Summary
Background/objective:
Hypodontia is often seen in people with Down syndrome (DS). In the normal population, persons with hypodontia have a shorter cranial base and a hypoplastic maxilla, leading to a skeletal Class III tendency and a reduced face height. The purpose of this study was to examine craniofacial morphology in patients with DS at different ages and the influence of hypodontia on their craniofacial morphology.
Materials and methods:
A comparative cross-sectional study was conducted in 63 children with DS (6–19 years old; 28 males and 35 females) at a Centre for Special Care Dentistry in Rotterdam, the Netherlands (CBT Rijnmond). Digital lateral cephalograms were obtained from all subjects and a cephalometric analysis was performed. The subjects were divided into a group with hypodontia (13 males and 25 females) and a group without hypodontia (15 males and 10 females).
Results:
Significant results included a decrease in antero-posterior relationship of upper and lower jaw (ANB angle −0.331° per year, P = 0.044) and a decrease in vertical dimension (S–N_Go–Gn angle −0.72° per year, P = 0.039) over the years in subjects with hypodontia compared to subjects without hypodontia.
Conclusion:
The process of growth in DS patients is towards a reversed overjet. Hypodontia seems to have an additional effect on this development. The management of hypodontia as part of the complete treatment of dental development in DS children is important because it strongly influences the jaw relationship.
Introduction
Down syndrome (DS), or trisomy 21, is the most common chromosomal disorder that is associated with intellectual disability. The degree of mental disability in DS can vary widely. The mean IQ is 50, ranging from 20 to 80 (1, 2). The prevalence of DS in the Netherlands is between 12.000 and 13.000. Yearly 275 babies in the Netherlands are born with DS; this is 1 in 700 newborns. The life-expectancy has improved significantly in the last decades, people with DS may now become 50–60 years (3, 4).
People with DS have a short stature, resulting from growth deficiency in infancy and early childhood (5–7). Medical conditions are common, such as congenital malformations (especially heart defects), immune, thyroid and haematologic disorders, and musculoskeletal and nervous system anomalies (2, 8–11).
Typical craniofacial characteristics are microcephalia, prominent epicanthic folds, a low general muscle tone, and a hypoplastic maxilla (12). In comparison to the normal population, low muscle tone of the face and tongue are often present, causing the characteristic protrusion of the tongue (13). A hypoplastic maxilla and a hypoplastic cranial base are already present at birth in children with DS (14, 15). Maxillary growth remains reduced and aggravates the midface hypoplasia over the years. Although the mandible is slightly smaller in children with DS, it has a normal growth pattern and rate (12, 14, 15). Due to these jaw developments, a Class III malocclusion often occurs in DS children.
Seventy-four per cent of DS patients have a malocclusion (13). There is a higher incidence of dental anomalies, such as taurodontism, conic teeth, and impacted teeth (16). Tooth eruption is delayed 2–3 years (1, 16).
Hypodontia is often seen in the DS population (10, 16–18): 50–60 per cent of persons with DS have one or more congenitally missing teeth; however, some studies show different prevalences in the range from 35 to 60 per cent because of unclarity about proven or suspected hypodontia. (16,19).
In the normal population, persons with hypodontia have a shorter cranial base length and a hypoplastic maxilla, leading to a skeletal Class III tendency and a reduced face height (20–23). A more reduced vertical jaw relation and a more prognathic mandible can be found as the number of missing teeth increases (24). It is possible that this tendency also affects people with DS and hypodontia, in that it reinforces the skeletal Class III tendency, already seen in DS children. The prevalence of hypodontia in the normal population is higher in girls than in boys; in Europe this prevalence is 4.6 per cent in males and 6.3 per cent in females (25, 26).
Malocclusions can have a considerable impact on the quality of life and cause problems in oral functioning, such as chewing, swallowing or speaking, and tooth wear. To overcome these problems, it is important to prevent and treat a Class III malocclusion in DS patients (1, 13, 27).
The purpose of this study was to examine craniofacial morphology in patients with DS at different ages and the influence of hypodontia on their craniofacial morphology.
Materials and methods
Subjects
The protocol for this research study was approved by the Research Ethical Committee (METC) of the Erasmus Medical Centre in Rotterdam (approval number: MEC-2009–400).
A prospective cross-sectional study was conducted on 63 children with DS [28 males (44.4 per cent) and 35 females (55.6 per cent)] at a Centre for Special Care Dentistry in Rotterdam (CBT Rijnmond). This study included all DS patients aged 6–19 who were under treatment of CBT Rijnmond in the period between January 2010 and March 2011. Before the age of 6 it is difficult to diagnose congenitally missing teeth. After the age of 19 no significant growth is to be expected. Patients who were unable to co-operate were excluded from this study, as well as patients who had had orthodontic treatment, jaw fracture or jaw surgery. An informed consent was obtained from the parents of all the subjects.
The DS subjects were divided into a hypodontia group and a control group without hypodontia. Hypodontia was determined by examining orthopantomograms and in certain cases by additional X-ray photographs and clinical inspection. This study defines hypodontia as a condition of one or more congenitally missing permanent teeth, excluding the third molars. Severe hypodontia or oligodontia is defined as six or more missing teeth.
Craniofacial measurements
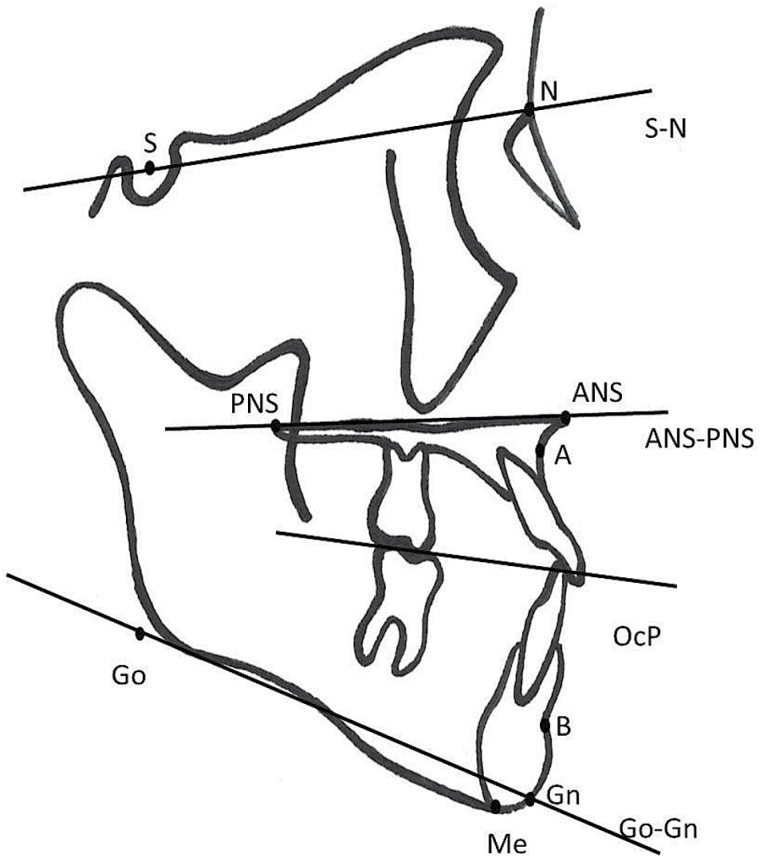
Digital lateral cephalometric radiographs were obtained from all subjects in a standardized position and were imported in a cephalometric measurement program (Viewbox version 3.1.1.12, dHAL Software, Kifissia, Greece) (28). Ten craniofacial landmarks and 8 measurements were used (29–31) (Figure 1). The measurements were performed by one observer.
Figure 1.
Cephalometric points, lines, and angles.
Statistics
Intra-observer agreement was tested by the Pearson correlation coefficient (32). Twenty randomly selected lateral cephalograms were measured twice by the same observer. To find systematic differences, a paired student t-test was used. A P-value of 0.05 was considered as statistically significant. The duplicate measurement error (DME) was determined by the standard deviation difference between first and second measurements divided by √2.
The effect of age on craniofacial growth patterns in DS patients was quantified by linear regression analyses (Statistical Package for the Social Sciences version 16.0, SPSS Inc., Chicago, IL, USA). For each of the cephalometric outcomes three models were made with the cephalometric value as dependent variable: one for the entire sample, one for the DS patients with hypodontia, and one for those without. In all models, age was the independent variable. A P-value of 0.05 was considered as statistically significant.
To test whether gender and the amount of missing teeth had a covariable effect on the cephalometric values, multiple regression analysis was performed. The cephalometric value was the dependent variable; the number of missing teeth, gender, and age were the independent variables. Multiple regression analysis was also repeated to test if oligodontia (yes/no) had an effect.
Results
Intra-observer agreement
Intra-observer reliability was good, in all instances a paired samples correlation of >0.9 was found. The DME indicated that the random error between the repeated measurements was acceptable, because it was considerably smaller than the range of the group measurements, by factor 3–5.
Hypodontia scores
The hypodontia group consisted of 38 subjects (60.3 per cent) and the group without hypodontia contained 25 subjects (39.7 per cent). The hypodontia group contained 13 males (20.6 per cent) and 25 females (39.7 per cent), and the control group without hypodontia consisted of 15 males (23.8 per cent) and 10 females (15.9 per cent). The hypodontia group consisted of 10 subjects with congenitally missing teeth in the anterior part of the jaws (26.3 per cent), 10 subjects with missing teeth in the posterior parts (26.3 per cent) and 18 subjects with missing teeth in anterior and posterior parts of the jaws (47.4 per cent).
The most common congenitally missing teeth were the upper lateral incisor, upper second premolar, both lower incisors, and lower second premolar (Table 1). The median of congenitally missing teeth in the hypodontia group was 4 with a range of 1–15. The median of congenitally missing teeth in the maxilla was 2 (range 0–7) and in the mandible it was 2 (range 0–8). In subjects with hypodontia, the maxilla showed a symmetrical pattern of congenitally missing teeth in 43.8 per cent; in the mandible this percentage was even higher with a percentage of 64.3.
Table 1.
Frequency table, division of missing elements by quadrant, and element number.
| Tooth number | Upper right | Upper left | Lower right | Lower left | Total |
|---|---|---|---|---|---|
| 1 (I1) | 0 | 0 | 9 | 11 | 20 |
| 2 (I2) | 21 | 18 | 8 | 10 | 57 |
| 3 (C) | 1 | 5 | 0 | 0 | 6 |
| 4 (P1) | 2 | 2 | 3 | 3 | 10 |
| 5 (P2) | 16 | 17 | 15 | 16 | 64 |
| 6 (M1) | 0 | 0 | 0 | 0 | 0 |
| 7 (M2) | 9 | 8 | 1 | 1 | 19 |
| Total | 49 | 50 | 36 | 41 | 176 |
Oligodontia was found in 23.8 per cent of the total study population; 7 males (46.7 per cent) and 8 females (53.3 per cent). The maximum amount of missing teeth per subject was 15.
Craniofacial measurements
The craniofacial morphology in each hypodontia group and in the non-hypodontia-group is described in Table 2. The results of the linear regression analysis for all cephalometric values for the total study population, for the hypodontia group and the non-hypodontia group are described in Tables 3–5.
Table 2.
Craniofacial morphology in each hypodontia group and the non-hypodontia group.
| Measurement | Total hypodontia group (A + P + AP) | Anterior (A) | Posterior (P) | Antero-posterior (AP) | Non-hypodontia group (NH) |
|---|---|---|---|---|---|
| SNA | 82.4±3.56 | 80.64±2.72 | 82.91±3.01 | 83.12±4.05 | 82.3±4.63 |
| SNB | 80.9±4.68 | 77.91±4.28 | 81.12±3.83 | 82.44±4.74 | 80.1±4.71 |
| ANB | 1.50±3.40 | 2.72±3.76 | 1.77±1.46 | 0.67±3.85 | 2.19±3.74 |
| ANS–Me/N–Me index | 57.7±2.48 | 57.46±1.66 | 58.01±3.71 | 57.54±2.14 | 58.3±2.52 |
| S–N_Go–Gn | 28.7±7.21 | 31.94±8.14 | 27.54±8.11 | 27.59±5.93 | 29.2±6.49 |
| ANS–PNS_S–N | 6.94±4.78 | 8.31±5.61 | 7.15±5.43 | 6.06±3.94 | 7.70±3.69 |
| OcP_ANS–PNS | 5.74±5.22 | 8.26±3.87 | 5.32±5.76 | 4.57±5.34 | 4.58±4.10 |
| OcP_Go–Gn | 16.1±5.62 | 15.38±3.94 | 15.08±8.15 | 16.96±4.86 | 17.0±4.74 |
| ANS–PNS_Go–Gn | 21.77±7.26 | 23.62±5.80 | 20.40±11.03 | 21.51±5.39 | 21.54±5.69 |
Hypodontia group (A = 10 + P = 10 + AP = 18): total number is 38 subjects. Non-hypodontia group: total number is 25 subjects. Values mentioned in this table are mean and standard deviation.
Table 3.
Effect per year for craniofacial measurements for the total Down syndrome (DS) population. CI, confidence interval; R 2 is r-square.
| Craniofacial measurement | N | Mean | SD | Effect per year | Significance (P-value) |
95% CI | R 2 |
|---|---|---|---|---|---|---|---|
| SNA | 63 | 82.4° | 3.99 | +0.397° | 0.011* | [0.094 to 0.701] | 0.101 |
| SNB | 63 | 80.6° | 4.67 | +0.561° | 0.002* | [0.214 to 0.907] | 0.147 |
| ANB | 63 | 1.78° | 3.52 | −0.159° | 0.261 | [−0.438 to 0.121] | 0.021 |
| ANS–Me/N–Me index | 63 | 57.9 | 2.50 | −0.042 | 0.676 | [−0.242 to 0.158] | 0.003 |
| S–N to Go–Gn | 63 | 28.9° | 6.89 | −0.598° | 0.028* | [−1.130 to −0.067] | 0.077 |
| ANS–PNS to S–N | 63 | 7.24° | 4.37 | −0.131° | 0.455 | [−0.480 to 0.218] | 0.009 |
| OcP to ANS–PNS | 63 | 5.28° | 4.81 | +0.254° | 0.187 | [−0.127 to 0.634] | 0.028 |
| OcP to Go–Gn | 63 | 16.41° | 5.27 | −0.211° | 0.318 | [−0.630 to 0.208] | 0.016 |
| Distance S–N | 63 | 60.0 | 9.32 | −0.70 | 0.853 | [−0.818 to 0.679] | 0.001 |
| ANS–PNS to Go–Gn | 63 | 21.68° | 6.63 | −0.465 | 0.078 | [−0.984 to 0.054] | 0.050 |
*Statistically significant (P < 0.05).
Table 5.
Effect per year for craniofacial measurements with hypodontia. CI, confidence interval; R 2, is r-square.
| Craniofacial measurement | N | Mean | SD | Effect per year | Significance (P-value) |
95% CI | R 2 |
|---|---|---|---|---|---|---|---|
| SNA | 38 | 82.4° | 3.56 | +0.104° | 0.556 | [−0.252 to 0.460] | 0.010 |
| SNB | 38 | 80.9° | 4.68 | +0.440° | 0.053 | [−0.005 to 0.886] | 0.100 |
| ANB | 38 | 1.50° | 3.40 | −0.331° | 0.044* | [−0.653 to 0.009] | 0.108 |
| ANS–Me/N–Me index | 38 | 57.7 | 2.48 | −0.144 | 0.241 | [−0.388 to 0.101] | 0.038 |
| S–N to Go–Gn | 38 | 28.7° | 7.21 | −0.720° | 0.039* | [−1.402 to 0.038] | 0.113 |
| ANS–PNS to S–N | 38 | 6.94° | 4.78 | −0.049° | 0.837 | [−0.528 to 0.431] | 0.001 |
| OcP to ANS–PNS | 38 | 5.74° | 5.22 | +0.250° | 0.333 | [−0.267 to 0.767] | 0.026 |
| OcP to Go–Gn | 38 | 16.1° | 5.62 | −0.420° | 0.127 | [−0.966 to 0.125] | 0.063 |
| Distance S–N | 38 | 58.0 | 10.77 | −0.297 | 0.580 | [−1.372 to 0.779] | 0.009 |
| ANS–PNS to Go–Gn | 38 | 21.77° | 7.26 | −0.670 | 0.057 | [−1.362 to 0.022] | 0.097 |
*Statistically significant (P < 0.05).
Table 4.
Effect per year for craniofacial measurements without hypodontia. CI, confidence interval; R 2, r-square.
| Craniofacial measurement | N | Mean | SD | Effect per year | Significance (P-value) |
95% CI | R 2 |
|---|---|---|---|---|---|---|---|
| SNA | 25 | 82.3° | 4.63 | +0.982° | 0.001* | [0.455 to 1.508] | 0.393 |
| SNB | 25 | 80.1° | 4.71 | +0.793° | 0.011* | [0.199 to 1.387] | 0.249 |
| ANB | 25 | 2.19° | 3.74 | +0.193° | 0.465 | [−0.345 to 0.731] | 0.023 |
| ANS–Me/N–Me index | 25 | 58.3 | 2.52 | +0.169 | 0.343 | [−0.191 to 0.529] | 0.039 |
| S–N to Go–Gn | 25 | 29.2° | 6.49 | −0.351° | 0.445 | [−1.285 to 0.583] | 0.026 |
| ANS–PNS to S–N | 25 | 7.70° | 3.69 | −0.287° | 0.269 | [−0.811 to 0.237] | 0.053 |
| OcP to ANS–PNS | 25 | 4.58° | 4.10 | +0.275° | 0.342 | [−0.311 to 0.861] | 0.039 |
| OcP to Go–Gn | 25 | 17.0° | 4.74 | +0.217° | 0.519 | [−0.467 to 0.901] | 0.018 |
| Distance S–N | 25 | 63.0 | 5.45 | 0.443 | 0.247 | [−0.328 to 1.214] | 0.058 |
| ANS–PNS to Go–Gn | 25 | 21.54° | 5.69 | −0.061 | 0.881 | [−0.890 to 0.768] | 0.001 |
*Statistically significant (P < 0.05).
The position of the maxilla in relation to the cranial base is represented by the SNA angle, which measures the antero-posterior position of point B (the deepest point on the contour of the mandible) in relation to the anterior cranial base. Age explained the significant increase of SNA by 0.397° per year in the total study population (P = 0.011). However, after separating the population into the two groups (with and without hypodontia), the significant increase per year was mostly explained by the group without hypodontia (0.982° per year, P = 0.001), whereas the hypodontia group showed no significant increase of SNA (0.104° per year, P = 0.556).
The position of the mandible in relation to the cranial base is represented by the SNB angle (SNB), which measures the antero-posterior position of point B (the deepest point on the contour of the mandible) in relation to the anterior cranial base. Age explained the significant increase of SNB by 0.561° per year in the total study population (P = 0.002). When separating the population in a group with hypodontia and a group without hypodontia, the significant increase per year was mostly explained by the group without hypodontia (0.793° per year, P = 0.011), whereas the hypodontia group showed no significant increase of SNB (0.440° per year, P = 0.053).
The ANB angle gives the relative position of point A (deepest point on the anterior contour of the maxilla) and point B (deepest point on the contour of the mandible) to each other. The ANB angle represents the inter-maxillary relationship. Age did not show an effect per year for ANB in the total study population (−0.159° per year, P = 0.261) and in the group without hypodontia (+0.193° per year, P = 0.465). However, isolating the hypodontia group, we found a significant decrease of ANB angle of 0.331° per year (P = 0.044). With increasing age, the difference in antero-posterior position of the jaws decreased in the hypodontia group, causing a greater tendency towards a Class III relationship of the jaws.
The angle between the lines S–N and Go–Gn provides an indication for the vertical dimensions of the mandible (SN–GoGn). Age explained the significant vertical decrease per year (0.598° effect per year, P = 0.028) of SN–GoGn in the total study population. Again, the decrease of SN–GoGn was mostly explained by the hypodontia group. The hypodontia group showed a significant decrease of SN–GoGn of 0.720° per year (P = 0.039), while the effect per year in the group without hypodontia was a non-significant decrease of 0.351° per year (P = 0.445).
All the other craniofacial measurements showed no significant effect per year in the total study population or after separating the population into the groups with and without hypodontia.
Discussion
The distinct craniofacial features in DS patients are different from the normal population (12–15). The hypoplastic maxilla is the most characteristic feature and defines the craniofacial characteristics. A shortened maxilla (ANS–PNS; ANS is the tip of the bony anterior nasal spine. PNS is the posterior end of the hard palate, if visible. Otherwise PNS is traced at the point of intersection of the dorsal maxillary contour and the soft palate contour.) is considered hypoplastic in the literature. The shortened cranial base length in DS can possibly explain why in our study population we found a nearly normal mean of SNA even though the maxilla is hypoplastic in DS. Similar results are found in the literature (15, 33).
Analysing our results, we found that with increasing age the significant increase of SNA and SNB was mostly explained by the group without hypodontia, whereas the hypodontia group showed no significant increase of SNA and SNB. Because this is not a longitudinal study, we cannot make conclusions for individual growth, but this suggests a significant mandibular and maxillary growth in the total DS population and the group without hypodontia and less forward growth of the jaws in the hypodontia group compared to the group without hypodontia.
In the hypodontia group, the maxillo-mandibular relationship of ANB decreased significantly compared to the total DS population and the group without hypodontia. These results suggest that there is a difference in phenotype between the group with hypodontia and the group without hypodontia. It is more and more assumed that a number of relevant genes lead to various phenotypes within DS (34, 35).
Compared to the total study population and the group without hypodontia, the hypodontia group also showed a significant decrease of mandibular vertical height. These changes support the view that hypodontia may lead to a smaller lower anterior face height and thereby an anti-clockwise rotation of the mandible. This finding that persons with hypodontia have a shorter cranial base length and a hypoplastic maxilla, leading to a skeletal Class III tendency and reduced face height, is in agreement with earlier findings in the normal population (20–23).
In our sample of DS patients we found hypodontia in 60 per cent of the DS patients. This is in agreement with the prevalences found in several other studies (16–18, 36, 37). DS patients are more affected by congenitally missing teeth (10, 16–18) and we think that this may explain the higher prevalence in DS syndrome patients of Class III jaw relationships.
We found that hypodontia more often occurs in girls than in boys; 65.8 per cent females versus 46.7 per cent males. This is in agreement with findings in the normal population (25, 26). In our study population girls are 1.92 times more affected with hypodontia than boys, and 1.14 times more affected by oligodontia. We have tested the effect of gender on craniofacial morphology in a multiregression analysis; however we did not find significant differences.
Endo et al. found that the location of the congenitally missing teeth have different effects on jaws (21). Unfortunately, our results (see Table 2) could not prove that anterior, posterior, and combined anterior and posterior hypodontia have different effects on the craniofacial measurements; this effect was too small to demonstrate in our study.
This prospective cross-sectional study is one of the largest in the world in DS patients. Although several large sample studies were performed, these studies did not take into account hypodontia and its influence on craniofacial form and growth. In our total group, assessment of our results resembles the data found in the other studies (12, 14, 15).
In our study, the ethnicity of the subjects was not taken into account.
A longitudinal study design is preferred over a cross-sectional study, in order to result in growth curves and better insights into craniofacial growth in DS children. Our findings indicate that the management of hypodontia as part of the complete treatment of dental development in DS children is important because it strongly influences the jaw relationship.
Conclusion
Determination of the risk of Class III malocclusion in DS seems to depend on both skeletal deviations on genetic basis and dentoalveolar deviations on the basis of local circumstances such as hypodontia. Over the years, both the difference in antero-posterior position of the jaws (ANB) and the vertical dimension (SN–GoGn) decrease significantly in patients with DS and hypodontia. DS children with hypodontia have a more obvious tendency in developing a Class III relationship of the jaws than DS children without hypodontia. This has to be taken into account when treating a DS patient.
Funding
Centre for Special Care Dentistry, CBT Rijnmond.
Acknowledgements
Special thanks to our patients who co-operated in this study, to the staff of CBT Rijnmond who made this study possible, and to Dr.ir. I.M. van Straten, Physicist at Department of Radiology, Erasmus MC, Rotterdam, The Netherlands.
References
- 1. Bouvy-Berends E.C. and Reuland-Bosma W (2006) ‘Emily goes to the dentist’. Oral care for individuals with Down syndrome. Nederlands Tijdschrift Voor Tandheelkunde, 113, 234–238. [PubMed] [Google Scholar]
- 2. Seagriff-Curtin P. Pugliese S. and Romer M (2006) Dental considerations for individuals with Down syndrome. The New York State Dental Journal, 72, 33–35. [PubMed] [Google Scholar]
- 3. Musich D.R. (2006) Orthodontic intervention and patients with Down syndrome. The Angle Orthodontist, 76, 734–735. [DOI] [PubMed] [Google Scholar]
- 4. Weijerman M.E. van Furth A.M. Vonk Noordegraaf A. van Wouwe J.P. Broers C.J. and Gemke R.J (2008) Prevalence, neonatal characteristics, and first-year mortality of Down syndrome: a national study. The Journal of Pediatrics, 152, 15–19. [DOI] [PubMed] [Google Scholar]
- 5. Barden H.S. (1983) Growth and development of selected hard tissues in Down syndrome: a review. Human Biology, 55, 539–576. [PubMed] [Google Scholar]
- 6. Cronk C.E. (1978) Growth of children with Down’s syndrome: birth to age 3 years. Pediatrics, 61, 564–568. [PubMed] [Google Scholar]
- 7. Myrelid A. Gustafsson J. Ollars B. and Annerén G (2002) Growth charts for Down’s syndrome from birth to 18 years of age. Archives of Disease in Childhood, 87, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roizen N.J. and Patterson D (2003) Down’s syndrome. Lancet (London, England), 361, 1281–1289. [DOI] [PubMed] [Google Scholar]
- 9. Wouters J., et al. (2009) Prospective human leukocyte antigen, endomysium immunoglobulin A antibodies, and transglutaminase antibodies testing for celiac disease in children with Down syndrome. The Journal of Pediatrics, 154, 239–242. [DOI] [PubMed] [Google Scholar]
- 10. Desai S.S. (1997) Down syndrome: a review of the literature. Oral surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 84, 279–285. [DOI] [PubMed] [Google Scholar]
- 11. Borstlap R. van Gameren-Oosterom H.B.M. Lincke C. Weijerman M.E. van Wieringen H. and van Wouwe J.P (2011) An Update of the Multidisciplinary Guideline for Medical Care of Children with Down Syndrome [in Dutch]. Nederlandse Vereniging van Kindergeneeskunde, Utrecht, The Netherlands; 21 p. [Google Scholar]
- 12. Fink G.B. Madaus W.K. and Walker G.F (1975) A quantitative study of the face in Down’s syndrome. American Journal of Orthodontics, 67, 540–553. [DOI] [PubMed] [Google Scholar]
- 13. Oliveira A.C. Paiva S.M. Campos M.R. and Czeresnia D (2008) Factors associated with malocclusions in children and adolescents with Down syndrome. American Journal of Orthodontics and Dentofacial Orthopedics: Official Publication of the American Association of Orthodontists, Its Constituent Societies, and the American Board of Orthodontics, 133, 489.e1–e8. [DOI] [PubMed] [Google Scholar]
- 14. Fischer-Brandies H. (1988) Cephalometric comparison between children with and without Down’s syndrome. European Journal of Orthodontics, 10, 255–263. [DOI] [PubMed] [Google Scholar]
- 15. Fischer-Brandies H. Schmid R.G. and Fischer-Brandies E (1986) Craniofacial development in patients with Down’s syndrome from birth to 14 years of age. European Journal of Orthodontics, 8, 35–42. [DOI] [PubMed] [Google Scholar]
- 16. de Moraes M.E. de Moraes L.C. Dotto G.N. Dotto P.P. and dos Santos L.R (2007) Dental anomalies in patients with Down syndrome. Brazilian Dental Journal, 18, 346–350. [DOI] [PubMed] [Google Scholar]
- 17. De Luca B. Massimino R. Materazzo S. Lombardo V. and Majorana M (1988) Skeletal alterations in Down’s syndrome: bibliographic review and case study contribution. Rays, 13, 49–59. [PubMed] [Google Scholar]
- 18. Reuland-Bosma W. Reuland M.C. Bronkhorst E. and Phoa K.H (2010) Patterns of tooth agenesis in patients with Down syndrome in relation to hypothyroidism and congenital heart disease: an aid for treatment planning. American Journal of Orthodontics and Dentofacial Orthopedics: Official Publication of the American Association of Orthodontists, Its Constituent Societies, and the American Board of Orthodontics, 137, 584.e1–9; discussion 584. [DOI] [PubMed] [Google Scholar]
- 19. Mestrović S.R. Rajić Z. and Papić J.S (1998) Hypodontia in patients with Down’s syndrome. Collegium Antropologicum, 22(Suppl), 69–72. [PubMed] [Google Scholar]
- 20. Bauer N. Heckmann K. Sand A. and Lisson J.A (2009) Craniofacial growth patterns in patients with congenitally missing permanent teeth. Journal of Orofacial Orthopedics = Fortschritte der Kieferorthopädie: Organ/Official Journal Deutsche Gesellschaft für Kieferorthopädie, 70, 139–151. [DOI] [PubMed] [Google Scholar]
- 21. Endo T. Ozoe R. Yoshino S. and Shimooka S (2006) Hypodontia patterns and variations in craniofacial morphology in Japanese orthodontic patients. The Angle Orthodontist, 76, 996–1003. [DOI] [PubMed] [Google Scholar]
- 22. Endo T. Yoshino S. Ozoe R. Kojima K. and Shimooka S (2004) Association of advanced hypodontia and craniofacial morphology in Japanese orthodontic patients. Odontology/the Society of the Nippon Dental University, 92, 48–53. [DOI] [PubMed] [Google Scholar]
- 23. Lisson J.A. and Scholtes S (2005) Investigation of craniofacial morphology in patients with hypo- and oligodontia. Journal of Orofacial Orthopedics = Fortschritte der Kieferorthopädie: Organ/Official Journal Deutsche Gesellschaft für Kieferorthopädie, 66, 197–207. [DOI] [PubMed] [Google Scholar]
- 24. Nodal M. Kjaer I. and Solow B (1994) Craniofacial morphology in patients with multiple congenitally missing permanent teeth. European Journal of Orthodontics, 16, 104–109. [DOI] [PubMed] [Google Scholar]
- 25. Polder B.J. Van’t Hof M.A. Van der Linden F.P. and Kuijpers-Jagtman A.M (2004) A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dentistry and Oral Epidemiology, 32, 217–226. [DOI] [PubMed] [Google Scholar]
- 26. Rølling S. and Poulsen S (2009) Agenesis of permanent teeth in 8138 Danish schoolchildren: prevalence and intra-oral distribution according to gender. International Journal of Paediatric Dentistry/The British Paedodontic Society [and] the International Association of Dentistry for Children, 19, 172–175. [DOI] [PubMed] [Google Scholar]
- 27. Musich D.R. (2006) Orthodontic intervention and patients with Down syndrome. The Angle Orthodontist, 76, 734–735. [DOI] [PubMed] [Google Scholar]
- 28. Halazonetis D.J. (1994) Computer-assisted cephalometric analysis. American Journal of Orthodontics and Dentofacial Orthopedics: Official Publication of the American Association of Orthodontists, Its Constituent Societies, and the American Board of Orthodontics, 105, 517–521. [DOI] [PubMed] [Google Scholar]
- 29. Ishikawa H. Nakamura S. Iwasaki H. Kitazawa S. Tsukada H. and Sato Y (1999) Dentoalveolar compensation related to variations in sagittal jaw relationships. The Angle Orthodontist, 69, 534–538. [DOI] [PubMed] [Google Scholar]
- 30. Athanasiou A.E. (1995) Orthodontic Cephalometry. Mosby Inc: London, UK. [Google Scholar]
- 31. Rakosi T. Jonas I. and Graber T.M (1993) Orthodontic Diagnosis. Thieme Medical Publishers Inc, New York, NY, 1st edn. 272 p. [Google Scholar]
- 32. Ongkosuwito E.M. Katsaros C. van‘t Hof M.A. Bodegom J.C. and Kuijpers-Jagtman A.M (2002) The reproducibility of cephalometric measurements: a comparison of analogue and digital methods. European Journal of Orthodontics 24:655–665. [DOI] [PubMed] [Google Scholar]
- 33. Suri S. Tompson B.D. and Cornfoot L (2010) Cranial base, maxillary and mandibular morphology in Down syndrome. The Angle Orthodontist, 80, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patterson D. (2007) Genetic mechanisms involved in the phenotype of Down syndrome. Mental Retardation and Developmental Disabilities Research Reviews, 13, 199–206. [DOI] [PubMed] [Google Scholar]
- 35. Patterson D. (2009) Molecular genetic analysis of Down syndrome. Human Genetics, 126, 195–214. [DOI] [PubMed] [Google Scholar]
- 36. Suri S. Tompson B.D. and Atenafu E (2011) Prevalence and patterns of permanent tooth agenesis in Down syndrome and their association with craniofacial morphology. The Angle Orthodontist, 81, 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kalf-Scholte S. and van Wijk A (2009) Agenesiepatronen in het blijvend gebit bij mensen met DS. Een analyse door middel van de TAC-procedure. Thesis, Academisch Centrum Tandheelkunde Amsterdam. [Google Scholar]