Abstract
The interaction between atovaquone and proguanil has never been studied against liver stage malaria, which is the main target of this drug combination when used for chemoprevention. Using human hepatocytes lacking cytochrome P450 activity, and thus avoiding proguanil metabolizing into potent cycloguanil, we show in vitro that the atovaquone-proguanil combination synergistically inhibits the growth of rodent Plasmodium yoelii parasites. These results provide a pharmacological basis for the high efficacy of atovaquone-proguanil used as malaria chemoprevention.
TEXT
The drug combination atovaquone-proguanil (AP) is an efficient drug for malaria chemoprevention and treatment. The rationale for combining these two drugs originated from the observation that they produce a synergistic inhibitory effect against replicating blood stage parasites in vitro (1–3). In the context of malaria chemoprevention, however, AP exerts its protective effect primarily during the hepatic phase of the parasite infection (4, 5), and whether the AP synergy is operating during this preerythrocytic phase has not been explored.
To test this directly, we sought to evaluate in vitro the interaction between atovaquone and proguanil against liver stage Plasmodium infection through a fixed-ratio isobologram method. In hepatocytes, proguanil is partially metabolized by host cytochrome P450 enzymes into cycloguanil (6), which potently inhibits the development of liver stage Plasmodium infection (7). To address this confounding factor, we used HepG2-CD81 human hepatoma cells that retain many liver-specific properties and can be infected by some Plasmodium species (8) while displaying impaired cytochrome P450 activity (9).
To confirm the lack of or very minimal proguanil metabolism, HepG2-CD81 cells were incubated in the presence of 100 μM proguanil for 2 days, and cell culture supernatants were collected at 24 and 48 h after treatment to quantify drug levels. Proguanil and its metabolite cycloguanil were separated and quantified on a liquid chromatography mass detection mass spectrometer (TSQ Quantum Ultra; Thermo Fisher, France) using an Atlantis dC18 column (100 by 2.1 mm, 3 μm; Waters, France) and a calibration curve. Cycloguanil was not detected in any of the collected samples from the 24- and 48-h time points (n = 8 values) at the 0.04 μM limit of our assay (proguanil was detected as expected). In contrast, cycloguanil was detected in the corresponding samples of primary human hepatocytes used as positive controls (median, 0.32 μM; minimum, 0.21 μM; maximum, 0.97 μM; n = 8 values; P < 0.001, Mann-Whitney test). The same observations regarding cycloguanil production in the two cell types were made when using 20 μM instead of 100 μM proguanil (P < 0.001, Mann-Whitney test).
Subsequently, Plasmodium-infected hepatocytes were treated with single agents, and then combination treatments were performed to assess drug interaction. Primary human hepatocytes were isolated as previously described (8). HepG2 cells were derived from the liver tissue of a 15-year-old boy with differentiated hepatocellular carcinoma (10) and modified to induce CD81 expression (11). Plasmodium yoelii sporozoites (BY265 strain) were obtained from infected salivary glands of Anopheles stephensi 14 to 21 days after an infective blood meal on a parasite-infected Swiss-Webster mouse. Using a 96-well plate, 15,000 sporozoites were added per well, containing an average of 80,000 HepG2-CD81 or primary human hepatocyte cells in a monolayer at a density of 250,000 cells/cm2. Drugs at various concentrations along with no-drug (solvent-only) controls were added together with sporozoites.
The drug-containing cell culture media were replaced 2 and 24 h after infection, and the development of liver stage parasites was stopped 48 h after infection by fixation with methanol. Liver stage parasites were stained with a polyclonal antibody specific for Plasmodium HSP70 (12), and host and parasite nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). Fluorescent labeling was visualized using a fluorescence microscope with ×400 magnification (Leica DMI4000 B), and parasites were counted visually. We measured the drug effects on parasite maturation into liver stage schizonts as a proxy for parasite growth. Only small parasites with a single nucleus were considered to display arrested maturation and thus were not counted as schizonts (Fig. 1A). We estimated the 50% inhibitory concentration (IC50) by plotting the relative reduction in the number of schizonts per well on the y axis against drug concentration on the x axis (13). Experiments were repeated at least three times with different sporozoite and hepatocyte batches. No hepatocyte toxicity was observed at any of the highest atovaquone and proguanil concentrations used in the study (0.039 and 154 μM, respectively). Two or three wells per drug concentration were used to analyze parasite counts in the IC50 and synergy experiments, respectively. The median numbers of schizonts per well in drug-free controls were 100 (minimum, 53; maximum, 319) and 505 (minimum, 311; maximum, 527) for the IC50 and synergy experiments, respectively.
FIG 1.
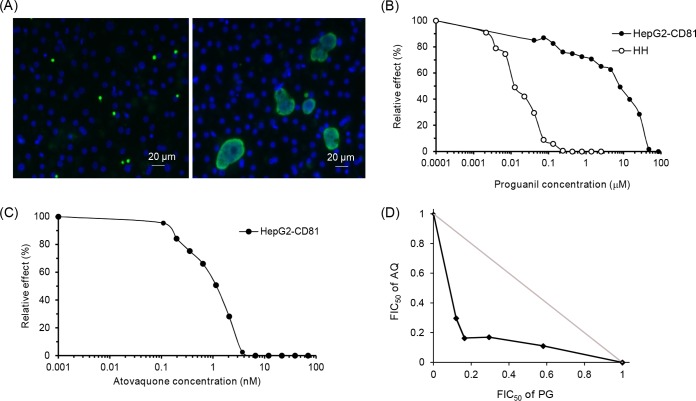
Inhibitory effect of atovaquone and proguanil on the development of liver stage rodent Plasmodium infection. P. yoelii sporozoites were added to HepG2-CD81 cell monolayers together with different drug concentrations. The drug-containing cell culture media were replaced 2 and 24 h after infection, and the development of liver stage parasites was stopped 48 h after infection by fixation with methanol. Liver stage parasites were stained with a polyclonal antibody specific for Plasmodium HSP70 (green), while host and parasite nuclei were stained with DAPI (blue). The numbers of arrested and nonarrested parasites were evaluated by microscopic examination. (A) Representative image of P. yoelii parasites 48 h after infection. Small arrested parasitic forms (<7 μM) with a single nucleus are shown in the left panel, and fully developed schizonts are displayed in the right panel. (B) Dose-response curves for proguanil against P. yoelii in HepG2-CD81 and primary human hepatocytes (HH). Data are representative of those from three independent assays. (C) Dose-response curve for atovaquone against P. yoelii in HepG2-CD81 cells. Data are representative of those from three independent assays. (D) Isobologram showing the interaction between atovaquone and proguanil against P. yoelii infecting HepG2-CD81 cells. Drug interactions are represented by the normalized FIC50s for atovaquone and proguanil plotted against each other. Data are representative of those from three independent assays. The gray line is the line of additivity.
We first compared the IC50s for proguanil against P. yoelii parasites obtained from HepG2-CD81 and human primary hepatocytes. The IC50s were about 100-fold higher in HepG2-CD81 (IC50s, 2.2, 3.2, and 18.4 μM; median, 3.2 μM) than in primary human hepatocytes (IC50s, 0.02, 0.03, and 0.04 μM; median, 0.03 μM) (Fig. 1B). Altogether, the drug dosage and IC50 measurements indicated a deficiency in proguanil metabolism in HepG2-CD81 cells. Regarding atovaquone, the median IC50 in HepG2-CD81 cells was 0.92 nM (IC50s, 0.76, 0.92, and 1.1 nM) (Fig. 1C). The proguanil and atovaquone IC50 values in HepG2-CD81 cells were consistent with those in a previous report (7).
Because we had evidence that cycloguanil production is dramatically reduced or prevented in HepG2-CD81 cells, we explored the effect of the AP combination against P. yoelii infection through a fixed-ratio isobologram method, as previously described (14). Briefly, six starting solutions containing six fixed atovaquone/proguanil molar ratios were prepared and then serially diluted 2-fold seven times. The starting solutions 1 to 6 were prepared at atovaquone/proguanil nanomolar concentrations of 6:0, 4.8:4,000, 3.6:8,000, 2.4:12,000, 1.2:16,000, and 0:20,000, respectively. The fractional inhibitory concentration (FIC) was calculated for each drug in each combination according to the following equation:
The isobologram curve was generated by plotting the FIC50 of atovaquone against the FIC50 of proguanil. The mean FIC50 index was calculated according to the following equation:
The median isobologram (of 3 independent experiments) for the interaction between atovaquone and proguanil is shown in Fig. 1D. The FIC50 index had a median value of 0.64 (0.62, 0.64, and 0.69), which together with the isobolograms indicated that the interaction between the two drugs is synergistic.
In conclusion, we report here the first in vitro study, to our knowledge, that formally investigated the effect of a drug combination on liver stages of malaria. We showed that the synergism between atovaquone and proguanil against rodent malaria parasites in vitro is conserved across liver and blood stages, which provides a pharmacological basis for the high efficacy of AP when used as malaria chemoprevention.
ACKNOWLEDGMENTS
We thank Jacques Le Bras, Gilles Cottrell, and Michel Cot for helpful discussions and suggestions and Sophie Adjalley and Rich Eastman for critical reading of the manuscript.
L.B. was supported by a DIM Malinf Fellowship. This work was supported by DIM Malinf Région Ile de France (to L.B., D.M., J.-F.F., and J.C.), the Fondation pour la Recherche Médicale (to D.M. and J.C.), and the Assistance Publique des Hôpitaux de Paris (P.H.).
Funding Statement
Neither funder had a role in study design, data analysis, data interpretation, or data reporting.
REFERENCES
- 1.Canfield CJ, Pudney M, Gutteridge WE. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol 80(3):373–381. doi: 10.1006/expr.1995.1049. [DOI] [PubMed] [Google Scholar]
- 2.Jones K, Ward SA. 2002. Biguanide-atovaquone synergy against Plasmodium falciparum in vitro. Antimicrob Agents Chemother 46(8):2700–2703. doi: 10.1128/AAC.46.8.2700-2703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava IK, Vaidya AB. 1999. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob Agents Chemother 43(6):1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman JD, Nielsen R, Chulay JD, Dowler M, Kain KC, Kester KE, Williams J, Whelen AC, Shmuklarsky MJ. 2001. Causal prophylactic efficacy of atovaquone-proguanil (Malarone) in a human challenge model. Trans R Soc Trop Med Hyg 95(4):429–432. doi: 10.1016/S0035-9203(01)90206-8. [DOI] [PubMed] [Google Scholar]
- 5.Deye GA, Miller RS, Miller L, Salas CJ, Tosh D, Macareo L, Smith BL, Fracisco S, Clemens EG, Murphy J, Sousa JC, Dumler JS, Magill AJ. 2012. Prolonged protection provided by a single dose of atovaquone-proguanil for the chemoprophylaxis of Plasmodium falciparum malaria in a human challenge model. Clin Infect Dis 54(2):232–239. doi: 10.1093/cid/cir770. [DOI] [PubMed] [Google Scholar]
- 6.Lu AH, Shu Y, Huang SL, Wang W, Ou-Yang DS, Zhou HH. 2000. In vitro proguanil activation to cycloguanil is mediated by CYP2C19 and CYP3A4 in adult Chinese liver microsomes. Acta Pharmacol Sin 21(8):747–752. [PubMed] [Google Scholar]
- 7.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. 2012. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med 9(2):e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, Hannoun L, Eling W, Levy S, Boucheix C, Mazier D. 2003. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med 9(1):93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 9.Donato MT, Lahoz A, Castell JV, Gomez-Lechon MJ. 2008. Cell lines: a tool for in vitro drug metabolism studies. Curr Drug Metab 9(1):1–11. doi: 10.2174/138920008783331086. [DOI] [PubMed] [Google Scholar]
- 10.Knowles BB, Howe CC, Aden DP. 1980. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 11.Yalaoui S, Zougbede S, Charrin S, Silvie O, Arduise C, Farhati K, Boucheix C, Mazier D, Rubinstein E, Froissard P. 2008. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain. PLoS Pathog 4(2):e1000010. doi: 10.1371/journal.ppat.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoudi N, Ciceron L, Franetich JF, Farhati K, Silvie O, Eling W, Sauerwein R, Danis M, Mazier D, Derouin F. 2003. In vitro activities of 25 quinolones and fluoroquinolones against liver and blood stage Plasmodium spp. Antimicrob Agents Chemother 47(8):2636–9. doi: 10.1128/AAC.47.8.2636-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Nagard H, Vincent C, Mentre F, Le Bras J. 2011. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput Methods Programs Biomed 104(1):10–18. doi: 10.1016/j.cmpb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Fivelman QL, Adagu IS, Warhurst DC. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob Agents Chemother 48(11):4097–4102. doi: 10.1128/AAC.48.11.4097-4102.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]