Abstract
Some pharmaceuticals and environmental chemicals bind the thyroid peroxidase (TPO) enzyme and disrupt thyroid hormone production. The potential for TPO inhibition is a function of both the binding affinity and concentration of the chemical within the thyroid gland. The former can be determined through in vitro assays, and the latter is influenced by pharmacokinetic properties, along with environmental exposure levels. In this study, a physiologically based pharmacokinetic (PBPK) model was integrated with a pharmacodynamic (PD) model to establish internal doses capable of inhibiting TPO in relation to external exposure levels predicted through exposure modeling. The PBPK/PD model was evaluated using published serum or thyroid gland chemical concentrations or circulating thyroxine (T4) and triiodothyronine (T3) hormone levels measured in rats and humans. After evaluation, the model was used to estimate human equivalent intake doses resulting in reduction of T4 and T3 levels by 10% (ED10) for 6 chemicals of varying TPO-inhibiting potencies. These chemicals were methimazole, 6-propylthiouracil, resorcinol, benzophenone-2, 2-mercaptobenzothiazole, and triclosan. Margin of exposure values were estimated for these chemicals using the ED10 and predicted population exposure levels for females of child-bearing age. The modeling approach presented here revealed that examining hazard or exposure alone when prioritizing chemicals for risk assessment may be insufficient, and that consideration of pharmacokinetic properties is warranted. This approach also provides a mechanism for integrating in vitro data, pharmacokinetic properties, and exposure levels predicted through high-throughput means when interpreting adverse outcome pathways based on biological responses.
Keywords: thyroid peroxidase, adverse outcome pathway, margin of exposure, PBPK/PD model, high-throughput in vitro assay.
The utility of the Adverse outcome pathway (AOP) framework lies in its ability to provide a biological context for facilitating the interpretation of data from high-throughput (HT) in vitro assays (Tollefsen et al., 2014). The AOP framework rests on the assumption that multiple chemicals can act through a common biological pathway that leads to a specific adverse outcome, such that chemical interactions with a specific molecular target identified in HT assays can be linked to health consequences for a whole organism or population (Ankley et al., 2010; Edwards et al., 2015). Although the coupling of an AOP with HT testing is useful for prioritizing chemicals in lower tier hazard assessment, higher tier risk assessment requires quantitative linkages between external exposure levels to internal doses able to perturb the molecular target (Groh et al., 2015). These linkages require the understanding of exposure pathways and pharmacokinetics (PKs) (absorption, distribution, metabolism, and excretion [ADME]). In data-poor situations, considering these pathways/processes qualitatively can aid in refining HT findings through identification of false positives (ie, in vitro active chemicals incapable of reaching the molecular target) and false negatives (ie, parents of active metabolites or in vivo active chemicals unable to be detected in vitro) (Phillips et al., 2016). In data-rich situations, computational models can be used to predict exposure doses and track chemical disposition in a biological system, allowing for the linking of an in vivo target tissue/blood concentration expected to perturb the molecular target, as identified through in vitro testing, to an external exposure level (in vitro to in vivo extrapolation [IVIVE]).
Such an IVIVE approach was applied by Judson et al. (2011) to estimate conazole fungicide concentrations sufficient enough to significantly alter the xenobiotic constitutive androstane receptor/pregnane X receptor signaling pathway in vivo. In their study, in vitro concentration–response relationships were used to derive a biological pathway altering concentration in blood at steady state. This blood concentration was then converted to a biological pathway-altering dose (BPAD) using a one compartment PK model assuming 100% oral bioavailability, and having only 2 ADME-related parameters, intrinsic clearance and fraction of the chemical unbound to plasma proteins (Judson et al., 2011). The BPAD for each fungicide was then compared with chronic exposure levels from food residues for HT risk assessment. When chemical-specific exposure or ADME data are lacking, such an approach is used mainly for chemical prioritization due to the uncertainties inherent in the accuracy of HT data or predictions.
On the other hand, when exposure and ADME data are sufficient, the IVIVE approach can be applied to estimate a margin of exposure (MOE) for assessing risk for individual chemicals. In the present a framework was constructed to apply the IVIVE approach. The AOP selected for use as a case study was the inhibition of thyroid peroxidase (TPO), a key synthesis enzyme for thyroid hormone production in the thyroid gland. This framework involved the integration of HT in vitro data targeting thyroid disruption, a physiologically based pharmacokinetic/pharmacodynamic (PBPK/PD) model that simulates changes in serum thyroid hormone levels following chemical exposures, and estimation of environmental exposure levels (either from literature or HT models) to estimate MOEs. Specifically, (1) a PBPK model was developed to predict target tissue (ie, the thyroid gland) dosimetry; (2) the PBPK model was coupled with a PD model to estimate a human equivalent oral dose as a function of target tissue dose and HT in vitro data for TPO inhibition; and (3) human equivalent doses and estimated environmental exposure levels were used to calculate a MOE for 6 potential TPO inhibitors. Although only 1 specific AOP is presented in this case study, the utility of this integrated framework lies in its versatility in being able to investigate multiple chemicals across various AOPs. This case study also highlights the value of chemical-specific exposure and ADME data when applying the integrative framework in chemical prioritization or risk-based decision making.
METHODS
Framework overview
Our framework integrated HT in vitro hazard data, literature-reported or HT in silico model-predicted exposure levels, and chemical-specific ADME characterization to prioritize chemicals based on distance between an intake equivalent dose expected to perturb a molecular target and an environmental concentration to which a population would likely be exposed. These individual components of the integrated framework (illustrated in Figure 1) are discussed in detail in following subsections. A case study that includes 6 chemicals with varying degrees of potency for TPO inhibition (Paul et al., 2014) was used to demonstrate the application of our framework.
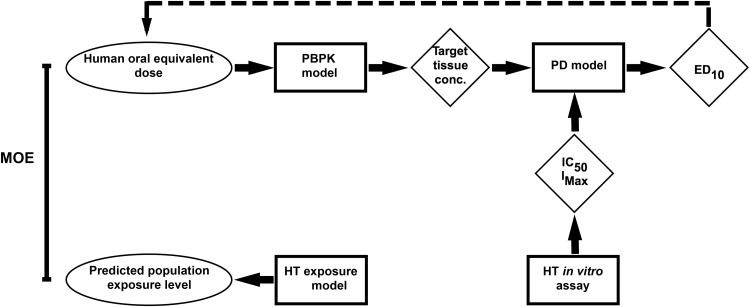
FIG. 1.
Workflow of the integrated modeling approach and its individual HT components. Squares represent processes, diamonds represent intermediates used as inputs into the next component, and ovals represent final outputs for decision making. The dashed line represents extrapolation of human equivalent dose from the combined PBPK and PD model.
HT in vitro assay
The experimental details for the in vitro HT TPO inhibition assay, as adapted from an existing rat thyroid microsome assay, are given in Paul et al. (2014). In this study, 6 chemicals were chosen from Paul et al. (2014) that ranged in potency from high (methimazole [MMI] and 6-propylthiouracil [PTU]) to moderate (benzophenone-2 [BP2] and 2-mercaptobenzothiazole [MBT]) to weak (triclosan [TCS] and resorcinol [RSC]). The concentration necessary to reduce TPO activity by 50% (IC50) and the maximum inhibitive activity (Imax) for each chemical were used as 2 of the inputs into the PD model, which is described below.
PD model
TPO plays a critical role in the synthesis of the thyroid hormones T4 and T3 through its conversion of iodide taken up from the blood into iodine by hydrogen peroxide-mediated oxidation (Jameson and Wheetman, 2001). Numerous xenobiotic chemicals have been identified to alter the biosynthesis, secretion, and regulation of thyroid hormones through feedback mechanisms related to the production and release of thyroid stimulating hormone (TSH), T4, and T3 (Crofton et al., 2005; Jugan et al., 2010). A quantitative model was developed by Ekerot et al. (2013) to predict the effects of TPO inhibitors on thyroid hormone homeostasis in humans. This model was modified in this study (Supplementary Figure S1) to describe changes in serum thyroid hormone (T3, T4, and TSH) concentrations in rats and humans following exposures to the 6 selected chemicals (Table 1). In this model, iodine is assumed to be readily available, and chemical effects are related only to TPO inhibition. The inhibitory effect of a chemical on TPO was described in our modified PD model as a function of Imax, IC50, (both were obtained from the in vitro study [Paul et al., 2014]), and the concentration of the chemical in thyroid tissue over time (CThy) derived from the PBPK model. Production rates of TSH and T4 were estimated from degradation rates of the 2 hormones and from their baseline levels in the absence of TPO inhibitors to ensure that TSH and T4 concentrations in plasma remain at their baseline values in the absence of chemical exposure. A similar approach was taken when estimating T3 production rates, although in this case, both the minor production of T3 in thyroid tissue, as well as the more abundant production via peripheral metabolic conversion of T4 into T3, were taken into account during model calibration. All other PD model parameters were taken from literature (see references in Table 1).
TABLE 1.
Thyroid Hormonea Elimination Rates, Generation Rates, Baseline Values, and Slope Parameters Used for the Rat and Human Thyroid Feedback PD Model
| Model | Parameter | Value | Units | Source |
|---|---|---|---|---|
| T4 Elimination | 9.63E-04 | 1/min | Modified from Silva et al. (1984) b | |
| T3 Elimination | 9.17E-03 | 1/min | Modified from Silva et al. (1984) b | |
| T4 Generation | 4.04E-03 | ug/dl/min | Modified from Ekerot et al. (2013) d | |
| Rat | T3 Generation | 1.70E-06c | ug/dl/min | Modified from Ekerot et al. (2013) d |
| TSH Generation | 1.22E-04 | ug/dl/min | Modified from Ekerot et al. (2013) d | |
| T4 Base Line | 4.2 | ug/dl | Huffman and Hedge (1986) | |
| T3 Base Line | 95.0 | ng/dl | Huffman and Hedge (1986) | |
| TSH Base Line | 5.0 | ng/ml | Choksi et al. (2003) | |
| fr | 0.215c | unitless | Silva et al. (1984) | |
| T4 Elimination | 7.78E-05 | 1/min | Nicoloff et al. (1972) | |
| T3 Elimination | 4.72E-04 | 1/min | Nicoloff et al. (1972) | |
| T4 Generation | 7.50E-03 | nM/min | Modified from Ekerot et al. (2013) d | |
| Human | T3 Generation | 7.78E-06c | nM/min | Modified from Ekerot et al. (2013) d |
| TSH Generation | 3.14E-05 | nM/min | Modified from Ekerot et al. (2013) d | |
| T4 Base Line | 96.5 | nmol/l | Ridgway et al. (1974) | |
| T3 Base Line | 3.2 | nmol/l | Ridgway et al. (1974) | |
| TSH Base Line | 0.129 | nmol/l | Odell et al. (1967) e | |
| fr | 0.20c | unitless | McGuire and Hays (1981) | |
| TSH Elimination | 2.43E-04 | 1/min | Ekerot et al. (2013) | |
| For all models | SF1 | 2.53 | unitless | Ekerot et al. (2013) |
| SF2 | 1.90 | unitless | Ekerot et al. (2013) | |
| SF3 | 0.11 | unitless | Ekerot et al. (2013) |
aT4, thyroxine, T3, triiodothyronine, and TSH, thyroid stimulating hormone.
bExtrapolated using the results of radiolabelled iodine tracer studies (Oppenheimer et al., 1972).
cThe 35% reduction in the fractional conversion of T4 to T3 caused by 6-propylthiouracil (21.5–14.0% in rats and 20–13% in humans) resulted in a T3 generation rate of 3.06E-04 in rats and 5.33E-04 in humans. The value provided in the table represents generation of T3 without this reduced fractional conversion and was used for all chemicals other than 6-propythiouracil.
dGeneration rates were derived from differential equations used to describe changes in time of TSH, T4, and T3 (Equations 3, 6, and 9 of Ekerot et al., 2013). Rather than examining changes in thyroid hormone concentrations, it was assumed that in absence of a chemical dose each respective hormone could be assigned its baseline concentration to simulate steady state.
eAssuming 1.5 U/mg and a molecular weight of 14 000 for the active alpha subunit of the TSH hormone, with a baseline concentration of 2.7 uU/ml for eurythyroid individuals (Odell et al. 1967).
PBPK model
The PBPK model for rats and humans included 10 perfusion-limited compartments: the thyroid, fat, brain, muscle, skin, kidney, liver, lumen and gastrointestinal (GI) tract, and slowly and rapidly perfused tissues (Supplementary Figure S2). In this PBPK model, only oral ingestion was simulated, as individuals are exposed to the 6 selected chemicals primarily through this route. Chemical-specific tissue-to-blood partition coefficients (PCs) were estimated based on common physicochemical properties (eg, solubility) (Rodgers et al., 2005; Rodgers and Rowland, 2006) through use of the proprietary software GastroPlus (Simulations Plus, Inc., Lancaster, California). As the generic GastroPlus module does not include a thyroid compartment, the same value as that of the kidney-to-blood PC was used for the thyroid lumen-to-blood PC. This approach was taken because the colloid lumen, or the region between the colloid and follicular cells, is the site at which thyroid hormone production occurs (Zoeller et al., 2007). According to a published PBPK model describing perchlorate kinetics in humans, the thyroid-to-blood PC is 0.13 for iodide and 0.15 for perchlorate, while the lumen-to-thyroid PC is 7.0 for both substrates (Merrill et al., 2005). The resulting lumen-to-blood PC is 1.05 for iodide and 0.91 for perchlorate, and these values are most similar to the kidney-to-blood PCs (1.09 for iodide and 0.99 for perchlorate) for all given tissue-to-blood PCs (Merrill et al., 2005).
The oral absorption rate for each chemical (min−1) was estimated from GastroPlus using the following equation:
| (1) |
where α is 2/R and R is the radius of each of the 9 GI compartments simulated in GastroPlus, Peff is the human-equivalent effective jejunal (source of the 9 compartments) permeability coefficient, and ka is the maximum absorption rate coefficient, as determined by plotting net ka versus time for the 9 GI compartments in GastroPlus. The initial oral dose was first introduced into the intestinal lumen, followed by the GI tract and liver, before entering into systemic circulation and distributing to body tissues. Physiological parameters used in the rat and human models were obtained from literature (Brown et al., 1997; Tables 2 and 3). The PBPK model was used to determine the concentration of each chemical within the thyroid compartment, and together with the PD model and using reverse dosimetry, the human equivalent intake dose resulting in a 10% reduction in circulating T4 and T3 levels due to TPO inhibition (ED10) was estimated. The 10% threshold was considered to be a conservative estimate for minimal adverse health effects according to United States Environmental Protection Agency guidelines (USEPA, 2011). All PBPK/PD model simulations were conducted in MATLAB (version R2014b; The MathWorks, Inc., Natick, Massachusetts).
TABLE 2.
Physiological Parameters Used for the Rat PBPK Model
| Parameter | Value | Source |
|---|---|---|
| BW (g)- assume 1 g = 1 ml | 250.0 | Nong et al. (2008) |
| Cardiac Output (Q_CO; ml/min) | 83.0 | Brown et al. (1997) |
| Tissue volume (as a fraction of BW) | ||
| Kidney (V_Kid) | 0.0073 | Brown et al. (1997) |
| Liver (V_Liv) | 0.0366 | Brown et al. (1997) |
| GI tract (V_GI) | 0.0269 | Brown et al. (1997) |
| Rest of body (V_Rap) | 0.0137 | Brown et al. (1997); remaining tissues |
| Muscle (V_Mus) | 0.404 | Brown et al. (1997) |
| Skin (V_Skin) | 0.190 | Brown et al. (1997) |
| Slowly perfused tissues (V_Slow)-for bone | 0.050 | Brown et al. (1997) |
| Adipose (V_Fat) | 0.064 | Brown et al. (1997) |
| Brain (V_Brn) | 0.0057 | Brown et al. (1997) |
| Thyroid (V_Thy) | 0.00005 | Brown et al. (1997) |
| Blood (V_Bld) | 0.070 | Brown et al. (1997) |
| Blood Flow (as a Fraction of Cardiac Output) | ||
| Kidney (Q_Kid) | 0.141 | Brown et al. (1997) |
| Liver (Q_Liv) | 0.183 | Brown et al. (1997) |
| Rest of body (Q_Rap) | Difference | Q_CO—blood flow of all other tissues |
| Muscle (Q_Mus) | 0.278 | Brown et al. (1997) |
| Skin (Q_Skin) | 0.058 | Brown et al. (1997) |
| Slowly perfused tissues (Q_Slow)- for bone | 0.122 | Brown et al. (1997) |
| Adipose (Q_Fat) | 0.070 | Brown et al. (1997) |
| Brain (Q_Brn) | 0.020 | Brown et al. (1997) |
| Thyroid (Q_Thy) | 0.00271 | Huffman and Hedge (1986) |
| Portal vein (Q_PV) | 0.153 | Brown et al. (1997) |
| Hepatic artery (Q_HA) | Difference | Q_Liv–Q_PV |
TABLE 3.
Physiological Parameters Used for the Human PBPK Model
| Parameter | Value | Source |
|---|---|---|
| BW(g)- assume 1 g = 1 ml | 70000.0 | Brown et al. (1997) |
| Cardiac Output (Q_CO; ml/min) | 5200.0 | Brown et al. (1997) |
| Tissue Volume (as a Fraction of BW) | ||
| Kidney (V_Kid) | 0.0040 | Brown et al. (1997) |
| Liver (V_Liv) | 0.026 | Brown et al. (1997) |
| GI tract (V_GI) | 0.0171 | Brown et al. (1997) |
| Rest of body (V_Rap) | 0.0493 | Brown et al. (1997); remaining tissues |
| Muscle (V_Mus)- for females | 0.290 | Brown et al. (1997) |
| Skin (V_Skin)- for females | 0.031 | Brown et al. (1997) |
| Slowly perfused tissues (V_Slow)- for bone | 0.049 | Brown et al. (1997) |
| Adipose (V_Fat)- for females | 0.327 | Brown et al. (1997) |
| Brain (V_Brn) | 0.020 | Brown et al. (1997) |
| Thyroid (V_Thy) | 0.0003 | Brown et al. (1997) |
| Blood (V_Bld) | 0.0714 | Brown et al. (1997) |
| Blood Flow (as a Fraction of Cardiac Output) | ||
| Kidney (Q_Kid) | 0.175 | Brown et al. (1997) |
| Liver (Q_Liv) | 0.227 | Brown et al. (1997) |
| Rest of body (Q_Rap) | Difference | Q_CO—blood flow of all other tissues |
| Muscle (Q_Mus) | 0.191 | Brown et al. (1997) |
| Skin (Q_Skin) | 0.058 | Brown et al. (1997) |
| Slowly perfused tissues (Q_Slow)- for bone | 0.042 | Brown et al. (1997) |
| Adipose (Q_Fat) | 0.085 | Brown et al. (1997) |
| Brain (Q_Brn) | 0.114 | Brown et al. (1997) |
| Thyroid (Q_Thy) | 0.016 | Brown et al. (1997) |
| Portal vein (Q_PV) | 0.181 | Brown et al. (1997) |
| Hepatic artery (Q_HA) | Difference | Q_Liv - Q_PV |
In addition to PCs, the other critical chemical-specific data required in a PBPK model is the determination of clearance of chemicals. Rather than using 2 distinct clearance terms for urinary excretion and hepatic clearance, one single rate was used to describe the total clearance of the chemical. In our study, total clearance for each chemical was taken as a function of both biological half-life and volume of distribution according to the first order kinetic rate equation:
| (2) |
where Vd (l/kg) is the volume of distribution, t1/2 (h) is the biological half-life, and ClTot is the total body clearance (ml/min) for each chemical.
Total clearance for MMI in rats was estimated from recovery of radio-labeled MMI from whole blood samples taken intermittently over 24 h after a single intravenous dosing of 20 mg/kg; the t1/2 of decay was 3.85 h, and Vd was 67 ml per 100 g rat BW (Sitar and Thornhill, 1973). Total clearance for PTU in rats was estimated from plasma concentrations of PTU taken intermittently within a 24-h period after a single intraperitoneal dose of 3.3 mg/kg PTU; the t1/2 was approximately 6 h, and Vd was 0.73 l/kg (Giles et al., 1982). Total clearance for TCS in rats was estimated from TCS concentrations in recovered plasma samples taken intermittently over a 48-h period after a single oral dose of 5 mg/kg; the t1/2 was 48.5 h, and Vd was 73.5 l/kg (Wu et al., 2009).
Total clearance for MMI in humans was calculated based on a Vd of 0.6 l/kg (Hengstmann and Hohn, 1985), approximating a total body water volume of 60–65%, and using a t1/2 of 6 h (Cooper et al., 1984a). Total clearance for PTU in humans was calculated based on a Vd of 0.42 l/kg (Kampmann and Hansen, 1981) and a t1/2 of 1.28 h (Kampmann and Skovsted, 1974). Total clearance for TCS in humans was estimated from measured plasma concentrations over 8 h for individuals given a single oral dose of 4 mg in 13 ml of a mouthwash solution after an overnight fasting period; the median t1/2 of TCS was 19 h, and Vd was 1.14 l/kg (Sandborgh-Englund et al., 2006). No literature-reported values of Vd could be obtained for the 3 remaining environmental chemicals, so these values were predicted using the ADMET Predictor (Simulations Plus, Inc., Lancaster, California). Predicted Vd values were 0.8 l/kg, 0.43 l/kg, and 0.25 l/kg for RSC, MBT, and BP2, respectively. The biological half-lives in rats are estimated to be 19 h for BP2 (Jeon et al., 2008), 6 h for MBT (Garcia, 2004), and 10 h for RSC (Merker et al., 1982). Rather than using reported half-lives for rodents, biological half-lives of RSC, MBT, and BPT were estimated from their log P values according to the equation of Sarver et al. (1997):
| (3) |
The t1/2 values for each chemical, as predicted using the equation above, were 4.65, 13.63, and 12.76 h for RSC, MBT, and BP2, respectively.
After parameterizing the PBPK model, the PBPK/PD model was evaluated by comparing model predictions against time course plasma or thyroid gland concentrations and/or thyroid hormone concentrations for PTU, MMI, and TCS measured in rats. The model-simulated time course concentrations of PTU, T3, and T4 in serum, and of PTU in the thyroid gland were compared against measured values in rats (200–250 g body weight [BW]) given 0.05% (0.5 mg/ml) PTU in drinking water for 7 days (Cooper et al., 1983). Assuming consumption of 25 ml drinking water (or approximately 10 ml/100 g) per day, which falls within the normal range of 20–50 ml/d (http://web.jhu.edu/animalcare/procedures/rat.html; Accessed February 19, 2016), the estimated dosage used in the model was 8.7 × 10−6 g/min. In our simulations, no dose was given in the first 2 days in order to demonstrate the persistence of baseline hormone levels. Then, the model was run to simulate daily dosing for 7 days, followed by no PTU dosing for a time sufficient enough to allow hormone levels to return to baseline concentrations. The model was also used to predict MMI concentrations in serum and in the thyroid gland for comparison against MMI concentrations measured in rats given a 0.05% dose in drinking water for 7 days (Cooper et al., 1984b). The third model evaluation case compared model simulations in rats against TCS concentrations in serum measured in rats given a single oral dose of 5 mg/kg in 1 ml corn oil (Wu et al., 2009), serum T3 and T4 in male Wistrar rats given daily oral doses of 0–300 mg/kg TCS for 31 days postweaning (Zorrilla et al., 2009), and serum T3 and T4 in female young Long-Evans rats given daily oral doses of 0–1000 mg/kg TCS for 4 days (Paul et al., 2010). The only measured human data available for evaluating our PBPK/PD model was of MMI concentrations in serum samples taken over 8 h from individuals that had fasted overnight and were given a single oral dose of 30 or 60 mg (Cooper et al., 1984a). Time-course serum concentration data from that study was digitized using the UN-SCAN-IT digitizing software (Silk Scientific, Inc., Orem, Utah), and model-predicted MMI concentrations in the blood were then compared against the measured concentrations at both doses.
Sensitivity analyses
The model’s system of equations can be written as:
| (4) |
where is the vector state of variables (eg, change in T4) and is the vector set of the parameters (eg, clearance), and the derivation of the sensitivity equations can be described as:
| (5) |
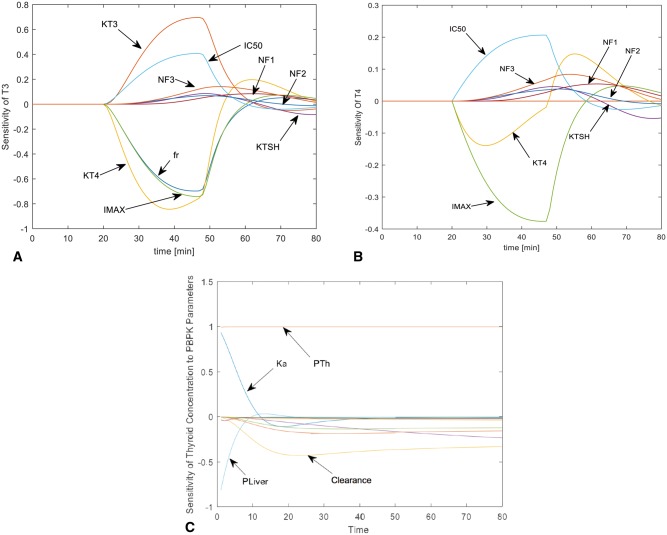
Switching the order of differentiation leads to a system of ordinary differential equations whose state variables () represent the sensitivity of the model states to each parameter. The formulation of the sensitivity equations was completed using the automatic differentiation package for MATLAB written by Martin Fink and Adam Attarian and available at MATLAB central (http://www.mathworks.com/matlabcentral/; Accessed February 19, 2016). The system of sensitivity equations was integrated with the model equations using MATLAB’s ODE solver. The sensitivity coefficients were normalized by dividing the value of the state variable, and multiplying by the parameter for which the derivative was being determined (eg, ). Normalizing the sensitivity coefficients ensures that observed changes are equivalent regardless of the magnitude of the parameter or the state variable. Results of the sensitivity analysis for chemical thyroid concentration and changes in T3 and T4 are shown below (Figs. 2A–C).
FIG. 2.
Results of the sensitivity analysis of physiological parameters influencing changes in T3 (A) and T4 (B) during evaluation of the PD model and chemical concentration in the thyroid gland compartment during evaluation of the PBPK model (C). Abbreviations are as follows: PTh, thyroid lumen-to-plasma PC; PLiv, liver-to-plasma PC; Ka, absorption rate; KT4, elimination rate of T4, KTSH, elimination rate of TSH, KT3, elimination rate of T3; fr, fraction of T4 peripherally converted to T3; NF1-NF3, slope factor constants 1–3 used in the PD model. Unlabeled lines in (C) represent influence of partition coefficients of remaining tissues.
Exposure level estimations and MOE
Environmental concentrations were found in literature for TCS, BP2, MBT, and RSC. For TCS, reported environmental concentrations ranged from 0.01 mg/kg/d (Allmyr et al., 2009) to 0.08 mg/kg/d (Queckenberg et al., 2010). For BP2, concentrations were found ranging from 0.0016 to 0.00492 mg/kg/d in food, water, and packaging (IARC, 2012). For MBT, concentrations were found ranging from 3.3e−4 to 0.00418 mg/kg/d in metal working agents or preservatives (USEPA, 1994). For RSC, concentrations were estimated to range from 0.1 to 0.8 mg/kg/d in those who consumed shrimp (EFSA, 2010).
In addition to literature-reported estimates, HT in silico models were used to predict distributions of exposure levels. As both maternal and fetal effects of thyroid hormone-perturbing chemicals are often of concern (Gilbert et al., 2012), our analysis focused on females of reproductive age. Exposure level estimates (mg/kg/d) at the 50th and 95th percentiles for the 4 environmental chemicals (MBT, RSC, BP2, and TCS) for females of reproductive age were predicted using the mechanistic High-Throughput Stochastic Human Exposure and Dose Simulation (SHEDS-HT) model (Isaacs et al., 2014). SHEDS-HT was used to simulate exposures for 100 000 simulated individuals to the environmental chemicals based on reported weight fractions (Goldsmith et al., 2014) and population use patterns (Isaacs et al., 2014) for approximately 200 consumer product types. SHEDS-HT estimated exposure levels via dermal, inhalation, and oral routes, and the oral route included both ingestion and transfer of chemical from the hands to the mouth. Dermal removal processes, as well as absorption fractions for the dermal and inhalation routes were applied as described in Isaacs et al. (2014). Absorption fractions for the oral route were not modeled in SHEDS-HT; rather, the chemical-specific absorption fractions were obtained from Gastroplus after a 24-h simulation with SHEDS-HT oral intake estimates. Total absorbed dose (in mg/kg/d) was then calculated as the sum of the absorbed doses from all routes. Because SHEDS-HT model was designed for prioritizing chemicals in consumer products, it cannot be used to predict exposure levels for the 2 drugs, PTU and MMI. Thus, population medians (mg/kg/d) of PTU and MMI for females of reproductive age were obtained from a published study that predicted distributions of human exposure levels of 7968 chemicals using a HT, heuristic model with 5 descriptors, including 4 use categories (Wambaugh et al., 2014).
The MOE for each chemical was calculated as the ratio of the ED10 and the estimated exposure levels obtained from either literature or the HT exposure models. MOEs were then used to calculate the ‘distance’ between estimated exposure levels and oral equivalent doses likely to inhibit T3 or T4 levels by 10% due to TPO inhibition (the ED10). To estimate the ED10, physiological parameters in the PBPK model were set specifically for females of reproductive age with a BW of 70 kg (Table 3), and thyroid hormone parameters in the PD model were set for individuals with normal thyroid hormone concentrations (Table 1).
RESULTS
Total Clearance of Chemicals
Total clearance values for MMI, PTU, and TCS in rats (needed for model evaluation), and total clearance values for all 6 chemicals in humans, are presented in Tables 4 and 5. TCS clearance in rats (4.38 ml/min) was about 10 times greater than the clearance for MMI (0.503 ml/min) and PTU (0.345 ml/min). PTU was estimated to have the largest clearance rate in humans (270 ml/min), followed by RSC (138.8 ml/min) and MMI (80.85 ml/min). The clearance rates for TCS (48.3 ml/min) and MBT (25.5 ml/min) were moderate, and BP2 had the slowest clearance rate of the 6 chemicals (15.8 ml/min) in humans.
TABLE 4.
Chemical-Specific Parameters for the Rat PBPK Model
|
Chemicala |
||||
|---|---|---|---|---|
| Parameter | MMI | PTU | TCS | Source |
| IC50 (μM) | 0.025 | 0.12 | 142 | Measured, Paul et al. (2014) |
| IMax (% activity) | 0.9084 | 0.9426 | 0.511 | Measured, Paul et al. (2014) |
| Clearance (ml/min) | 0.5025 | 0.345 | 4.38 | Measured, See text |
| Absorption rate constant (ka; (1/min) | 0.0252 | 0.018 | 0.0288 | Predicted, GastroPlus |
| Tissue to Plasma PCs | ||||
| Kidney (P_Kid) | 0.81 | 0.41 | 7.48 | Predicted, GastroPlus |
| Liver (P_Liv) | 0.75 | 0.36 | 8.20 | Predicted, GastroPlus |
| Rest of body (P_Rap)- for lung | 0.82 | 0.48 | 9.39 | Predicted, GastroPlus |
| Muscle (P_Mus) | 0.77 | 0.33 | 4.55 | Predicted, GastroPlus |
| Skin (P_Skin) | 0.69 | 0.48 | 10.56 | Predicted, GastroPlus |
| Yellow marrow (P_Slow) | 0.16 | 0.20 | 59.61 | Predicted, GastroPlus |
| Adipose (P_Fat) | 0.16 | 0.20 | 59.61 | Predicted, GastroPlus |
| Brain (P_Brain) | 0.87 | 0.45 | 19.70 | Predicted, GastroPlus |
| Thyroid (P_Thyroid) | 2.80 | 2.21 | 7.48 | Predicted, GastroPlus or fittedb |
aChemical abbreviations: MMI, methimazole; PTU, 6-propylthiouracil; TCS, triclosan.
bThe thyroid to plasma PC for TCS was set to that of kidney to plasma PC with the observation that these values are similar (Merrill et al. 2005). For MMI and PTU, this value was fitted in order to simulate the higher concentration of chemical in tissue compared with plasma, as shown by measured data.
TABLE 5.
Chemical-Specific Parameters for the Human PBPK Model
|
Chemicala |
|||||||
|---|---|---|---|---|---|---|---|
| Parameter | MMI | PTU | BP2 | MBT | TCS | RSC | Source |
| IC50 (μM) | 0.025 | 0.12 | 0.16 | 0.45 | 142 | 253 | Measured, Paul et al. (2014) |
| IMax (% activity) | 0.9084 | 0.9426 | 0.9059 | 0.9684 | 0.511 | 0.229 | Measured, Paul et al. (2014) |
| Clearance (ml/min) | 80.9 | 270.0 | 15.8 | 25.5 | 48.3 | 139.1 | Measured and predictedb |
| Absorption rate constant (ka; 1/min) | 0.0087 | 0.0078 | 0.009 | 0.012 | 0.0438 | 0.0096 | Predicted, GastroPlus |
| Tissue to Plasma PCs | |||||||
| Kidney (P_Kid) | 0.81 | 0.42 | 0.20 | 0.88 | 3.98 | 0.82 | Predicted, GastroPlus |
| Liver (P_Liv) | 0.80 | 0.42 | 0.18 | 1.28 | 6.55 | 0.88 | Predicted, GastroPlus |
| Rest of body (P_Rap)- for lung | 0.82 | 0.43 | 0.23 | 0.41 | 0.91 | 0.76 | Predicted, GastroPlus |
| Muscle (P_Mus) | 0.78 | 0.36 | 0.13 | 0.83 | 4.02 | 0.79 | Predicted, GastroPlus |
| Skin (P_Skin) | 0.75 | 0.51 | 0.35 | 1.14 | 4.99 | 0.81 | Predicted, GastroPlus |
| Yellow marrow (P_Slow) | 0.21 | 0.21 | 0.18 | 1.79 | 23.85 | 0.37 | Predicted, GastroPlus |
| Adipose (P_Fat) | 0.21 | 0.21 | 0.18 | 1.79 | 23.85 | 0.37 | Predicted, GastroPlus |
| Brain (P_Brain) | 0.87 | 0.47 | 0.20 | 1.92 | 10.48 | 1.03 | Predicted, GastroPlus |
| Thyroid (P_Thyroid) | 2.80 | 2.21 | 0.20 | 0.88 | 3.98 | 0.82 | Predicted, GastroPlus or fittedc |
aChemical abbreviations: MMI, methimazole; PTU, 6-propylthiouracil; BP2, benzophenone-2; MBT, 2-mercatobenzothiazole; TCS, triclosan; RSC, resorcinol.
bTotal clearance was taken for each chemical as a function of t1/2 and Vd. Clearance for MMI, PTU, and TCS used measured values for t1/2 and Vd. Clearance for BP2, RSC, and MBT used predicted Vd from ADMET Predictor software and t1/2 predicted using the equation from Sarver et al. (1997). See text for additional details.
cThe thyroid to plasma PC was set to that of kidney to plasma PC with the observation that these values are similar (Merrill et al., 2005). For MMI and PTU, this value was adjusted to that in the rat used to fit measured data showing higher concentrations of the drugs in the thyroid compared with within blood.
Although PK data are readily available for drugs such as PTU and MMI, it is often lacking for environmental chemicals. A review of literature and technical documents failed to reveal information pertaining to human t1/2, Vd, or clearance values for BP2, MBT, and RSC. In a prior unpublished analysis not related to this study, measured t1/2 values of 670 chemicals were compared against Sarver-predicted values (Equation 3), and the Sarver equation was most appropriate between log P values of −4 and 4 (70% within a factor of 5). The log P values of BP2 and MBT were approximately 2.3, while the log P value of RCS was approxiamately 1. Although an ideal scenario remains the use of known clearance measurements for chemicals of interest when available, these Sarver-predicted half-lives were within 2-fold difference from those values measured in rats (Garcia, 2004; Jeon et al., 2008; Merker et al., 1982).
Model Evaluation in Rats
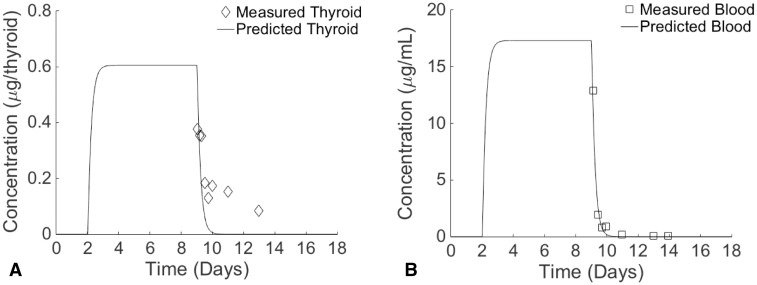
For both PTU and MMI, the thyroid lumen-to-blood PC required adjustment from a value similar to kidney-to-blood PC to a value above unity (Table 4) in order to fit data (Cooper et al., 1983, 1984b) that showed higher concentrations in the rat thyroid gland than in blood (Figs. 3a and 4a). These 2 drugs exhibit a longer residence time in the thyroid gland than in blood (Marchant et al., 1972). Both drugs were shown to leave the blood immediately after dosing in the rat model, and this behavior agrees with measured data (Figs. 3b and 4b). In contrast, measured data suggest that both chemicals remain in the thyroid compartment for a longer period of time compared with model simulations (Figs. 3a and 4a). Both MMI and PTU are drugs likely to remain in the thyroid for a duration sufficient to exert their therapeutic effects (Cooper, 2005). This persistence is possibly due to binding or other mechanisms not described in the present model, in which the thyroid gland represents a generic perfusion-limited compartment (Skellern et al., 1980; Wartofsky and Ingbar, 1971) that is generalizable for a wide variety of chemicals rather than for MMI or PTU specifically.
FIG. 3.
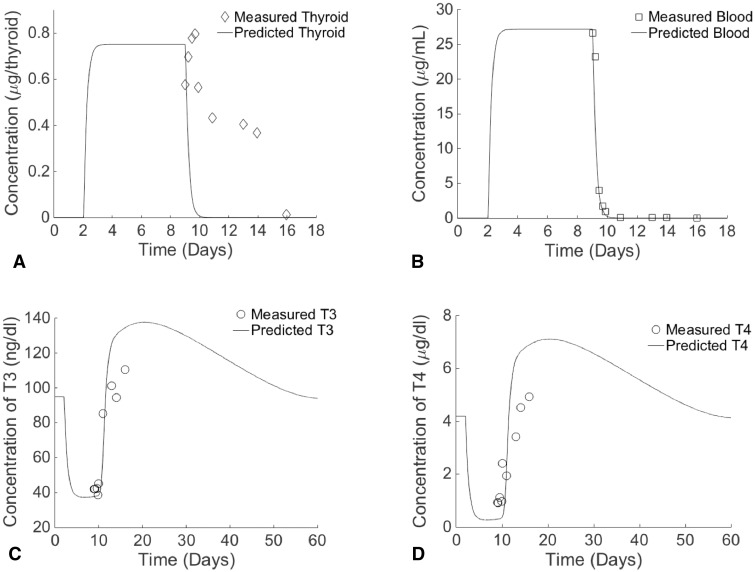
Time-course measured and predicted concentrations of 6-PTU in the thyroid gland tissue (A) and blood (B) of rats provided 0.05% PTU in drinking water for 7 days and PTU-induced changes in predicted and measured rat T3 (C) and T4 (D) serum concentrations. Measured data taken from Cooper et al. (1983).
FIG. 4.
Time-course measured and predicted concentrations of MMI in the thyroid gland tissue (A) and blood (B) of rats provided 0.05% MMI in drinking water for 7 days. Measured data taken from Cooper et al. (1984b).
Both PTU and MMI were predicted to reach peak blood concentrations 65 h after dosing, peak thyroid concentrations 70 h after dosing, and then to remain steady until stopping dosage after 7 days. These peak concentrations were 27.2 μg/ml for serum PTU and 0.751 μg/tissue for thyroid PTU, and were 17.3 μg/ml for serum MMI and 0.606 μg/tissue for thyroid MMI. The measured peak concentration of PTU in blood (26.65 μg/ml) was observed immediately after stoppage of the daily 7-day doses (Fig. 3b), while the peak measured concentration in the thyroid gland (0.77 μg/tissue) was observed 17 h after stopping dosage (Fig. 3a). There was a 2 and 26% difference between measured and predicted concentrations of PTU in the blood and thyroid gland, respectively, at the first measured time point. For MMI, the first measured time point occurred 0.6 h after stoppage of the daily 7-day doses, and at this time, concentrations were at their peak in the blood (12.9 μg/ml; Fig. 4b) and in the thyroid gland (0.376 μg/tissue; Fig. 4a). At this time point, predicted blood concentrations had dropped to 11.91 μg/ml, resulting in a percent difference between predicted and measured values of 8%. The percent difference between measured and predicted thyroid concentrations at this time point was 41.1%.
At the end of the 7-day simulated dosing with PTU (on days 6 and 7), predicted levels of both T3 (Fig. 3c) and T4 (Fig. 3d) were at their minimum. Two days after the final dosing, both hormones rose above their baseline levels, and both hormones reached their maximum levels approximately 2 weeks after stopping dosage. Seven weeks after stopping dosage, all hormones had returned to their baseline levels. Hormonal changes induced by simulated MMI exposure also resulted in similar trends, though it only required 6 weeks to return to baseline levels (data not shown). Although the PBPK model predicted a more rapid disappearance of MMI and PTU from the thyroid compartment, the accompanying PD model was able to predict hormone concentrations in blood that agreed with measured T3 (Fig. 3c) and T4 (Fig. 3d) data observed in recovering rats after dosing with PTU for 7 days (Cooper et al., 1983).
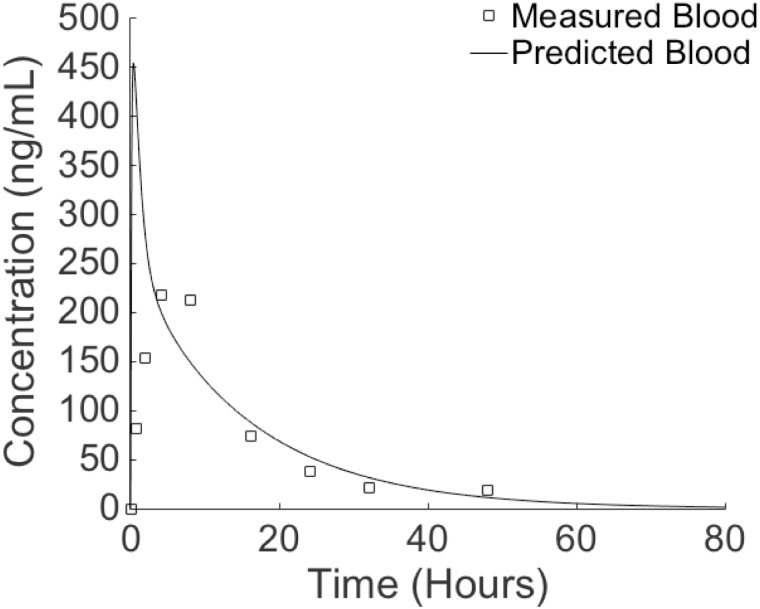
Predicted blood concentrations of TSC in rats reached their peak (454 ng/ml) just 24 min after dosing and then began to decline (Fig. 5). Measured serum concentrations of TCS reached their peak 4 h after dosing. The predicted blood TCS concentration was 5 times higher than the measured concentration at the first measured time point of 43 min (421 vs 81 ng/ml). All other predicted values fell within a 2-fold difference of measured data and appeared to have no systematic errors. All predicted hormonal concentrations at this single oral dosage changed <1% (data not shown). The overall agreement between predicted and measured TCS concentrations suggests that the estimated clearance for TCS is reasonable.
FIG. 5.
Time-course measured and predicted concentrations of TCS in the blood of rats provided a single oral dose of 5 mg/kg in 1 ml corn oil. Measured data taken from Wu et al. (2009).
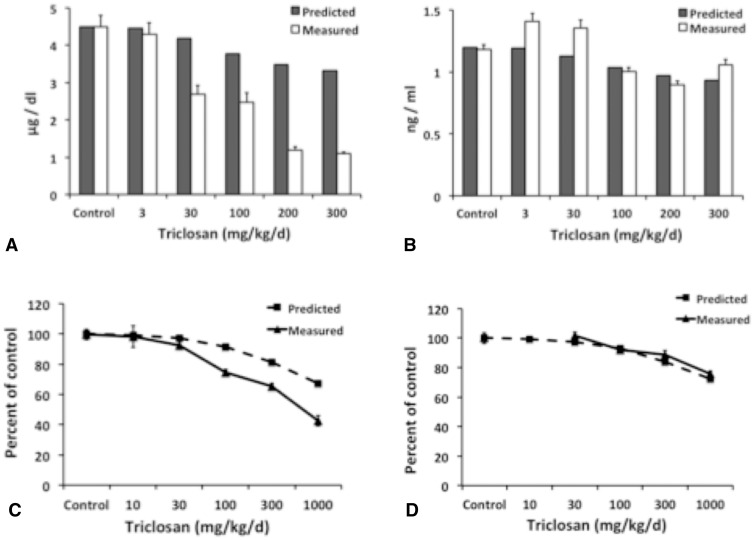
Predicted levels of T4 were consistent with results observed by Zorrilla et al. (2009) and Paul et al. (2010) at lower doses (Figs. 6a and c). Predicted levels of T4, however, were higher than measured values at doses above 30 mg/kg/d. These discrepancies were 2–3 times greater than measured levels for Wistrar rats (Fig. 6a), and were 1.5 times greater than measured data for Long-Evans rats (Fig. 6c). As expected, the smaller decrease in predicted T4 levels seen at higher doses likely reflects influence from only TPO inhibition in our model, whereas the more pronounced decline in measured data is due to thyroid disruption in general. For example, TCS is thought to result in increased metabolism (eg, sulfation and glucuronidation) of thyroid hormones (Paul et al., 2010). In contrast, there was little difference between predicted and measured levels of T3 (Figs. 6b and d).
FIG. 6.
Measured and predicted changes in T4 (A) and T3 (B) levels in the serum of male Wistrar rats after 31 days of dosing, and percent change from the control for T4 (C) and T3 (D) in the serum of female Long-Evans rats after 4 days of dosing. Measured data taken from Zorrilla et al. (2009) and Paul et al. (2010). Error bars of measured data represent SE.
Model Evaluation in Humans
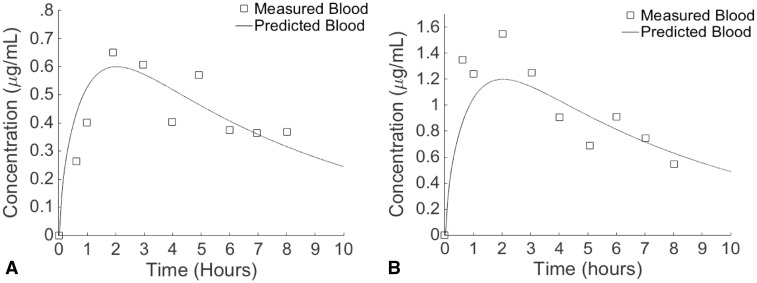
The modified thyroid lumen-to-blood PCs in rats were used in the human model when simulating exposures to MMI and PTU (Table 5). Predicted and measured serum concentrations of MMI after a single oral 30 or 60 mg dose in humans both reached their peaks 2 h after dosing and then declined (Figs. 7a and b). At this time, predicted and measured MMI concentrations at the 30 mg dose were nearly identical (0.60 and 0.651 μg/ml, respectively), while the predicted value at the 60 mg dose (0.773 μg/ml) was 1.29 times less than the measured value (1.20 μg/ml). At both doses, all predicted values fell within a 2-fold difference of measured values and tended to overpredict for 44% of the 9 time points at the 30 mg dose and for 67% of the time points at the 60 mg dose. Hormones returned to their baseline levels 52 days after dosing, after surpassing these levels 21 days after dosing (data not shown). Based upon the agreement between predicted and measured serum concentrations in humans and rats, as well as thyroid hormone levels in rats, the combined PBPK/PD model is appropriate for estimating oral doses of chemicals that can lead to thyroid concentrations capable of inhibiting TPO.
FIG. 7.
Time-course measured and predicted concentrations of MMI in the blood of humans provided a single oral dose of 30 mg (A) or 60 mg (B). Measured data taken from Cooper et al. (1984a).
MOE for TPO Inhibitors
The IC50 values obtained from the HT in vitro study for the 6 selected chemicals ranged from 0.025 to 253 μmol/l, while IMax ranged from 23 to 97% inhibition (Table 5). Despite the high potencies of MMI and PTU, their exposure estimates are negligible at the HT in silico model predicted median, leading to MOE estimates above 1000 (Table 6). The predicted exposure levels for MBT were the lowest among the 4 environmental chemicals, but MBT was of medium concern at the 95th percentile exposure level due to its estimated clearance rate, which was second lowest among the 6 chemicals. Literature-reported environmental concentrations of MBT (USEPA, 1994), however, were at least 2 orders of magnitude greater than the predicted distribution of exposure levels, leading to MOE estimates <50 (Tables 6 and 7) and suggesting that MBT is of greater concern than indicated using predicted exposure levels.
TABLE 6.
MOE Based on the Target-Tissue Concentrations of TPO Inhibiting Compoundsa Expected to Result in a 10% Decline in Circulating T4 and T3 Levels Compared with Predicted Environmental Exposures at the 50th Percentile of the Population and Low End Literature-Reported Values
| Chemical | IC50 | T3 ED10 (mg/kg/d) | 50th percentile (mg/kg/d) | MOE | Measured (mg/kg/d)b | MOE |
| TCS | 142 | 4.19 | 1.98E-02 | 211 | 1.00E-02 | 419 |
| MMI | 0.025 | 2.86E-04 | 4.18E-08 | 6840 | N/A | Not calculated |
| PTU | 0.12 | 1.38E-02 | 1.31E-07 | 105,000 | N/A | Not calculated |
| RSC | 253 | 130 | 2.60E-06 | 50 000 000 | 1.00E-01 | 1300 |
| BP2 | 0.16 | 1.09E-02 | Below detection | Not calculated | 1.60E-03 | 7 |
| MBT | 0.45 | 7.06E-03 | Below detection | Not calculated | 3.30E-04 | 21 |
| Chemical | IC50 | T4 ED10 (mg/kg/d) | 50th percentile (mg/kg/d) | MOE | Measured (mg/kg/d)b | MOE |
| TCS | 142 | 4.12 | 1.98E-02 | 209 | 1.00E-02 | 412 |
| MMI | 0.025 | 2.84E-04 | 4.18E-08 | 6790 | N/A | Not calculated |
| PTU | 0.12 | 8.24E-03 | 1.31E-07 | 62 900 | N/A | Not calculated |
| RSC | 253 | 128.17 | 2.60E-06 | 49 300 000 | 1.00E-01 | 1282 |
| BP2 | 0.16 | 1.08E-02 | Below detection | Not calculated | 1.60E-03 | 7 |
| MBT | 0.45 | 7.01E-03 | Below detection | Not calculated | 3.30E-04 | 21 |
aChemical abbreviations: MMI, methimazole; PTU, 6-propylthiouracil; BP2, benzophenone-2; MBT, 2-mercatobenzothiazole; TCS, triclosan; RSC, resorcinol.
bNo measured concentrations could be obtained from the literature for MMI and PTU. See text for references for the measured exposure levels of the 4 environmental chemicals.
TABLE 7.
MOE Based on the Target-Tissue Concentrations of TPO Inhibiting Compoundsa Expected to Result in a 10% Decline in Circulating T4 and T3 Levels Compared with Predicted Environmental Exposures at the 95th Percentile of the Population and High End Literature-Reported Values
| Chemical | IC50 | T3ED10 (mg/kg/d) | 95th percentile (mg/kg/d) | MOE | Measured (mg/kg/d) | MOE |
| BP2 | 0.16 | 1.09E-02 | 5.38E-03 | 2 | 4.92E-03 | 2 |
| TCS | 142 | 4.19 | 9.61E-02 | 42 | 8.40E-02 | 50 |
| MBT | 0.45 | 7.06E-03 | 4.74E-06 | 1496 | 4.18E-03 | 2 |
| RSC | 253 | 130 | 1.15E-02 | 11 607 | 8.00E-01 | 163 |
| Chemical | IC50 | T4ED10 (mg/kg/d) | 95th percentile (mg/kg/d) | MOE | Measured (mg/kg/d) | MOE |
| BP2 | 0.16 | 1.08E-02 | 5.38E-03 | 2 | 4.92E-03 | 2 |
| TCS | 142 | 4.12 | 9.61E-02 | 42 | 8.40E-02 | 49 |
| MBT | 0.45 | 7.01E-03 | 4.74E-06 | 1485 | 4.18E-03 | 2 |
| RSC | 253 | 128.17 | 1.15E-02 | 11 443 | 8.00E-01 | 160 |
aChemical abbreviations: BP2, benzophenone-2; MBT, 2-mercatobenzothiazole; TCS, triclosan; RSC, resorcinol. No predictions at this percentile were available for the 2 drugs MMI or 6-PTU.
bNo measured concentrations could be obtained from the literature for MMI and PTU, and predictions were not available at the 95th percentile for these 2 drugs. See text for references for the measured exposure levels of the 4 environmental chemicals.
Model-predicted exposure levels for BP2, RSC, and TCS at the 95th percentile ranged from 0.01 to 0.1 mg/kg/d, or about 5 orders of magnitude greater than those of the other 3 chemicals (Table 7). TCS was also the only chemical that had an appreciable exposure level predicted at the 50th percentile (0.02 mg/kg/d). Thus, despite its low potency, the high predicted exposure levels of TCS resulted in a MOE of approximately 200 at the predicted population exposure level at the 50th percentile (Table 6) and a MOE below 50 at the 95th percentile (Table 7). In addition, the MOE for TCS remained below 50 at the high end of the literature-reported exposure level (Table 7) and increased to 400 at the low end of the literature-reported exposure level (Table 6).
Both the high predicted exposure level at the 95th percentile and slow clearance rate of BP2 resulted in it having a MOE below 10, which is similar to that using literature-reported exposure levels (Table 7). The predicted exposure level at the 50th percentile, however, is much lower than literature-reported values, making the MOE at this predicted exposure level highly uncertain (Table 6). For RSC, predicted exposure levels at both 50th and 95th percentiles were much lower than literature-reported values. MOE estimates fall from above 1000 using predicted exposure levels to below 1000 at the lowest literature-reported concentration (Table 6) and below 100 at the highest literature-reported concentration (Table 7). This finding suggests that RSC also might be of greater concern than indicated using predicted exposure levels.
DISCUSSION
In humans, thyroid binding globulin is abundant and has a high affinity for thyroid hormones, while this serum protein is absent in rats (Oppenheimer, 1968; Refetoff, 1970). This species difference should be considered when thyroid hormone kinetics and diffusion to tissues are investigated. However, in our analyses, total hormone levels, including both free and bound, in blood were the endpoints of interest. Therefore, differences in binding between species should not affect the predictions of hormone levels, or subsequently, the estimates of MOEs. In addition, the agreement observed between measured and predicted blood concentrations of PTU, MMI, and TCS in rats, and of MMI in humans, also suggests that exclusion of species-dependent plasma protein binding mechanisms in our PBPK model is nonproblematic.
The 2 most potent (ie, lowest IC50 values) chemicals within this study, MMI and PTU, are drugs used to treat mild to severe thyrotoxicosis (Carroll and Matfin, 2010). Within the thyroid compartment itself, MMI is generally irreversibly bound to the TPO enzyme (Cooper, 2005). In contrast, PTU exerts its efficacy through its structural similarity to the iodide substrate and so easily and reversibly binds to the TPO enzyme. There are likely additional mechanisms at work that are influencing the accumulation of these 2 drugs in the thyroid gland, such as the influence of the sodium/iodide basolateral symporter protein (Pesce and Kopp, 2014). Without data, however, to support the incorporation of these other mechanisms into our PBPK model, a simpler adjustment of the thyroid lumen-to-blood PC was a reasonable approach to simulate the disposition of these 2 drugs. In MOE estimation for MMI and PTU, their high potencies were countered by low exposure level predictions (Wambaugh et al., 2014). It is reasonable to assume that exposure to these 2 drugs occurs primarily to individuals taking the medications and that little enters into the environment. Therefore, even with the uncertainties in estimating thyroid concentrations of these 2 drugs, there is low concern of TPO inhibition from exposures to these 2 drugs in the environment.
TCS was originally used as a negative control in the HT in vitro study (Paul et al., 2014) because of previously reported negative TPO activity (Crofton et al., 2007; Paul et al., 2013). In contrast, Paul et al. (2014) concluded that TCS may inhibit TPO weakly, evident with the enhanced sensitivity of the Amplex UltraRed-based assay. Although it can be debated whether TCS does in fact inhibit the TPO enzyme, this chemical’s thyroid-disrupting properties cannot be argued (Crofton et al., 2007; Paul et al., 2010; Stoker et al., 2010). Due to its high exposure potential (Thompson et al., 2005), TCS appears to be of high concern based on its MOE, even though its very low potency leads to the second lowest ED10 among the 6 selected chemicals. This result by no means suggests that TCS should be prioritized as a TPO inhibitor. However, given the knowledge of TCS as a thyroid-disrupting chemical in general and its abundance in consumer products, further studies may be warranted in order to ascertain its TPO-inhibiting potential.
The uncertainty associated with the estimated MOE values is inversely proportional to estimated exposure concentrations and directly proportional to the ED10. The large discrepancies between literature-reported and model-predicted exposure levels and resultant MOE estimates for the 4 environmental chemicals illustrate the large uncertainty inherent in both the heuristics-based model (Wambaugh et al., 2014) and the SHEDS-HT. These models were developed for exposure-based prioritization of chemicals, and thus, by design these models take limited amounts of data (eg, chemical or product use descriptors) to rapidly produce exposure estimates with large margins of error. For example, SHEDS-HT was designed to be conservative with respect to several model parameters, such as the number of product types in which chemicals are found, chemical concentrations in various formulations, and differential consumer product use patterns. However, even with large uncertainties, these models can provide useful metrics for discriminating among chemicals when environmental data are lacking. The MOE values presented here do not represent a rank-ordering of risk per se, but rather should be used to separate chemicals into bins of low, medium, and high concerns. For example, a MOE of 1000 or 10 000 will both be considered as low concern; while a MOE of 2 or 100 will both be considered as high concern. Improving these HT exposure models and reducing the uncertainty in their predictions to support the development of risk-based metrics is an area of ongoing research. Results from published studies often reflect exposure to those groups most highly exposed (eg, pesticide applicators), which can further explain the large differences between predicted and literature-reported exposure values. It should be noted that measured exposure levels also hold a certain degree of uncertainty due to the difficulty in measuring external exposure concentrations from all sources (eg, food, water, product use). Such uncertainty in exposure concentration is likely more challenging to characterize than the uncertainty associated with PK and PD parameters.
The other source of uncertainty in MOE calculations lies in the estimate of clearance rates, as the ED10 is partly a function of the clearance rate. The clearance rates for MMI, PTU, and TCS were determined using literature-reported half-lives, so these estimates should be reasonably accurate. Using the half-lives of BP2, MBT, and RSC estimated in rats (Garcia, 2004; Jeon et al., 2008; Merker et al., 1982) to compare with the use of half-lives predicted from the Sarver equation (Equation 3), it was found that MOE estimates are much more robust to uncertainty in clearance, compared with uncertainty in exposure estimates. For example, replacing the Sarver-predicted t1/2 value with the rat value (Jeon et al., 2008) for BP2 resulted in a slight decrease in clearance rate from 15 to 10 ml/min, and the MOE estimate continues to indicate high concern (below 10). The rat t1/2 of MBT (Garcia, 2004) is approximately 2 times lower than the Sarver-predicted value, and predicted estimates of clearance are more conservative (25 vs 60 ml/min). Despite this difference, the MOE estimate for MBT remains above 1000 and continues to indicate low concern. In contrast to MBT, the rat t1/2 of RSC (Merker et al., 1982) is approximately 2 times lower than the Sarver-predicted value, and predicted estimates are less conservative (139 vs 65 ml/min). RSC continues to exhibit the highest clearance rate among the selected chemicals, despite this apparent fall in rate, and the MOE estimates continue to remain very high (>5000), indicating the very low concern for this chemical.
Coupling HT in vitro toxicity data with appropriately parameterized computational models that quantitatively link exposure to internal tissue dose-response can generate regulatory-relevant predictions of biological responses and adverse outcomes for reducing uncertainties in risk assessment. When multiple computational models are used in concert to derive values that allow comparisons among chemicals, such as MOE estimates, confidence in the results is limited by the weakest model in the series. When chemical-specific data on exposure levels and ADME characteristics are available, more reliable MOE estimates may be achieved. Such an approach is limited; however, when data for calibrating or evaluating predictive models is missing or when high degrees of uncertainties are present in toxicity data or exposure/dosimetry predictions. Although the framework presented in this study may be used as a screening tool to prioritize chemicals in a HT manner, our recommendation is to use this MOE approach primarily when some chemical-specific data is available to evaluate predictions of exposure potentials, hazard potentials, and ADME behaviors. Incorporating the relationship between environmental exposure and target tissue dose, through consideration of related ADME properties and within the context of the biological events described by the AOP of concern, offers the potential for reducing uncertainty in chemical prioritization and risk assessment.
CONCLUSIONS
This study utilizes known biological and chemical-specific information to design and implement a combined PBPK/PD model to examine a chemical’s potential for TPO inhibition. Such an effort will aid decision makers in their prioritization of chemicals screened in a higher throughput manner. Integration of hazard data from HT in vitro tests, HT exposure predictions or literature-reported levels, and ADME-related considerations can aid in the screening of chemicals of concern, as demonstrated from this study using chemicals with varying degrees of TPO inhibiting activity, exposure levels, and ADME properties. There is clear evidence that simply assessing the health risk of a chemical by its potency, as determined solely through in vitro testing, may be misleading and that both exposure and consideration of ADME-related properties to determine internal dosimetry would reduce uncertainty and increase confidence in the chemical screening process.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
Disclaimer
This article has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
FUNDING
Jeremy Leonard was funded through the Oak Ridge Institute for Science and Education Research Participation Program at the U.S. EPA.
Supplementary Material
REFERENCES
- Allmyr M., Panagiotidis G., Sparve E., Diczfalusy U., Sandborgh-Englund G. (2009). Human exposure to triclosan via toothpaste does not change CYP3a4 activity or plasma concentrations of thyroid hormones. Basic Clin. Pharmacol. Toxicol. 105, 339–344. [DOI] [PubMed] [Google Scholar]
- Ankley G. T., Bennett R. S., Erickson R. J., Hoff D. J., Hornung M. W., Johnson R. D., Mount D. R., Nichols J. W., Russom C. L., Schmieder P. K, et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. SETAC 29, 730–741. [DOI] [PubMed] [Google Scholar]
- Brown R. P., Delp M. D., Lindstedt S. L., Rhomberg L. R., Beliles R. P. (1997). Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 13, 407–484. [DOI] [PubMed] [Google Scholar]
- Carroll R., Matfin G. (2010). Endocrine and metabolic emergencies: thyroid storm. Ther. Adv. Endocrinol. Metab. 1, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi N. Y., Jahnke G. D., St Hilaire C., Shelby M. (2003). Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res. B Dev. Reprod. Toxicol. 68, 479–491. [DOI] [PubMed] [Google Scholar]
- Cooper D. S. (2005). Antithyroid Drugs. N. Engl. J. Med 352, 905–917. [DOI] [PubMed] [Google Scholar]
- Cooper D. S., Bode H. H., Nath B., Saxe V., Maloof F., Ridgway E. C. (1984a). Methimazole pharmacology in man: studies using a newly developed radioimmunoassay for methimazole. J. Clin. Endocrinol. Metab 58, 473–479. [DOI] [PubMed] [Google Scholar]
- Cooper D. S., Kieffer J. D., Halpern R., Saxe V., Mover H., Maloof F., Ridgway E. C. (1983). Propylthiouracil (PTU) pharmacology in the rat. II. Effects of PTU on thyroid function. Endocrinology 113, 921–928. [DOI] [PubMed] [Google Scholar]
- Cooper D. S., Kieffer J. D., Saxe V., Mover H., Maloof F., Ridgway E. C. (1984b). Methimazole pharmacology in the rat: studies using a newly developed radioimmunoassay for methimazole. Endocrinology 114, 786–793. [DOI] [PubMed] [Google Scholar]
- Crofton K. M., Craft E. S., Hedge J. M., Gennings C., Simmons J. E., Carchman R. A., Carter W. H., DeVito M. J. (2005). Thyroid-Hormone–Disrupting Chemicals: Evidence for dose-dependent additivity or synergism. Environ. Health Perspect. 113, 1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton K. M., Paul K. B., DeVito M. J., Hedge J. M. (2007). Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol. 24, 194–197. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Tan Y. M., Villeneuve D. L., Meek M. E., Bette, McQueen C. A. (2015). Adverse outcome pathways – Organizing toxicological information to improve decision making. J. Pharmacol. Exp. Ther. Accessed November 4, 2015. doi:10.1124/jpet.115.228239. [DOI] [PubMed] [Google Scholar]
- Ekerot P., Ferguson D., Glämsta E. L., Nilsson L. B., Andersson H., Rosqvist S., Visser S. A. G. (2013). Systems pharmacology modeling of drug-induced modulation of thyroid hormones in dogs and translation to human. Pharm. Res. 30, 1513–1524. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA). (2010). Scientific opinion on the use of resorcinol as a food additive. EFSA J. 8, 41. [Google Scholar]
- Garcia H. (2004). Spacecraft Water Exposure Guidelines for Selected Contaminants: Volume 1, Appendix 5: 2-Mercaptobenzothiazole. National Academies Press, Washington, DC: Accessed December 3, 2015. [Google Scholar]
- Gilbert M. E., Rovet J., Chen Z., Koibuchi N. (2012). Developmental thyroid hormone disruption: Prevalence, environmental contaminants and neurodevelopmental consequences. NeuroToxicology 33, 842–852. [DOI] [PubMed] [Google Scholar]
- Giles H. G., Long J. P., Orrego H., Sellers E. M. (1982). Mechanism of alterations in propylthiouracil disposition after long-term therapy. Clin. Pharmacol. Ther. 31, 559–563. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. R., Grulke C. M., Brooks R. D., Transue T. R., Tan Y. M., Frame A., Egeghy P. P., Edwards R., Chang D. T., Tornero-Velez R, et al. (2014). Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem. Toxicol. 65, 269–279. [DOI] [PubMed] [Google Scholar]
- Groh K. J., Carvalho R. N., Chipman J. K., Denslow N. D., Halder M., Murphy C. A., Roelofs D., Rolaki A., Schirmer K., Watanabe K. H. (2015). Development and application of the Adverse Outcome Pathway framework for understanding and predicting chronic toxicity: I. Challenges and research needs in ecotoxicology. Chemosphere 120, 764–777. [DOI] [PubMed] [Google Scholar]
- Hengstmann J. H., Hohn H. (1985). Pharmacokinetics of methimazole in humans. Klin. Wochenschr 63, 1212–1217. [DOI] [PubMed] [Google Scholar]
- Huffman L., Hedge G. A. (1986). Neuropeptide control of thyroid blood flow and hormone secretion in the rat. Life Sci. 39, 2143–2150. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). (2012). Some Chemicals Present in Industrial and Consumer Products, Food, and Drinking Water: Benzophenone. Volume 101 Available at: http://monographs.iarc.fr/ENG/Monographs/Vol15101/index.php. Accessed December 3, 2015.
- Isaacs K. K., Glen W. G., Egeghy P., Goldsmith M. R., Smith L., Vallero D., Brooks R., Grulke C. M., Özkaynak H. (2014). SHEDS-HT: an integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ. Sci. Technol. 48, 12750–12759. [DOI] [PubMed] [Google Scholar]
- Jameson J. L, Wheetman A. P. (2001). Disorders of the thyroid gland In Harrison’s Principles of Internal Medicine (Braunwald E., Fauci A. S., Kasper D. L., Hauser S. L., Longo D. L., Jameson J. L., Eds.), pp. 2048–2060. McGraw-Hill, New York, NY. [Google Scholar]
- Jeon H. K., Sarma S. N., Kim Y. J., Ryu J. C. (2008). Toxicokinetics and metabolism of benzophenone-type UV filters in rats. Toxicology 248, 89–95. [DOI] [PubMed] [Google Scholar]
- Judson R. S., Kavlock R. J., Setzer R. W., Hubal E. A. C., Martin M. T., Knudsen T. B., Houck K. A., Thomas R. S., Wetmore B. A., Dix D. J. (2011). Estimating toxicity-related biological pathway altering doses for high-throughput chemical risk assessment. Chem. Res. Toxicol. 24, 451–462. [DOI] [PubMed] [Google Scholar]
- Jugan M. L., Levi Y., Blondeau J. P. (2010). Endocrine disruptors and thyroid hormone physiology. Biochem. Pharmacol. 79, 939–947. [DOI] [PubMed] [Google Scholar]
- Kampmann J. P., Hansen J. M. (1981). Clinical pharmacokinetics of antithyroid drugs. Clin. Pharmacokinet. 6, 401–428. [DOI] [PubMed] [Google Scholar]
- Kampmann J., Skovsted L. (1974). The Pharmacokinetics of Propylthiouracil. Acta Pharmacol. Toxicol. (Copenh.) 35, 361–369. [DOI] [PubMed] [Google Scholar]
- Marchant B., Alexander W. D., Lazarus J. H., Lees J., Clark D. H. (1972). The acclumulation of 35 S-antithyroid drugs by the thyroid gland. J. Clin. Endocrinol. Metab. 34, 847–851. [DOI] [PubMed] [Google Scholar]
- McGuire R. A., Hays M. T. (1981). A kinetic model of human thyroid hormones and their conversion products. J. Clin. Endocrinol. Metab. 53, 852–862. [DOI] [PubMed] [Google Scholar]
- Merker P. C., Yeung D., Doughty D., Nacht S. (1982). Pharmacokinetics of resorcinol in the rat. Res. Commun. Chem. Pathol. Pharmacol. 38, 367–388. [PubMed] [Google Scholar]
- Merrill E. A., Clewell R. A., Robinson P. J., Jarabek A. M., Gearhart J. M., Sterner T. R., Fisher J. W. (2005). PBPK model for radioactive iodide and perchlorate kinetics and perchlorate-induced inhibition of iodide uptake in humans. Toxicol. Sci. 8, 25–43. [DOI] [PubMed] [Google Scholar]
- Nicoloff J. T., Low J. C., Dussault J. H., Fisher D. A. (1972). Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J. Clin. Invest. 51, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong A., Teeguarden J. G., Clewell H. J., Dorman D. C., Andersen M. E. (2008). Pharmacokinetic modeling of manganese in the rat IV: Assessing factors that contribute to brain accumulation during inhalation exposure. J. Toxicol. Environ. Health Part A 71, 413–426. [DOI] [PubMed] [Google Scholar]
- Odell W. D., Utiger R. D., Wilber J. F., Condliffe P. G. (1967). Estimation of the secretion rate of thyrotropin in man. J. Clin. Invest. 46, 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H. (1968). Role of plasma proteins in the binding, distribution and metabolism of the thyroid hormones. N. Engl. J. Med. 278, 1153–1162. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. (1972). Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J. Clin. Invest. 51, 2493–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K. B., Hedge J. M., DeVito M. J., Crofton K. M. (2010). Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young Long-Evans rats. Toxicol. Sci. 113, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K. B., Hedge J. M., Macherla C., Filer D. L., Burgess E., Simmons S. O., Crofton K. M., Hornung M. W. (2013). Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology 312, 97–107. [DOI] [PubMed] [Google Scholar]
- Paul K. B., Hedge J. M., Rotroff D. M., Hornung M. W., Crofton K. M., Simmons S. O. (2014). Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem. Res. Toxicol. 27, 387–399. [DOI] [PubMed] [Google Scholar]
- Pesce L., Kopp P. (2014). Iodide transport: implications for health and disease. Int. J. Pediatr. Endocrinol. 2014, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. B., Leonard J. A., Grulke C. M., Chang D., Edwards S. W., Brooks R., Goldsmith M. R., El-Masri H. A., Tan Y. M. (2016). A workflow to investigate exposure and pharmacokinetics influences on high-throughput in vitro chemical screening based on Adverse Outcome Pathways. Environ. Health Perspect. 124, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queckenberg C., Meins J., Wachall B., Doroshyenko O., Tomalik-Scharte D., Bastian B., Abdel-Tawab M., Fuhr U. (2010). Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob. Agents Chemother. 54, 570–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S., Robin N. I., Fang V. S. (1970). Parameters of thyroid function in serum of 16 selected vertebrate species: a study of PBI, serum T4, free T4, and the pattern of T4 and T3 binding to serum proteins. Endocrinology 86, 793–805. [DOI] [PubMed] [Google Scholar]
- Ridgway E. C., Weintraub B. D., Maloof F. (1974). Metabolic clearance and production rates of human thyrotropin. J. Clin. Invest. 53, 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers T., Leahy D., Rowland M. (2005). Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 94, 1259–1276. [DOI] [PubMed] [Google Scholar]
- Rodgers T., Rowland M. (2006). Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 95, 1238–1257. [DOI] [PubMed] [Google Scholar]
- Sandborgh-Englund G., Adolfsson-Erici M., Odham G., Ekstrand J. (2006). Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health A 69, 1861–1873. [DOI] [PubMed] [Google Scholar]
- Sarver J. G., White D., Erhardt P., Bachmann K. (1997). Estimating xenobiotic half-lives in humans from rat data: influence of log P. Environ. Health Perspect. 105, 1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Gordon M. B., Crantz F. R., Leonard J. L., Larsen P. R. (1984). Qualitative and quantitative differences in the pathways of extrathyroidal triiodothyronine generation between euthyroid and hypothyroid rats. J. Clin. Invest. 73, 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitar D. S., Thornhill D. P. (1973). Methimazole: Absorption, metabolism and excretion in the albino rat. J. Pharmacol. Exp. Ther. 184, 432–439. [PubMed] [Google Scholar]
- Skellern G. G., Knight B. I., Low C. K., Alexander W. D., McLarty D. G., Kalk W. J. (1980). The pharmacokinetics of methimazole after oral administration of carbimazole and methimazole, in hyperthyroid patients. Br. J. Clin. Pharmacol. 9, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker T. E., Gibson E. K., Zorrilla L. M. (2010). Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol. Sci. Off. J. Soc. Toxicol. 117, 45–53. [DOI] [PubMed] [Google Scholar]
- Thompson A., Griffin P., Stuetz R., Cartmell E. (2005). The fate and removal of triclosan during wastewater treatment. Water Environ. Res. 77, 63–67. [DOI] [PubMed] [Google Scholar]
- Tollefsen K. E., Scholz S., Cronin M. T., Edwards S. W., de Knecht J., Crofton K., Garcia-Reyero N., Hartung T., Worth A., Patlewicz G. (2014). Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA). Regul. Toxicol. Pharmacol. 70, 629–640. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA). (1994). Reregistration eligibility decision: Sodium and zinc salts of 2-mercaptobenzothiazole. Department of Prevention, Pesticides, and Toxic Substances. 738-R-94-027. p. 168. Available at: http://archive.epa.gov/pesticides/reregistration/web/pdf/2380.pdf. Accessed December 7, 2015.
- United States Environmental Protection Agency (USEPA). (2011). Exposure factors handbook. National Center for Environmental Assessment, Office of Research and Development, U. S. Environmental Protection Agency. EPA/600/R-090/052F. Available at: http://pbadupws.nrc.gov/docs/ML1400/ML14007A666.pdf. Accessed February 18, 2016.
- Wambaugh J. F., Wang A., Dionisio K. L., Frame A., Egeghy P., Judson R., Setzer R. W. (2014). High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ. Sci. Technol. 48, 12760–12767. [DOI] [PubMed] [Google Scholar]
- Wartofsky L., Ingbar S. H. (1971). Estimation of the rate of release of non-thyroxine iodine from the thyroid glands of normal subjects and patients with thyrotoxicosis. J. Clin. Endocrinol. Metab. 33, 488–500. [DOI] [PubMed] [Google Scholar]
- Wu J., Yue H., Cai Z. (2009). Investigation on metabolism and pharmacokinetics of triclosan in rat plasma by using UPLC-triple quadrupole MS. Chin. J. Chromatogr. 27, 724–730. [PubMed] [Google Scholar]
- Zoeller R. T., Tan S. W., Tyl R. W. (2007). General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit. Rev. Toxicol. 37, 11–53. [DOI] [PubMed] [Google Scholar]
- Zorrilla L. M., Gibson E. K., Jeffay S. C., Crofton K. M., Setzer W. R., Cooper R. L., Stoker T. E. (2009). The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol. Sci. 107, 56–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.