Abstract
Little is known about the effect of antibiotics on eradication of carriage and development of resistance in Haemophilus influenzae in individuals with chronic obstructive pulmonary disease (COPD). Our goals were to assess antibiotic susceptibilities, prevalence of resistance genes, and development of resistance in H. influenzae and to evaluate the effect of macrolide and fluoroquinolone administration on H. influenzae eradication. Data were from a 15-year longitudinal study of COPD. Genome sequence data were used to determine genotype and identify resistance genes. MICs of antibiotics were determined by reference broth microdilution. Generalized linear mixed models were used to evaluate associations between antibiotic use and H. influenzae eradication. We examined 267 H. influenzae isolates from 77 individuals. All newly acquired H. influenzae isolates were susceptible to azithromycin. Five of 27 (19%) strains developed 4-fold increases in azithromycin MICs and reached or exceeded the susceptibility breakpoint (≤4 μg/ml) during exposure. H. influenzae isolates were uniformly susceptible to ciprofloxacin, levofloxacin, and moxifloxacin (MIC90s of 0.015, 0.015, and 0.06, respectively); there were no mutations in quinolone resistance-determining regions. Fluoroquinolone administration was associated with increased H. influenzae eradication compared to macrolides (odds ratio [OR], 16.67; 95% confidence interval [CI], 2.67 to 104.09). There was no difference in H. influenzae eradication when comparing macrolide administration to no antibiotic (OR, 1.89; 95% CI, 0.43 to 8.30). Fluoroquinolones are effective in eradicating H. influenzae in individuals with COPD. Macrolides are ineffective in eradicating H. influenzae, and their use in COPD patients may lead to decreased macrolide susceptibility and resistance.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects approximately 24 million people in the United States and is the third leading cause of death worldwide (1, 2). Disease is characterized by intermittent acute exacerbations of COPD (AECOPD) that result in missed work, clinic visits, emergency room visits, hospital admissions, and respiratory failure requiring mechanical ventilation. Approximately half of AECOPD cases are caused by bacterial infection, with nontypeable Haemophilus influenzae being the most common pathogen (3, 4). H. influenzae also colonizes the lower airways of individuals with COPD for extended periods and contributes to inflammation, impaired pulmonary function, and increases in daily respiratory symptoms (3–6).
Clinical studies indicate that antibiotic therapy results in accelerated recovery and reduced complications of moderate and severe exacerbations (7, 8). Thus, antibiotics, particularly oral macrolides and fluoroquinolones, are commonly prescribed for treatment of AECOPD (4, 8–10). Several recent clinical trials demonstrated that daily azithromycin results in fewer exacerbations and improved quality of life, and thus, azithromycin is increasingly administered prophylactically to many COPD patients (11–13).
In spite of widespread administration of antibiotics for treatment and prophylaxis of infection in COPD, remarkably little is known about the effect of antibiotics on carriage and eradication of respiratory tract pathogens, particularly H. influenzae. Prior studies suggested that fluoroquinolones were superior to macrolides in eradicating H. influenzae (14–17). However, a significant limitation of these studies is that eradication was assumed if the patient was unable to produce sputum and/or was determined by a single posttreatment sputum culture. This study design misses the possibility of H. influenzae persistence in spite of negative culture, a scenario known to be common in COPD (3). When microbiologic confirmation was obtained, the strains were not genotyped, precluding determination of whether a positive culture represented unsuccessful bacterial eradication or acquisition of a new strain. Furthermore, relationships between antibiotic susceptibility and eradication were not investigated.
Longitudinal studies of COPD that include serial cultures to examine acquisition, eradication, and long-term carriage of pathogens are critical to assess the effect of antibiotics on bacterial pathogens in the respiratory tract. We performed a 15-year longitudinal study of individuals with COPD that included monthly sputum cultures and genotyping of each strain of H. influenzae isolated. Many of the study participants received repeated courses of macrolides and/or fluoroquinolones as part of their clinical management, thus providing a unique opportunity to rigorously assess the effect of antibiotic therapy on H. influenzae in individuals with COPD. The goals of this study were to use this unique set of 267 H. influenzae isolates with corresponding epidemiologic and clinical data to (i) define azithromycin and fluoroquinolone susceptibility in H. influenzae isolates, (ii) determine the prevalence of known resistance-determining genes to macrolides and fluoroquinolones, (iii) assess the effect of antibiotic administration in individuals with COPD on development of resistance in persistent strains of H. influenzae, and (iv) assess the effect of administration of macrolides and fluoroquinolones on eradication of H. influenzae from the respiratory tract of individuals with COPD.
MATERIALS AND METHODS
The Institutional Review Boards of the University at Buffalo and the Western New York VA Healthcare System approved this study. All study participants provided written informed consent prior to enrollment.
COPD study clinic.
Detailed data regarding demographic and clinical characteristics, antibiotic use, and H. influenzae strains were collected in the COPD study clinic. Detailed methods of the COPD study clinic are provided elsewhere (10). Briefly, the COPD study clinic is a prospective study that started in 1994 at the Buffalo VA Medical Center. Subjects had monthly clinic visits and were also seen at unscheduled visits at the onset of suspected acute exacerbations. Expectorated sputum samples were collected at each visit. Sputum samples were also collected from subjects at their homes at 2-week intervals between monthly clinic visits by a courier (i.e., courier visits) during selected years of the study. The current study includes participants enrolled from April 1994 through March 2009 who had three or more clinic visits. Dates and types of antibiotic use were determined by self-report at clinic visits and medical record review. Standard dosage regimens were used for each antibiotic prescribed.
H. influenzae strains.
H. influenzae strains were identified using standard techniques and differentiated from Haemophilus haemolyticus using a P6-specific monoclonal antibody (18). H. influenzae strains were classified as cleared if they were isolated at a single monthly clinic visit and were not isolated again at subsequent monthly clinic visits. Strains were classified as persistent if they were isolated from a study participant multiple times at monthly clinic visits and were shown to be the same strain by multilocus sequence typing (MLST). This study included cleared H. influenzae strains and the first detected H. influenzae isolate and final detected H. influenzae isolate of persistent strain pairs. MICs of azithromycin, ciprofloxacin, moxifloxacin, and levofloxacin were determined by reference broth microdilution using Clinical and Laboratory Standards Institute (CLSI) standards (19). CLSI interpretative antimicrobial sensitive breakpoints were utilized for azithromycin (≤4 μg/ml), ciprofloxacin (≤1 μg/ml), levofloxacin (≤2 μg/ml), and moxifloxacin (≤2 μg/ml). We considered ≥4-fold changes in MIC that reached or exceeded the CLSI breakpoints as clinically significant.
Identification of multilocus STs and antibiotic resistance genes.
Genomic DNA was extracted and sequenced on the Illumina platform. Raw sequencing data were filtered to retain high-quality reads and error-corrected using btrim (20). Sequences were aligned to the H. influenzae 86-028NP genome (GenBank accession no. CP000057.2) using bwa (21). Reads mapping to the seven MLST genes (22) were extracted from bam files using SAMtools (23). Read alignments were constructed, and the consensus sequences were called using an in-house program. Sequences were submitted to the MLST database (www.mlst.net), and a sequence type (ST) was assigned to each strain.
H. influenzae resistance to macrolides has been linked to chromosomal mutations in genes encoding L4 and L22 ribosomal proteins and in 23S rRNA (24, 25). The sequences of L4, L22, and domains II and V of 23S rRNA were determined by mapping reads to H. influenzae 86-028NP. Macrolide resistance has also been associated with horizontally transferred erm genes, which encode rRNA methylases that inhibit macrolide binding of the 23S rRNA target, as well as mef genes, which encode alternate efflux pumps (24, 25). Resistance genes mefA, ermA, ermB, ermC, and ermF were mapped to GenBank accession numbers NC_023287.1, CP003585.1, NC_014959.1, AJ888003.1, and JQ707297, respectively. Fluoroquinolone resistance occurs via mutations within the quinolone resistance-determining regions (QRDRs) in genes that encode DNA gyrase (gyrA and gryB) and topoisomerase IV (parC and parE) (26–28). The sequences of gyrA, gyrB, parC, and parE were determined by mapping reads to H. influenzae 86-028NP. Genes were screened for mutations using Sequencher 5.3 (Gene Codes, Ann Arbor, MI).
Statistical analyses.
Analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC). Unadjusted associations between patient characteristics, antibiotic prescriptions within 5 days of a clinic visit, and H. influenzae detection were evaluated by χ2 test or Student's t test as appropriate. The duration of carriage of individual H. influenzae strains was determined by calculating the number of days between the first visit date when the strain was detected and the last visit date when the strain was detected. We used a generalized linear mixed model, which is a logistic regression model with fixed and random effects, to evaluate the association between antibiotic use, occurring within 5 days of the visit in which the strain was first detected, and the outcome of H. influenzae eradication, defined as confirmed eradication of H. influenzae within 6 weeks of the initial visit. This modeling approach was used to take into account multiple samples contributed by individual patients. The 6-week cutoff was chosen due to the study design, which included monthly visits plus a 2-week scheduling window.
RESULTS
Study population.
During the study period, 157 participants had sputum samples taken during monthly clinic visits. Of these, seven had fewer than three visits and were dropped, leaving 150 participants. Demographic and clinical characteristics of the study participants are in Table 1. The majority of study participants were white and male. A total of 167 independent H. influenzae strains were identified from sputum samples from 77 participants by MLST. Study subjects from whom H. influenzae was isolated were enrolled for significantly longer periods and had more clinic visits and a greater number of AECOPD episodes (Table 1). Study participants were colonized with between one and 11 independent H. influenzae strains. Of the 167 independent isolates, 67 strains were isolated at only one study visit and 100 isolates were isolated at more than one monthly visit. Thus, the entire strain collection included 267 isolates comprised of 67 cleared strains and 100 persistent strain pairs. Of the 267 H. influenzae isolates collected, 209 (78%) were from regularly scheduled clinic visits, 46 (17%) were from unscheduled exacerbation visits, and 12 (4%) were courier samples. The remaining analyses were restricted to the 77 participants who carried H. influenzae at some point during the study period.
TABLE 1.
Characteristics of study participants
| Characteristic | All patients |
Haemophilus influenzae status |
P valueb | |
|---|---|---|---|---|
| Negative | Positive | |||
| Patients (n) | 150 | 73 | 77 | |
| Age (yr) | 0.38 | |||
| Mean (SD) | 66.6 (9.6) | 67.3 (9.8) | 65.9 (9.5) | |
| Range | 45–85 | 47–85 | 45–84 | |
| Race (n [%])a | 0.57 | |||
| White | 131 (87.3) | 65 (89.0) | 66 (85.7) | |
| Nonwhite | 18 (12.0) | 8 (11.0) | 10 (13.0) | |
| Sex (n [%]) | 0.16 | |||
| Male | 148 (98.7) | 73 (100) | 75 (97.4) | |
| Female | 2 (1.3) | 0 | 2 (2.6) | |
| Current smoker (n [%]) | 0.10 | |||
| Yes | 51 (34.0) | 20 (27.4) | 31 (40.3) | |
| No | 99 (66.0) | 53 (72.6) | 46 (59.7) | |
| Pack yrsc | 0.83 | |||
| Mean (SD) | 81.2 (38.2) | 80.5 (42.0) | 81.8 (34.4) | |
| Range | 10–185 | 10–185 | 10–160 | |
| Enrollment duration (yr) | <0.0001 | |||
| Mean (SD) | 4.1 (3.6) | 2.3 (2.1) | 5.8 (3.8) | |
| Range | 0.10–15.0 | 0.10–8.4 | 0.5–15.0 | |
| FEV1d at enrollment | 0.65 | |||
| Mean (SD) | 1.63 (0.68) | 1.60 (0.70) | 1.65 (0.67) | |
| Range | 0.47–4.07 | 0.53–3.96 | 0.47–4.07 | |
| Clinic visits (no.) | <0.0001 | |||
| Mean (SD) | 43 (40) | 23 (21) | 62 (45) | |
| Range | 3–176 | 3–85 | 6–176 | |
| Exacerbation visits (no.) | <0.0001 | |||
| Mean (SD) | 8 (9) | 4 (4) | 11 (11) | |
| Range | 0–42 | 0–16 | 0–42 | |
| Courier samples (no.) | 0.11 | |||
| Mean (SD) | 10 (15) | 8 (13) | 12 (17) | |
| Range | 0–48 | 0–44 | 0–48 | |
One subject with H. influenzae was missing data on race.
P values are from chi-square tests for race, sex, and current smoker and from t tests for all other variables. Boldface indicates a significant P value.
Pack years were calculated by multiplying the number of packs of cigarettes smoked by the number of years the participant smoked.
FEV1, median forced expiratory volume in 1 s.
Antibiotic use in patients from whom H. influenzae was cultured during the study period.
During the study period, the 77 participants from whom H. influenzae was isolated were exposed to a significant number of antibiotics. Only five of the 77 never had an AECOPD episode, and as a group, they experienced a total of 882 AECOPD episodes. Within 5 days of these 882 exacerbation visits, a macrolide was administered in 21.9%, a fluoroquinolone was administered in 23.2%, and an “other antibiotic,” which includes cephalosporins, penicillins, sulfonamides, and all others, was administered in 32.7%. These 77 participants received a total of 218 courses of macrolides at some time during the study with a mean (standard deviation) of 2.8 (3.7) courses per person and a range of 0 to 15 courses per person. Of the 218 macrolides administered, 96.8% were azithromycin, 1.8% were clarithromycin, and 1.4% were erythromycin. There were 240 courses of fluoroquinolones administered at some time during the study with a mean of 3.1 (4.5) courses per person and a range of 0 to 24 courses per person. Of the 240 fluoroquinolones administered, 41.2% were levofloxacin, 20.4% were ciprofloxacin, 17.1% were gatifloxacin, 11.7% were moxifloxacin, 9.2% were ofloxacin, and 0.4% were gemifloxacin.
Susceptibility of H. influenzae to macrolide and fluoroquinolones.
The susceptibilities to azithromycin, ciprofloxacin, levofloxacin, and moxifloxacin of the initial (first-detected) H. influenzae isolates and the final H. influenzae isolates from persistent strains are shown in Table 2. All initial H. influenzae isolates were susceptible to azithromycin using the CLSI breakpoint of ≤4 μg/ml (MIC50, 1 μg/ml; MIC90, 4 μg/ml; range, 0.06 to 4 μg/ml) (Table 2). One H. influenzae strain developed resistance (MIC, 16 μg/ml) to azithromycin during long-term persistence. All of the H. influenzae isolates were susceptible to ciprofloxacin (MIC50, 0.008 μg/ml; MIC90, 0.015 μg/ml; range, 0.004 to 1 μg/ml), levofloxacin (MIC50, 0.008 μg/ml; MIC90, 0.015 μg/ml; range, 0.008 to 1 μg/ml), and moxifloxacin (MIC50, 0.015 μg/ml; MIC90, 0.06 μg/ml; range, 0.004 to 0.5 μg/ml) (Table 2).
TABLE 2.
Activity of macrolides and fluoroquinolones against initial and final persistent H. influenzae isolates
| H. influenzae strain group (n) and antimicrobial agent | MIC (μg/ml) |
% susceptible | ||
|---|---|---|---|---|
| 50% | 90% | Range | ||
| Initial (167 strains) | ||||
| Azithromycin | 1 | 4 | 0.06–4 | 100 |
| Ciprofloxacin | 0.008 | 0.015 | 0.004–1 | 100 |
| Levofloxacin | 0.008 | 0.015 | 0.008–1 | 100 |
| Moxifloxacin | 0.015 | 0.06 | 0.004–0.5 | 100 |
| Final persistent (100 strain pairs) | ||||
| Azithromycin | 2 | 4 | 0.25–16 | 99 |
| Ciprofloxacin | 0.008 | 0.015 | 0.00–0.06 | 100 |
| Levofloxacin | 0.008 | 0.015 | 0.008–0.25 | 100 |
| Moxifloxacin | 0.015 | 0.06 | 0.004–0.12 | 100 |
Relationship between macrolide and fluoroquinolone administration and H. influenzae persistence: paired analysis of strains.
Next, we assessed the effect of macrolide and fluoroquinolone administration on 100 persistent strain pairs. Table 3 shows the H. influenzae strain, multilocus ST, duration of persistence, number of courses of macrolides, and initial and final azithromycin MICs for 27 H. influenzae persistent pairs from study subjects who received azithromycin. H. influenzae strains were carried for 17 to 1,422 days. Each strain persisted despite at least one and up to seven courses of a macrolide.
TABLE 3.
Haemophilus influenzae strain pairs from study participants who received macrolide(s) during the time that they carried H. influenzae (n = 27)
| Study identifier | Strain first isolated | Strain last isolated | Multilocus ST | Strain duration (days)a | Rx no.b | Azithromycin MIC (μg/ml) |
|
|---|---|---|---|---|---|---|---|
| Initial | Final | ||||||
| 5 | 5P28H1 | 5P54H1c | 103 | 819 | 4 | 2 | 16 |
| 13 | 13P24H2 | 13P36H1 | 57 | 355 | 1 | 2 | 2 |
| 19 | 19P49H1 | 19P94H1 | 243 | 1,422 | 5 | 1 | 2 |
| 24 | 24P44H4 | 24P45H1c | 680 | 17 | 1 | 0.5 | 4 |
| 33 | 33P110H3 | 33P125H1 | 136 | 329 | 1 | 4 | 4 |
| 45 | 45P9H2 | 45P11H1 | 3 | 29 | 1 | 1 | 2 |
| 48 | 48P106H1 | 48P153H1 | 155 | 993 | 2 | 1 | 2 |
| 48 | 48P160H1 | 48P162H1 | 196 | 28 | 1 | 1 | 1 |
| 49 | 49P5H1 | 49P21H1 | 85 | 672 | 1 | 1 | 1 |
| 56 | 56P1H1 | 56P16H1 | 265 | 469 | 1 | 2 | 1 |
| 67 | 67P38H1 | 67P56H1c | 156 | 570 | 2 | 1 | 4 |
| 67 | 67P10H5 | 67P50H1 | 411 | 1,380 | 1 | 1 | 1 |
| 73 | 73P2H1 | 73P39H1 | 14 | 1,175 | 1 | 4 | 4 |
| 84 | 84P18H1 | 84P20H1 | 66 | 77 | 1 | 4 | 4 |
| 87 | 87P128H2 | 87P138H1 | 389 | 140 | 2 | 4 | 4 |
| 91 | 91P109H1 | 91P114H1 | 98 | 70 | 1 | 1 | 0.5 |
| 91 | 91P3H3 | 91P4H1c | 411 | 29 | 1 | 1 | 4 |
| 94 | 94P17H1 | 94P20H1 | 139 | 98 | 1 | 0.5 | 2 |
| 99 | 99P41H1 | 99P50H1 | 3 | 212 | 1 | 1 | 1 |
| 101 | 101P3H1 | 101P19H1 | 12 | 474 | 2 | 2 | 4 |
| 102 | 102P20H1 | 102P32H1c | 159 | 364 | 1 | 0.5 | 4 |
| 103 | 103P3H1 | 103P20H1 | 155 | 565 | 2 | 1 | 0.5 |
| 107 | 107P50H1 | 107P75H1 | 215 | 560 | 7 | 2 | 4 |
| 122 | 122P4H1 | 122P13H1 | 11 | 254 | 1 | 2 | 4 |
| 126 | 126P11H1 | 126P26H1 | 66 | 344 | 1 | 0.5 | 2 |
| 128 | 128P1H1 | 128P56H1 | 98 | 932 | 3 | 2 | 4 |
| 138 | 138P2H1 | 138P39H1 | 203 | 721 | 1 | 1 | 2 |
Strain duration is defined as the number of days between the first date that the strain was identified and the last date that the strain was identified.
Rx no., number of courses of macrolides administered while the strain was carried.
MIC of azithromycin increased 4-fold or more and reached or exceeded the CLSI breakpoint (≤4 μg/ml) during carriage.
One of the strain pairs (5P28H1 and 5P54H1) demonstrated a remarkable shift in MIC from susceptible to nonsusceptible during the course of macrolide therapy and persistence (Table 3). This strain demonstrated a susceptible azithromycin MIC of 2 μg/ml when first detected (isolate 5P28H1), and the final isolate had a resistant azithromycin MIC of 16 μg/ml (isolate 5P54H1). The study participant received four courses of macrolides during 819 days of strain carriage. We identified a known macrolide resistance mutation in L22 in isolate 5P54H1, a switch from glycine to aspartic acid at amino acid 91 (G91D) (25). In four instances, the azithromycin MIC increased 4-fold during persistence and reached the CLSI breakpoint of 4 μg/ml (Table 3). A G91D mutation in L22 was present in one of these strains. The initial isolate, 24P44H4, had an azithromycin MIC of 0.5 mg/ml, and the final detected isolate, 24P45H1, had an azithromycin MIC at the upper limit of susceptibility following exposure to one course of macrolides over 17 days of carriage. We did not detect mutations in L4 or in domains II and V of 23S rRNA in any of the isolates. Nor did we detect mefA, ermA, ermB, ermC, and ermF in any of the 100 persistent pairs, including the 27 persistent pairs that were exposed to macrolides. There were no significant shifts (i.e., ≥4-fold change that reached or exceeded the CLSI breakpoint of ≤4 μg/ml) in the MICs of persistent H. influenzae strains from patients who were not exposed to macrolides during carriage (data not shown).
Table 4 shows the H. influenzae strain, multilocus ST, duration of persistence, number of fluoroquinolone prescriptions, and initial and final levofloxacin MICs for three H. influenzae persistent pairs from study subjects who received fluoroquinolones. H. influenzae strains were carried for 305 to 993 days. In contrast to the 27 instances where strains persisted through macrolide administration, there were only three H. influenzae strains that persisted despite exposure to a fluoroquinolone (Table 4). Each of these strains had low MICs of levofloxacin (MIC, ≤0.015 μg/ml), ciprofloxacin (MIC, ≤0.015 μg/ml), and moxifloxacin (MIC, ≤0.015 μg/ml). Moreover, there were no significant changes in fluoroquinolone MICs and the strains remained highly susceptible during persistence (Table 4). Consistent with the MIC data, mutations were not detected in gyrA, gyrB, parC, or parE for any H. influenzae isolates.
TABLE 4.
Haemophilus influenzae strain pairs from study participants who received courses of fluoroquinolones during the time that they carried H. influenzae (n = 3)
| Study identifier | Strain first isolated | Strain last isolated | Multilocus ST | Strain duration (days)a | Rx no.b | Levofloxacin MIC (μg/ml)c |
|
|---|---|---|---|---|---|---|---|
| Initial | Final | ||||||
| 39 | 39P1H1 | 39P18H1 | 10002 | 529 | 1 | 0.008 | 0.015 |
| 48 | 48P106H1 | 48P153H1 | 155 | 993 | 1 | 0.008 | 0.008 |
| 54 | 54P24H7 | 54P33H2 | 107 | 305 | 1 | 0.008 | 0.008 |
Strain duration is defined as the number of days between the first date that the strain was identified and the last date that the strain was identified.
Rx no., number of prescriptions taken for fluoroquinolones within 5 days of any clinic visit while the strain was carried.
Data are presented for levofloxacin only; ciprofloxacin and moxifloxacin MICs were <0.03 μg/ml for both initial and last strains.
Effect of antibiotic administration on eradication of a newly acquired H. influenzae strain.
Figure 1 shows the relationship between antibiotic administration for macrolides, fluoroquinolones, or other antibiotics within 5 days of the first visit when an H. influenzae strain was identified and confirmed eradication of the same strain within 6 weeks. We eliminated 14 strains from this analysis. There were 13 strains that we could not confirm as recent-acquisition isolates; 11 were present at the first study clinic visit, and two were isolated from participants with more than three missing visits prior to strain isolation. One additional strain was eliminated because the patient did not have a clinic visit after the strain was identified. No difference was observed in the likelihood of H. influenzae eradication at 6 weeks when comparing macrolide administration and no antibiotic (Table 5; Fig. 1). In contrast, fluoroquinolone administration was associated with a >30-fold-greater likelihood of H. influenzae eradication at 6 weeks compared to no antibiotic (Table 5). A separate generalized linear mixed model also indicated that fluoroquinolone administration was associated with increased H. influenzae eradication compared to macrolide administration (odds ratio [OR], 16.67; 95% confidence interval [95% CI], 2.67 to 104.09).
FIG 1.
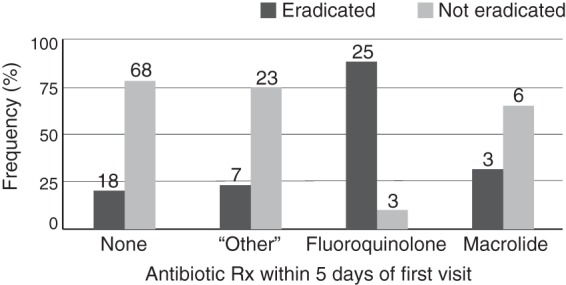
Confirmed eradication of a newly acquired H. influenzae strain at 6 weeks. Graph showing the relationship between antibiotic administration within 5 days of the first visit when a newly acquired H. influenzae strain was identified (n = 153 strains) and eradication within 6 weeks of the first visit. Antibiotic categories were as follows: none (n = 86); “other” (n = 30), including cephalosporins, penicillins, sulfa drugs, and all others; fluoroquinolones (n = 28); and macrolides (n = 9). Bars indicate the proportion of strains in each antibiotic category where the strain was eradicated (dark gray) or was not eradicated (light gray) within 6 weeks of the first visit. Numbers of samples in each category are shown above bars. We eliminated from analysis 14 strains that we could not confirm as recent-acquisition isolates; 11 were present at the first study clinic visit, and two were isolated from participants with more than three missing visits prior to strain isolation. One additional strain was eliminated because the participant did not have a follow-up visit.
TABLE 5.
Estimated odds ratios for administration of specific antibiotics within 5 days of the first visit when an H. influenzae strain was identified (n = 153b) and eradication of the strain within 6 weeks
| Antibiotic administered | Odds ratio | 95% confidence interval |
|---|---|---|
| No antibiotic (reference) | ||
| Macrolide | 1.89 | 0.43–8.30 |
| Fluoroquinolone | 31.48 | 8.53–116.14 |
| Other antibiotica | 1.15 | 0.43–3.10 |
Other antibiotics included cephalosporins, penicillins, sulfa drugs, and all others.
We eliminated from analysis 14 strains that we could not confirm as recent-acquisition isolates; 11 were present at the first study clinic visit, and two were isolated from participants with more than three missing visits prior to strain isolation. One additional strain was eliminated because the participant did not have a follow-up visit.
DISCUSSION
This 15-year prospective study of H. influenzae carriage and infection demonstrates striking differences between the effects of macrolides and those of fluoroquinolones in eradication of H. influenzae from the respiratory tract of individuals with COPD. Macrolide administration resulted in eradication of a newly acquired H. influenzae strain in only 33% of patients, which was no different from those patients who received no antibiotic (P = 0.40) (Fig. 1). Administration of a fluoroquinolone resulted in eradication of the strain in 89% of patients, which was significantly different from patients who received no antibiotic (P < 0.0001). Furthermore, 27 strains persisted in the airways of study participants while they were receiving macrolides (Table 3). In contrast, only three strains persisted through courses of fluoroquinolones (Table 4). Of concern, five of 27 strains of H. influenzae that persisted during macrolide administration showed a 4-fold rise in MIC and reached or exceeded the upper limit of susceptibility. In contrast, H. influenzae isolates were uniformly susceptible to all fluoroquinolones.
This study used a rigorous approach to assess eradication of H. influenzae. Pooled analyses of four randomized controlled trials showed that eradication of H. influenzae was higher for patients treated with moxifloxacin than for those receiving macrolides (93.0% versus 73.2%, respectively; P = 0.001) (17). These prior studies have been limited by employing a single culture to assess eradication or by assuming that the bacterial strain was eradicated if a patient was unable to produce sputum, missing the common occurrence of intermittent positive and negative cultures during persistent carriage of H. influenzae (3). Molecular typing enabled us to distinguish true eradication from clearance and reacquisition of another strain, another common occurrence in individuals with COPD (10).
The optimal antibiotic for treatment of COPD is one that would effectively eradicate H. influenzae and minimize development of antibiotic resistance (29). Data from our study indicate that macrolide treatment has limited effectiveness in eradicating H. influenzae in COPD patients. With regard to the potential development of antibiotic resistance, the observed increases in MICs are particularly worrisome. The CLSI breakpoint for azithromycin is the wild-type cutoff value for H. influenzae and reflects the intrinsic activity of azithromycin when it was introduced; the clinical relevance of azithromycin MICs at the upper edge of the susceptibility breakpoint is a matter of debate (24, 30). Azithromycin accumulates intracellularly, and extracellular serum concentrations generally do not exceed 0.5 μg/ml, which is well below the CLSI breakpoint of 4.0 μg/ml (31–33). Thus, one might expect azithromycin failures at MIC values below the CLSI breakpoint. Azithromycin has a long elimination half-life of approximately 66 h (34). Importantly, this half-life is longer for azithromycin than for all other antibiotics utilized in COPD patients and may lead to drug concentrations well below the MIC for up to 30 days (34, 35). Prolonged and subinhibitory antibiotic concentrations are likely to provide an optimal environment for the amplification of antibiotic-resistant subpopulations (36). A randomized placebo-controlled trial of daily azithromycin to prevent AECOPD showed that while patients receiving azithromycin were less likely to be colonized with bacterial pathogens, they were more likely to be colonized with macrolide-resistant bacteria than were patients receiving placebo (11). The widespread use of azithromycin for treatment and prevention of AECOPD, combined with the large number of individuals with COPD, indicates that such use may lead to increased levels of resistance in H. influenzae. The widespread use of azithromycin has also been linked to increases in resistance in Streptococcus pneumoniae, another important COPD pathogen, and also in the commensal flora (37).
Our data demonstrated that fluoroquinolones were effective in eradicating H. influenzae in individuals with COPD and that all of the isolates were susceptible to fluoroquinolones. The prevalence of fluoroquinolone resistance in H. influenzae is low in the United States (24, 37). Mutations in gyrA occur as a first step toward development of resistance and facilitate additional mutations in gyrB, parC, or parE that can further increase resistance (38). Analysis of genes that mediate fluoroquinolone resistance indicated an absence of mutations that lead to resistance in all 267 H. influenzae strains examined. In a study where six 5-day courses of a fluoroquinolone were administered over 48 weeks to a large cohort of COPD patients to prevent exacerbations, no emergence of fluoroquinolone-resistant H. influenzae was described, consistent with our observations (39). The prevalence of resistance to fluoroquinolones has also remained low in S. pneumoniae (37). While these data are reassuring, caution is warranted with fluoroquinolone use as COPD is a risk factor for acquisition of fluoroquinolone-resistant H. influenzae (40).
Nine courses of macrolides, 28 courses of fluoroquinolones, and 30 courses of “other antibiotics” were administered in association with acquisition of a new H. influenzae strain (Fig. 1 and Table 4). The number of macrolides associated with new acquisitions of H. influenzae was low given the high frequency of macrolide use throughout the study period (218 macrolides versus 240 fluoroquinolones). We evaluated this observation further in post hoc analyses and believe that this occurred due to clinician prescribing behavior and the severity of COPD associated with H. influenzae. AECOPD episodes were associated with detection of H. influenzae, which was isolated in 19.4% of exacerbation visits, 14.9% of nonexacerbation visits, and 6.1% of courier samples (χ2 test, P < 0.0001). We used a generalized linear mixed model in analyses stratified by exacerbation status to examine the odds of a particular class of antibiotic being used within 5 days of a clinic visit and the outcome of whether any H. influenzae strain was detected at that clinic visit (yes/no). During exacerbation visits, fluoroquinolone administration (compared to no antibiotic prescribed as the reference group) was significantly associated with isolation of H. influenzae (OR, 3.23; 95% CI, 1.76 to 5.94). “Other antibiotic” administration was also significantly associated with isolation of H. influenzae (OR, 3.80; 95% CI, 2.16 to 6.68). In contrast, there was no difference in the odds of macrolide administration when H. influenzae was detected (OR, 1.90; 95% CI, 1.00 to 3.63). Current treatment guidelines recommend macrolides for moderate to severe AECOPD in uncomplicated COPD and fluoroquinolones and amoxicillin-clavulanate for AECOPD in complicated COPD (4). The clinicians in this study prescribed antibiotics empirically. These data support the idea that H. influenzae is associated with more severe COPD and that the doctors in this study were adjusting their prescribing behavior to reflect this.
Strengths of the study include the longitudinal design, examination of study participants during AECOPD and stable disease, the incorporation of both susceptibility and genome sequence data, and accurate assessment of eradication. A limitation of our study is that we did not identify the mechanism that allowed susceptible H. influenzae to persist despite antibiotic treatment; susceptible H. influenzae may persist due to biofilm formation. We did not identify a mechanism for the 4-fold increases in azithromycin MICs for three of the five strains. Furthermore, we were unable to make observations regarding other classes of antibiotics due to the heterogeneity in mechanisms of resistance and range of other antibiotics administered. While relying on antibiotic prescriptions to determine exposure is a limitation, study participants received their health care at the Buffalo VA Medical Center, where the study was performed, and were followed monthly, providing careful monitoring of antibiotic administration and symptoms.
In summary, our data indicate that compared to macrolides, the fluoroquinolones were significantly more effective in eradicating H. influenzae from the respiratory tract of adults with COPD. We also demonstrate that 19% of H. influenzae strains that were exposed to macrolides and persisted developed 4-fold increases in azithromycin MICs that reached or exceeded CLSI susceptibility breakpoints. The widespread use of azithromycin for treatment of AECOPD and for maintenance therapy, combined with the large number of individuals with COPD, indicates that such use may lead to increased levels of resistance in H. influenzae. Monitoring of antimicrobial susceptibility of bacteria isolated from individuals with COPD who are receiving chronic azithromycin will be critical. Moreover, the development of macrolide resistance may not simply be confined to H. influenzae in COPD patients, as it has been suggested that widespread macrolide use may result in collateral damage and decreases in susceptibility in a range of pathogens (41).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. 2006. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Kim V, Criner GJ. 2013. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 187:228–237. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. 2004. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 170:266–272. doi: 10.1164/rccm.200403-354OC. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, Murphy TF. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 5.White AJ, Gompertz S, Bayley DL, Hill SL, O'Brien C, Unsal I, Stockley RA. 2003. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 58:680–685. doi: 10.1136/thorax.58.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai H, Eschberger K, Wrona C, Grove L, Agrawal A, Grant B, Yin J, Parameswaran GI, Murphy T, Sethi S. 2014. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 11:303–309. doi: 10.1513/AnnalsATS.201310-350OC. [DOI] [PubMed] [Google Scholar]
- 7.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. 2013. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 8.Saint S, Bent S, Vittinghoff E, Grady D. 1995. Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysis. JAMA 273:957–960. [PubMed] [Google Scholar]
- 9.Miravitlles M. 2007. Moxifloxacin in the management of exacerbations of chronic bronchitis and COPD. Int J Chron Obstruct Pulmon Dis 2:191–204. [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi S, Evans N, Grant BJ, Murphy TF. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 11.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR, COPD Clinical Research Network. 2011. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365:689–698. doi: 10.1056/NEJMoa1104623 (Erratum, 366:1356, 2012.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van't Veer NE, Ermens AA, Pelle AJ, Hoogsteden HC, Aerts JG, van der Eerden MM. 2014. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2:361–368. doi: 10.1016/S2213-2600(14)70019-0. [DOI] [PubMed] [Google Scholar]
- 13.Berkhof FF, Doornewaard-ten Hertog NE, Uil SM, Kerstjens HA, van den Berg JW. 2013. Azithromycin and cough-specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respir Res 14:125. doi: 10.1186/1465-9921-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson R, Kubin R, Ballin I, Deppermann KM, Bassaris HP, Leophonte P, Schreurs AJ, Torres A, Sommerauer B. 1999. Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother 44:501–513. doi: 10.1093/jac/44.4.501. [DOI] [PubMed] [Google Scholar]
- 15.Chodosh S, Schreurs A, Siami G, Barkman HW Jr, Anzueto A, Shan M, Moesker H, Stack T, Kowalsky S. 1998. Efficacy of oral ciprofloxacin vs. clarithromycin for treatment of acute bacterial exacerbations of chronic bronchitis. The Bronchitis Study Group Clin Infect Dis 27:730–738. doi: 10.1086/514934. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FJ, Grossman RF, Zadeikis N, Fisher AC, Walker K, Ambruzs ME, Tennenberg AM. 2005. Patient stratification in the management of acute bacterial exacerbation of chronic bronchitis: the role of levofloxacin 750 mg. Eur Respir J 25:1001–1010. doi: 10.1183/09031936.05.00106404. [DOI] [PubMed] [Google Scholar]
- 17.Niederman MS, Anzueto A, Sethi S, Choudhri S, Kureishi A, Haverstock D, Perroncel R. 2006. Eradication of H. influenzae in AECB: a pooled analysis of moxifloxacin phase III trials compared with macrolide agents. Respir Med 100:1781–1790. doi: 10.1016/j.rmed.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ. 2007. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis 195:81–89. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M100-S21, vol 31 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Kong Y. 2011. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98:152–153. doi: 10.1016/j.ygeno.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, Kroll JS, Popovic T, Spratt BG. 2003. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol 41:1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tristram S, Jacobs MR, Appelbaum PC. 2007. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev 20:368–389. doi: 10.1128/CMR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogdanovich T, Bozdogan B, Appelbaum PC. 2006. Effect of efflux on telithromycin and macrolide susceptibility in Haemophilus influenzae. Antimicrob Agents Chemother 50:893–898. doi: 10.1128/AAC.50.3.893-898.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies TA, Kelly LM, Hoellman DB, Ednie LM, Clark CL, Bajaksouzian S, Jacobs MR, Appelbaum PC. 2000. Activities and postantibiotic effects of gemifloxacin compared to those of 11 other agents against Haemophilus influenzae and Moraxella catarrhalis. Antimicrob Agents Chemother 44:633–639. doi: 10.1128/AAC.44.3.633-639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgiou M, Munoz R, Roman F, Canton R, Gomez-Lus R, Campos J, De La Campa AG. 1996. Ciprofloxacin-resistant Haemophilus influenzae strains possess mutations in analogous positions of GyrA and ParC. Antimicrob Agents Chemother 40:1741–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Martinez JM, Lopez L, Garcia I, Pascual A. 2006. Characterization of a clinical isolate of Haemophilus influenzae with a high level of fluoroquinolone resistance. J Antimicrob Chemother 57:577–578. doi: 10.1093/jac/dki488. [DOI] [PubMed] [Google Scholar]
- 29.Rennie RP, Ibrahim KH. 2005. Antimicrobial resistance in Haemophilus influenzae: how can we prevent the inevitable? Commentary on antimicrobial resistance in H influenzae based on data from the TARGETed surveillance program. Clin Infect Dis 41(Suppl 4):S234–S238. [DOI] [PubMed] [Google Scholar]
- 30.Dagan R, Leibovitz E. 2002. Bacterial eradication in the treatment of otitis media. Lancet Infect Dis 2:593–604. doi: 10.1016/S1473-3099(02)00394-8. [DOI] [PubMed] [Google Scholar]
- 31.Drusano GL, Craig WA. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J Chemother 9(Suppl 3):S38–SS44. [PubMed] [Google Scholar]
- 32.Foulds G, Shepard RM, Johnson RB. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 25(Suppl A):73–82. [DOI] [PubMed] [Google Scholar]
- 33.Amsden GW, Nafziger AN, Foulds G. 1999. Pharmacokinetics in serum and leukocyte exposures of oral azithromycin, 1,500 milligrams, given over a 3- or 5-day period in healthy subjects. Antimicrob Agents Chemother 43:163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nightingale CH. 1997. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr Infect Dis J 16:438–443. doi: 10.1097/00006454-199704000-00027. [DOI] [PubMed] [Google Scholar]
- 35.Crokaert F, Hubloux A, Cauchie P. 1998. A phase I determination of azithromycin in plasma during a 6-week period in normal volunteers after a standard dose of 500mg once daily for 3 days. Clin Drug Invest 16:161–166. doi: 10.2165/00044011-199816020-00009. [DOI] [PubMed] [Google Scholar]
- 36.Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 37.Pfaller MA, Farrell DJ, Sader HS, Jones RN. 2012. AWARE Ceftaroline Surveillance Program (2008–2010): trends in resistance patterns among Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States. Clin Infect Dis 55(Suppl 3):S187–S193. doi: 10.1093/cid/cis561. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Mariano N, Rahal JJ, Urban CM, Drlica K. 2004. Quinolone-resistant Haemophilus influenzae: determination of mutant selection window for ciprofloxacin, garenoxacin, levofloxacin, and moxifloxacin. Antimicrob Agents Chemother 48:4460–4462. doi: 10.1128/AAC.48.11.4460-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sethi S, Jones PW, Theron MS, Miravitlles M, Rubinstein E, Wedzicha JA, Wilson R, PULSE Study Group. 2010. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res 11:10. doi: 10.1186/1465-9921-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazir J, Urban C, Mariano N, Burns J, Tommasulo B, Rosenberg C, Segal-Maurer S, Rahal JJ. 2004. Quinolone-resistant Haemophilus influenzae in a long-term care facility: clinical and molecular epidemiology. Clin Infect Dis 38:1564–1569. doi: 10.1086/420820. [DOI] [PubMed] [Google Scholar]
- 41.Serisier DJ. 2013. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir Med 1:262–274. doi: 10.1016/S2213-2600(13)70038-9. [DOI] [PubMed] [Google Scholar]


