Abstract
Nicotine exposure during pregnancy leads to placental insufficiency impairing both fetal and neonatal development. Previous studies from our laboratory have demonstrated that in rats, nicotine augmented endoplasmic reticulum (ER) stress in association with placental insufficiency; however, the underlying mechanisms remain elusive. Therefore, we sought to investigate the possible direct effect of nicotine on ER stress in Rcho-1 rat placental trophoblast giant (TG) cells during differentiation. Protein and/or mRNA expression of markers involved in ER stress (eg, phosphorylated PERK, eIF2α, CHOP, and BiP/GRP78) and TG cell differentiation and function (eg, Pl-1, placental growth factor [Pgf], Hsd11b1, and Hsd11b2) were quantified via Western blot or real-time polymerase chain reaction. Nicotine treatment led to dose-dependent increases in the phosphorylation of PERK[Thr981] and eIF2α[Ser51], whereas pretreatment with a nicotinic acetylcholine receptor (nAChR) antagonist (mecamylamine hydrochloride) blocked the induction of PERK phosphorylation, verifying the direct involvement of nicotine and nAChR binding. We next investigated select target genes known to play essential roles in placental TG cell differentiation and function (Pl-1, Pgf, Hsd11b1, and Hsd11b2), and found that nicotine significantly augmented the mRNA levels of Hsd11b1 in a dose-dependent manner. Furthermore, using tauroursodeoxycholic acid, a safe bile acid known to improve protein chaperoning and folding, we were able to prevent nicotine-induced increases in both PERK phosphorylation and Hsd11b1 mRNA levels, revealing a potential novel therapeutic approach to reverse the deleterious effects of nicotine exposure in pregnancy. Collectively, these results implicate that nicotine, acting through its receptor, can directly augment ER stress and impair placental function.
Keywords: nicotine, placenta, endoplasmic reticulum stress, tauroursodeoxycholic acid, Rcho-1, trophoblast giant cell.
Nicotine exposure during pregnancy remains prevalent worldwide. Studies report that approximately 10%–28% of pregnant women smoke (Cui et al., 2014; Dhalwani et al., 2013; Tong et al., 2013), but exposure to nicotine may also occur through nicotine replacement therapies (NRTs), electronic (e)-cigarettes, and other forms of noncombustible tobacco products (Carroll Chapman and Wu, 2014; Myung et al., 2012). While the risks in pregnancy associated with NRTs or e-cigarettes have been long overlooked in comparison to the known hazards of cigarette smoking, recent evidence from animal studies warrant a careful reevaluation of the safety of nicotine exposure alone on pregnancy outcomes (De Long et al., 2014).
During pregnancy, the placenta sustains the health and development of the fetus through finely balanced nutrient exchange, waste removal, immune and barrier protection, and endocrine regulation (Burton and Fowden, 2015). Interestingly, the lipid-soluble nature of nicotine allows it to rapidly traverse past the membrane barriers to enter into the placenta where it competes with endogenous acetylcholine for binding to nicotinic acetylcholine receptors (nAChRs). Many nAChR subtypes are expressed in both human and rat placentas, and nAChRs in rat placental trophoblast cells are found to be responsive to nicotine doses within the average serum concentrations (25 nM–25 μM) of moderate to heavy cigarette smokers and/or NRT users (Holloway et al., 2014; Lips et al., 2005; Machaalani et al., 2014). nAChR signaling governs many functions, including cell differentiation, migration, viability, and transmitter release, thus augmented activation due to nicotine binding may elicit alternative, and potentially pathological, effects to impair placental development and function (Albuquerque et al., 2009). Indeed, many animal studies reveal that nicotine exposure in utero can lead to placental insufficiency, as seen through structural, morphological, and functional defects in vivo, alongside various developmental and long-term health consequences in the offspring (Bruin et al., 2010; De Long et al., 2014; Genbacev et al., 1995; Gruslin et al., 2009; Holloway et al., 2014). However, the cellular mechanisms underlying this nicotine-induced placental insufficiency have yet to be fully explored.
Endoplasmic reticulum (ER) stress, an intracellular perturbation involving the accumulation of misfolded or unfolded proteins, was recently proposed to underlie placental insufficiency associated with intrauterine growth restriction (IUGR; Yung et al., 2008, 2012a,b , 2014). In response to ER stress, the unfolded protein response (UPR) is activated to alleviate protein misfolding through 3 major signaling pathways that decrease global protein translation and increase protein folding capacity (Chambers and Marciniak, 2014). While the placenta normally exhibits low basal levels of ER stress due to high protein secretory activity, chronically unresolved ER stress leads to downstream apoptosis, which can impair placental development in vivo and in vitro (Kawakami et al., 2014;Yung et al., 2007, 2008). We recently reported that maternal nicotine exposure leads to augmented ER stress in the rat placenta (Wong et al., 2015), revealing a potential cellular mechanism that may underlie nicotine-induced placental insufficiency. However, due to the broad scope of indirect effects that nicotine may also elicit in vivo (eg, vasoconstriction leading to hypoxia and amino acid starvation [Koumenis et al., 2002; Lo et al., 2015; Pastrakuljic et al., 1999; Wong et al., 2015]), it is difficult to determine from in vivo studies if the ability of nicotine to cause ER stress results from a direct effect on placental cells.
To address this, mechanistic in vitro studies using placental trophoblast cells are necessary. The trophoblast giant (TG) cell serves as a relevant cell lineage to target due to its large population in the rat placenta, cardinal involvements in early establishment of pregnancy through uterine invasion and anastomosis of maternal blood supply, and later maintenance of healthy gestation through key endocrine roles in local and systemic physiological adaptations (Fonseca et al., 2012; Hu and Cross, 2010; Soares et al., 1996). Differentiated Rcho-1 TG cells in vitro have been very well characterized and determined to exhibit many similarities to the true placental TG cells in vivo in terms of cell cycle regulation, differentiation, gene transcription profile, transport processes, hormone production, and others (please refer to Sahgal et al., 2006 for full reference list on conducted studies). Therefore, utilizing differentiated Rcho-1 TG cells would provide better translatability to the mechanisms that might be occurring in vivo. Given that nicotine exposure has been found to negatively impact TG cell function and differentiation (Holloway et al., 2014), our current study was designed to determine the direct role of nicotine on placental ER stress. Moreover, we were also interested in whether nicotine-induced ER stress could be prevented with the use of tauroursodeoxycholic acid (TUDCA), a taurine-conjugated ursodeoxycholic bile acid endogenously produced by intestinal bacteria (Vang et al., 2014). TUDCA has previously relieved ER stress and stabilized UPR activation in several cell and tissue types (Berger and Haller, 2011; Ozcan et al., 2006; Xie et al., 2002), however, its efficacy as a therapeutic agent in the placenta and in nicotine-induced ER stress has yet to be examined.
MATERIALS AND METHODS
Cell culture and differentiation
Rcho-1, a placental trophoblast cell line derived from rat choriocarcinoma, was used as a model of placental TG cells. Rcho-1 cells can be maintained in either proliferative trophoblast stem (TS) cell or differentiated TG cell states based on the culture conditions (Faria and Soares, 1991). Rcho-1 TS cells were plated at 1.5 × 106 cells/ml and cultured at 37°C in 5% CO2/95% atmospheric air (Faria and Soares, 1991). Proliferation was maintained by growing Rcho-1 TS cells in RPMI-1640 media (Gibco) supplemented with 20% fetal bovine serum (Gibco), 50 µM 2-mercaptoethanol (Gibco), 1 mM sodium pyruvate (Gibco), and 0.04% gentamicin (10 mg/ml; Gibco). TG cell differentiation was induced as previously described (Sahgal et al., 2006). Briefly, at 80%–90% confluency (or after 3 days of proliferation), Rcho-1 TS cells were exposed to NCTC-135 media (Gibco) supplemented with 1% horse serum (Gibco), 50 µM 2-mercaptoethanol (Gibco), 1mM sodium pyruvate (Gibco), and 0.04% gentamicin (10 mg/ml; Gibco) for 10 days, with daily media changes. Removal of essential nutrients in the fetal bovine serum halted proliferation and promoted TG cell differentiation (Sahgal et al., 2006). NCTC-135 media was used during these differentiation conditions as it provided better pH regulation and decreased toxicity (Faria and Soares, 1991). Rcho-1 cells between passages 26–30 were used for all experiments.
Nicotine treatments
After 10 days of differentiation, Rcho-1 TG cells were treated with vehicle or increasing doses of nicotine (0.1–100 μM; Sigma-Aldrich) for 6 or 24 h. The nicotine doses encompassed the average serum concentrations of nicotine (25 nM–25 μM) previously reported in pharmacokinetic studies of cigarette smoking and/or NRTs (Armitage et al., 1975; DeVeaugh-Geiss et al., 2010; Massadeh et al., 2009; McNabb et al., 1982; Oncken et al., 1997; Russell et al., 1980). The 6 h time-point ensured detection of rapid protein phosphorylation events (eg, phosphorylation of PERK[Thr981] and eIF2α[Ser51]) and the 24 h time-point allowed detection of changes in protein and mRNA expression. Activation of the UPR indicated the presence of ER stress (Schroder and Kaufman, 2005).
Mecamylamine hydrochloride (nAChR inhibitor) treatments
After 10 days of differentiation, Rcho-1 TG cells were pretreated for 1 h with mecamylamine hydrochloride (MH; 10 μM; Santa Cruz), a noncompetitive, total nAChR inhibitor, and then exposed to nicotine (10 μM) for 6 h. The dose of MH was chosen based on previously published findings demonstrating effective nAChR blocking against nicotine in neuronal cells (Collo et al., 2013; Rao et al., 2003; Ridley et al., 2002). We selected PERK phosphorylation as a marker to assess the effect of nAChR antagonism on ER stress response induction due to the robust increase observed in response to nicotine.
TUDCA treatments
After 10 days of differentiation, Rcho-1 TG cells were pretreated for 1 h with TUDCA (100 μM; Sigma-Aldrich), and then treated with 10 μM nicotine for 6 or 24 h. The dose of TUDCA was chosen based on previously published findings that demonstrated effective amelioration of ER stress and UPR activation in vitro (Yin et al., 2012; Zhu et al., 2013).
RNA extraction and real time-polymerase chain reaction
Total RNA was extracted from cells using TRIzol reagent (Invitrogen). Chloroform (Sigma-Aldrich) was added to the solution, and then centrifuged at change to 16 500 g rpm. Supernatant was transferred to a fresh tube with an equal volume of isopropanol (Sigma-Aldrich) and centrifuged again at 16 500 g rpm. Total RNA was then collected from the pellet and dissolved in DEPC-treated water. Deoxyribonuclease I, Amplification Grade (Invitrogen) was added to the RNA to digest contaminating single- and double-stranded DNA. Four micrograms of RNA were reverse-transcribed to cDNA using random hexamers and Superscript II Reverse Transcriptase (Invitrogen). Primer sets directed against gene targets of interest were designed through National Center for Biotechnology Information’s primer designing tool and generated via Invitrogen Custom DNA Oligos (Table 1). Quantitative analysis of mRNA expression was performed via real time-polymerase chain reaction (RT-PCR) using fluorescent nucleic acid dye SsoFast EvaGreen supermix (BioRad) and BioRad CFX384 Real Time System. The cycling conditions were 95 °C for 10 min, followed by 43 cycles of 95 °C for 15 s and 60 °C for 30 s and 72 °C for 30 s. The cycle threshold was set so that exponential increases in amplification were approximately level between all samples. Relative fold changes were calculated using the comparative cycle times (Ct) method, normalizing all values to the geometric mean of 2 housekeeping genes (β-actin and Gapdh). Suitable housekeeping genes were determined using algorithms from GeNorm (Vandesompele et al., 2002), Normfinder (Andersen et al., 2004), BestKeeper (Pfaffl et al., 2004), and the comparative ΔCt method (Silver et al., 2006) to provide an overall ranking of the most stable housekeeping genes. Given all primer sets had equal priming efficiency, the ΔCt values for each primer set were calibrated to the average of all control Ct values, and the relative abundance of each primer set compared with calibrator was determined by the formula 2ΔΔCt, in which ΔΔCt was the normalized value.
TABLE 1.
Forward and Reverse Sequences for the Primers Used for RT-PCR
| Gene | Forward | Reverse | GenBank/Reference |
|---|---|---|---|
| Pl-1 | TGACTTTGACTCTTTCGGGCT | GCTCTGAATACACCGAGAGCG | Dai et al. (1996) |
| Pgf | GTGAGTATGCTGAGCCTAAGGG | AGACCTTACAAGACATGGATTCCC | NM_053595.2 |
| Hsd11b1 | GTCTCGGTAGGAGATGCTCAGG | GTAAGAGGCAACTTCCAGATGGC | NM_017080.2 |
| Hsd11b2 | TCGGCATCAGCAGTAGAGG | ACAACCCAGGACCCAAAC | Xu et al. (2012) |
| β-Actin | CACAGCTGAGAGGGAAAT | TCAGCAATGCCTGGGTAC | NM_031144 |
| Gapdh | GGATACTGAGAGCAAGAGAGAGG | TCCTGTTGTTATGGGGTCTGG | NM_017008.4 |
Protein extraction and Western blot
Cells were homogenized in RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P40, 0.25% C24H39NaO4, supplemented with phosphatase inhibitors (20 mM NaF, 40 mM Na-pyrophosphate, 40 mM Na3VO4, 200 mM β-glycerophosphate disodium salt hydrate), and a protease inhibitor cocktail (Roche). The solution was sonicated at 30% amplitude for 5 s total, 1 s per pulse. It was then mixed in a rotator for 10 min at 4 °C and centrifuged at 16 000 × g for 20 min at 4 °C. The resulting supernatant was collected as the total cellular protein extract and quantified by colorimetric DC protein assay (BioRad). Loading samples were prepared with fresh total protein extract (avoiding repeated freeze-thaw cycles), NuPAGE LDS Sample Buffer (4×) (Invitrogen), NuPAGE Reducing Agent (10×) (Invitrogen), and deionized water, and heated at 70 °C for 10 min to denature the proteins. Proteins (20 μg/well) were separated by size via gel electrophoresis in gradient polyacrylamide gels (Novex), and transferred onto polyvinylidene difluoride membrane (Millipore). Membranes were blocked in 1× Tris-buffered saline-Tween 20 buffer with 5% nonfat milk (blocking solution), and then probed using primary antibodies of the protein targets of interest, diluted in the blocking solution (Table 2). Secondary antibodies were used to detect the species-specific portion of the primary antibody, diluted in the blocking solution (Table 3). Immunoreactive bands were visualized using SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific). Relative band intensity was calculated using ImageLab software (BioRad) and normalized to the quantified total protein on each respective membrane, as determined through Amido black staining (Aldridge et al., 2008).
TABLE 2.
Western Blot Primary Antibodies, Dilutions Used in Experiments, and Company and Catalogue Information
| Antibody Name | Source | Dilution | Company (Catalogue Number) |
|---|---|---|---|
| P-PERK[Thr981] | Rabbit polyclonal | 1:800 | Santa Cruz Biotechnology Inc., Santa Cruz, California (sc-32577) |
| PERK (D11A8) | Rabbit monoclonal | 1:500 | Cell Signaling Technology Inc., Danvers, Massachusetts (5683) |
| P-eIF2α[Ser51] (119A11) | Rabbit monoclonal | 1:1000 | Cell Signaling Technology Inc., Danvers, Massachusetts (3597) |
| eIF2α | Rabbit monoclonal | 1:1000 | Cell Signaling Technology Inc., Danvers, Massachusetts (9722) |
| CHOP (D46F1) | Rabbit monoclonal | 1:500 | Cell Signaling Technology Inc., Danvers, Massachusetts (5554) |
| BiP (GRP78) | Rabbit polyclonal | 1:1000 | Cell Signaling Technology Inc., Danvers, Massachusetts (3183) |
| PDI (C81H6) | Rabbit monoclonal | 1:1000 | Cell Signaling Technology Inc., Danvers, Massachusetts (3501) |
TABLE 3.
Western Blot Secondary Antibodies, Dilutions Used in Experiments, and Company and Catalogue Information
| Antibody name | Dilution | Company (Catalogue Number) |
|---|---|---|
| Donkey Anti-Rabbit IgG (H+L) | 1:10 000 | Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania (711-001-003) |
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5 software. All results were expressed as means of normalized values ± SEM. All experiments were replicated 4 times (n = 4). Each replicate represents an independent experiment initiated from a different frozen vial of cells. The significance of the differences (P < .05) between normalized mean values were then evaluated using 1-way ANOVA followed by Tukey’s posttest.
RESULTS
Determination of Rcho-1 TG Cell Differentiation
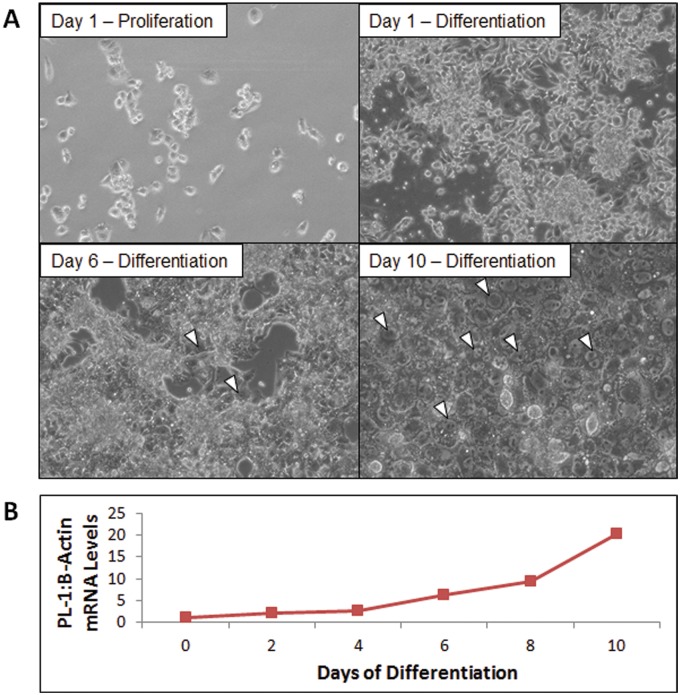
Rcho-1 TS cells were cultured for 10 days in NCTC-135 media + 1% horse serum to achieve large populations of actively differentiating TG cells as previously described (Sahgal et al., 2006). Successful differentiation of Rcho-1 TG cells was determined via phase contrast microscopic imaging of distinct morphological traits (ie, multiple nuclei, large cell body) (Figure 1A) and quantification of placental lactogen-I (Pl-1) mRNA levels (Figure 1B), which are uniquely expressed by TG cells (Faria and Soares, 1991).
FIG. 1.
Different methods used to detect the presence of differentiated Rcho-1 TG cells. A, Phase contrast microscopic images (10×). White triangles identify several representative differentiated TG cells. B, Steady-state mRNA levels of Pl-1 as measured through RT-PCR. RT-PCR: real time-polymerase chain reaction
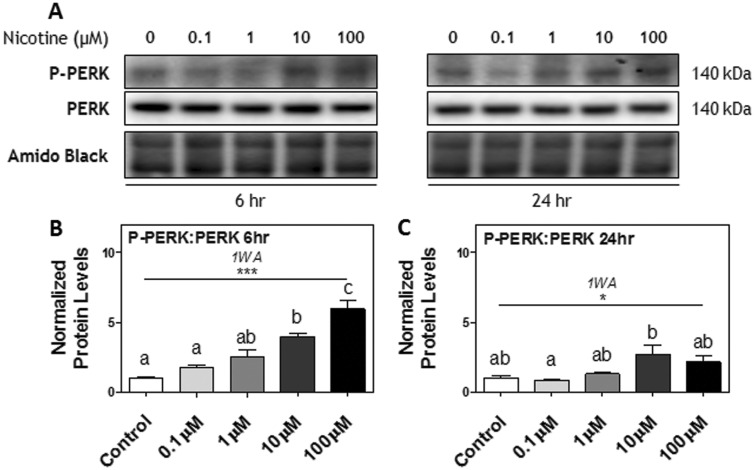
Nicotine Treatments Increased PERK Phosphorylation in a Dose-Dependent Manner
Rcho-1 TG cells were treated with vehicle or increasing doses of nicotine (0.1–100 μM) for 6 or 24 h. We had previously demonstrated that this range of nicotine does not cause overt toxicity nor affect viability in Rcho-1 cells (Holloway et al., 2014). Nicotine treatment led to PERK activation of the UPR in a dose-dependent manner. At 6 h, the ratio of phosphorylated (P)-PERK[Thr981]: PERK protein was significantly increased by nicotine treatment; post hoc testing showed a significant effect at 10 and 100 μM nicotine compared to the control (P < .05, P < .001, respectively; Figs. 2A and B). At 24 h, the ratio of P-PERK[Thr981]: PERK protein remained significantly increased by nicotine (P < .01), however this effect did not exhibit a dose-dependent relationship (Figs. 2A and C).
FIG. 2.
The effect of nicotine exposure (0.1–100 μM) on phosphorylation of PERK after 6 and 24 h in Rcho-1 TG cells. A, Specific targeted protein bands as detected by respective antibodies via Western blot. B, Protein levels of the ratio of P-PERK[Thr981]: PERK at 6 h and C, 24 h of nicotine exposure. All arbitrary values were expressed as means normalized to Amido Black ± SEM. All experiments were performed in quadruplicates (n = 4). Significant differences between treatment groups as determined by 1-way ANOVA (1WA) indicated by ** (P < .01) or *** (P < .001). Different letters represent means that are significantly different from one another according to Tukey’s posttest (P < .05). Nonsignificant differences (P > .05) indicated by n.s.
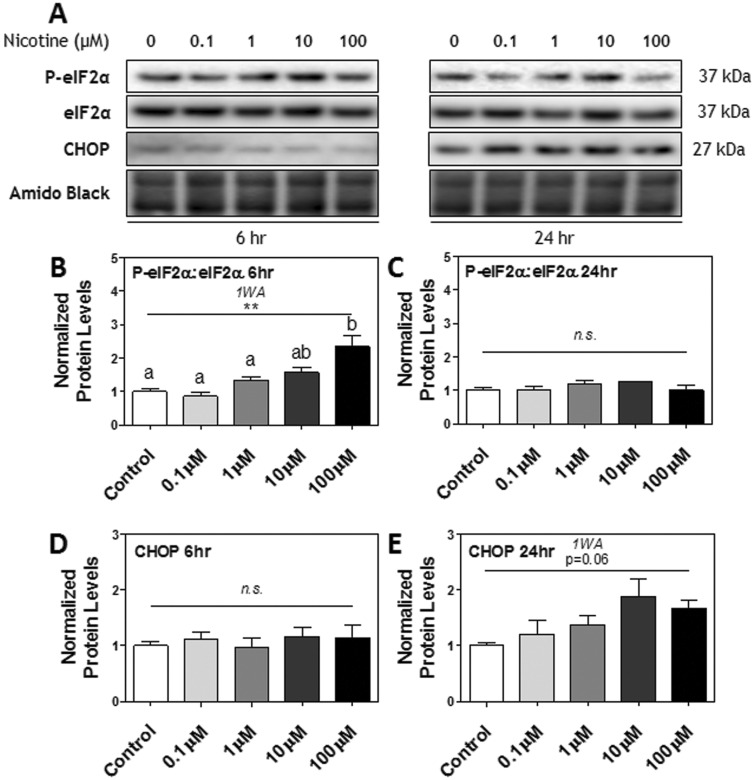
The Effect of Nicotine Treatments on Downstream Targets of the PERK Pathway
Downstream of PERK in the UPR pathway, a similar response to nicotine treatments was exhibited in the phosphorylation of eIF2α. At 6 h, the ratio of P-eIF2α[Ser51]: eIF2α was significantly increased by nicotine treatment; post hoc testing showed a significant effect at 100 μM nicotine compared to control (P < .01; Figs. 3A and B). However, protein levels were no longer significantly different from one another at 24 h (Figs. 3A and C). Protein levels of CHOP, a transcription factor downstream of P-eIF2α[Ser51] involved in activating ER stress-related apoptotic pathways during chronic ER stress (Matsumoto et al., 1996; Oyadomari and Mori, 2004), remained unaltered at 6 h, although it appeared to be trending toward an increase at 24 h (P = .06; Figs. 3A, D, and E).
FIG. 3.
The effect of nicotine exposure (0.1–100 μM) on downstream targets of the PERK pathway after 6 and 24 h in Rcho-1 TG cells. A, Specific targeted protein bands as detected by respective antibodies via Western blot. B, Protein levels of the ratio of P-eIF2α[Ser51]: eIF2α at 6 h and C, 24 h of nicotine exposure. D, Protein levels of CHOP at 6 h and (E) 24 h of nicotine exposure. All arbitrary values were expressed as means normalized to Amido Black ± SEM. All experiments were performed in quadruplicates (n = 4). Significant differences between treatment groups as determined by 1-way ANOVA (1WA) indicated by ** (P < .01) or *** (P < .001). Different letters represent means that are significantly different from one another according to Tukey’s posttest (P < .05). Nonsignificant differences (P > .05) indicated by n.s.
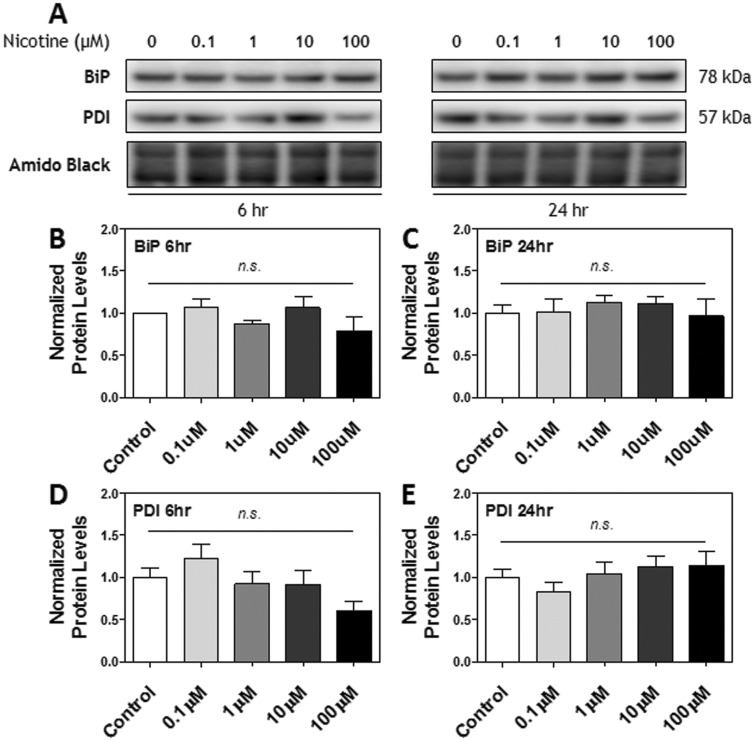
To investigate other aspects of the UPR, we also measured protein levels of 2 chaperone proteins: BiP/GRP78, a chaperone protein upregulated during ER stress to assist with protein refolding (Lee, 2005), and PDI, a key enzyme and chaperone protein involved in disulfide bond formation during protein folding (Benham et al., 2013; Frand and Kaiser, 1999; Wang and Tsou, 1993; Zhang et al., 2014). Nicotine treatment did not significantly alter the protein expression of either marker at any time or dose tested (Figs. 4A–E).
FIG. 4.
The effect of nicotine exposure (0.1–100 μM) on BiP and PDI after 6 and 24 h in Rcho-1 TG cells. A, Specific targeted protein bands as detected by respective antibodies via Western blot. B, Protein levels of BiP at 6 h and C, 24 h of nicotine exposure. D, Protein levels of PDI at 6 h and E, 24 h of nicotine exposure. All arbitrary values were expressed as means normalized to Amido Black ± SEM. All experiments were performed in quadruplicates (n = 4). Non-significant differences (P > .05) indicated by n.s.
Pretreatment With nAChR Antagonist Blocked Nicotine-Induced PERK Phosphorylation
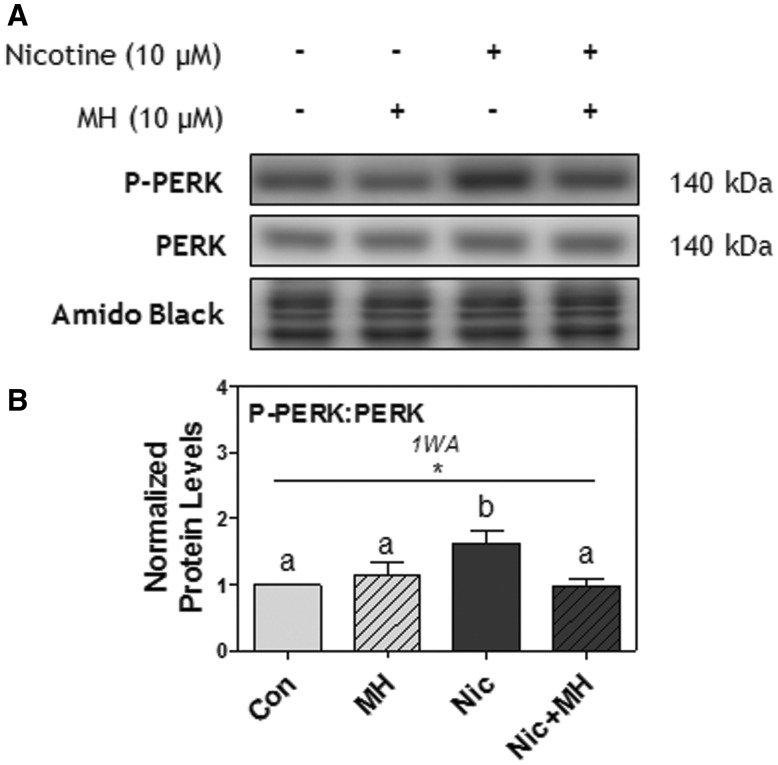
To identify whether the effect of nicotine on UPR activation occurred via nAChR signaling, Rcho-1 TG cells were pretreated for 1 h with mecamylamine hydrochloride (MH; 10 μM), and then treated with nicotine (10 μM) for 6 h. Nicotine significantly increased PERK phosphorylation (P < .05), an effect that was completely blocked with MH pretreatment (P < .05; Figs. 5A and B). MH alone did not affect PERK phosphorylation.
FIG. 5.
Pretreatment with MH (10 μM) blocked nicotine-induced PERK phosphorylation after 6 h in Rcho-1 TG cells. A, Specific targeted protein bands as detected by respective antibodies via Western blot. B, Protein levels of the ratio of P-PERK[Thr981]: PERK at 6 h. All arbitrary values were expressed as means normalized to Amido Black ± SEM. All experiments were performed in quadruplicates (n = 4). Significant differences between treatment groups as determined by 1-way ANOVA (1WA) indicated by * ( P < .05). Different letters represent means that are significantly different from one another according to Tukey’s posttest (P < .05).
Nicotine Treatments Increased 11β-Hydroxysteroid Dehydrogenase (Hsd11b) 1 Expression
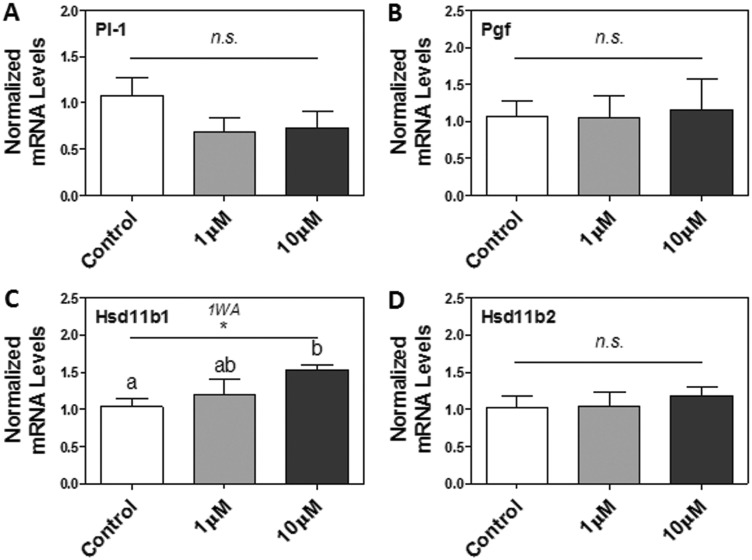
Given that nicotine can directly induce ER stress and UPR activation in Rcho-1 TG cells, we were next interested in assessing its impact on select markers of placental TG cell differentiation (ie, Pl-1 and placental growth factor [Pgf] [Faria and Soares, 1991; Vrachnis et al., 2013]) and function (ie, Hsd11b1 and Hsd11b2 for placental steroid metabolism [Chapman et al., 2013]). We measured the steady-state mRNA levels of Pl-1, Pgf, Hsd11b1, and Hsd11b2 via RT-PCR. While nicotine treatments did not significantly affect Pl-1, Pgf, and Hsd11b2 mRNA levels after 24 h (Figs. 6A, B, and D), it did significantly increase Hsd11b1 mRNA levels in a dose-dependent manner (P < .05; Figure 6C).
FIG. 6.
The effect of nicotine exposure (1–10 μM) on markers of placental TG cell differentiation and function after 24 h in Rcho-1 TG cells. mRNA levels of A, Pl-1, B, Pgf, C, Hsd11b1, and D, Hsd11b2. All arbitrary values were expressed as means normalized to the geometric mean of β-Actin and Gapdh ± SEM. All experiments were performed in quadruplicates (n = 4). All experiments were performed in quadruplicates (n = 4). Significant differences between treatment groups as determined by 1-way ANOVA (1WA) indicated by * (P < .05). Different letters represent means that are significantly different from one another according to Tukey’s posttest (P < .05). Non-significant differences (P > .05) indicated by n.s.
Pretreatment With TUDCA Prevented the Effects of Nicotine on PERK Phosphorylation and Hsd11b1 Expression
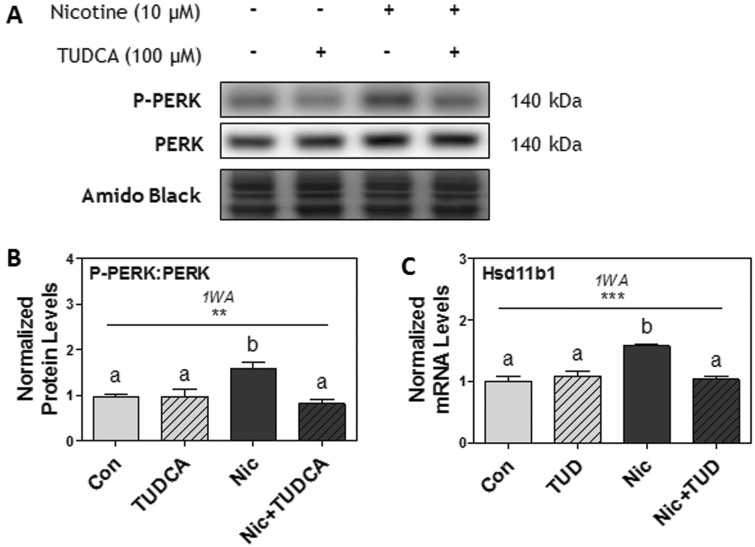
Lastly, Rcho-1 TG cells were pretreated for 1 h with TUDCA (100 μM), and then treated with nicotine (10 μM) for 6 or 24 h. Nicotine significantly increased PERK phosphorylation (P < .05), and pretreatment with TUDCA (100 μM) completely abrogated this effect (P < .05; Figs. 7A and B). Furthermore, nicotine treatment significantly increased Hsd11b1 mRNA levels, and pretreatment with TUDCA (100 μM) also completely inhibited nicotine-induced increases in Hsd11b1 mRNA levels (P < .001; Figure 7C). TUDCA pretreatment alone did not induce any effects on PERK phosphorylation nor Hsd11b1 mRNA levels.
FIG. 7.
Pretreatment with TUDCA (TUD; 100 μM) prevented the effects of nicotine on PERK phosphorylation and Hsd11b1 expression after 6 h in Rcho-1 TG cells. A, Specific targeted protein bands as detected by respective antibodies via Western blot. B, Protein levels of the ratio of P-PERK[Thr981]: PERK at 6 h. All arbitrary values were expressed as means normalized to Amido Black ± SEM. All experiments were performed in quadruplicates (n = 4). Significant differences between treatment groups determined by 1-way ANOVA (1WA) indicated by * (P < .05) or *** (P < .001). Different letters represent means that are significantly different from one another according to Tukey’s posttest ( P < .05).
DISCUSSION
We recently demonstrated that maternal nicotine exposure augments ER stress in the rat placenta in vivo (Wong et al., 2015). However, chronic maternal exposure to nicotine in this study also led to increased placental hypoxia (eg, HIF-1α), which is an indirect trigger for ER stress (Koritzinsky et al., 2013; Romero-Ramirez et al., 2004; Wong et al., 2015). Thus, the direct effects of nicotine on placental ER stress could not be ascertained. In this study, we now demonstrate that nicotine can directly augment ER stress and UPR activation in differentiating Rcho-1 TG cells, as indicated through the dose-dependent increases in PERK and eIF2α phosphorylation. The involvement of nAChR activation was further verified by the inhibition of nicotine-induced PERK phosphorylation using MH, a total nAChR antagonist. However, dissimilar to our previous in vivo results, the effects of nicotine on CHOP and PDI were far less prominent amidst strong PERK and eIF2α activation (Wong et al., 2015). It is likely that longer exposures and/or stronger doses of nicotine may have been required to alter these downstream targets in vitro (eg, 10 mM nicotine was used to increase CHOP expression in periodontal ligament cells in vitro [Lee et al., 2012]). We further revealed that nicotine treatments can augment the expression of Hsd11b1 in a dose-dependent manner, in association with augmented ER stress, suggesting possible alterations in placental steroid metabolism. Subsequently, pre-treatment of Rcho-1 TG cells with TUDCA ameliorated the effects of nicotine on ER stress in placental TG cells, and consequentially, on Hsd11b1 expression, suggesting nicotine-induced ER stress may mediate its augmented expression.
Consistent with our findings, Repo et al. (2014) provided preliminary evidence that nicotine (15 μM) can increase the expression of 2 ER stress markers, IRE1 and BiP, in BeWo choriocarcinoma cells. To comprehensively build upon these results, we utilized an actively differentiating placental TG cell model, a broad range of nicotine doses, along with inhibitors of nAChR activation and ER stress in order to provide a more robust, mechanistic assessment of the direct effects of nicotine on ER stress in the placenta. Rcho-1 cells are remarkable in their versatile ability to actively differentiate into TG cells, providing a more physiologically representative cellular model of the placenta (Faria and Soares, 1991). Rcho-1 cells have also been extensively validated and exhibit many similarities to true placental TG cells in cell cycle regulation, differentiation, gene transcription, transport processes, hormone production, and others (please refer to Sahgal et al., 2006 for reference list on characterization studies). Furthermore, the use of rat placental trophoblast cells complement what we previously reported in the rat placenta in vivo, allowing us to further elucidate the underlying mechanisms involved in nicotine-induced placental dysfunction (Wong et al., 2015).
Considering that nicotine directly increased placental ER stress, we were next interested in investigating the influence of nicotine and ER stress on select placental target genes. The increases seen in Hsd11b1 mRNA levels in Rcho-1 TG cells are important to note as the 11β-HSD family of enzymes are essential for steroid metabolism in the placenta (Chapman et al., 2013). While 11β-HSD2 (the protein product of Hsd11b2 transcript) is known for inactivating maternal glucocorticoids entering the placenta, 11β-HSD1 (the protein product of Hsd11b1 transcript), increases bioactive glucocorticoid production (Patel et al., 1999; Yang et al., 1994). Prenatal nicotine exposure has been associated with elevated glucocorticoid levels, low birth weight, and IUGR in rats (Chen et al., 2007; Feng et al., 2014), and Chen et al. (2007) further demonstrated that this is associated with decreased placental Hsd11b2 mRNA levels in rats. The lack of change seen in Hsd11b2 mRNA levels in our experiments may perhaps be attributed to the acute duration of our nicotine treatments (6, 24 h) in comparison to their chronic nicotine exposures (7 days); however, Benediktsson et al. (1997) also reported that nicotine exposure does not alter 11β-HSD2 activity in LLC-PK1 kidney-derived cells after 24 h, thus there may be discrepancies between the in vivo and in vitro effects. On the other hand, our finding that nicotine increased Hsd11b1 mRNA levels was consistent with a past study demonstrating that prenatal nicotine exposure led to increased Hsd11b1 mRNA levels in fetal rat hippocampus, liver, and gastrocnemius muscle in vivo (Xu et al., 2012). It is noteworthy that nicotine increased 11β-HSD1 expression, given that bioactive glucocorticoids in the placenta have been demonstrated to act in an autocrine manner to reduce prostaglandin breakdown and promote premature parturition (Chapman et al., 2013). This reveals an important area for research considering that smoking and/or nicotine exposure in pregnancy is associated with increased risk of preterm birth or IUGR in humans and rodents, respectively (Fantuzzi et al., 2007; Feng et al., 2014; Jaddoe et al., 2008). Yet, the involvement of placental 11β-HSD1 has yet to be examined in these studies, thus future studies are required to investigate the changes in placental glucocorticoid metabolism upon exposure to nicotine and/or increased ER stress to further supplement these results. The specific involvement of various nAChRs, as activated by nicotine, would also be of great interest in future studies. Moreover, there are no interventions currently available to target the deleterious impact of nicotine on placental glucocorticoid metabolism. Our findings revealing a dose-dependent association of nicotine with ER stress contribute insight into potential therapeutic options.
Therefore, we next investigated the use of TUDCA in ameliorating nicotine-induced placental ER stress, and the downstream effects on Hsd11b1 expression. Findings from our study are the first to reveal that TUDCA can prevent nicotine-induced PERK activation in placental TG cells. The ability of TUDCA to prevent nicotine-induced increases in Hsd11b1 mRNA levels also suggests that ER stress may play a role in altering placental glucocorticoid metabolism, though more studies would need to be conducted to confirm this mechanism, as TUDCA is not specific to only ER stress. TUDCA is suggested to ameliorate ER stress either through direct assistance with protein folding or by increasing the expression of endogenous molecular chaperones (Berger and Haller, 2011). Exogenous supplementation of TUDCA has provided beneficial effects in treating animal models of protein misfolding disorders such as obesity, type 2 diabetes, and Alzheimer’s disease (Ozcan et al., 2006; Sola et al., 2003). Recent developmental studies further demonstrated that postnatal TUDCA injections can reverse prenatal ethanol-induced ER stress damage in liver and skeletal muscle of rat offspring (Yao et al., 2013, 2014). Alongside the favourable effects against ER stress, TUDCA may also inhibit ROS production and protect against mitochondrial-mediated and caspase-mediated apoptosis (Miller et al., 2007; Rodrigues et al., 2003; Sokol et al., 2005), perhaps collectively enriching global cellular health. These properties of TUDCA may be especially desirable during pregnancy, as the placenta is particularly susceptible to augmented oxidative and ER stress due to its high protein folding and secretory activity (Yung et al., 2012b). Despite the fact that TUDCA, and UDCA (the non-taurine-conjugated counterpart of TUDCA), are approved by the FDA for treating primary biliary cirrhosis (Engin and Hotamisligil, 2010; Larghi et al., 1997), the safety of maternal usage during pregnancy has yet to be explored in vivo. It will be of great interest to examine the potential benefits of TUDCA on nicotine-induced ER stress in the placenta in vivo, and ultimately, as a potential novel therapeutic agent to remedy the consequences of maternal nicotine exposure in the clinical setting (please refer to Cortez and Sim (2014) for a useful review on the speculated therapeutic potential of TUDCA).
In conclusion, our current in vitro study provides strong mechanistic insight on the direct effects of nicotine exposure on placental ER stress at the cellular level. Our findings further demonstrate that TUDCA supplementation may indeed be a promising therapeutic option to consider for treating the negative outcomes of maternal nicotine exposure, although more studies are warranted to assess its safety and efficacy in pregnancy. With the high rates of maternal nicotine exposure that continue to occur worldwide, research providing intervention strategies are urgently required.
ACKNOWLEDGMENTS
The authors would like to thank Dr Patrick Lajoie for generously providing the TUDCA.
FUNDING
This work was supported by the Canadian Institutes of Health Research [MOP 111001 to D.B.H.].
REFERENCES
- Albuquerque E. X., Pereira E. F., Alkondon M., Rogers S. W. (2009). Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge G. M., Podrebarac D. M., Greenough W. T., Weiler I. J. (2008). The use of total protein stains as loading controls: An alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods 172, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen C. L., Jensen J. L., Orntoft T. F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250. [DOI] [PubMed] [Google Scholar]
- Armitage A. K., Dollery C. T., George C. F., Houseman T. H., Lewis P. J., Turner D. M. (1975). Absorption and metabolism of nicotine from cigarettes. Br. Med. J. 4, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson R., Magnusdottir E. M., Seckl J. R. (1997). Lack of effect of nicotine or ethanol on the activity of 11beta-hydroxysteroid dehydrogenase type 2. J. Steroid Biochem. Mol. Biol. 63, 303–307. [DOI] [PubMed] [Google Scholar]
- Benham A. M., van Lith M., Sitia R., Braakman I. (2013). Ero1-PDI interactions, the response to redox flux and the implications for disulfide bond formation in the mammalian endoplasmic reticulum. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 368, 20110403 10.1098/rstb.2011.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E., Haller D. (2011). Structure-function analysis of the tertiary bile acid TUDCA for the resolution of endoplasmic reticulum stress in intestinal epithelial cells. Biochem. Biophys. Res. Commun. 409, 610-615.. [DOI] [PubMed] [Google Scholar]
- Bruin J. E., Gerstein H. C., Holloway A. C. (2010). Long-term consequences of fetal and neonatal nicotine exposure: A critical review. Toxicol. Sci. 116, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. J., Fowden A. L. (2015). The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 370, 20140066.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll Chapman S. L., Wu L. T. (2014). E-cigarette prevalence and correlates of use among adolescents versus adults: a review and comparison. J. Psychiatr. Res. 54, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. E., Marciniak S. J. (2014). Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 2. Protein misfolding and ER stress. Am. J. Physiol. Cell Physiol. 307, C657–C670. 10.1152/ajpcell.00183.2014. [DOI] [PubMed] [Google Scholar]
- Chapman K., Holmes M., Seckl J. (2013). 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 93(3), 1139–1206. 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wang T., Liao Z. X., Pan X. L., Feng Y. H., Wang H. (2007). Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Exp. Toxicol. Pathol. 59, 245–251. 10.1016/j.etp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Collo G., Bono F., Cavalleri L., Plebani L., Mitola S., Merlo Pich E., Millan M. J., Zoli M., Maskos U., Spano P., Missale C. (2013). Nicotine-induced structural plasticity in mesencephalic dopaminergic neurons is mediated by dopamine D3 receptors and Akt-mTORC1 signaling. Mol. Pharmacol. 83, 1176–1189. 10.1124/mol.113.084863. [DOI] [PubMed] [Google Scholar]
- Cortez L., Sim V. (2014). The therapeutic potential of chemical chaperones in protein folding diseases. Prion 8, 197–202. 10.4161/pri.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Shooshtari S., Forget E. L., Clara I., Cheung K. F. (2014). Smoking during pregnancy: findings from the 2009–2010 Canadian Community Health Survey. PLoS One 9, e84640 10.1371/journal.pone.0084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G., Imagawa W., Liu B., Szpirer C., Levan G., Kwok S. C., Soares M. J. (1996). Rcho-1 trophoblast cell placental lactogens: complementary deoxyribonucleic acids, heterologous expression, and biological activities. Endocrinology 137, 5020–5027. 10.1210/endo.137.11.8895376. [DOI] [PubMed] [Google Scholar]
- De Long N. E., Barra N. G., Hardy D. B., Holloway A. C. (2014). Is it safe to use smoking cessation therapeutics during pregnancy? Expert Opin. Drug Saf. 13, 1721–1731. 10.1517/14740338.2014.973846. [DOI] [PubMed] [Google Scholar]
- DeVeaugh-Geiss A. M., Chen L. H., Kotler M. L., Ramsay L. R., Durcan M. J. (2010). Pharmacokinetic comparison of two nicotine transdermal systems, a 21-mg/24-hour patch and a 25-mg/16-hour patch: a randomized, open-label, single-dose, two-way crossover study in adult smokers. Clin. Therap. 32, 1140–1148. 10.1016/j.clinthera.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Dhalwani N. N., Tata L. J., Coleman T., Fleming K. M., Szatkowski L. (2013). Completeness of maternal smoking status recording during pregnancy in United Kingdom primary care data. PLoS One 8, e72218 10.1371/journal.pone.0072218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F., Hotamisligil G. S. (2010). Restoring endoplasmic reticulum function by chemical chaperones: An emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 12(Suppl 2), 108–115. 10.1111/j.1463-1326.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G., Aggazzotti G., Righi E., Facchinetti F., Bertucci E., Kanitz S., Barbone F., Sansebastiano G., Battaglia M. A., Leoni V., et al. (2007). Preterm delivery and exposure to active and passive smoking during pregnancy: A case-control study from Italy. Paediatr. Perinat. Epidemiol. 21, 194–200. 10.1111/j.1365-3016.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- Faria T. N., Soares M. J. (1991). Trophoblast cell differentiation: Establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology 129, 2895–2906. 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- Feng J. H., Yan Y. E., Liang G., Liu Y. S., Li X. J., Zhang B. J., Chen L. B., Yu H., He X. H., Wang H. (2014). Maternal and fetal metabonomic alterations in prenatal nicotine exposure-induced rat intrauterine growth retardation. Mol. Cell. Endocrinol. 394, 59–69. 10.1016/j.mce.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Fonseca B. M., Correia-da-Silva G., Teixeira N. A. (2012). The rat as an animal model for fetoplacental development: A reappraisal of the post-implantation period. Reprod. Biol. 12, 97–118. 10.1016/s1642-431x(12)60080-1. [DOI] [PubMed] [Google Scholar]
- Frand A. R., Kaiser C. A. (1999). Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell 4, 469–477. [DOI] [PubMed] [Google Scholar]
- Genbacev O., Bass K. E., Joslin R. J., Fisher S. J. (1995). Maternal smoking inhibits early human cytotrophoblast differentiation. Reprod. Toxicol. 9, 245–255. [DOI] [PubMed] [Google Scholar]
- Gruslin A., Cesta C. E., Bell M., Qing Q., Petre M. A., Holloway A. C. (2009). Effect of nicotine exposure during pregnancy and lactation on maternal, fetal, and postnatal rat IGF-II profile. Reprod. Sci. 16, 875–882. 10.1177/1933719109337038. [DOI] [PubMed] [Google Scholar]
- Holloway A. C., Salomon A., Soares M. J., Garnier V., Raha S., Sergent F., Nicholson C. J., Feige J. J., Benharouga M., Alfaidy N. (2014). Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. Am. J. Physiol., Endocrinol. Metab. 306, E443–E456. 10.1152/ajpendo.00478.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Cross J. C. (2010). Development and function of trophoblast giant cells in the rodent placenta. Int. J. Dev. Biol. 54, 341–354. 10.1387/ijdb.082768dh. [DOI] [PubMed] [Google Scholar]
- Jaddoe V. W., Troe E. J., Hofman A., Mackenbach J. P., Moll H. A., Steegers E. A., Witteman J. C. (2008). Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: The Generation R Study. Paediatr. Perinat. Epidemiol. 22, 162–171. 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Yoshimi M., Kadota Y., Inoue M., Sato M., Suzuki S. (2014). Prolonged endoplasmic reticulum stress alters placental morphology and causes low birth weight. Toxicol. Appl. Pharmacol. 275, 134–144. 10.1016/j.taap.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Koritzinsky M., Levitin F., van den Beucken T., Rumantir R. A., Harding N. J., Chu K. C., Boutros P. C., Braakman I., Wouters B. G. (2013). Two phases of disulfide bond formation have differing requirements for oxygen. J. Cell Biol. 203, 615–627. 10.1083/jcb.201307185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumenis C., Naczki C., Koritzinsky M., Rastani S., Diehl A., Sonenberg N., Koromilas A., Wouters B. G. (2002). Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2. Mol. Cell. Biol. 22(21), 7405–7416, 10.1128/mcb.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larghi A., Crosignani A., Battezzati P. M., De Valle G., Allocca M., Invernizzi P., Zuin M., Podda M. (1997). Ursodeoxycholic and tauro-ursodeoxycholic acids for the treatment of primary biliary cirrhosis: A pilot crossover study. Aliment Pharmacol. Ther. 11, 409–414. [DOI] [PubMed] [Google Scholar]
- Lee A. S. (2005). The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35, 373–381. 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lee S. I., Kang K. L., Shin S. I., Herr Y., Lee Y. M., Kim E. C. (2012). Endoplasmic reticulum stress modulates nicotine-induced extracellular matrix degradation in human periodontal ligament cells. J. Periodontal Res. 47, 299–308. 10.1111/j.1600-0765.2011.01432.x. [DOI] [PubMed] [Google Scholar]
- Lips K. S., Bruggmann D., Pfeil U., Vollerthun R., Grando S. A., Kummer W. (2005). Nicotinic acetylcholine receptors in rat and human placenta. Placenta 26, 735–746. 10.1016/j.placenta.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Lo J. O., Schabel M. C., Roberts V. H., Morgan T. K., Rasanen J. P., Kroenke C. D., Shoemaker S. R., Spindel E. R., Frias A. E. (2015). Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am. J. Obstet. Gynecol. 212, 370.e1–370.e8. 10.1016/j.ajog.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaalani R., Ghazavi E., Hinton T., Waters K. A., Hennessy A. (2014). Cigarette smoking during pregnancy regulates the expression of specific nicotinic acetylcholine receptor (nAChR) subunits in the human placenta. Toxicol. Appl. Pharmacol. 276, 204–212. 10.1016/j.taap.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Massadeh A. M., Gharaibeh A. A., Omari K. W. (2009). A single-step extraction method for the determination of nicotine and cotinine in Jordanian smokers' blood and urine samples by RP-HPLC and GC-MS. J. Chromatogr. Sci. 47, 170–177. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Minami M., Takeda K., Sakao Y., Akira S. (1996). Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 395, 143–147. [DOI] [PubMed] [Google Scholar]
- McNabb M. E., Ebert R. V., McCusker K. (1982). Plasma nicotine levels produced by chewing nicotine gum. Jama 248, 865–868. [PubMed] [Google Scholar]
- Miller S. D., Greene C. M., McLean C., Lawless M. W., Taggart C. C., O'Neill S. J., McElvaney N. G. (2007). Tauroursodeoxycholic acid inhibits apoptosis induced by Z alpha-1 antitrypsin via inhibition of Bad. Hepatology 46, 496–503. 10.1002/hep.21689. [DOI] [PubMed] [Google Scholar]
- Myung S. K., Ju W., Jung H. S., Park C. H., Oh S. W., Seo H., Kim H., and Korean Meta-Analysis Study Group. (2012). Efficacy and safety of pharmacotherapy for smoking cessation among pregnant smokers: A meta-analysis. BJOG 119, 1029–1039. 10.1111/j.1471-0528.2012.03408.x. [DOI] [PubMed] [Google Scholar]
- Oncken C. A., Hardardottir H., Hatsukami D. K., Lupo V. R., Rodis J. F., Smeltzer J. S. (1997). Effects of transdermal nicotine or smoking on nicotine concentrations and maternal-fetal hemodynamics. Obstet. Gynecol. 90(4 Pt 1), 569–574. [DOI] [PubMed] [Google Scholar]
- Oyadomari S., Mori M. (2004). Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389. 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Gorgun C. Z., Hotamisligil G. S. (2006). Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140, 10.1126./science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrakuljic A., Derewlany L. O., Koren G. (1999). Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta 20, 499–512. 10.1053/plac.1999.0418. [DOI] [PubMed] [Google Scholar]
- Patel F. A., Sun K., Challis J. R. (1999). Local modulation by 11beta-hydroxysteroid dehydrogenase of glucocorticoid effects on the activity of 15-hydroxyprostaglandin dehydrogenase in human chorion and placental trophoblast cells. J. Clin. Endocrinol. Metab. 84, 395–400. 10.1210/jcem.84.2.5442. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W., Tichopad A., Prgomet C., Neuvians T. P. (2004). Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515. [DOI] [PubMed] [Google Scholar]
- Rao T. S., Adams P. B., Correa L. D., Santori E. M., Sacaan A. I., Reid R. T., Suto C. M., Michel Vernier J. (2003). In vitro pharmacological characterization of (±)-4-[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride (SIB-1553A), a nicotinic acetylcholine receptor ligand. Brain Res. 981, 85–98. 10.1016/s0006-8993(03)02979-2. [DOI] [PubMed] [Google Scholar]
- Repo J. K., Pesonen M., Mannelli C., Vahakangas K., Loikkanen J. (2014). Exposure to ethanol and nicotine induces stress responses in human placental BeWo cells. Toxicol. Lett. 224, 264–271. 10.1016/j.toxlet.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Ridley D. L., Pakkanen J., Wonnacott S. (2002). Effects of chronic drug treatments on increases in intracellular calcium mediated by nicotinic acetylcholine receptors in SH-SY5Y cells. Br. J. Pharmacol. 135, 1051–1059. 10.1038/sj.bjp.0704508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues C. M., Sola S., Sharpe J. C., Moura J. J., Steer C. J. (2003). Tauroursodeoxycholic acid prevents Bax-induced membrane perturbation and cytochrome C release in isolated mitochondria. Biochemistry 42, 3070–3080. 10.1021/bi026979d. [DOI] [PubMed] [Google Scholar]
- Romero-Ramirez L., Cao H., Nelson D., Hammond E., Lee A. H., Yoshida H., Mori K., Glimcher L. H., Denko N. C., Giaccia A. J., et al. (2004). XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res 64, 5943–5947. 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- Russell M. A., Jarvis M., Iyer R., Feyerabend C. (1980). Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br. Med. J. 280, 972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal N., Canham L. N., Canham B., Soares M. J. (2006). Rcho-1 trophoblast stem cells: a model system for studying trophoblast cell differentiation. Methods Mol. Med. 121, 159–178. [PubMed] [Google Scholar]
- Schroder M., Kaufman R. J. (2005). ER stress and the unfolded protein response. Mutat. Res. 569, 29–63. 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Silver N., Best S., Jiang J., Thein S. L. (2006). Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 7, 33 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M. J., Chapman B. M., Rasmussen C. A., Dai G., Kamei T., Orwig K. E. (1996). Differentiation of trophoblast endocrine cells. Placenta 17, 277–289. [DOI] [PubMed] [Google Scholar]
- Sokol R. J., Dahl R., Devereaux M. W., Yerushalmi B., Kobak G. E., Gumpricht E. (2005). Human hepatic mitochondria generate reactive oxygen species and undergo the permeability transition in response to hydrophobic bile acids. J. Pediatr. Gastroenterol. Nutr. 41, 235–243. [DOI] [PubMed] [Google Scholar]
- Sola S., Castro R. E., Laires P. A., Steer C. J., Rodrigues C. M. (2003). Tauroursodeoxycholic acid prevents amyloid-beta peptide-induced neuronal death via a phosphatidylinositol 3-kinase-dependent signaling pathway. Mol. Med. 9, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong V. T., Dietz P. M., Morrow B., D'Angelo D. V., Farr S. L., Rockhill K. M., England L. J. (2013). Trends in smoking before, during, and after pregnancy—pregnancy risk assessment monitoring system, United States, 40 Sites, 2000–2010. MMWR Surveill. Summ. 62(6), 1–19. [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang S., Longley K., Steer C. J., Low W. C. (2014). The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of non-liver diseases. Glob. Adv. Health Med. 3, 58–69. 10.7453/gahmj.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrachnis N., Kalampokas E., Sifakis S., Vitoratos N., Kalampokas T., Botsis D., Iliodromiti Z. (2013). Placental growth factor (PlGF): A key to optimizing fetal growth. J. Matern. Fetal Neonatal Med. 26(10), 995–1002, 10.3109/14767058.2013.766694. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Tsou C. L. (1993). Protein disulfide-isomerase is both an enzyme and a chaperone. FASEB J. 7, 1515–1517. [DOI] [PubMed] [Google Scholar]
- Wong M. K., Nicholson C. J., Holloway A. C., Hardy D. B. (2015). Maternal nicotine exposure leads to impaired disulfide bond formation and augmented endoplasmic reticulum stress in the rat placenta. PLoS One 10, e0122295 10.1371/journal.pone.0122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q., Khaoustov V. I., Chung C. C., Sohn J., Krishnan B., Lewis D. E., Yoffe B. (2002). Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36, 592–601. 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- Xu D., Liang G., Yan Y. E., He W. W., Liu Y. S., Chen L. B., Magdalou J., Wang H. (2012). Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic alterations in fetal rats. Toxicol. Lett. 209, 282–290. 10.1016/j.toxlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Yang K., Berdusco E. T., Challis J. R. (1994). Opposite effects of glucocorticoid on hepatic 11 beta-hydroxysteroid dehydrogenase mRNA and activity in fetal and adult sheep. J. Endocrinol. 143, 121–126. [DOI] [PubMed] [Google Scholar]
- Yao X. H., Nguyen H. K., Nyomba B. L. (2013). Prenatal ethanol exposure causes glucose intolerance with increased hepatic gluconeogenesis and histone deacetylases in adult rat offspring: reversal by tauroursodeoxycholic acid. PLoS One 8, e59680 10.1371/journal.pone.0059680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. H., Nguyen K. H., Nyomba B. L. (2014). Reversal of glucose intolerance in rat offspring exposed to ethanol before birth through reduction of nuclear skeletal muscle HDAC expression by the bile acid TUDCA. Physiol. Rep. 2, 10.14814/phy2.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q. Q., Dong C. F., Dong S. Q., Dong X. L., Hong Y., Hou X. Y., Luo D. Z., Pei J. J., Liu X. P. (2012). AGEs induce cell death via oxidative and endoplasmic reticulum stresses in both human SH-SY5Y neuroblastoma cells and rat cortical neurons. Cell. Mol. Neurobiol. 32, 1299–1309. 10.1007/s10571-012-9856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung H. W., Atkinson D., Campion-Smith T., Olovsson M., Charnock-Jones D. S., Burton G. J. (2014). Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J. Pathol. 234(2), 262–276, 10.1002/path.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung H. W., Calabrese S., Hynx D., Hemmings B. A., Cetin I., Charnock-Jones D. S., Burton G. J. (2008). Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 173, 451–462. 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung H. W., Cox M., Tissot van Patot M., Burton G. J. (2012a). Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J. 26(5), 1970–1981, 10.1096/fj.11-190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung H. W., Hemberger M., Watson E. D., Senner C. E., Jones C. P., Kaufman R. J., Charnock-Jones D. S., Burton G. J. (2012b). Endoplasmic reticulum stress disrupts placental morphogenesis: Implications for human intrauterine growth restriction. J. Pathol. 228, 554–564. 10.1002/path.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung H. W., Korolchuk S., Tolkovsky A. M., Charnock-Jones D. S., Burton G. J. (2007). Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 21, 872–884. 10.1096/fj.06-6054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Niu Y., Zhu L., Fang J., Wang X., Wang L., Wang C. C. (2014). Different interaction modes for protein-disulfide isomerase (PDI) as an efficient regulator and a specific substrate of endoplasmic reticulum oxidoreductin-1alpha (Ero1alpha). J. Biol. Chem. 289, 31188–31199. 10.1074/jbc.M114.602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Zhong J. J., Jin J. F., Yin X. M., Miao H. (2013). Tauroursodeoxycholate, a chemical chaperone, prevents palmitate-induced apoptosis in pancreatic beta-cells by reducing ER stress. Exp. Clin. Endocrinol. Diabetes 121, 43–47. 10.1055/s-0032-1321787. [DOI] [PubMed] [Google Scholar]