI] [PubMed] [Google Scholar]
- 3.Webb HE, Granier SA, Marault M, Millemann Y, den Bakker HC, Nightingale KK, Bugarel M, Ison SA, Scott HM, Loneragan GH. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:144–145. doi: 10.1016/S1473-3099(15)00538-1. [DOI] [PubMed] [Google Scholar]
- 4.Arcilla MS, van Hattem JM, Matamoros S, Melles DC, Penders J, de Jong MD, Schultsz C; COMBAT consortium. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:147–149. doi: 10.1016/S1473-3099(15)00541-1. [DOI] [PubMed] [Google Scholar]
- 5.Haenni M, Poirel L, Kieffer N, Châtre P, Saras E, Métayer V, Dumoulin R, Nordmann P, Madec JY. 2016. Co-occurrence of extended spectrum β lactamase and MCR-1 encoding genes on plasmids. Lancet Infect Dis 16:281–282. doi: 10.1016/S1473-3099(16)00007-4. [DOI] [PubMed] [Google Scholar]
- 6.Zurfuh K, Poirel L, Nordmann P, Nüesch-Inderbinen M, Hächler H, Stephan R. 2016. Occurrence of the plasmid-borne mcr-1 colistin resistance gene in ESBL-producing Enterobacteriaceae in river water and imported vegetable samples in Switzerland. Antimicrob Agents Chemother 60:2594–2595. doi: 10.1128/AAC.00066-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falgenhauer L, Waezsada SE, Yao Y, Imirzalioglu C, Käsbohrer A, Roesler U, Michael GB, Schwarz S, Werner G, Kreienbrock L, Chakraborty T; RESET consortium . 2016. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis 16:282–283. doi: 10.1016/S1473-3099(16)00009-8. [DOI] [PubMed] [Google Scholar]
- 8.Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agersø Y, Zankari E, Leekitcharoenphon P, Stegger M, Kaas RS, Cavaco LM, Hansen DS, Aarestrup FM, Skov RL. 2015. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill 20(49). doi: 10.2807/1560-7917.ES.2015.20.49.30085. [DOI] [PubMed] [Google Scholar]
- 9.Olaitan AO, Chabou S, Okdah L, Morand S, Rolain JM. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:147. doi: 10.1016/S1473-3099(15)00540-X. [DOI] [PubMed] [Google Scholar]
- 10.Peirano G, van der Bij AK, Freeman JL, Poirel L, Nordmann P, Costello M, Tchesnokova VL, Pitout JD. 2014. Characteristics of Escherichia coli sequence type 131 isolates that produce extended-spectrum β-lactamases: global distribution of the H30-Rx sublineage. Antimicrob Agents Chemother 58:3762–3767. doi: 10.1128/AAC.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirel L, Dortet L, Bernabeu S, Nordmann P. 2011. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrob Agents Chemother 55:5403–5407. doi: 10.1128/AAC.00585-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel L, Kieffer N, Liassine N, Thanh D, Nordmann P. 2016. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect Dis 16:281. doi: 10.1016/S1473-3099(16)00006-2. [DOI] [PubMed] [Google Scholar]
- 13.Catry B, Cavaleri M, Baptiste K, Grave K, Grein K, Holm A, Jukes H, Liebana E, Lopez Navas A, Mackay D, Magiorakos AP, Moreno Romo MA, Moulin G, Muñoz Madero C, Matias Ferreira Pomba MC, Powell M, Pyörälä S, Rantala M, Ružauskas M, Sanders P, Teale C, Threlfall EJ, Törneke K, van Duijkeren E, Torren Edo J. 2015. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): development of resistance in animals and possible impact on human and animal health. Int J Antimicrob Agents 46:297–306. doi: 10.1016/j.ijantimicag.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Poirel L, Savov E, Nazli A, Trifonova A, Todorova I, Gergova I, Nordmann P. 2014. Outbreak caused by NDM-1- and RmtB-producing Escherichia coli in Bulgaria. Antimicrob Agents Chemother 58:2472–2474. doi: 10.1128/AAC.02571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Stokes MO, Abuoun M, Umur S, Wu G, Partridge SR, Mevius DJ, Coldham NG, Fielder MD. 2013. Complete sequence of pSAM7, an IncX4 plasmid carrying a novel blaCTX-M-14b transposition unit isolated from Escherichia coli and Enterobacter cloacae from cattle. Antimicrob Agents Chemother 57:4590–4594. doi: 10.1128/AAC.01157-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra-Kumar S, Xavier BB, Das AJ, Lammens C, Butaye P, Goossens H. 2016. Colistin resistance gene mcr-1 harboured on a multidrug resistant plasmid. Lancet Infect Dis 16:283–284. doi: 10.1016/S1473-3099(16)00012-8. [DOI] [PubMed] [Google Scholar]
- 18.Cattoir V, Poirel L, Aubert C, Soussy CJ, Nordmann P. 2008. Unexpected occurrence of plasmid-mediated quinolone resistance determinants in environmental Aeromonas spp. Emerg Infect Dis 14:231–237. doi: 10.3201/eid1402.070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Martinez JM, Poirel L, Canton R, Nordmann P. 2006. Common region CR1 for expression of antibiotic resistance genes. Antimicrob Agents Chemother 50:2544–2546. doi: 10.1128/AAC.00609-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solà-Ginés M, Cameron-Veas K, Badiola I, Dolz R, Majó N, Dahbi G, Viso S, Mora A, Blanco J, Piedra-Carrasco N, González-López JJ, Migura-Garcia L. 2015. Diversity of multi-drug resistant avian pathogenic Escherichia coli (APEC) causing outbreaks of colibacillosis in broilers during 2012 in Spain. PLoS One 10:e0143191. doi: 10.1371/journal.pone.0143191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia X, Meng J, McDermott PF, Zhao S. 2011. Escherichia coli from retail meats carry genes associated with uropathogenic Escherichia coli, but are weakly invasive in human bladder cell culture. J Appl Microbiol 110:1166–1176. doi: 10.1111/j.1365-2672.2011.04978.x. [DOI] [PubMed] [Google Scholar]
- 22.Djamdjian L, Naas T, Tandé D, Cuzon G, Hanrotel-Saliou C, Nordmann P. 2011. CTX-M-93, a CTX-M variant lacking penicillin hydrolytic activity. Antimicrob Agents Chemother 55:1861–1866. doi: 10.1128/AAC.01656-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel L, Nordmann P. 29 March 2016. Emerging plasmid-encoded colistin resistance; the animal world as the culprit? J Antimicrob Chemother doi: 10.1093/jac/dkw074. [DOI] [PubMed] [Google Scholar]