Abstract
Acetamido derivatives of the naturally antibacterial non-β-lactam lactivicin (LTV) have improved activity against their penicillin binding protein targets and reduced hydrolysis by β-lactamases, but penetration into Gram-negative bacteria is still relatively poor. Here we report that modification of the LTV lactone with a catechol-type siderophore increases potency 1,000-fold against Stenotrophomonas maltophilia, a species renowned for its insusceptibility to antimicrobials. The MIC90 of modified lactone compound 17 (LTV17) against a global collection of extensively drug-resistant clinical S. maltophilia isolates was 0.063 μg · ml−1. Sideromimic modification does not reduce the ability of LTVs to induce production of the L1 and L2 β-lactamases in S. maltophilia and does not reduce the rate at which LTVs are hydrolyzed by L1 or L2. We conclude, therefore, that lactivicin modification with a siderophore known to be preferentially used by S. maltophilia substantially increases penetration via siderophore uptake. LTV17 has the potential to be developed as a novel antimicrobial for treatment of infections by S. maltophilia. More generally, our work shows that sideromimic modification in a species-targeted manner might prove useful for the development of narrow-spectrum antimicrobials that have reduced collateral effects.
INTRODUCTION
Lactivicin (LTV) is highly unusual in that it is the only non-β-lactam natural product known to target penicillin binding proteins (PBPs). Unlike the β-lactams, which remain the most important antimicrobial class, lactivicin contains cycloserine and γ-lactam motifs, but like the β-lactams, lactivicin reacts covalently with PBPs to form a stable acyl-enzyme complex (1) (Fig. 1A and B). However, lactivicin has poor penetration into Gram-negative bacteria and is susceptible to at least some β-lactamase enzymes (2–4). A deeper understanding of the interactions between lactivicin and its derivatives and their various enzyme targets has led to the rational design of synthetic derivatives with higher potency against bacteria and reduced susceptibility to β-lactamases (1, 5, 6), including LTV13, which has the “ATMO”-type side chain (6) (Fig. 1C). Recently, it has been shown that sideromimic modification of the LTV γ-lactone (4) results in more-favorable 50% inhibitory concentrations (IC50s) against Pseudomonas aeruginosa PBPs and improved penetration into P. aeruginosa strain PAO1 via interaction with the siderophore receptors and uptake systems of this strain. One of these lactivicin derivatives is phthalimide-conjugated compound 17 (here referred to as LTV17) (Fig. 1C) (6).
FIG 1.
Structures and mechanism of action of β- and γ-lactams. (A) Structures of β-lactam antibiotics and the cycloserine derivative lactivicin. (B) Outline of mechanism of action of LTV derivatives against penicillin binding proteins. (C) Structures of LTV13 and LTV17 used in the current study.
Here we describe the activity of lactivicin derivatives against Stenotrophomonas maltophilia, which is an important nosocomial pathogen, primarily causing bloodstream and respiratory tract infections in severely debilitated patients. S. maltophilia also causes sporadic urinary tract and ocular infections and is a colonizer of the lungs of a significant proportion of adult patients with cystic fibrosis. Clinical isolates of S. maltophilia are notoriously resistant to antimicrobial drugs, with resistance to most β-lactams, quinolones, and aminoglycosides being an intrinsic property of the species (7, 8). Intrinsic resistance mechanisms in S. maltophilia include the expression of antibiotic-modifying enzymes, e.g., two β-lactamases, L1 and L2, which together can hydrolyze all known β-lactams (9–11), and of multidrug efflux pumps, most notably SmeDEF (12, 13), SmeVWX (14, 15), and SmeYZ and SmeIJK (16).
MATERIALS AND METHODS
Bacterial isolates and materials.
S. maltophilia clinical isolates used in this study either originated from the SENTRY antimicrobial resistance survey, as previously described (17), or were isolated from patients being treated at the Bristol Oncology Centre (18). Isolates of other species were obtained from type strain collections or were clinical isolates collected by SENTRY and provided as gifts by Mark Toleman, Cardiff University, or have previously been described (19). Growth media were from Oxoid. Chemicals were from Sigma, unless otherwise stated. LTV13 was synthesized according to the literature protocol (6). LTV17 was kindly supplied by Pfizer.
β-Lactamase induction and measurement of β-lactamase activity in cell extracts.
MICs were determined using CLSI broth microtiter assays (20) and interpreted using published breakpoints (21). For β-lactamase induction assays, an overnight culture of bacteria was diluted to an optical density at 600 nm (OD600) of 0.1 in nutrient broth and grown at 37°C until the OD600 was 0.4. Inducer (100 μg · ml−1 cefoxitin, 10 μg · ml−1 imipenem, 50 μg · ml−1 LTV13, and 0.35 μg · ml−1 LTV17) was then added and incubation continued for 2 h before cell extracts were prepared and levels of β-lactamase activity in cell extracts measured as described previously (22) using 100 μM meropenem as a substrate. Protein concentrations were determined using Bio-Rad protein assay dye reagent concentrate, and an extinction coefficient at 299 nm of 9,600 absorbance units (AU)/M/cm for meropenem was used to calculate the specific meropenem hydrolyzing activity in each cell extract.
Expression and purification of L1 and L2.
Recombinant L1 protein was produced in Escherichia coli and purified as previously described (23). For L2 protein production, the putative signal sequence (residues 1 to 27) was “removed” by amplifying positions 82 to 912 from the L2 gene of S. maltophilia K279a genomic DNA with forward and reverse primers 5′-AAGTTCTGTTTCAGGGCCCGGCGGGCAAGGCCAC-3′ and 5′-ATGGTCTAGAAAGCTTTATCCGATCAACCGGTCGGC-3′. Primer sequences included extensions which allowed recombination into pOPINF vector (24), resulting in a construct encoding L2 with an N-terminal hexa-His tag, with the tag being cleavable with rhinovirus 3C protease. The resultant plasmid was designated pOPINF-L2Δ27. For protein overproduction, E. coli SoluBL21 (DE3) cells (Genlantis) bearing pOPINF-L2Δ27 were grown in 2× tryptone-yeast extract (TY) medium containing ampicillin (50 μg · ml−1) to an OD600 of 0.9 at 37°C. Protein production was induced with 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) at 18°C for 16 h. All subsequent purification steps were performed at 4°C. Cells were harvested by centrifugation (6,500 × g, 10 min) and resuspended in 50 mM Tris (pH 7.5)–150 mM NaCl–1 mM Tris(2-carboxyethyl)phosphine (TCEP) supplemented with EDTA-free protease inhibitor (Roche). Cells were lysed by two 30,000-lb/in2 passages through a cell disruptor. After centrifugation at 100,000 × g for 45 min, the supernatant was incubated for 1.5 h with nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen). The resin was washed with buffer A (50 mM Tris [pH 7.5], 400 mM NaCl, 1 mM TCEP) plus 10 mM imidazole, then with buffer A plus 0.1% (vol/vol) Triton X-100, and then with buffer A plus 20 mM imidazole. Protein was eluted with 50 mM Tris (pH 7.5)–200 mM NaCl–400 mM imidazole–1 mM TCEP. Imidazole and NaCl concentrations were reduced to 5 mM and 150 mM, respectively, in an Amicon 10-molecular-weight-cutoff (mwco) concentrator, and the His tag was then removed by overnight incubation with His-tagged 3C protease. The digestion mixture was then incubated with Ni-NTA resin for 30 min, and the flowthrough containing purified L2 was collected and concentrated to 30 mg · ml−1 using a 10-mwco Amicon concentrator.
Assay of lactivicin hydrolysis by NMR.
1H nuclear magnetic resonance (NMR) spectra were acquired at 298 K on a Bruker AVIII 700 spectrometer with a 1H/13C/15N TCI CryoProbe. The data were recorded employing a pulse sequence with water suppression (excitation sculpting with gradients using perfect echo) (25, 26). Spectra were collected with a sweep width of 11,161 Hz, a relaxation delay of 2 s, 65,536 data points, and 16 scans. For data processing, line broadening of 0.3 Hz was applied. The NMR samples were prepared in 50 mM Tris-d11 buffer (pH 7.5), supplemented with 10% D2O. LTV13 was prepared as a 100 mM stock mixed in H2O and LTV17 as a 25 mM stock mixed in 50% H2O–50% dimethyl sulfoxide (DMSO) and diluted to a final concentration of 0.4 mM. The final concentrations of L1 and L2 were 150 nM.
RESULTS AND DISCUSSION
MICs of lactivicin derivatives against S. maltophilia clinical isolates.
MICs for LTV13 and LTV17 were determined against a selection of clinical isolates representing key Gram-negative species whose multidrug resistance is frequently a problem. These data confirmed the antibacterial potential of LTV17; its MIC against multidrug-resistant S. maltophilia bloodstream isolate K279a (18) is remarkably low, and it is more than 1,000-fold more potent than LTV13, an improvement in potency greater than that against any other test species (Table 1).
TABLE 1.
MICs of lactivicin derivatives against clinical Gram-negative isolates
| Isolate | MIC (μg · ml−1) |
|
|---|---|---|
| LTV13 | LTV17 | |
| Klebsiella pneumoniae NCTC5055 | 2 | 0.5 |
| Citrobacter freundii D571 | 512 | 16 |
| Enterobacter cloacae 30950 | 512 | 16 |
| Enterobacter aerogenes 15-8358A | 64 | 4 |
| Serratia marcescens NCTC1041 | 4 | 4 |
| Pseudomonas aeruginosa NCTC10662 | 256 | 1 |
| Stenotrophomonas maltophilia K279a | 64 | ≤0.0625 |
| Acinetobacter baumannii 39-4034C | 256 | 8 |
According to published CLSI susceptibility testing performance standards, there are only six antimicrobials that have potential for treatment of S. maltophilia and for which resistance breakpoints have been defined (21). The current drug of choice is trimethoprim-sulfamethoxazole (SXT), and there are five alternatives: ceftazidime, ticarcillin-clavulanate, minocycline, levofloxacin, and chloramphenicol. We used the CLSI performance standards to define resistance phenotypes for a collection of 50 clinical S. maltophilia isolates from around the world (11, 13, 17). Initially, the isolates were divided into two groups: 23/50 that are STX resistant and 27/50 that are STX sensitive. The two groups were then subdivided based on how many alternative antimicrobials they remain sensitive to. Finally, the MICs of LTV13 and LTV17 were determined against the 50 isolates using standard CLSI broth microdilution methodology (Table 2). Clearly, LTV17 is very potent against all of these S. maltophilia clinical isolates, including extensively drug-resistant strains. The highest MIC seen was 0.25 μg · ml−1, and the MIC90 for the 50 isolates was 0.063 μg · ml−1.
TABLE 2.
MICs of lactivicin derivatives against S. maltophilia clinical isolates having different profiles of resistance to last-resort antibiotics
| Resistance phenotype | No. of isolates with indicated MIC (μg · ml−1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTV13 |
LTV17 |
||||||||
| 16 | 32 | 64 | 128 | 256 | ≤0.031 | 0.063 | 0.125 | 0.25 | |
| SXTr; sensitive to 0 to 2 alternatives | 0 | 0 | 3 | 5 | 1 | 5 | 3 | 0 | 1 |
| SXTr; sensitive to 3 to 5 alternatives | 1 | 0 | 7 | 6 | 0 | 13 | 1 | 0 | 0 |
| SXTs; sensitive to 1 to 3 alternatives | 0 | 0 | 4 | 9 | 1 | 8 | 3 | 3 | 0 |
| SXTs; sensitive to 4 or 5 alternatives | 0 | 5 | 3 | 5 | 0 | 12 | 1 | 0 | 0 |
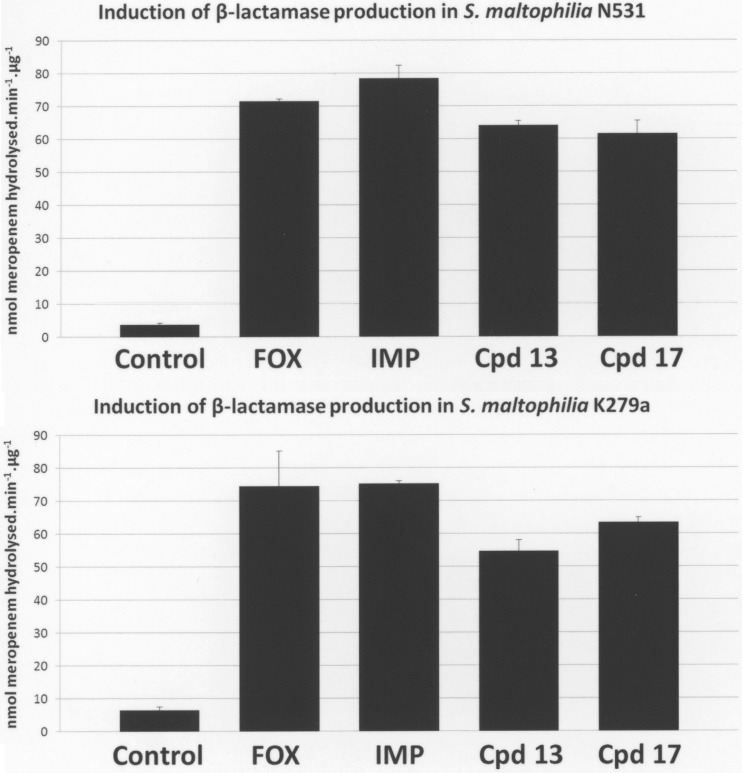
Induction of β-lactamase production in S. maltophilia by lactivicin derivatives.
One of the reasons for the initial failure of lactivicin and its early derivatives as antibiotics was susceptibility to β-lactamases, coupled with an ability to induce β-lactamase production in bacteria where inducible enzymes exist (2–4). S. maltophilia has two inducible β-lactamases, L1 and L2, which are coordinately controlled by an AmpR-type transcriptional regulator (27). Genetic disruption of one of the main targets of lactivicin, PBP1A, has been shown to constitutively activate β-lactamase production in S. maltophilia (22, 28). As expected, we found that treatment of two well-characterized S. maltophilia clinical isolates, K279a and N531 (18), with LTV13 or LTV17 induced β-lactamase production to a similar extent as treatment with the β-lactam antibiotics cefoxitin and imipenem when the antibiotics were added to growing cells at concentrations proportionate to the compounds' relative MICs (Fig. 2).
FIG 2.
Effect of β-lactams and lactivicin derivatives on the production of β-lactamase by S. maltophilia clinical isolates N531 (upper panel) and K279a (lower panel). Bacteria were incubated in the presence of inducer (100 μg · ml−1 cefoxitin, 10 μg · ml−1 imipenem, 50 μg · ml−1 LTV13, and 0.35 μg · ml−1 LTV17), cell extracts were prepared, and β-lactamase activity in cell extracts was measured as described previously (22) using 100 μM meropenem as the substrate. Protein concentrations were determined using Bio-Rad protein assay dye reagent concentrate, and an extinction coefficient at 299 nm of 9,600 AU/M/cm for meropenem was used to calculate the specific meropenem hydrolyzing activity in each cell extract. Data presented are means ± standard errors of the means; n = 3.
Breakdown of lactivicin derivatives by S. maltophilia β-lactamases.
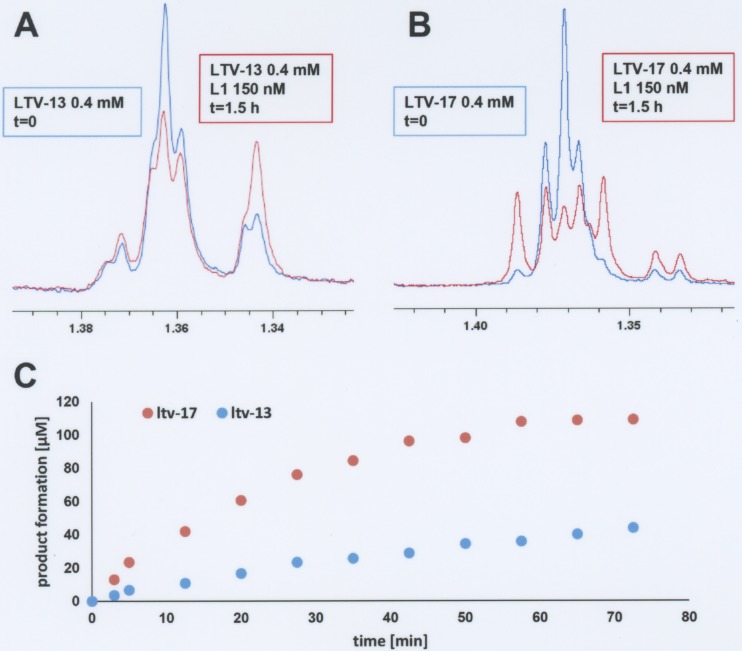
Since LTV13 and LTV17 both strongly induce L1 and L2 β-lactamase production, one explanation for the increased potency of LTV17 versus LTV13 is that LTV17 is a poorer substrate than LTV13 for the S. maltophilia β-lactamases. To test this hypothesis, NMR spectroscopy was used to evaluate the time-dependent hydrolysis of LTV13 and LTV17 by purified recombinant L1 and L2 β-lactamases. L2 was able to totally hydrolyze 400 μM ampicillin in less than 5 min (data not shown), while ∼95% of the LTV13 and LTV17 remained intact. Longer incubation times confirmed that LTV13 and LTV17 are not substrates for L2. They were both found to be substrates for L1 β-lactamase, however, and underwent enzyme-catalyzed hydrolysis (Fig. 3). LTV17 was broken down faster than LTV13, so reduced susceptibility to the L1 β-lactamase does not explain the increased potency of LTV17 against S. maltophilia.
FIG 3.
L1 catalyzed turnover of lactivicin derivatives as observed by 1H NMR spectrum analysis. (A) Overlay of 1H NMR spectra of LTV13 before addition of L1 and after 1.5 h of incubation with L1. (B) Overlay of 1H NMR spectra of LTV17 before addition of L1 and after 1.5 h of incubation with L1. (C) Time course of L1-mediated hydrolysis of LTV13 and LTV17.
Protection of S. maltophilia from lactivicin derivatives by the activity of β-lactamases.
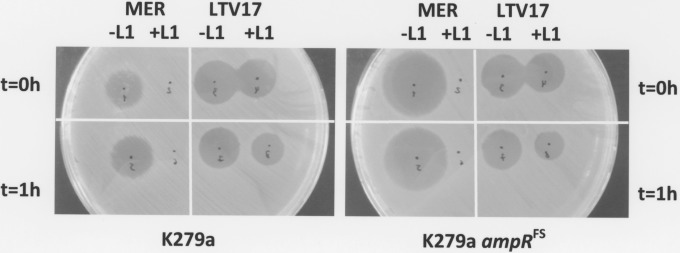
While they are clearly substrates, the rate of LTV17 and LTV13 hydrolysis by L1 was very low compared with that of meropenem (L1 was able to totally hydrolyze 400 μM meropenem in less than 5 min using similar assay conditions when ∼95% and 90% of LTV13 and 17 were still intact—data not shown), so we hypothesized that LTV13 and LTV17 actually kill S. maltophilia, even though they induce β-lactamase production, simply because cellular β-lactamase hydrolysis is too slow to protect the cells. To test this hypothesis, we incubated LTV17 with or without purified L1 and spotted the two mixtures onto a lawn of S. maltophilia K279a. In parallel, we used meropenem as a control (Fig. 4). Incubation with L1 does not significantly reduce the ability of LTV17 (or of LTV13—data not shown) to kill S. maltophilia K279a, whereas meropenem is rendered totally ineffective by L1. Preincubation of LTV17 with L1 for 1 h prior to spotting onto K279a did reduce the zone of clearing, suggestive of modest destruction of LTV17 as shown in the NMR experiments. Importantly, the inhibition zone diameter for LTV17 (and LTV13—data not shown) is the same for K279a as it is for the ampR frameshift mutant (FS) derivative K279a::ampRFS, which cannot induce β-lactamase production (27), even though the latter is far more sensitive to meropenem (Fig. 4). These results imply that even though L1 can break down LTV17 at a relatively modest rate (Fig. 3), its induction by LTV17 is not sufficient to protect the cell. This also explains why S. maltophilia clinical isolates in our worldwide collection that are known to express β-lactamase constitutively at high levels (11, 22) are no less susceptible to the lactivicin derivatives than are isolates with normally inducible β-lactamases (Table 2). Indeed, to confirm this, we tested four L1/L2-hyperproducing mutants previously derived from S. maltophilia K279a (22, 27, 29) and found that the MICs of both lactivicin derivatives against K279a and these mutants are the same (32 and ≤0.031 μg · ml−1, respectively, for LTV13 and LTV17).
FIG 4.
Inhibition zone produced by LTV17 and meropenem with and without L1 β-lactamase. A lawn of S. maltophilia K279a (left) or the ampR frameshift mutant (ampRFS) (right) was spread as if for CLSI disc susceptibility testing (using a 108 CFU/ml suspension of bacteria). In all cases, 2 μl of reaction mixture (50 mM Tris [pH 7.5]) with meropenem (MER, 12 mM) or LTV17 (0.4 mM) mixed (+L1) or not mixed (−L1) with purified L1 β-lactamase (1 μM final) was immediately spotted onto the lawn (t = 0 h) (top panels) or was spotted following 1 h of preincubation at 37°C (t = 1 h) (bottom panels). The plate was then incubated for 18 h at 37°C. This figure is representative of the results determined for three biological replicates.
Conclusions.
The reason for the dramatically increased potency of LTV17 versus LTV13 against S. maltophilia is not its relatively limited ability to induce L1/L2 β-lactamase production or its relatively slow hydrolysis by either of these β-lactamases. Both LTV17 and LTV13 are hydrolyzed by L1 β-lactamase slowly and not at a level that is detectable by L2, so β-lactamase production by S. maltophilia is not actually protective against either lactivicin derivative. Accordingly, while we have not excluded the possibility that there is some increased affinity for its PBP target(s), the 1,000-fold increased potency of LTV17 over that of LTV13 is most likely due to an increased rate of entry into S. maltophilia. The major difference between LTV13 and LTV17 is the presence of a catechol-type siderophore on the lactone ring of LTV17 (6). Notably, all S. maltophilia clinical isolates previously tested, including the K279a isolate used here, exclusively produced catechol-type siderophores (30). Thus, it is reasonable to infer that they preferentially take up this type of siderophore and the antibiotics conjugated to them. Accordingly, it would appear that the siderophore used for LTV17 particularly favors uptake by S. maltophilia, explaining its remarkable potency against this otherwise extensively drug-resistant bacterium. This observation is important because it implies that side-chain modification of the core fused bicyclic non β-lactam ring system of the lactivicins has the potential to improve activity in the same way as it has done for the β-lactams, e.g., for BAL30072, which is in the early phase of clinical development. This mechanism can operate by increasing potency versus PBPs and/or reducing β-lactamase susceptibility and, in addition, by improving uptake (31). Moreover, the results presented in Table 1 suggest that siderophore-mediated uptake is not a general effect, equally seen in all species. It may be that the apparent species specificity of the effect seen is dependent on the conjugation of a particular siderophore preferentially used by a particular species. In an era of improved diagnostics for infection (32), the routine use of narrow-spectrum antimicrobials is becoming a realistic proposition, and the benefit would be reduced collateral damage to the host microbiome and cross-selection for the acquisition of resistant isolates of other species of bacteria.
ACKNOWLEDGMENTS
K.C. was in receipt of a postgraduate scholarship from SENESCYT, Ecuador.
We are grateful to Pfizer for providing LTV17 for use in this study.
REFERENCES
- 1.Macheboeuf P, Fischer DS, Brown T Jr, Zervosen A, Luxen A, Joris B, Dessen A, Schofield CJ. 2007. Structural and mechanistic basis of penicillin-binding protein inhibition by lactivicins. Nat Chem Biol 3:565–569. doi: 10.1038/nchembio.2007.21. [DOI] [PubMed] [Google Scholar]
- 2.Nozaki Y, Katayama N, Ono H, Tsubotani S, Harada S, Okazaki H, Nakao Y. 1987. Binding of a non-β-lactam antibiotic to penicillin-binding proteins. Nature 325:179–180. doi: 10.1038/325179a0. [DOI] [PubMed] [Google Scholar]
- 3.Nozaki Y, Katayama N, Harada S, Ono H, Okazaki H. 1989. Lactivicin, a naturally occurring non-β-lactam antibiotic having β-lactam-like action: biological activities and mode of action. J Antibiot (Tokyo) 42:84–93. doi: 10.7164/antibiotics.42.84. [DOI] [PubMed] [Google Scholar]
- 4.Tamura N, Matsushita Y, Kawano Y, Yoshioka K. 1990. Synthesis and antibacterial activity of lactivicin derivatives. Chem Pharm Bull (Tokyo) 38:116–122. doi: 10.1248/cpb.38.116. [DOI] [PubMed] [Google Scholar]
- 5.Brown T Jr, Charlier P, Herman R, Schofield CJ, Sauvage E. 2010. Structural basis for the interaction of lactivicins with serine β-lactamases. J Med Chem 53:5890–5894. doi: 10.1021/jm100437u. [DOI] [PubMed] [Google Scholar]
- 6.Starr J, Brown MF, Aschenbrenner L, Caspers N, Che Y, Gerstenberger BS, Huband M, Knafels JD, Lemmon MM, Li C, McCurdy SP, McElroy E, Rauckhorst MR, Tomaras AP, Young JA, Zaniewski RP, Shanmugasundaram V, Han S. 2014. Siderophore receptor-mediated uptake of lactivicin analogues in Gram-negative bacteria. J Med Chem 57:3845–3855. doi: 10.1021/jm500219c. [DOI] [PubMed] [Google Scholar]
- 7.Ryan RP, Monchy S, Cardinale M, Taghavi S, Crossman L, Avison MB, Berg G, van der Lelie D, Dow JM. 2009. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat Rev Microbiol 7:514–525. doi: 10.1038/nrmicro2163. [DOI] [PubMed] [Google Scholar]
- 8.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh TR, Hall L, Assinder SJ, Nichols WW, Cartwright SJ, MacGowan AP, Bennett PM. 1994. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim Biophys Acta 1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TR, MacGowan AP, Bennett PM. 1997. Sequence analysis and enzyme kinetics of the L2 serine β-lactamase from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 41:1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould VC, Okazaki A, Avison MB. 2006. β-Lactam resistance and β-lactamase expression in clinical Stenotrophomonas maltophilia isolates having defined phylogenetic relationships. J Antimicrob Chemother 57:199–203. doi: 10.1093/jac/dki453. [DOI] [PubMed] [Google Scholar]
- 12.Alonso A, Martínez JL. 2000. Cloning and characterization of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 44:3079–3086. doi: 10.1128/AAC.44.11.3079-3086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould VC, Avison MB. 2006. SmeDEF-mediated antimicrobial drug resistance in Stenotrophomonas maltophilia clinical isolates having defined phylogenetic relationships. J Antimicrob Chemother 57:1070–1076. doi: 10.1093/jac/dkl106. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Huang CC, Chung TC, Hu RM, Huang YW, Yang TC. 2011. Contribution of resistance-nodulation-division efflux pump operon smeU1-V-W-U2-X to multidrug resistance of Stenotrophomonas maltophilia. Antimicrob Agents Chemother 55:5826–5833. doi: 10.1128/AAC.00317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-León G, Ruiz de Alegría Puig C, García de la Fuente C, Martínez-Martínez L, Martínez JL, Sánchez MB. 2015. High-level quinolone resistance is associated with the overexpression of smeVWX in Stenotrophomonas maltophilia clinical isolates. Clin Microbiol Infect 21:464–467. doi: 10.1016/j.cmi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Gould VC, Okazaki A, Avison MB. 2013. Coordinate hyperproduction of SmeZ and SmeJK efflux pumps extends drug resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 57:655–657. doi: 10.1128/AAC.01020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toleman MA, Bennett PM, Bennett DM, Jones RN, Walsh TR. 2007. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis 13:559–565. doi: 10.3201/eid1304.061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avison MB, von Heldreich CJ, Higgins CS, Bennett PM, Walsh TR. 2000. A TEM-2 β-lactamase encoded on an active Tn1-like transposon in the genome of a clinical isolate of Stenotrophomonas maltophilia. J Antimicrob Chemother 46:879–884. doi: 10.1093/jac/46.6.879. [DOI] [PubMed] [Google Scholar]
- 19.Avison MB, Underwood S, Okazaki A, Walsh TR, Bennett PM. 2004. Analysis of AmpC β-lactamase expression and sequence in biochemically atypical ceftazidime-resistant Enterobacteriaceae from paediatric patients. J Antimicrob Chemother 53:584–591. doi: 10.1093/jac/dkh151. [DOI] [PubMed] [Google Scholar]
- 20.CLSI. 2015. M07-A10: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard— 10th ed CLSI, Wayne, PA. [Google Scholar]
- 21.CLSI. 2015. M100-S25: performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI, Wayne, PA. [Google Scholar]
- 22.Talfan A, Mounsey O, Charman M, Townsend E, Avison MB. 2013. Involvement of mutation in ampD I, mrcA, and at least one additional gene in β-lactamase hyperproduction in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 57:5486–5491. doi: 10.1128/AAC.01446-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ullah JH, Walsh TR, Taylor IA, Emery DC, Verma CS, Gamblin SJ, Spencer J. 1998. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 Å resolution. J Mol Biol 284:125–136. doi: 10.1006/jmbi.1998.2148. [DOI] [PubMed] [Google Scholar]
- 24.Berrow NS, Alderton D, Sainsbury S, Nettleship J, Assenberg R, Rahman N, Stuart DI, Owens RJ. 2007. A versatile ligation-independent cloning method suitable for high-throughput expression screening applications. Nucleic Acids Res 35:e45. doi: 10.1093/nar/gkm047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams RW, Holroyd CM, Aguilar JA, Nilsson M, Morris GA. 2013. “Perfecting” WATERGATE: clean proton NMR spectra from aqueous solution. Chem Commun (Camb) 49:358–360. doi: 10.1039/C2CC37579F. [DOI] [PubMed] [Google Scholar]
- 26.Hwang TL, Shaka AJ. 1998. Multiple-pulse mixing sequences that selectively enhance chemical exchange or cross-relaxation peaks in high-resolution NMR spectra. J Magn Reson 135:280–287. doi: 10.1006/jmre.1998.1598. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki A, Avison MB. 2008. Induction of L1 and L2 β-lactamase production in Stenotrophomonas maltophilia is dependent on an AmpR-type regulator. Antimicrob Agents Chemother 52:1525–1528. doi: 10.1128/AAC.01485-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CW, Lin HC, Huang YW, Chung TC, Yang TC. 2011. Inactivation of mrcA gene derepresses the basal-level expression of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. J Antimicrob Chemother 66:2033–2037. doi: 10.1093/jac/dkr276. [DOI] [PubMed] [Google Scholar]
- 29.Avison MB, Higgins CS, Ford PJ, von Heldreich CJ, Walsh TR, Bennett PM. 2002. Differential regulation of L1 and L2 β-lactamase expression in Stenotrophomonas maltophilia. J Antimicrob Chemother 49:387–389. doi: 10.1093/jac/49.2.387. [DOI] [PubMed] [Google Scholar]
- 30.García CA, Passerini De Rossi B, Alcaraz E, Vay C, Franco M. 2012. Siderophores of Stenotrophomonas maltophilia: detection and determination of their chemical nature. Rev Argent Microbiol 44:150–154. [PubMed] [Google Scholar]
- 31.Page MG. 2013. Siderophore conjugates. Ann N Y Acad Sci 1277:115–126. doi: 10.1111/nyas.12024. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]