Abstract
Background. Nonstandardized specimen-transport logistics, lack of laboratory personnel to transport specimens, lack of standard specimen containers, and long turnaround time (TAT) hindered access to quality laboratory services. The objective of the Becton, Dickinson, and Company (BD)–US President's Emergency Plan for AIDS Relief (PEPFAR) Public-Private Partnership (PPP) was to support country-specific programs to develop integrated laboratory systems, services, and quality improvement strategies, with an emphasis on strengthening the specimen-referral system (SRS).
Methods. In 2007, through the Centers for Disease Control and Prevention (CDC), the Ethiopian Public Health Institute (EPHI) joined with the BD-PEPFAR PPP to strengthen laboratory systems. A joint planning and assessment committee identified gaps in the SRS for prioritization and intervention and piloted the system in Addis Ababa and Amhara Region.
Results. The PPP established standardized, streamlined specimen logistics, using the Ethiopian Postal Service Enterprise to support a laboratory network in which 554 facilities referred specimens to 160 laboratories. The PPP supported procuring 400 standard specimen containers and the training of 586 laboratory personnel and 81 postal workers. The average TAT was reduced from 7 days (range, 2–14 days) to 2 days (range, 1–3 days) in Addis Ababa and from 10 days (range, 6–21 days) to 5 days (range, 2–6 days) in Amhara Region.
Conclusions. This study highlights the feasibility and untapped potential of PPPs to strengthen laboratory systems. This planned and structured approach to improving specimen referral enhanced access to quality laboratory services.
Keywords: specimen-referral system, training, public-private partnership, postal system, turn around time
In sub-Saharan Africa, the AIDS epidemic exposed the fragile and fragmented specimen-referral networks within the health system. The lack of these networks resulted in unacceptably long turnaround times for laboratory results. Lower-tier laboratories in health facilities were not equipped to perform all of the necessary laboratory tests, and this required referring patient samples to higher-tiered laboratories that were equipped to do so. Timely access to and wide availability of human immunodeficiency virus (HIV) and tuberculosis diagnosis, treatment, and monitoring services are key components of improving and prolonging the lives of patients, as well as a path toward attaining the goal of an AIDS-free generation [1–3]. Laboratory services, which have a key role in disease diagnosis, control, and prevention, have been found to be one of the weakest areas in healthcare-delivery systems in developing countries [4–6]. Through the Maputo Declaration, ministries of health (MOH) agreed that the creation of national laboratory networks is essential to achieve access to quality laboratory services [7, 8].
Increasingly, national laboratory strategic planning efforts recognize the need for strengthening laboratory systems, including human resource and infrastructure development, quality management, supply chain management, specimen referral, and results-reporting and laboratory information systems, in an integrated and coordinated laboratory network [9, 10]. Transportation systems in resource-limited settings are either nonexistent or insufficient and, in most cases, do not address the need to transport patient specimens from the district healthcare centers to higher-level testing facilities [11].
Ethiopia's laboratory structure is well aligned with the structure of the healthcare-delivery system. The laboratories at each of the tiers have different testing capacities, with health centers being the smallest facilities and, therefore, having the smallest testing capacity, in the national laboratory system. To increase access to specialized tests, it is important to develop a reliable laboratory network with a strong specimen-referral system. A specimen-referral system was first established in 2008 by the Ethiopian Public Health Institute (EPHI) in collaboration with the Centers for Disease Control and Prevention (CDC) under the US President's Emergency Plan for AIDS Relief (PEPFAR) and several implementing partners, such as Johns Hopkins University (JHU), Columbia University (CU-ICAP), the University of Washington International Training and Education Center for Health (I-TECH), and the University of California–San Diego (UCSD), to support the massive scale-up of antiretroviral treatment (ART) to >330 000 patients. The referral system focused on collecting specimens from patients receiving ART, for CD4+ T-cell, chemical, hematological monitoring, and collecting blood specimens from infants, for early diagnosis of HIV infection by the dried blood spot (DBS) technique. Four hundred twenty laboratories were referring specimens for ART monitoring to the healthcare facility at next higher level or to regional laboratories. There was no dedicated specimen courier system, with specimens being transported through partner vehicles or public transportation, integrated with mentoring and supportive supervision visits, leading to significant delay in results delivery. However, the presence of the specimen-referral network supported by partners made it easier to implement the public-private partnership (PPP). The specimen-referral program was transitioned to the regional health bureaus in 2010, in line with the decentralization of services, and financed by the EPHI, using PEPFAR/CDC cooperative agreement funds.
The PEPFAR-supported supply chain management system worked with the procurement section of the Ethiopian MOH, the Pharmaceutical Fund and Supply Agency, for regular monitoring and forecasting of laboratory commodities, including specimen-collection tubes and infection-prevention materials. However, the system was challenged by an underdeveloped transportation system, a lack of specimen-packaging logistics, and long turnaround times. Additionally, in most instances, laboratory staff were deployed to transport specimens to testing facilities, thereby leaving laboratories understaffed or unattended. This was a major problem at health center laboratories in remote sites, which were usually staffed by 1 or 2 laboratory personnel.
There are examples from some organizations that have described approaches to overcome challenges in implementing quality-assured and timely laboratory services under a well-coordinated network [12]. However, these organizations have not successfully addressed these multifaceted challenges comprehensively, despite the significant resources and efforts invested [13]. Addressing the healthcare needs of people in the developing world requires cooperation, partnerships, and coordination among various sectors [14]. There was the recognition that the PPP would build local capacity and contribute to the long-term sustainability of programs, thus demonstrating the important role that the private sector can have in strengthening health systems.
The goal of the BD-PEPFAR PPP with the EPHI/MOH was to address key gaps in the existing specimen-referral network, with particular focus on reducing turnaround times, introducing standard specimen-transport logistics, and increasing access to quality laboratory services.
METHODS
Assessment and Planning
Prior to the PPP intervention, with the exception of blood specimens collected for analysis by the DBS technique for early diagnosis of HIV infection in infants, specimens were transported by laboratory personnel or implementing partners, using public transportation. The samples obtained for analysis by the DBS technique were transported using the postal services network. The PPP worked closely with the country program in a consultative manner to identify priorities. The core country program team involved the EPHI and the CDC and its implementing partners (ie, JHU, CU-ICAP, I-TECH, and UCSD). BD, in collaboration with the country team, conducted a baseline assessment using a structured checklist, with particular emphasis on specimen referral. Additionally, using findings from past assessments of the national laboratory services and considering strategic initiatives in the National Laboratory Strategic Master Plan, including informed discussions with the Ethiopian MOH, the country program team and BD identified key gaps in the system to address and strengthen the existing specimen-referral and result reporting system.
A comprehensive work plan was developed to address the identified gaps in the existing system, with roles and responsibilities of the different parties clearly defined. Training laboratory and postal service personnel, ensuring an adequate supply of standard specimen-transport materials, improving physical specimen logistics, and addressing laboratory safety were considered important priorities under the PPP. In addition, mentoring the facility staff, using geographic information system (GIS) software and geographic positioning system (GPS) devices, and monitoring and evaluating the specimen-referral network were part of the scope of work for the PPP. The PPP focused on strengthening the existing specimen-referral systems by directly working with facilities and partners. Through the leadership role provided by the EPHI and CDC-Ethiopia, the PPP involved other partners, such as the Clinton Health Access Initiative (CHAI), Direct Relief International, and the CDC implementing partners JHU, CU-ICAP, ITECH, the supply chain management system, and UCSD.
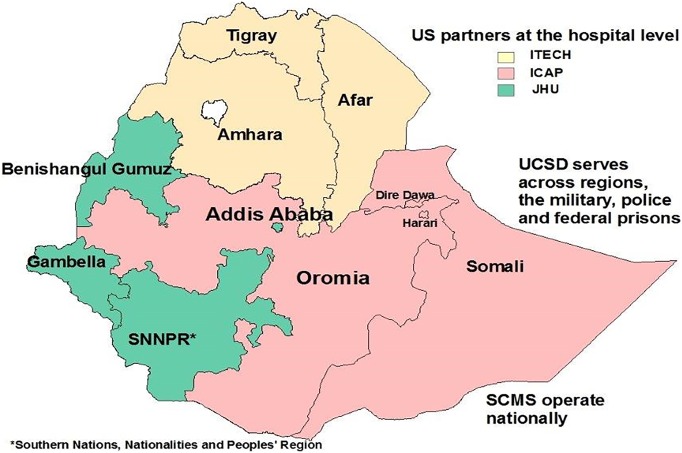
The nonoverlapping coverage of geographic zones by partners helped to better coordinate specimen referral across regions (Figure 1). The PPP supported consolidation and integration of all available specimens and results-reporting mechanisms into a unified system. However, a phased implementation approach was deemed logical, and it was initiated in Addis Ababa, the capital of Ethiopia, and later expanded to Amhara Region. The successful implementation of the pilot in Addis Ababa and Amhara Region and the associated lessons learned informed plans for national scale-up.
Figure 1.
Regional placement of partners providing site-level technical support for laboratory-system strengthening, including specimen-referral testing, in Ethiopia. Abbreviations: ICAP, Columbia University International Center for AIDS Care and Treatment Program; I-TECH, University of Washington International Training & Education Center for Health; JHU, Johns Hopkins University; SCMS, supply chain management system; UCSD, University of California, San Diego.
Sample Referral Mapping and Training
The EPHI mapped out all specimen-referring sites, specimen types, and linkages to laboratories where tests would be performed. This was primarily along regional boundaries, and the mapping had a key role in establishing a memorandum of understanding with the EPSE. Under the PPP, the EPHI signed a memorandum of understanding with the EPSE to transport all specimens in Addis Ababa and samples obtained for analysis by the DBS technique nationwide. Under this agreement, the EPSE, instead of laboratory personnel, transported all samples from and results to referring facilities enrolled in the pilot program. Local post offices and referring heath facilities established a special delivery route for laboratory specimens. The arrangement was such that the post office worker would visit the health facility twice weekly to pick up specimens that were available for transport. Appointments for patients usually occurred during Monday–Wednesday mornings to avoid specimen transport near the weekend, and the testing load at facilities was managed by overtime work and assignment of additional laboratory personnel based on specimen flow. On these days, the laboratory personnel collected the specimens, packaged them properly, and made them ready for pickup by the postal office. Postal office workers were required to report any incident during specimen transport for proper corrective actions and use in improving training or refresher trainings of postal workers. Training materials on specimen referral were developed by BD and customized by the country team for both laboratory personnel and postal workers. Standard operating procedures, transport logs, and safety procedures during transport were developed. A standard training consisted of an initial assessment of trainees, training on documentation and biosafety, and hands-on instruction with an emphasis on packaging, handling, and tracking, to ensure the creation of an audit trail. A training-of-trainers course was provided to establish personnel capacity and a pool in each of the regions, and a training rollout was conducted in 2 regions by the PPP in collaboration with the EPHI and the CDC and its implementing partners. The country team subsequently conducted training rollout to all other regions. Standard specimen-transport materials (BD isothermal containers [catalog no. 368 562]) were procured by BD through Direct Relief International and distributed to facilities by the EPHI. GIS software, laptops, and a server were procured and installed at the EPHI. Two sessions of training on GIS/GPS were conducted for EPHI staff, with full implementation of GIS planned for the second phase.
Tools were developed for regular monitoring and evaluation of program implementation, with major indicators being turnaround time, frequency of rejecting transported samples because they were inadequate for analysis, availability of standard specimen-transport containers at referring sites, and the number of laboratory and postal service personnel trained. Turnaround time was defined as the time from referral of specimens collected from ART recipients to a laboratory (for monitoring CD4+ T-cell, chemical, and hematological parameters) to receipt of results by the referring facility. Turnaround time for early diagnosis of HIV infection in infants was not part of this analysis.
RESULTS
Activities Before PPP Formation
Prior to the collaborative efforts between the PPP, CDC, and EPHI, 420 sites were referring specimens obtained from patients receiving ART for CD4+ T-cell, chemical, and hematological monitoring at testing laboratories (Table 1). The average time for transporting specimens was 4 hours for Addis Ababa and 6 hours for other regions of Ethiopia, depending on the locations of the referring and testing sites. There was a need to procure >2000 standard specimen carriers, following World Health Organization (WHO) specifications. Only 85 laboratory personnel and no postal workers were trained in conserving the integrity of specimens and in protecting themselves from blood-borne diseases. The average turnaround time for results of tests among ART recipients (for CD4+ T-cell, chemical, hematological monitoring) was 7 days (range, 2–14 days) in Addis Ababa and 10 days (range, 6–21 days) elsewhere. There was no standard specimen-transport option; use of the public transportation system was the only option. The availability of standardized and safe containers for packaging and transporting specimens was inadequate. Laboratory personnel were transporting specimens through the public transportation system in all regions.
Table 1.
Results of Key Interventions Used in Strengthening and Monitoring the Specimen-Referral System in Ethiopia Under the Public-Private Partnership (PPP) Between Becton, Dickinson, and Company and the US President's Emergency Plan for AIDS Relief
| Key Indicators | Before the PPP (2008–2010) | After the PPP (2010–2012) |
|---|---|---|
| Sites referring specimens | 420 | 554 |
| Sample transportation mechanism | Not standardized, laboratory personnel transport the specimen | Ethiopian Postal System, no laboratory personnel transport specimen in pilot sites |
| Time needed to transport specimen for ART-related monitoring after collection | 4 h (range, 2–6 h) in Addis Ababa and >6 h (range, 3–14 h) in other regions | 1 h in Addis Ababa (range, 0.5–2 h) and 4 h (range, 1–5 h) in other regions |
| Specimen-transport containers with triple-packaging capacity, no, | 100 | 2132 (400 procured by BD and the rest procured by PEPFAR) |
| People trained on specimen-referral system, no. | 85 laboratory personnel only | 667, including laboratory personnel (n = 586) and postal workers (n = 81) |
| Training coverage | No structured training in any region except for demonstration of specimen-transport and standard operating procedures | Standard training module available for all regions |
| Turnaround timea for ART-related monitoring | 7 d (range, 2–14 d) in Addis Ababa and 10 d (range, 6–21 d) in Amhara Region | 2 d (range, 1–3 d) in Addis Ababa and 5 d (range, 2–6 d) in Amhara Region |
| GIS mapping of sites | No system | System installed, with provision of initial trainingb |
Abbreviations: ART, antiretroviral therapy; GIS, geographic information system; PEPFAR, US President's Emergency Plan for AIDS Relief.
a Defined as the time from referral of specimens collected from ART recipients to a laboratory (for monitoring CD4+ T-cell, chemical, and hematological parameters) to receipt of results by the referring facility. It does not account for the time needed to collect specimens.
b Full implementation and evaluation of GIS mapping is planned for the second phase of the PPP.
Activities After PPP Formation
Training
The EPHI, CDC-Ethiopia, and partners collaborated with BD to successfully design a tailor-made course for proper packaging and transport of infectious materials such as blood and sputum. This course was for laboratory personnel and postal staff. The initial 2 regional trainings were supported by BD, but the overall regional training rollout in all regions was conducted by the country team. There was adequate local capacity established with the initial training of trainers, which helped yield 30 trained individuals. Over 2 years, the team of facilitators, composed of staff from BD, the EPHI, regional laboratories, and partners, trained 667 participants (586 laboratory personnel and 81 postal workers) in 11 regions. There was increased awareness by both laboratory and postal workers on biosafety and occupational exposure preventions. A GIS/GPS system was established at the EPHI. Fifteen personnel were trained from the EPHI and the Ethiopian MOH on laboratory, survey and surveillance, and public health emergency management programs.
Specimen-Transport Network and Turnaround Time
The EPSE's services were used in 2 regions, Addis Ababa and Amhara, to transport all specimens. In these 2 regions, laboratory personnel were no longer involved in transporting specimens. This allowed them to focus on providing continuous laboratory services for clients. In addition, the PPP contributed by procuring 400 of the 2132 standard triple-packaged specimen containers per WHO specifications, while PEPFAR procured the remaining 1732. Following the training intervention, mentorship, and transitioning the responsibility of specimen transport to the postal system, specimens collected for ART monitoring now arrived at the closest laboratory within 1 hour after collection (range, 0.5–2 hours) in Addis Ababa, compared with 4 hours (range, 2–6 hours) before the intervention. Similarly, a specimen obtained for ART monitoring from any referring site in the region now took an average of 4 hours (range, 1–5 hours) to reach its closest testing laboratory, compared with 6 hours (range, 3–14 hours) before the intervention. Following training of laboratory and postal workers on specimen referral, the turnaround time was improved to an average of 2 days (range, 1–3 days), compared with 7 days (range, 2–14 days) before the intervention, for specimens referred in Addis Ababa (Table 1). In other regions where similar trainings were conducted and specimen transport and results feedback were provided by the postal system, preliminary data indicated that, on average, turnaround time decreased from 10 days (range, 6–21 days) before the intervention to 5 days (range, 2–6 days) after the intervention.
Number of Referring Sites
The main goal of the PPP was to improve the specimen-referral system in the existing facilities. However, it also helped to enroll more facilities into the specimen-referral network. Prior to the creation of the PPP, engagement of stakeholders, and implementation of the training intervention, only 420 sites referred specimens. However, following the intervention, >554 laboratories referred specimens to 160 testing laboratories for ART monitoring (Table 1). Effective networking of the 554 health centers and hospital laboratories is ongoing and includes continued use of GIS and GPS technology so that the post office can be instrumental in the timely collection of specimens from facilities in remote areas, the transport of these specimens to specialized testing laboratories, and the return of the results back to each of the referring sites. To establish local capacity, the PPP customized a curriculum to address the gaps in the existing specimen referral and results-reporting system for laboratory and postal workers. The training materials are a valuable resource for the country team to train laboratory and postal service personnel in future efforts to expand specimen referral to more facilities.
DISCUSSION
Through the PPP, we documented the feasibility of standardizing the specimen-transport system in Ethiopia by using a unified approach for specimen transport through the EPSE and by providing standard packaging materials. Laboratory and postal office staff were successfully trained on safe sample packaging and transportation, to protect staff and ensure specimen integrity for accurate diagnosis. Furthermore, we demonstrated that enhanced local capacity and increased awareness among service providers led to a significant reduction in turnaround times. Because testing capacities of laboratories across the laboratory tiered system are not the same, establishing a functional and reliable laboratory network is critical to improve patient outcomes. The specimen-referral system was established to support laboratory-based monitoring of ART.
Ethiopia is a vast country striving to increase access to quality-assured, integrated laboratory services as part of the health sector development plan. The EPHI has developed an integrated national laboratory strategic master plan to coordinate and guide the strengthening of laboratory systems [15]. One of the objectives of the strategic plan was to put in place a reliable specimen-referral system across all regions. The success of the PPP was based on its strong leadership and emphasis on the host country's ownership of joint program planning, implementation, and evaluation among the parties [16]. By being introduced to the Ethiopian MOH through CDC-Ethiopia, BD built on the existing partnership and close working relationship between the CDC, the Ethiopian MOH, and local partners. The CDC's prior experience in the implementation of the national laboratory strategic master plans and in working with the Ethiopian MOH and ensuring country ownership was key to the success of the program. Furthermore, the effective country engagement through a local supporter at the EPHI, the leveraging of core competencies of each partner, and the leadership and stewardship on the national, regional, and district levels were crucial for program execution for a better health system [17].
Maintaining the safety and integrity of specimens is a critical element in transporting biological specimens for analysis and accurate and reliable diagnosis. The importance of awareness and training to prevent healthcare worker exposure to biological materials and infection cannot be overemphasized [18]. WHO guidelines recommend that packaging of infectious materials for transport must adhere to standards and be designed to minimize the potential for damage during transportation. In addition, the packaging must ensure the integrity of the materials and timely processing of specimens [19]. The PPP took the lead in conceptualizing and designing a tailor-made course for the laboratory and postal staff and training them on biosafety and accurate packaging and transport practices for infectious materials. The PPP also provided standard transport containers to ensure the safety and integrity of specimens.
The prompt diagnosis and initiation of ART among patients with HIV/AIDS reduces transmission, morbidity, and mortality [20–22]. The laboratory has an important role in ensuring that quality results are available in a timely manner to enable care providers to initiate treatment. The PPP enhanced capacity to receive specimens transported under optimum conditions and for timely and accurate reports of patient results. One of the benefits of the PPP's specimen-referral program was the reduction in turnaround time, which improved the timely initiation and cost-effectiveness of treatment. In particular, this increased access to CD4+ T-cell testing, for staging and early initiation of ART, and to laboratory-based ART monitoring. As greater access to quality laboratory services continues to increase, an efficient specimen-referral system, along with innovative technology, such as point-of-care testing, would further decrease turnaround time and increase patient access to treatment.
The successful implementation of the PPP led to the establishment of the first innovative and sustainable transport chain by using the postal system to send specimens from remote sites to specialized laboratories and then return the results to the point of care. Ethiopia used the PPP collaboration to develop a system to refer multiple types of specimens. The PPP built on the success the EPSE used throughout the country to transport specimens for evaluation by the DBS technique for early diagnosis of HIV infection in infants.
The increased availability of GIS technology among EPHI staff improved the institution's ability to create maps and visualize epidemiological data. Use of the GIS system would further streamline efficient sample referral because it would be based on relatively shorter distances between the referring and testing sites as against regional boundaries. There has been a carryover effect of the GIS program in strengthening local capacity for disease surveillance and for public health emergency management.
Despite the successful piloting and roll out of an efficient sample-referral system, the PPP faced both health system–associated and country-specific challenges. The lack of adequate resources in terms of workforce and budget allocations to cover the national needs was an important limitation, including operational budgets for specimen-referral and GIS programs. The PPP-supported trainings were delivered in English, which presented a significant language barrier to many trainees, especially postal workers in areas where the predominant language is Amharic. Although Ethiopia has focused on making the program sustainable, through training-of-trainer's workshops for local capacity building and engagement of regional health bureaus and partners to increase country ownership, longer-term sources of funding for all PPP activities will be key to scaling up these interventions to optimize HIV services delivery and health systems strengthening. The effective use of the EPSE with offices and services in 800 districts thus bringing it closer to most referring facilities at reasonable cost. Furthermore, more engagement of regional health bureaus to coordinate the referral network, integration of the specimen-referral system, translation of standard operating procedures and training materials to local languages, and discovery of local and alternative financing schemes are critical for the sustainability of the specimen-referral system. A limitation of our study was the nonsystematic collection of data prior to and after the intervention on the rate of sample rejection, which would have allowed analysis of the effect of the intervention on the rejection rate. However, it is noteworthy that the other key indicators that were assessed all showed improvements following intervention by the PPP.
In summary, the integration of technology, local workforce training, and the healthcare infrastructure significantly enhanced specimen referral and results-reporting networks by increasing the number of referring sites and decreasing turnaround times for ART-related monitoring. Joint planning among the Ethiopian MOH/EPHI, the CDC, and BD, in addition to strong leadership provided by the EPHI and the clear outline of objectives by the national laboratory strategic master plan, was crucial for improving the specimen-referral system and increasing access to quality laboratory services. The successful implementation of this initiative may be relevant to other areas of laboratory-system strengthening and may encourage governments and businesses to increasingly support the creation of more PPPs to strengthen healthcare systems.
Notes
Acknowledgments. We thank the management and technical staff involved in this project; the EPSE and the management and laboratory personnel at each facility supported by this project; the Centers for Disease Control and Prevention (CDC) implementing partners, for providing strong site-level laboratory technical support to make this collaboration a success; and the management and laboratory team of the 4 US university partners (CU-ICAP, I-TECH, JHU, and UCSD).
Becton, Dickinson, and Company staff engaged in the Ethiopia project were Adam Yeung, Adrian Calderon, Belinda Ngongo, Calin Yuan, David Carr, Debbie Redondo, Diane Kawa, Geoffrey Dugue, Justin Wright, Maarten Van Eynde, Nuphar Rozen-Adler, Richard Scott, Stacy Stanton, Susan Chambers, and Ubaldo Barbosa.
Disclaimer. The findings and conclusions in this report are the views of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by the US President's Emergency Plan for AIDS Relief, through the CDC (grant U2GPS000825).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Alemnji G, Nkengasong JN, Parekh BS. HIV testing in developing countries: what is required? Indian J Med Res 2011; 134:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PEPFAR. Remarks on “Creating an AIDS-Free Generation”. http://wwwstategov/secretary/20092013clinton/rm/2011/11/176810htm Accessed 24 June 2015.
- 3.Nsubuga P, Nwanyanwu O, Nkengasong JN, Mukanga D, Trostle M. Strengthening public health surveillance and response using the health systems strengthening agenda in developing countries. BMC Public Health 2010; 10(suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birx D, de Souza M, Nkengasong JN. Laboratory challenges in the scaling up of HIV, TB, and malaria programs: The interaction of health and laboratory systems, clinical research, and service delivery. Am J Clin Pathol 2009; 131:849–51. [DOI] [PubMed] [Google Scholar]
- 5.Westerman LE, Kohatsu L, Ortiz A et al. A quality management systems approach for CD4 testing in resource-poor settings. Am J Clin Pathol 2010; 134:556–67. [DOI] [PubMed] [Google Scholar]
- 6.Fonjungo PN, Kebede Y, Messele T et al. Laboratory equipment maintenance: a critical bottleneck for strengthening health systems in sub-Saharan Africa? J Public Health Policy 2012; 33:34–45. [DOI] [PubMed] [Google Scholar]
- 7.WHO. The Maputo Declaration on Strengthening of Laboratory Systems. http://wwwwhoint/diagnostics_laboratory/Maputo-Declaration_2008pdf Accessed 24 June 2015.
- 8.Gershy-Damet GM, Rotz P, Cross D et al. The World Health Organization African Region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am J Clin Pathol 2010; 134:393–400. [DOI] [PubMed] [Google Scholar]
- 9.Nkengasong JN, Nsubuga P, Nwanyanwu O et al. Laboratory systems and services are critical in global health: time to end the neglect? Am J Clin Pathol 2010; 134:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nkengasong JN, Mesele T, Orloff S et al. Critical role of developing national strategic plans as a guide to strengthen laboratory health systems in resource-poor settings. Am J Clin Pathol 2009; 131:852–7. [DOI] [PubMed] [Google Scholar]
- 11.Nkengasong JN. Strengthening laboratory services and systems in resource-poor countries. Am J Clin Pathol 2009; 131:774. [DOI] [PubMed] [Google Scholar]
- 12.Nkengasong JN. A shifting paradigm in strengthening laboratory health systems for global health: acting now, acting collectively, but acting differently. Am J Clin Pathol 2010; 134:359–60. [DOI] [PubMed] [Google Scholar]
- 13.May D. Going public, and private: collaboration proves effective and can even lead to some lofty results. Mod Healthc 2012; 42:26. [PubMed] [Google Scholar]
- 14.Philpott TG. Public-private partnerships: a Canadian hospital's perspective. Healthc Manage Forum 2007; 20:33–6. [DOI] [PubMed] [Google Scholar]
- 15.Ethiopian Ministry of Health. Master plan for the public health laboratory system in Ethiopia 2009–2013. 2nd ed Addis Ababa, Ethiopia: Federal Ministry of Health, 2009. [Google Scholar]
- 16.Ghebreyesus TA. Achieving the health MDGs: country ownership in four steps. Lancet 2010; 376:1127–8. [DOI] [PubMed] [Google Scholar]
- 17.Sturchio JL, Cohen GM. How PEPFAR's public-private partnerships achieved ambitious goals, from improving labs to strengthening supply chains. Health Aff (Millwood) 2012; 31:1450–8. [DOI] [PubMed] [Google Scholar]
- 18.CDC. Update: human immunodeficiency virus infections in health-care workers exposed to blood of infected patients. MMWR Morb Mortal Wkly Rep 1987; 36:285–9. [PubMed] [Google Scholar]
- 19.WHO. Guidelines for the safe transport of infectious substances and diagnostic specimens. http://wwwwhoint/csr/emc97_3pdf Accessed 24 June 2015.
- 20.Mphatswe W, Blanckenberg N, Tudor-Williams G et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS 2007; 21:1253–61. [DOI] [PubMed] [Google Scholar]
- 21.Violari A, Cotton MF, Gibb DM et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters PJ, Moore DM, Mermin J et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int 2008; 74:925–9. [DOI] [PubMed] [Google Scholar]