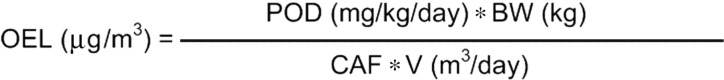Abstract
In the pharmaceutical industry, genotoxic drug substances are developed for life-threatening indications such as cancer. Healthy employees handle these substances during research, development, and manufacturing; therefore, safe handling of genotoxic substances is essential. When an adequate preclinical dataset is available, a risk-based decision related to exposure controls for manufacturing is made following a determination of safe health-based limits, such as an occupational exposure limit (OEL). OELs are calculated for substances based on a threshold dose-response once a threshold is identified. In this review, we present examples of genotoxic mechanisms where thresholds can be demonstrated and OELs can be calculated, including a holistic toxicity assessment. We also propose a novel approach for inhalation Threshold of Toxicological Concern (TTC) limit for genotoxic substances in cases where the database is not adequate to determine a threshold.
Keywords: occupational exposure limit, genotoxicity, threshold, acceptable risk, TTC
For decades, the risks of occupational exposure in pharmaceutical manufacturing have been successfully managed by evaluating pharmacological and toxicological data of individual drug substances (DS) to enable determination of data driven threshold levels. These thresholds are used in calculating toxicological health-based limits, such as occupational exposure limits (OEL) for DS, when an adequate dataset is available to derive such a limit (Galer et al., 1992; Sargent and Kirk, 1988). In the early stages of pharmaceutical development when only limited preclinical test results are available, these test results may not provide sufficient information for the calculation of health-based limits. In these cases, the occupational exposure controls are established using occupational exposure bands, based on the intrinsic hazard properties, the mechanism of action, and the predicted potency of the DS. The occupational exposure band determines predefined strategies which are known to provide the necessary degree of control to protect both employees and the environment (Naumann et al., 1996).
A qualified toxicologist proficient in the field of hazard assessment is needed to accurately collect and analyze all available physicochemical, preclinical, and human data in order to determine critical effect(s) for the calculation of an OEL. The critical effect is often that which occurs at the lowest dose (most sensitive adverse effect) if it is to be considered relevant to the target population (Naumann and Sargent, 2007; Sargent et al., 2013). For a DS with a narrow therapeutic index, one of the critical effects is often the minimal recommended therapeutic dose needed to achieve the intended pharmacological activity. Critical effects from preclinical studies are also considered in the evaluation. As the basic rule in occupational health, both intended pharmacology and unintended toxicity are considered adverse to the population of healthy employees (Bercu et al., 2016). Once critical effects are identified, the dose-response relationships of the individual critical effects are evaluated to determine points of departure (PODs) for calculation of the OELs (Nielsen and Øvrebø, 2008). The PODs represent the lowest dose at which the critical effect was observed and are based on the no-observed-(adverse-) effect level (NO(A)EL) or the low-observed-(adverse-) effect level (LO(A)EL) from preclinical, clinical, or epidemiologic data (Bercu et al., 2016). When assessing phase IIb, phase III, and marketed DS, the dataset is sufficient to select several PODs. They can be used to identify effect or toxicity yielding the most relevant or conservative OEL, after extrapolating and adjusting the data for the intended target population of healthy employees, who are potentially exposed by inhalation during manufacturing of DS. Adjustment factors (AFs) are applied to each selected POD to account for uncertainties and pharmacokinetic adjustments are made for inhalation exposure (Reichard et al., 2016). Frequently applied AFs are those accounting for interspecies variability, study duration, intraspecies variability (also referred to as interindividual variability), LOAEL-to-NOAEL conversion, the severity of effect, and database completeness (EMA, 2014; ICH, 1998; ISPE, 2010; Sargent et al., 2013; Sussman et al., 2016; U.S. EPA, 2002; WHO, 2005). Multiplication of all AFs, including the pharmacokinetic adjustments, generates composite adjustment factor (CAF). CAF represents a margin of safety (MOS) between the dose causing the critical effect and the safe dose for the employees (see Fig. 1 for calculation). The selection of AFs depends on the selected end point of the study from which the POD is determined. The most sensitive and reliable OEL value with relevance for healthy employees is selected as the basis for exposure controls.
FIG. 1.
The derivation of occupational exposure limit is based on the equation published by Galer et al. (1992), Naumann and Weideman (1995), Sargent and Kirk (1988), and Schwartz (1995). Body weight (BW) of a healthy worker is set at 70 kg and volume of air (V) that a healthy worker breathes in a normal working day is 10m3. CAF = Composite Adjustment Factor.
Preclinical and clinical data available for DS is not normally available for pharmaceutical intermediates (IMs) and therefore no OELs can be calculated for this class of compounds. IMs are materials produced during steps of the synthesis that undergo further chemical transformation to make a DS (ICH, 2006) and are generally not pharmacologically active (Faria et al., 2016).
INTERPRETATION OF GENOTOXIC DOSE-RESPONSE EFFECTS FOR DS
Drugs are pharmacologically active substances with a generally well-known mechanism of action and effects on target and off target tissues. These effects are well characterized and used in the hazard assessment for occupational health. Genotoxicity is an endpoint which needs additional expert consideration with respect to the underlying mechanism of genotoxic effects since they may or may not be a part of the intended effects of the drugs. The term “genotoxic” has been used broadly to describe toxicological endpoints such as mutagenicity, structural chromosomal damage (clastogenic activity), and numerical chromosome aberrations (aneugenic activity), as well as end points not routinely used such as sister chromatid exchanges or unscheduled DNA synthesis (Gould et al., 2016). Within this context, the term mutagenicity more specifically describes DNA-reactive substances that have a potential to directly cause DNA damage, often even when present at low levels, leading to damage and therefore potentially initiating cancer (eg, alkylating agents or some DNA intercalators). This type of mutagenic activity is usually detected in a bacterial reverse mutation (mutagenicity) assay (Ames test, OECD TG 471) (ICHM7, 2014). On the contrary, non-DNA-reactive genotoxins are generally detected by cytogenetic methods. For such compounds, it has been accepted that biologically plausible threshold mechanisms can exist (Crebelli, 2000; Elhajouji et al., 2011; Henderson et al., 2000; ICH, 2014; Kirsch-Volders et al., 2000), resulting from the redundancy of the cellular target and the fact that a certain concentration of the genotoxin is required to exert a biological effect. For DNA-interactive compounds, where the biological target is unique for each cell, a threshold must be demonstrated. There have been recent examples showing that biologically plausible and experimentally demonstrable thresholds can exist below which no cellular effect is occurring (eg, Doak et al., 2007; Müller et al., 2006).
In general, an OEL can only be based on effects for which nonlinear, dose–response relationships with safe dose or exposure levels apply (Zielhuis and Notten, 1979). For genotoxic compounds, several examples demonstrate limits below which no genotoxic effect was observed (Elhajouji et al. 2011; Guérard et al., 2014; Kirsch-Volders et al., 2000). Mechanistic hypotheses were formulated when safe exposure levels could be justified. As an example, for spindle fiber function inhibitors, an activity that typically leads to an aneugenic effect, threshold levels have been established (Elhajouji et al., 2011). Recently, however, examples have also been presented for DNA-reactive agents where a threshold was convincingly demonstrated (Bryce et al., 2010; Cao et al., 2014; Doak et al., 2007; Dobo et al., 2011; Müller et al., 2006; Olipiltz et al., 2012; Parry et al., 2000; Pottenger et al., 2009). These thresholds were usually based on a nonlinear DNA repair activity with a kinetic characteristic that leads to an efficient and essentially error-free repair of DNA lesions at low levels and to a biologically significant increase in unrepaired DNA damage only at levels that exhaust these systems (Thomas et al., 2013).
It is a matter of debate how much data are required to confirm a threshold mode of action (MOA) for a genotoxic DS or IM. If such an MOA can be identified, the measured or conservatively estimated genotoxicity NOAEL can be used to derive the OEL.
In an ideal case, relevant in vivo genotoxicity dose–response data to determine the POD are available, using appropriate extrapolation methods in combination with human exposure data (Gollapudi et al., 2013; Johnson et al., 2014; MacGregor et al., 2015a,b). Alternatively, if no human exposure data are available, allometric dose translation from animals to humans using body surface area normalization can be used (Reagan-Shaw et al., 2007). If a substance exerts other effects at lower dose levels (eg, reproductive toxicity), which can be more critical than the genotoxic effect, these need to be taken into consideration when identifying appropriate POD and AFs.
CALCULATING AN OEL FOR GENOTOXIC SUBSTANCES WITH A CLEAR THRESHOLD DOSE
When evaluating genotoxic substances, the genotoxic MOA and quality of all available study results are first evaluated. Subsequently, the PODs, based on critical effects, are selected and OELs calculated, provided that the data allow derivation of a threshold POD on the basis of quantitative and mechanistic information. Here we demonstrate the derivation of OELs from human and preclinical data using 2 well-characterized genotoxic drugs: colchicine and paclitaxel.
Colchicine is not directly genotoxic, but as a mitotic spindle poison induces aneuploidy, ie, a numerical chromosome aberration in the affected cells. As expected, for a molecule not directly interacting with DNA, the Ames test was negative with or without the presence of metabolic activation. A chromosome aberration assay in human white blood cells (enriched lymphocytes) was negative at doses up to those that disrupted mitosis. At concentrations above those, an increased proportion of cells exhibited mitotic disruption in the form of an elevated mitotic index (mitotic arrest) and centromeric disruption (dissociated chromatids) (Wang et al., 2006). The threshold for chromosome loss in vivo was 0.49 mg/kg (Cammerer et al., 2010). It has to be noted that lower “no-genotoxic-effect” levels have been reported for nondisjunction chromosome loss, (Kirsch-Volders et al., 2003) although the difference is typically about 2-fold (Elhajouji et al., 1997).
In this example, OELs can be derived from 2 end points: genotoxicity data with a NOAEL from an in vivo study and the lowest oral human therapeutic dose of 0.6 mg/day for treatment of gout (Cammerer et al., 2010; Guérard et al. 2014), where the POD is considered a LOAEL and an AF was added to extrapolate to NOAEL (for details see Table 1). The interspecies AF of 12 was used for extrapolation from mice to humans, whereas the intraspecies AF value of 10 was used for both PODs. For the POD derived from the mouse data, an AF of 10 was used to adjust for the different duration of exposure, which was not required for the therapeutic dose. Further, AFs were applied for (1) the severity of effects (a factor of 5 for potential effects on reproduction or genotoxicity was applied to the human POD) and (2) an AF of 2 for the extrapolation from oral administration in gout treatment to inhalation exposure in manufacturing exposure. No bioavailability adjustment was required from intraperitoneal exposure in mice to inhalation exposure. Overall, the OEL calculated from the critical effects of chromosome loss in mice, with a CAF of 1200 applied to the data obtained from the genotoxicity study, was 6-fold higher than the one applied to the data from human therapeutic dose with a CAF of 200 (Table 1). In addition, the calculated OEL value of 0.3 µg/m3 based on a therapeutic dose can be considered protective of potential genotoxic effects with a 10-fold MOS compared to the OEL of 3 µg/m3 calculated from the genotoxic POD.
TABLE 1.
Comparison of the Adjustment Factors (AFs) Used for Different PODs for the Calculation of OEL for Colchicine Based on the Critical Effects from Human and Preclinical Data
| Critical effect | Gastro intestinal (GI) symptoms | Aneuploidy |
|---|---|---|
| POD: | Lowest oral human therapeutically active dose (used for gout treatment) | NOAEL for aneuploidy in vivo with IP administration in mice |
| POD value | 0.6 mg/day | 0.49 mg/kg |
| Adjustment factors | GI symptoms | Aneuploidy | References |
|---|---|---|---|
| Interspecies variability | |||
| Value | 1 | 12 | EMA (2014) and ICH (2011) |
| Remarks | Default values for extrapolation between species. | ||
| Intraspecies variability | |||
| Value | 10 | 10 | EFSA Scientific Committee (2012), EMA (2014), U.S. EPA (2002), WHO (2001) |
| Remarks | Extrapolation of pharmacokinetic and pharmacodynamic differences within the population. 10 is a conservative approach considering worker population. | ||
| LOAEL to NOAEL | |||
| Value | 2 | 1 | BAuA (2014), ECETOC (2010), ECHA (2012), ISPE (2010), Naumann and Weiderman (1995), Sussman et al. (2016) |
| Remarks | The human therapeutic dose is considered a LOAEL as it has certain effects in patients. The value is selected depending on the severity of toxicity and therapeutic index. | A NOAEL was identified in the MN study, no extrapolation is required. | |
| Duration of exposure | |||
| Value | 1 | 10 | ICH (1998), Naumann and Weiderman (1995), Sussman et al. (2016) |
| Remarks | Derivation from human therapy does not require an additional factor. | A factor of 10 is used for extrapolation of preclinical studies of short duration to chronic exposure. | |
| Database completeness | |||
| Value | 1 | 1 | EMA (2014), ISPE (2010) |
| Remarks | Database is complete, data are reliable. | ||
| Severity of effect | |||
| Value | 5 | 1 | ISPE (2010), Sussman et al. (2016) |
| Remarks | An additional factor is applied for potential reprotoxic and genotoxic effects which may not be covered with the POD derived from human therapeutic dose. | The critical effect is covered with the in vivo MN study. Therefore no adjustment is required. | |
| Bioaccumulation | |||
| Value | 1 | 1 | Reichard et al. (2016) |
| Remarks | Applied if bioaccumulation is expected; no additional factor is required for this substance. | ||
| Bioavailability | |||
| Value | 2 | 1 | Reichard et al. (2016) |
| Remarks | The POD was with oral administration in therapy; in the absence of bioavailability a factor of 2 is applied for the extrapolation from oral to inhalation administration as a default in the absence of oral bioavailability data. | IP administration required no adjustment as bioavailability with IP administration is considered the same as with inhalation exposure. | |
| CAF | 200 | 1200 | |
| OEL (µg/m3) | 0.3 | 3 | |
CAF, composite adjustment factor; IP, intraperitoneal; OEL, occupational exposure limit; POD, points of departure.
Our second example is paclitaxel: a drug used in tumor therapy. In vitro, paclitaxel enhances the polymerization of tubulin to stable microtubules and also interacts directly with microtubules and the centrosome, stabilizing them against depolymerization. The fact that the drug has a specific binding site on the microtubule polymer makes it unique among chemotherapeutic agents, and the ability of paclitaxel to polymerize tubulin in the absence of co-factors like guanosine triphosphate and microtubule-associated proteins is unusual. As a result, paclitaxel blocks cells in the G2/M phase of the cell cycle and cells are unable to form a normal mitotic apparatus (Cunha et al., 2001; Horwitz 1994). In accordance with this MOA, paclitaxel was genotoxic in vivo micronucleus (MN) test in mice but it did not induce mutagenicity in the Ames test or the Chinese hamster ovary/hypoxantine-guanidine phosphoribosyl transferase (CHO/HGPRT) gene mutation assay (FDA, 2011).
For paclitaxel, 3 OELs were identified based on 3 critical effects: reproduction, genotoxicity, and effects on gastrointestinal tract and blood cell parameters. Of these, the OEL value of 0.9 µg/m3, based on the critical effects seen in the rat fertility study of 1 mg/kg/day is the most conservative. AFs for interspecies variability of 5, study duration of 10, intraspecies default factor of 10, LOAEL to NOAEL AF of 3, and severity of effect AF of 5 were used, resulting in a CAF of 7500. Such a large CAF indicates there are considerable uncertainties and the calculated OEL value may be over conservative. The MN study was not readily available for full interpretation. Therefore, the POD was considered less reliable. The CAF applied to the data from the mouse MN study was 3600 resulting in an OEL of 2 µg/m3 (see Table 2 for details). The selected OEL based on animal data is 100-fold lower than that based on human data and being 2-fold lower than that from the mouse MN data provides protection of employees from the potential genotoxic effects.
TABLE 2.
Comparison of the Adjustment Factors Used for Different PODs for the Calculation of OEL for Paclitaxel Based on the Critical Effects from Human and Preclinical Data
| Critical effect | Anemia, neutropenia, thrombocytopenia, alopecia, GI symptoms | Positive MN effects with dose-response | Impairment of fertility in both sexes |
|---|---|---|---|
| POD: | Lowest human therapeutic intravenous (IV) dose | LOAEL in the IV in vivo mouse MN study | LOAEL in rat fertility study (presumably IV) |
| POD value: | 135 mg/m2 = 270 mg/day | 1 mg/kg/day | 1 mg/kg/day |
| Adjustment factors | Blood and GI symptoms | MN effects | Impaired fertility | References |
|---|---|---|---|---|
| Interspecies variability | ||||
| Value | 1 | 12 | 5 | ICH (2011), EMA (2014) |
| Remarks | Default values for extrapolation between species. | |||
| Intraspecies variability | ||||
| Value | 10 | 10 | 10 | WHO (2001), U.S. EPA (2002), EFSA Scientific Committee (2012), EMA (2014 |
| Remarks | Extrapolation of pharmacokinetic and pharmacodynamic differences within the population. 10 is a conservative approach considering worker population. | |||
| LOAEL to NOAEL | ||||
| Value | 3 | 3 | 3 | Naumann and Weiderman (1995), ISPE (2010), ECETOC (2010), ECHA (2012), BAuA (2014), Sussman et al. (2016) |
| Remarks | A factor of 3 is assigned for extrapolation from LOAEL to NOAEL for all 3 PODs. | |||
| Duration of exposure | ||||
| Value | 1 | 10 | 10 | Naumann and Weiderman (1995), ICH (1998), Sussman et al. (2016) |
| Remarks | Derivation from human therapy does not require an additional factor. | A factor of 10 is used for extrapolation of preclinical studies of short duration to chronic exposure. | ||
| Database completeness | ||||
| Value | 1 | 1 | 1 | ISPE (2010), EMA (2014) |
| Remarks | Database is complete. | |||
| Severity of effect | ||||
| Value | 10 | 1 | 5 | ISPE (2010), Sussman et al. (2016) |
| Remarks | Factor applied to human therapeutic dose to cover potential fertility and genotoxic effects. | POD covers all critical effects. | AF of 5 is applied for uncertainty with regard to genotoxicty in rat fertility study. | |
| Bioaccumulation | ||||
| Value | 1 | 1 | 1 | Reichard et al. (2016) |
| Remarks | Applied if bioaccumulation is expected; no additional factor is required for this substance. | |||
| Bioavailability | ||||
| Value | 1 | 1 | 1 | Reichard et al. (2016) |
| Remarks | The POD was with 100% bioavailability (IV administration). | |||
| CAF | 300 | 3600 | 7500 | |
| OEL (µg/m3) | 90 | 2 | 0.9 | |
CAF, composite adjustment factor; OEL, occupational exposure limit; POD, points of departure.
CALCULATING AN OEL FOR GENOTOXIC SUBSTANCES WITHOUT A THRESHOLD DOSE
In contrast to the previous examples, where an accepted threshold mechanism allowed the derivation of a mechanistically supported MOS, here we propose an approach where this is not the case. Gemcitabine is a nucleoside used in cancer therapy that is an analog of deoxycytidine. It is a pro-drug that is intracellularly phosphorylated by deoxycytidine kinase to the active forms gemcitabine diphosphate and gemcitabine triphosphate, both of which inhibit DNA synthesis. Incorporation of gemcitabine triphosphate into DNA is most likely the major mechanism by which gemcitabine causes cell death. After incorporation of active gemcitabine at the end of the elongating DNA strand, one more deoxynucleotide is added and thereafter the DNA polymerases are unable to proceed and proof-reading enzymes are unable to remove gemcitabine from this position. Furthermore, the unique actions that gemcitabine metabolites exert on cellular regulatory processes serve to enhance the overall inhibitory activities on cell growth. Gemcitabine induced forward mutations in vitro in a mouse lymphoma (L5178Y) assay and was clastogenic in an in vivo mouse micronucleus assay. Gemcitabine was negative when tested in the Salmonella reverse mutation test, the in vivo sister chromatid exchange and the in vitro chromosomal aberration assay. It did not cause unscheduled DNA synthesis in vitro (Aydemir et al., 2005). Although few assays were negative, which may be in part due to an inappropriate specificity of cellular kinases for the phosphorylation of gemcitabine (eg, in Ames test), clearly positive routine tests indicate a relevant genotoxic potential. Therefore, threshold could not be clearly established. In this case, an OEL is not calculated and an acceptable excess cancer risk-based approach for protecting employees during manufacturing is proposed unless the calculated value is more conservative. This judgment should be revised when mechanism of genotoxic effects for gemcitabine is better understood.
In previous 2 examples, the CAF was large due to uncertainties in the dataset and adjustments due to variability when extrapolating the data to desired target population. United States Environmental Protection Agency (U.S. EPA) recommends limiting the total AF applied to a chronic reference value (not taking into account the AF for bioavailability or accumulation potential) for any particular substance to 3000 (U.S. EPA, 2002). In paclitaxel example, a factor of 10 was used to extrapolate from shorter studies in animals to chronic exposure. Therefore, CAF used in this example can be considered within the reasonable range. Further work is required to justify reduction of uncertainty to allow for fewer AFs.
When the dataset is adequate, there are several possible improvements in the calculation: a chemical specific AF (WHO, 2001) can be applied instead of the default factor of 10 for intraspecies extrapolation and a benchmark dose modeling can be applied to derive a POD. Benchmark dose has a lot of future potential; however, it is of limited value for substances with data limitations and constraints of the underlying statistical methods. Additional possibility which is already applied for other genotoxins would be to calculate No Observed Genotoxic Effect Level. No Observed Genotoxic Effect Level is defined as the highest tested dose for which no statistically significant increase in the incidence of the genotoxic effect is observed relative to an appropriate untreated control (ie, background). OELs can then be developed if an appropriate dataset including a dose-response for genotoxic effects is available (Gollapudi et al., 2013; Johnson et al., 2014).
ACCEPTABLE CANCER RISK IN HEALTHY EMPLOYEES EXPOSED WITH INHALATION
Several guidance documents describe the tolerable and acceptable excess cancer risk in manufacturing (BaUA, 2014; ECHA, 2012; NIOSH, 2013; SUVA, 2005). Generally acceptable cancer risk for working lifetime (40 years) used for employees in the European Union is around 1:10 000 but higher or lower levels have been considered to be tolerable under certain circumstances (ECHA, 2012). For example, Occupational Safety and Health Administration has used a risk level at about 1:1000 in setting Permissible Exposure Limits (PEL) for carcinogens such as benzene in healthy employees (Fiori et al., 2002), and Swiss Accident Insurance Fund (Schweizerische Unfallversicherungsanstalt, SUVA) has used the same risk level for asbestos (SUVA, 2004). Fiori et al. (2002) using a maximum daily exposure for working lifetime of 20 years with 250 8-h workdays/year and a 10m3 volume of inhaled air in 8 h, calculated an airborne limit of 5 µg/m3 for carcinogens and 50 µg/m3 for mutagens at a risk level at about 1:1000. It should be noted that 40 years is considered a standard work lifetime duration (ECHA, 2012) and the use of 20 years as working lifetime is not appropriate.
In other regulations, such as ICH M7 for mutagenic impurities (ICH, 2014), the concept of the Threshold of Toxicological Concern (TTC) is applied. In brief, this concept accepts the elevated cancer risk of 1:100 000 associated with lifetime exposure of patients to an impurity in pharmaceuticals with unknown toxicological properties including mutagenicity, when the intake level does not exceed 1.5 µg/day. A number of structural classes of particularly high mutagenic or carcinogenic potency (the so-called Cohort of Concern) for which lower limits may apply are exempted. For shorter exposure durations, accordingly higher limits are applicable to result in an equally minimal risk. Here, we propose to apply this concept for occupational health purposes, using an analogous argumentation as for mutagenic impurities. Extrapolating the acceptable intake of 1.5 µg/day × (365 days × 75 years = 25 550 days)/total number of exposure days the exposure for employees can be calculated as follows: 5 working days/week × 48 working weeks/year × 40 years = 9600 days, which is based on approach used by ECHA (2012) and is more conservative than used by Fiori et al (2012). In such a case, the accepted daily intake for a 1:100 000 risk would be 4 µg/day. By applying the common assumptions for exposure through inhalation including 10 m3 of air breathed during 8h working day (Galer et al., 1992; Sargent and Kirk 1988), and an excess risk of 1:25 000 would result in an acceptable level of 1.6 µg/m3 (rounded to 2 µg/m3). Thus, the inhalation concentration of 2 µg/m3 can be assumed to be generically sufficiently protective for all substances of unknown toxicological properties including genotoxic effects.
The TTC limit of 2 µg/m3 is in many cases higher than calculated values based on experimental data. It can be argued that despite the perhaps large CAF that would be applied for genotoxic substances without sufficient explanation of the genotoxic mechanism, it would be more appropriate to apply a calculated value. In general, robust control strategies to protect the employees in the manufacturing are applied; hence values which are within the order of magnitude may not change the risk at the workplace.
FUTURE DIRECTIONS
In occupational health settings, exposure limits are generally determined on the basis of the most sensitive endpoint considered relevant for healthy employees. This has not been possible for genotoxic agents which directly interact with DNA because of the classical interpretation that even a minimal hazard to genomic DNA can have potentially severe consequences. Such assumption is based on a simplistic view that genomic DNA is a single molecule target for which there is no cellular redundancy as for other macromolecules, so that the inactivation of a single molecule can have dramatic consequences. In contrast, for genotoxins acting primarily via non-DNA mechanisms such as binding to microtubules have been accepted. As a result, observed NOELs are utilized and appropriate MOS applied for risk assessment purposes. However, examples have been presented where the DNA-interacting genotoxins biologically plausible NOELs and thresholds for genotoxic effects have been found and PODs determined using dose-response modeling (Gollapudi et al., 2013; Johnson et al., 2014). In most cases, this was the consequence of nonlinear DNA repair capacities, acting in a way that at low levels of DNA damage a quasi-perfect repair of the damage was achieved. At supra-threshold levels, an exhaustion of the repair capacity became evident, leading to the manifestation of permanent genotoxic effects. In addition, by the introduction of the TTC concept in the regulation of mutagenic impurities in DSs, the principle of a threshold level was introduced below which no biologically relevant risk is to be expected even for directly DNA-interactive mutagens.
Principles have been introduced to be utilized for occupational health protection of employees when handling genotoxic substances. These principles have combined setting of a mechanistically and experimentally supported NOAEL or, alternatively, the TTC-compliant maximum daily intake, with assumptions on intake under workplace-relevant exposure scenarios. By this, we present a strategy to quantitatively limit the exposure to genotoxic agents in the workplace using holistic hazard assessment criteria accepted in genotoxicity risk assessment, such as the assessment of pharmaceuticals for patient safety. A toxicological hazard assessment followed by a calculation of an OEL must include all toxicological and clinical end-points. It can be expected that with the increasing interest in quantitative risk assessment of genotoxic effects more mechanisms will be described that allow to postulate thresholds for such toxicities. Therefore, these examples will allow the acceptance of our proposed approach which may reduce the uncertainty in quantifying the risk associated with genotoxic agents in the workplace while protecting the employees’ health.
FUNDING
No funding was received for this study.
ACKNOWLEDGMENTS
The authors acknowledge that they are employed by Novartis.
REFERENCES
- Aydemir N., Celikler S., Bilaloğlu R. (2005). In vitro genotoxic effects of the anticancer drug gemcitabine in human lymphocytes. Mutat. Res. 582, 35–41. [DOI] [PubMed] [Google Scholar]
- Bercu J., Morinello E., Sehner C., Shipp B., Weideman P. (2016). Point of departure (PoD) selection for the derivation of acceptable daily exposure (ADE) value for Active Pharmaceutical Ingredients (APIs). Regul. Toxicol. Pharmacol. [DOI] [PubMed] [Google Scholar]
- Bryce S. M., Avlasevich S. L., Bemis J. C., Phonethepswath S., Dertinger S. D. (2010). Miniaturized flow cytometric in vitro micronucleus assay represents an efficient tool for comprehensively characterizing genotoxicity dose-response relationships. Mutat. Res. 703, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA) (2014). Techniche Regeln fur Gefarstoffe, TRGS 910: Risikobezogenes Maßnahmenkonzept für Tätigkeiten mit krebserzeugenden Gefahrstoffen. GMBI 64, 1313 [Google Scholar]
- Cammerer Z., Schumacher M. M., Kirsch-Volders M., Suter W., Elhajouji A. (2010). Flow cytometry peripheral blood micronucleus test in vivo: Determination of potential thresholds for aneuploidy induced by spindle poisons. Environ. Mol. Mutagen. 51, 278–284. [DOI] [PubMed] [Google Scholar]
- Cao X., Mittelstaedt R. A., Pearce M. G., Allen B. C., Soeteman-Hernandez L. G., Johnson G. E., Bigger C. A., Heflich R. H. (2014). Quantitative dose–response analysis of ethyl methanesulfonate genotoxicity in adult gpt-delta transgenic mice. Environ. Mol. Mutagen. 55, 385–399. [DOI] [PubMed] [Google Scholar]
- Crebelli R. (2000). Threshold-mediated mechanisms in mutagenesis: Implications in the classification and regulation of chemical mutagens. Mutat. Res. 464, 129–135. [DOI] [PubMed] [Google Scholar]
- Cunha K. S., Reguly M. L., Graf U., de Andrade H. H. (2001). Taxanes: The genetic toxicity of paclitaxel and docetaxel in somatic cells of Drosophila melanogaster. Mutagenesis 16, 79–84. [DOI] [PubMed] [Google Scholar]
- Doak S. H., Jenkins G. J. S., Johnson G. E., Quick E., Parry E. M., Parry J. M. (2007). Mechanistic influences for mutation induction curves after exposure to DNA-reactive carcinogens. Cancer Res. 67, 3904. [DOI] [PubMed] [Google Scholar]
- Dobo K. L., Fiedler R. D., Gunther W. C., Thiffeault C. J., Cammerer Z., Coffing S. L., Shutsky T., Schuler M. (2011). Defining EMS and ENU dose–response relationships using the Pig-a mutation assay in rats. Mutat. Res. 725, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhajouji A., Lukamowicz M., Cammerer Z., Kirsch-Volders M. (2011). Potential thresholds for genotoxic effects by micronucleus scoring. Mutagenesis 26, 199–204. [DOI] [PubMed] [Google Scholar]
- European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) (2010). Guidance on Assessment Factors to Derive a DNEL, Technical Report No. 110. ECETOC AISBL, 110. Available at: http://www.ecetoc.org/index.php?mact=Newsroom,cntnt01,details,0&cntnt01documentid=146&cntnt01returnid=76. Accessed October 25, 2015.
- European Chemicals Agency (ECHA) (2012). Guidance on Information Requirements and Chemical Safety Assessment Chapter R.8: Characterisation of Dose [Concentration]-Response for Human Health ECHA-2010-G-19-EN Available at: https://echa.europa.eu/documents/10162/13632/information_requirements_r8_en.pdf. Accessed October 25, 2015.
- European Food Safety Authority (EFSA) (2012). Scientific opinion: Guidance on selected default values to be used by the EFSA Scientific Committee, scientific panels and units in the absence of actual measured data. EFSA J. 10, 1–32. [Google Scholar]
- European Medicines Agency (EMA) (2014). Guideline on Setting Health Based Exposure Limits for Use in Risk Identification in the Manufacture of Different Medicinal Products in Shared Facilities EMA/CHMP/CVMP/SWP/169430/2012 Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/11/WC500177735.pdf. Accessed October 25, 2015.
- Faria E. C., Bercu J., Dolan D. G., Morinello E., Pecquet A. M., Seaman C., Sehner C., Weideman P. (2016). Using default methodologies to derive an Acceptable Daily Exposure (ADE). Regul. Toxicol. Pharmacol. [DOI] [PubMed] [Google Scholar]
- Food and Drug Safety Agency (FDA) (2011). Taxol (Paclitaxel) Injection, FDA Prescribing Information Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf. Accessed October 25, 2015.
- Galer D. M., Leung H. W., Sussman R. G., Trzos R. J. (1992). Scientific and practical considerations for the development of occupational exposure limits (OELs) for chemical substances. Regul. Toxicol. Pharmacol. 15, 291–306. [DOI] [PubMed] [Google Scholar]
- Gollapudi B. B., Johnson G. E., Hernandez L. G., Pottenger L. H., Dearfield K. L., Jeffrey A. M., Julien E., Kim J. H., Lovell D. P., MacGregor J. T., et al. (2013). Quantitative approaches for assessing dose-response relationships in genetic toxicology studies. Environ. Mol. Mutagen. 54, 8–18. [DOI] [PubMed] [Google Scholar]
- Gould J., Callis C., Dolan D. G., Stanard B., Weideman P. (2016). Special endpoint considerations in pharmaceutical ADE derivation. Regul. Toxicol. Pharmacol. [DOI] [PubMed] [Google Scholar]
- Guérard M., Baum M., Bitsch A., Eisenbrand G., Elhajouji A., Epe B., Habermeyer M., Kaina B., Martus H. J., Pfuhler S., et al. (2015). Assessment of mechanism driving non-linear dose–response 4 relationships in genotoxicity testing. Mutat. Res. 763, 181–201. [DOI] [PubMed] [Google Scholar]
- Henderson L., Albertini S., Aardema M. (2000). Thresholds in genotoxicity responses. Mutat. Res. 464, 123–128. [DOI] [PubMed] [Google Scholar]
- Horwitz S. B. (1994). Taxol (paclitaxel): Mechanisms of action. Ann. Oncol. 5(Suppl 6), 3–6. [PubMed] [Google Scholar]
- International Conference on Harmonisation (ICH) (1998). ICH Topic Q 3 C (R3), Impurities: Residual Solvents, CPMP/ICH/283/95 Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002979.pdf. Accessed October 25, 2015.
- International Conference on Harmonisation (ICH) (2006). Impurities in New Drug Substances Q3A (R2). ICH Harmonised Tripartite Guideline International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3A_R2/Step4/Q3A_R2__Guideline.pdf. Accessed November 28, 2015.
- International Conference on Harmonisation (ICH) (2011). Impurities: Guideline for Residual Solvents Q3C(R5) Step 4 Version February 4, 2011. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3C/Step4/Q3C_R5_Step4.pdf. Accessed October 25, 2015.
- International Conference on Harmonisation (ICH) (2014). ICH M7 guideline, Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk, Step 4 Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm347725.pdf. Accessed October 25, 2015.
- International Society for Pharmaceutical Engineering (ISPE) (2010). Risk-Based Manufacture of Pharmaceutical Products, 1st ed., vol. 7. International Society for Pharmaceutical Engineering, Tampa, FL. Available at: http://www.ispe.org/baseline-guides/risk-mapp. Accessed October 25, 2015.
- Johnson G. E., Soeteman-Hernández L. G., Gollapudi B. B., Bodger O. G., Dearfield K. L., Heflich R. H., Hixon J. G., Lovell D. P., MacGregor J. T., Pottenger L. H., et al. (2014). Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment. Environ. Mol. Mutagen. 55, 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Volders M., Aardema M., Elhajouji A. (2000). Concepts of threshold in mutagenesis and carcinogenesis. Mutat. Res. 464, 3–11. [DOI] [PubMed] [Google Scholar]
- Kirsch-Volders M., Vanhauwaert A., Eichenlaub-Ritter U., Decordier I. (2003). Indirect mechanisms of genotoxicity. Toxicol. Lett. 140/141, 63–74. [DOI] [PubMed] [Google Scholar]
- MacGregor J. T., Frötschl R., White P. A., Crump K. S., Eastmond D. A., Fukushima S., Guérard M., Hayashi M., Soeteman-Hernández L. G., Kasamatsu T., et al. (2015a). IWGT report on quantitative approaches to genotoxicity risk assessment I. Methods and metrics for defining exposure–response relationships and points of departure (PoDs). Mutat. Res. 783, 55–65. [DOI] [PubMed] [Google Scholar]
- MacGregor J. T., Frötschl R., White P. A., Crump K. S., Eastmond D. A., Fukushima S., Guérard M., Hayashi M., Soeteman-Hernández L. G., Kasamatsu T., et al. (2015b). IWGT report on quantitative approaches to genotoxicity risk assessment II. Use of point-of-departure (PoD) metrics in defining acceptable exposure limits and assessing human risk. Mutat. Res. 783, 66–78. [DOI] [PubMed] [Google Scholar]
- Müller L., Gocke E., Lavé T., Pfister T. (2006). Ethyl methanesulfonate toxicity in Viracept—A comprehensive human risk assessment based on threshold data for genotoxicity. Toxicol. Lett. 3, 317–329. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) (2013). Current Intelligence Bulletin: Update of NIOSH Carcinogen Classification and Target Risk Level Policy for Chemical Hazards in the Workplace. Available at: http://www.cdc.gov/niosh/docket/review/docket240A/pdf/EID-CIB-11052013.pdf. Accessed October 25, 2013.
- Naumann B. D., Sargent E. V. (1997). Setting occupational exposure limits for pharmaceuticals. Occup. Med. 12, 67–80. [PubMed] [Google Scholar]
- Naumann B. D., Sargent E. V., Starkman B. S., Fraser W. J., Becker G. T., Kirk G. D. (1996). Performance-based exposure control limits for pharmaceutical active ingredients. AIHA. J. 57, 33–42. [DOI] [PubMed] [Google Scholar]
- Nielsen G. D., Ovrebo S. (2008). Background, approaches and recent trends for setting health-based occupational exposure limits: A minireview. Regul. Toxicol. Pharmacol. 51, 253–269. [DOI] [PubMed] [Google Scholar]
- Parry J. M., Jenkins G. J. S., Haddad F., Bourner R., Parry E. M. (2000). In vitro and in vivo extrapolations of genotoxin exposures: Consideration of factors which influence dose–response thresholds. Mutat. Res. 464, 53–63. [DOI] [PubMed] [Google Scholar]
- Pottenger L. H., Schisler M. R., Zhang F., Bartels M. J., Fontaine D. D., McFadden L. G., Gollapudi B. B. (2009). Dose response and operational thresholds/NOAELs for in vitro mutagenic effects from DNA-reactive mutagens, MMS and MNU. Mutat. Res. 678, 138–147. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S., Nihal M., Ahmad N. (2007). Dose translation from animal to human studies revisited. FASEB J. 22, 659–661. [DOI] [PubMed] [Google Scholar]
- Reichard J. F., Maier M. A., Naumann B. D., Pfister T., Sandhu R., Sargent E. V., Streeter A. J., Willis A. M., Weideman P. (2016). Toxicokinetic and toxicodynamic considerations when deriving health-based exposure limits for pharmaceuticals. Regul. Toxicol. Pharmacol. [DOI] [PubMed] [Google Scholar]
- Sargent E., Kirk G. (1988). Establishing airborne exposure control limits in the pharmaceutical industry. Am. Ind. Hyg. Assoc. J. 49, 309–313. [DOI] [PubMed] [Google Scholar]
- Sargent E. V., Faria E., Pfister T., Sussman R. G. (2013). Guidance on the establishment of acceptable daily exposure limits (ADE) to support Risk-Based Manufacture of Pharmaceutical Products. Regul. Toxicol. Pharmacol. 65, 242–250. [DOI] [PubMed] [Google Scholar]
- Schwartz C. S. (1995). A semiquantitative method for selection of safety factors in establishing OELs for pharmaceutical compounds. Hum. Ecol. Risk Assess. 1, 527–543. [Google Scholar]
- Schweizerishe Unfallversicherungsanstalt (SUVA) (2004). Grenzwerte am Arbetsplatz 2005. Schweiz 2004. Available at: http://www2.isaac.supsi.ch/ISAAC/Studi%20Ambientali/Formazione/Formazione%20continua/Corso%20DACD%20E22%20-%20Inquinamento%20indoor/Materiale%20corso/limiti%20suva%20d.pdf. Accessed October 25 2015.
- Sussman R., Naumann B., Pfister T., Sehner C., Seaman C., Weideman P. (2016). A harmonization effort for acceptable exposure methodology—considerations for application of adjustment factors. Regul. Toxicol. Pharmacol. [DOI] [PubMed] [Google Scholar]
- Thomas A. D., Jenkins G. J., Kaina B., Bodger O. G., Tomaszowski K. H., Lewis P. D., Doak S. H., Johnson G. E. (2013). Influence of DNA repair on nonlinear dose-responses for mutation. Toxicol. Sci. 132, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). (2002). A Review of the Reference Dose and Reference Concentration Processes. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, EPA/630/P-02/002F, 2002. Available at: http://www2.epa.gov/osa/review-reference-dose-and-reference-concentration-processes. Accessed October 25 2015.
- Wang Y. J., Zhang L. S. (2006). Evaluation of the genotoxicity of vincristine and colchicine using mouse lymphoma tk mutation assay. Wei Sheng Yan Jiu 35, 179–181. [PubMed] [Google Scholar]
- World Health Organization (WHO) (2005). Harmonization Project Document No.2: Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration—Response Assessment Available at: http://www.inchem.org/documents/harmproj/harmproj/harmproj2.pdf. Accessed October 25, 2015.
- Zielhuis R. L., Notten W. R. F. (1979). Permissible levels for occupational exposure: Basic concepts. Int. Arch. Occup. Environ. Health 42, 269–281. [DOI] [PubMed] [Google Scholar]