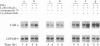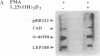Abstract
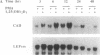
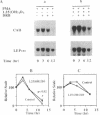
Carbonic anhydrase II (CAII) is highly expressed in the osteoclast, where it is involved in the process of extracellular acidification required for bone resorption. We have previously shown that 1 alpha,25-dihydroxyvitamin D3 [1,25(OH)2D3], a steroid hormone that regulates the differentiation of macrophages and osteoclasts, induces the expression of CAII mRNA and protein in avian bone marrow cells. To determine whether this regulation occurred at the gene level, we have studied the effects of 1,25(OH)2D3 on CAII expression in a transformed myelomonocytic avian cell line (BM2). As observed in nontransformed cells, 1,25(OH)2D3 markedly increased CAII biosynthesis and mRNA levels. The increase in CAII mRNA was detected as early as 3 hr after adding the hormone (1.9-fold) and reached 4.7-fold by 48 hr. These effects were completely blocked by actinomycin D, and nuclear run-on analysis confirmed that 1,25(OH)2D3 increased the rate of CAII gene transcription. In contrast, induction of CAII mRNA expression was not affected by inhibition of protein synthesis with cycloheximide, and no significant changes in mRNA stability were seen. Thus, 1,25(OH)2D3 modulates CAII gene expression at the transcriptional level, and this effect does not require de novo synthesis of other gene products. These results suggest that activation of the CAII gene occurs early in the differentiation events triggered by vitamin D3 in myelomonocytic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Schraer H., Gay C. V. Ultrastructural immunocytochemical localization of carbonic anhydrase in normal and calcitonin-treated chick osteoclasts. Anat Rec. 1982 Sep;204(1):9–20. doi: 10.1002/ar.1092040103. [DOI] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Baron R., Neff L., Roy C., Boisvert A., Caplan M. Evidence for a high and specific concentration of (Na+,K+)ATPase in the plasma membrane of the osteoclast. Cell. 1986 Jul 18;46(2):311–320. doi: 10.1016/0092-8674(86)90748-8. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Billecocq A., Emanuel J. R., Levenson R., Baron R. 1 alpha,25-dihydroxyvitamin D3 regulates the expression of carbonic anhydrase II in nonerythroid avian bone marrow cells. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6470–6474. doi: 10.1073/pnas.87.16.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelvi Z. S., Studzinski G. P. Changes in the expression of oncogenes encoding nuclear phosphoproteins but not c-Ha-ras have a relationship to monocytic differentiation of HL 60 cells. J Cell Biol. 1986 Jun;102(6):2234–2243. doi: 10.1083/jcb.102.6.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Gay C. V. Effects of parathyroid hormone and calcitonin on carbonic anhydrase location in osteoclasts of cultured embryonic chick bone. Experientia. 1985 Nov 15;41(11):1472–1474. doi: 10.1007/BF01950043. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demay M. B., Gerardi J. M., DeLuca H. F., Kronenberg H. M. DNA sequences in the rat osteocalcin gene that bind the 1,25-dihydroxyvitamin D3 receptor and confer responsiveness to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Jan;87(1):369–373. doi: 10.1073/pnas.87.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret J. M., Brun P., Perret C., Lomri N., Thomasset M., Cuisinier-Gleizes P. Transcriptional and post-transcriptional regulation of vitamin D-dependent calcium-binding protein gene expression in the rat duodenum by 1,25-dihydroxycholecalciferol. J Biol Chem. 1987 Dec 5;262(34):16553–16557. [PubMed] [Google Scholar]
- Fambrough D. M., Takeyasu K., Lippincott-Schwarz J., Siegel N. R. Structure of LEP100, a glycoprotein that shuttles between lysosomes and the plasma membrane, deduced from the nucleotide sequence of the encoding cDNA. J Cell Biol. 1988 Jan;106(1):61–67. doi: 10.1083/jcb.106.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gay C. V., Mueller W. J. Carbonic anhydrase and osteoclasts: localization by labeled inhibitor autoradiography. Science. 1974 Feb 1;183(4123):432–434. doi: 10.1126/science.183.4123.432. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Moscovici C., Moscovici M. G., Samarut J. Response of hemopoietic cells to avian acute leukemia viruses: effects on the differentiation of the target cells. Cell. 1979 Mar;16(3):627–638. doi: 10.1016/0092-8674(79)90036-9. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hall G. E., Kenny A. D. Role of carbonic anhydrase in bone resorption: effect of acetazolamide on basal and parathyroid hormone-induced bone metabolism. Calcif Tissue Int. 1987 Apr;40(4):212–218. doi: 10.1007/BF02556624. [DOI] [PubMed] [Google Scholar]
- Harrison J. R., Petersen D. N., Lichtler A. C., Mador A. T., Rowe D. W., Kream B. E. 1,25-Dihydroxyvitamin D3 inhibits transcription of type I collagen genes in the rat osteosarcoma cell line ROS 17/2.8. Endocrinology. 1989 Jul;125(1):327–333. doi: 10.1210/endo-125-1-327. [DOI] [PubMed] [Google Scholar]
- Hillstrom Shapiro L., Venta P. J., Yu Y. S., Tashian R. E. Carbonic anhydrase II is induced in HL-60 cells by 1,25-dihydroxyvitamin D3: a model for osteoclast gene regulation. FEBS Lett. 1989 Jun 5;249(2):307–310. doi: 10.1016/0014-5793(89)80647-7. [DOI] [PubMed] [Google Scholar]
- Hott M., Marie P. J. Carbonic anhydrase activity in fetal rat bone resorbing cells: inhibition by acetazolamide infusion. J Dev Physiol. 1989 Nov;12(5):277–281. [PubMed] [Google Scholar]
- Ikeda K., Lu C., Weir E. C., Mangin M., Broadus A. E. Regulation of parathyroid hormone-related peptide gene expression by cycloheximide. J Biol Chem. 1990 Apr 5;265(10):5398–5402. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N., Gluck S., Roodman G. D. Sequential expression of phenotype markers for osteoclasts during differentiation of precursors for multinucleated cells formed in long-term human marrow cultures. Endocrinology. 1990 Dec;127(6):3215–3221. doi: 10.1210/endo-127-6-3215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Linser P. J., Perkins M. S., Fitch F. W., Moscona A. A. Comparative characterization of monoclonal antibodies to carbonic anhydrase. Biochem Biophys Res Commun. 1984 Dec 14;125(2):690–697. doi: 10.1016/0006-291x(84)90594-1. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Couto E. O. The nature of anion inhibition of human red cell carbonic anhydrases. Arch Biochem Biophys. 1979 Sep;196(2):501–510. doi: 10.1016/0003-9861(79)90302-3. [DOI] [PubMed] [Google Scholar]
- Minkin C., Yu X. H. Calcitonin receptor expression and its regulation by 1 alpha-25-dihydroxyvitamin D3 during de novo osteoclast formation in organ cultures of fetal mouse metatarsals. Bone Miner. 1991 Jun;13(3):191–200. doi: 10.1016/0169-6009(91)90068-b. [DOI] [PubMed] [Google Scholar]
- Morrison N. A., Shine J., Fragonas J. C., Verkest V., McMenemy M. L., Eisman J. A. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989 Dec 1;246(4934):1158–1161. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- Noda M., Yoon K., Rodan G. A. Cyclic AMP-mediated stabilization of osteocalcin mRNA in rat osteoblast-like cells treated with parathyroid hormone. J Biol Chem. 1988 Dec 5;263(34):18574–18577. [PubMed] [Google Scholar]
- Ozono K., Sone T., Pike J. W. The genomic mechanism of action of 1,25-dihydroxyvitamin D3. J Bone Miner Res. 1991 Oct;6(10):1021–1027. doi: 10.1002/jbmr.5650061002. [DOI] [PubMed] [Google Scholar]
- Roodman G. D., Ibbotson K. J., MacDonald B. R., Kuehl T. J., Mundy G. R. 1,25-Dihydroxyvitamin D3 causes formation of multinucleated cells with several osteoclast characteristics in cultures of primate marrow. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8213–8217. doi: 10.1073/pnas.82.23.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Lettieri D., Sherwood L. M. Suppression by 1,25(OH)2D3 of transcription of the pre-proparathyroid hormone gene. Endocrinology. 1986 Dec;119(6):2864–2866. doi: 10.1210/endo-119-6-2864. [DOI] [PubMed] [Google Scholar]
- Schüle R., Umesono K., Mangelsdorf D. J., Bolado J., Pike J. W., Evans R. M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990 May 4;61(3):497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- Silver J., Naveh-Many T., Mayer H., Schmelzer H. J., Popovtzer M. M. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986 Nov;78(5):1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. U., Hsu T., Begley D. A., Mitchell B. S., Alizadeh B. N. Transcriptional regulation of the c-myc protooncogene by 1,25-dihydroxyvitamin D3 in HL-60 promyelocytic leukemia cells. J Biol Chem. 1987 Mar 25;262(9):4104–4108. [PubMed] [Google Scholar]
- Sly W. S., Hewett-Emmett D., Whyte M. P., Yu Y. S., Tashian R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983 May;80(9):2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds G., Klempnauer K. H., Evan G. I., Bishop J. M. Induced differentiation of avian myeloblastosis virus-transformed myeloblasts: phenotypic alteration without altered expression of the viral oncogene. Mol Cell Biol. 1984 Dec;4(12):2587–2593. doi: 10.1128/mcb.4.12.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Sasaki T., Nicholson G. C., Moseley J. M., Martin T. J., Suda T. Induction of calcitonin receptors by 1 alpha, 25-dihydroxyvitamin D3 in osteoclast-like multinucleated cells formed from mouse bone marrow cells. Endocrinology. 1988 Sep;123(3):1504–1510. doi: 10.1210/endo-123-3-1504. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Mundy G. R., Kuehl T. J., Roodman G. D. Osteoclast-like cell formation in fetal and newborn long-term baboon marrow cultures is more sensitive to 1,25-dihydroxyvitamin D3 than adult long-term marrow cultures. J Bone Miner Res. 1987 Aug;2(4):311–317. doi: 10.1002/jbmr.5650020408. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vänänen H. K., Parvinen E. K. High active isoenzyme of carbonic anhydrase in rat calvaria osteoclasts. Immunohistochemical study. Histochemistry. 1983;78(4):481–485. doi: 10.1007/BF00496199. [DOI] [PubMed] [Google Scholar]
- Yoshihara C. M., Federspiel M., Dodgson J. B. Isolation of the chicken carbonic anhydrase II gene. Ann N Y Acad Sci. 1984;429:332–334. doi: 10.1111/j.1749-6632.1984.tb12357.x. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]