Abstract
Fatty acid esters of 3-chloro-1, 2-propanediol (3-MCPD esters) are a group of processing induced food contaminants with nephrotoxicity but the molecular mechanism(s) remains unclear. This study investigated whether and how the JNK/p53 pathway may play a role in the nephrotoxic effect of 3-MCPD esters using 3-MCPD 1-palmitate (MPE) as a probe compound in Sprague Dawley rats. Microarray analysis of the kidney from the Sprague Dawley rats treated with MPE, using Gene Ontology categories and KEGG pathways, revealed that MPE altered mRNA expressions of the genes involved in the mitogen-activated protein kinase (JNK and ERK), p53, and apoptotic signal transduction pathways. The changes in the mRNA expressions were confirmed by qRT-PCR and Western blot analyses and were consistent with the induction of tubular cell apoptosis as determined by histopathological, TUNEL, and immunohistochemistry analyses in the kidneys of the Sprague Dawley rats. Additionally, p53 knockout attenuated the apoptosis, and the apoptosis-related protein bax expression and cleaved caspase-3 activation induced by MPE in the p53 knockout C57BL/6 mice, whereas JNK inhibitor SP600125 but not ERK inhibitor U0126 inhibited MPE-induced apoptosis, supporting the conclusion that JNK/p53 might play a critical role in the tubular cell apoptosis induced by MPE and other 3-MCPD fatty acid esters.
Keywords: acute tubular cell injury, apoptosis, p53, JNK, ERK.
Fatty acid esters of 3-chloro-1, 2-propanediol (3-MCPD) have been detected in many foods, and food ingredients especially in refined edible oils (Hamlet and Sadd, 2004; Svejkovská et al., 2004; Zelinková et al., 2006). The concentrations of 3-MCPD esters found in food are in the range of 0.14–27 mg/kg (Larsen, 2009; Svejkovská et al., 2004). A 90-day toxicology study using 3-MCPD di-palmitic esters at the doses of 9.78, 39.19, and 156.75 mg/kg body weight (BW) per day by daily oral gavage in Wistar rats led to the tubular epithelial degeneration in a dose-dependent manner (Barocelli et al., 2011). Another 90-day study compared the toxicity of 3-MCPD 1-palmitic, dipalmitic, and dioleic esters at the equal molar concentrations. The three 3-MCPD esters appeared to exert subchronic kidney toxicity at a similar degree (Onami et al., 2014). Also, our recent study found that 3-MCPD palmitic monoester and di-esters induced acute tubular cell injury and protein cast in a 14-day acute oral toxicity study using Swiss mice (Liu et al., 2012). Hence, kidney may be a critical target organ for 3-MCPD esters. However, the molecular mechanisms behind the kidney injury induced by 3-MCPD esters remain unclear.
Apoptotic cell death has been proposed to be a major intracellular mechanism involved in the nephrotoxin induced toxicity (Hortelano et al., 2000; Quiros et al., 2013; Servais et al., 2008). The signaling pathways that led to apoptosis have been well defined, and the proteins associated with the apoptosis may include mitogen-activated protein kinase (MAPK) and p53. P53 is a well-known and first identified protein that controls apoptosis through modulating the proapoptotic protein bax expression. Bax interacts with the antiapoptotic member bcl-2 and induces apoptosis (Mikhailov et al., 2003; Moll et al., 2005).
This study was designed to test the hypothesis that the nephrotoxicity induced by 3-MCPD fatty acid esters is mediated through activating the MAPK and p53 related apoptosis pathway in vivo using 3-MCPD 1-palmitic ester (MPE) as the probe compound, because MPE is relatively more toxic than other tested 3-MCPD esters on a per weight concentration basis in our previous study (Liu et al., 2012). The results from this study may advance our understanding of the molecular mechanisms involved in the nephrotoxicity induced by 3-MCPD fatty acid esters and lay foundation for safety evaluation of 3-MCPD fatty acid esters.
MATERIALS AND METHODS
Antibodies and Reagents
The following antibodies were used for Western blot: phosphorylated- (p-)ERK (Thr202/Tyr204) (4370), ERK (4695), p-c-Jun (Ser63) (2361), p-JNK (Thr183/Tyr185) (4668), JNK (9258), p-p38 (Thr180/Tyr182) (4511), p38 (8690), p-p53 (Ser15) (9284), p53 (2524), bax (2772), bcl-2 (2870), cleaved caspase-3 (9664), and β-actin (4970) antibodies were purchased from Cell Signaling Technology (Beverly, Massachusetts). Kim-1 (ab47634) antibody was purchased from Abcam (Cambridge, Massachusetts). The following antibodies were used for immunohistochemistry (IHC): p-JNK (Thr183/Tyr185) (4668), p-c-Jun (Ser63) (2361), p-p53 (Ser15) (12571), and cleaved caspase-3 (9664) were purchased from Cell Signaling Technology. P53 (ab131442) was obtained from Abcam. The SignalStain Boost IHC Detection Reagents (HRP, anti-Mouse 8125; HRP, anti-Rabbit 8114) and DAB substrate kit (8059) were also purchased from Cell Signaling Technology. ERK inhibitor U0126 and JNK inhibitor SP600125 were purchased from Selleck Chemicals China (Shanghai, China). 3-MCPD 1-palmitic ester (MPE) was synthesized in our lab following a published protocol (Kaze et al., 2011) with purity greater than 98%.
Animals and MPE Treatment
Male Sprague Dawley rats (200 ± 20 g, 6–8 weeks) were purchased from Shanghai SLAC Laboratory Animal Co Ltd (Shanghai, China). Male C57BL/6 mice (18 ± 2 g, 6–8 weeks) were obtained from Beijing Wei Tong Li Hua Laboratory (Beijing, China). The heterozygous p53 knockout (p53+/−) mice were purchased from the Model Animal Resource Information Platform of Nanjing University (Nanjing, Jiangsu, China), and homozygotes p53 knockout (p53−/−) mice were generated by mating between p53+/− mice. All procedures requiring the use of animal were conducted in accordance with the Animal Care and Welfare Committee of the Laboratory Animal Center of Shanghai Jiao Tong University. Rodent laboratory chow and tap water were provided ad libitum and maintained under controlled conditions with a temperature of 24°C ± 1°C, humidity of 40%–80%, and a 12-h light/12-h dark cycle. Animals were acclimatized for 1 week before assigned into experimental groups.
MPE was dissolved in olive oil and administrated to animals through gavage. The dose was 1 g/kg BW and volume was 5 ml/kg BW for SD rats. For C57BL/6 mice, the dose was 1.5 g/kg BW based on the body surface area conversion and preliminary results, and dose volume was 0.2 ml/10 g BW. JNK inhibitor SP600125 and ERK inhibitor U0126 were dissolved in olive oil and administrated to C57BL/6 mice through intraperitoneal injection 1 h prior to MPE treatment with the doses of 10 or 30 mg/kg BW (0.1 ml/10 g BW).
Experimental Design
Experiment 1- effect of MPE on kidney in SD rats
SD rats were divided into the control and the MPE treated groups. The control and the treated groups were divided into subgroups-A, -B, -C, and -D (n = 8/subgroup), respectively. SD rats in the treatment subgroups were given MPE (1 g/kg BW) in vehicle (5 ml/kg BW), while rats in the control subgroups received olive oil only at the same dose volume, for a period of 6, 12, 24, and 48 h, respectively. Rats in the 48 h group were placed in the metabolism cage individually and urine samples were collected at 6, 12, 24, and 48 h on ice without any protease inhibitors, respectively.
Experiment 2- role of p53 in the regulation of kidney toxicity induced by MPE
Twelve wild type (WT) C57BL/6 mice and 12 homozygotes p53 knockout (p53−/−) mice were used to further confirm the target role of p53. WT and p53−/− mice were randomly assigned to 2 subgroups (control and MPE treated), respectively, and administrated with MPE at a dose of 1.5 g/kg BW through intragavage with olive oil as the vehicle for 24 h (Wang et al., 2014).
Experiment 3- effect of JNK and ERK inhibitor on the kidney toxicity induced by MPE
C57BL/6 mice were given a single dose of 1.5 g/kg BW MPE, and the vehicle control animal received same volume of olive oil (n = 6/group). One hour before MPE administration, vehicle (olive oil), SP600125, or U0126 was injected intraperitoneally at doses of 10 or 30 mg/kg BW (n = 6/group). Mice were sacrificed 24 h after MPE treatment (Jo et al., 2005; Yoshikane et al., 2015).
Clinical Chemistry
Animals were anesthetized by carbon dioxide and the blood samples were collected from retro-orbital. Anticoagulants used were K2/ethylenediaminetetraacetic acid for hematology samples. The serum was obtained by centrifugation at 3500 rpm for 15 min at 4°C.
The following hematology parameters in the plasma were determined by Adicon Clinical Laboratories, Inc (Shanghai, China): white blood cells (WBC), red blood cells (RBC), red cell distribution width-coefficient of variation (RDW-CV), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean platelet volume (MPV), platelet distribution width (PDW), total platelet count (PLT), plateletcrit (PCT), and differential fractions and absolute leukocyte counts for neutrophils, lymphocytes, monocytes (MONO), eosinophils (EO), basophils, and reticulocyte count.
Serum urea nitrogen and creatinine levels were also measured by Adicon Clinical Laboratories, Inc.
The following urine parameters were determined by Adicon Clinical Laboratories, Inc: bilirubin (BIL), blood (BLD), glucose (GLU), ketone (KET), nitrite (NIT), PH, protein (PRO), specific gravity (SG), urobilinogen (URO), and WBC.
Serum and urine kidney injury molecular 1 (Kim-1) levels were determined using a commercial testing kit (catalog RKM100) from the R&D Systems China Co Ltd (Shanghai, China) according to the manufacturer’s instructions.
Microarray Analysis of mRNA Expression
Total RNA was isolated using the TRIzol reagent (Thermo Fisher Scientific China, Shanghai, China) according to the manufacturer’s instructions and purified using RNeasy Mini Kit from Qiagen China Co, Ltd (Shanghai, China). Purified RNAs were used to generate biotinylated cRNA targets for the Affymetrix Rats Genome 230 2.0 Array, which contained 31 042 probe sets. The biotinylated cRNA targets were hybridized with the microarray. After hybridization, arrays were stained in the Fluidics Station 450 and scanned on the Affymetrix Scanner 3000. The microarray experiments were performed by following the protocol of Affymetrix Inc at Shanghai Biotechnology Corporation (Shanghai, China).
RNA Isolation and quantitative Real-time-PCR
Total RNA was isolated from kidney cortex using the TRIzol reagent (Thermo Fisher Scientific China), followed by the reverse transcription cDNA using the IScript Advanced cDNA Synthesis kit. Real-time PCR was carried out on an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, California) using AB Power SYBR Green PCR Master Mix. The following amplification parameters were used for PCR: 50°C for 2 min, 95°C for 10 min, and 46 cycles of amplification at 95°C for 15 s and 60°C for 1 min (Shi et al., 2012). Primers for genes were: Kim-1 (for 5′-CCTCCACTCCTCCAACATCTACA-3′, rev 5′-TGGGATGGGATTCCTGTCA-3′); bax (for 5′-GCAGAGGATGATTGCTGATG-3′, rev 5′-CTCAGCCCATCTTCTTCCAG-3′); bcl-2 (for 5′-TCCATTATAAGCTGTCACAG-3′, rev 5′-GAAGAGTTCCTCCACCAC-3′); p53 (for 5′-GCTGAGTATCTGGACGACA-3′, rev 5′-CAGGCACAAACACGAACC-3′); caspase-3 (for 5′-CCTTGCCTCAAACAAAGGTT-3′, rev 5′-CCTGAGTGGGTTCACAATGTT-3′); and β-actin (for 5′-TTGTAACCAACTGGGACGATATGG-3′, Rev 5′-GATCTTGATCTTCATGGTGCTAGG-3′).
Western Blot Analysis
For Western blot analysis, kidney cortex samples (30 mg) were homogenized in a RIPA buffer (Beyotime, Nantong, Jiangsu, China): 50 mM Tris, 150 mM NaCl, 10% glycerol, 0.1% SDS, 1% Triton X-100, 1 mM EDTA, 0.5% deoxycholate, 1% protease inhibitor mix, pH 7.5 and lysed for 30 min on ice. The homogenate was centrifuged at 14 000 × g (4°C) for 10 min. The supernatants were removed for protein determination using the BCA kit from Thermo Fisher Scientific China (catalog 23227) with BSA as the standard. Subsequently, equal amounts (20–80 μg) of protein samples were separated on 12% SDS-PAGE and transferred to a PVDF membrane (Bio-Rad Laboratories, Inc, Hercules, California). After incubating for 1.5 h in 5% nonfat milk, the membranes were incubated with primary antibodies overnight at 4°C, followed by washing 4 times with TBST, and incubated with a HRP conjugated secondary polyclonal antibody at room temperature for 1.5 h. Blots were developed using Bio-Rad Clarity Western ECL (Hercules). The signal intensities of the bands were estimated using a ChemiDoc XRS+ System (Hercules) and quantified using an Image Lab Software (Hercules).
Histology Examination and IHC
Whole kidney was removed and fixed in 4% paraformaldehyde overnight at 4°C. Fixed kidney samples were embedded in paraffin and sectioned at 5 μm. All the kidney sections were deparaffinized, hydrated through a graded series of xylene, ethanol, and deionized water for hematoxylin and eosin (HE) staining, IHC, and TUNEL assays. For pathological examination, the kidney sections were stained with HE using standard techniques (Liu et al., 2012). The modified 0–5 Jablonsky grading scale was used for histopathological evaluation of the kidney injury (Islam et al., 1997): 0 represents normal; 1 represents occasional degenerative and necrosis of individual cells; 2 represents degenerative cells and necrosis of individual tubules; 3 represents degeneration and necrosis of all cells in adjacent proximal convoluted tubules with survival of surrounding tubules; 4 represents necrosis confined to the distal third of the proximal convoluted tubules, with a band of necrosis extending across the inner cortex; and 5 represents necrosis affecting all 3 segments of the proximal convoluted tubules.
For IHC, the kidney sections were treated to block endogenous peroxidase activity for 10 min with 3% H2O2/H2O solution. Antigen retrieval was performed by heating slides in 10 mM sodium citrate buffer (pH 6.0) at a subboiling temperature for 10 min. Nonspecific antibody binding was blocked with 5% normal goat serum for 1 h at room temperature, the sections were then incubated with primary antibodies against p-JNK (1:50 dilution), p-c-Jun (1:50 dilution), p53 (1:50 dilution), p-p53 (1:50 dilution), or cleaved caspase-3 (1:2000 dilution) overnight at 4°C. Then, the sections were incubated with a HRP linked anti-mouse or rabbit secondary antibody in a humidified chamber for 30 min at room temperature. After staining with DAB, the slides were visualized with microscope (Olympus, Tokyo, Japan) and photographed using a digital camera (Wang et al., 2014).
TUNEL Assay
Animals were killed 6, 12, 24, and 48 h after MPE administration, respectively, and whole kidney samples were fixed with 4% paraformaldehyde in phosphate-buffer saline (PBS) (pH 7.4). Apoptosis of tubular cells was examined by the TUNEL assay using a commercial kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. After that, kidney sections were stained with 4 ng/ml DAPI solution (Sigma-Aldrich, St Louis, Missouri) at 4°C for 5–10 min. TUNEL-positive apoptotic and DAPI positive cells were counted 10 fields at ×200 magnification with a fluorescence microscopy, respectively. Apoptotic index was calculated as TUNEL positive cells/DAPI positive cells (Wang et al., 2014).
Statistical Analysis
All data were expressed as the means ± SD using SPSS version 18.0 (SPSS Inc, Chicago, Illinois). Statistical analyses were performed using 1-way analysis of variance (ANOVA) and Tukey’s test. P < .05 indicates statistically significant difference.
RESULTS
MPE Induced Kidney Injury in Sprague Dawley Rats
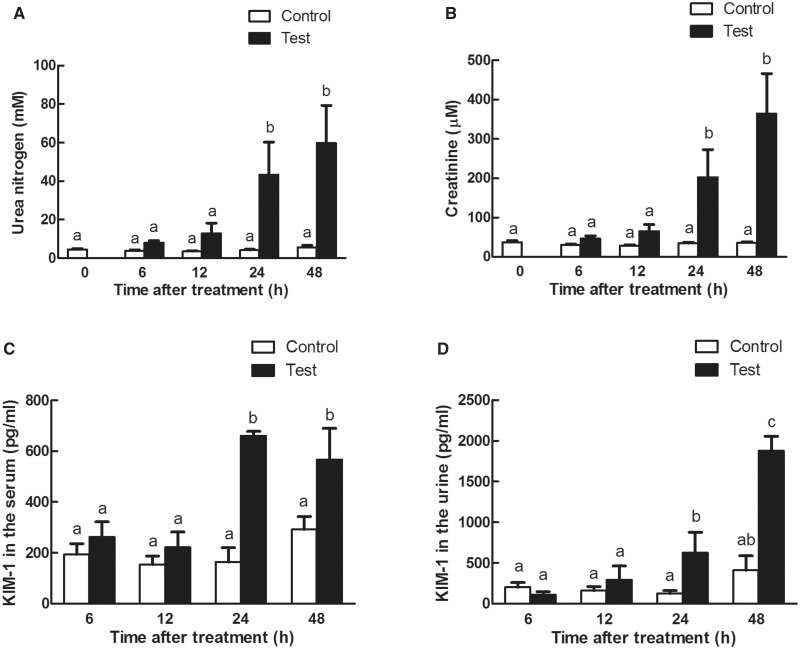
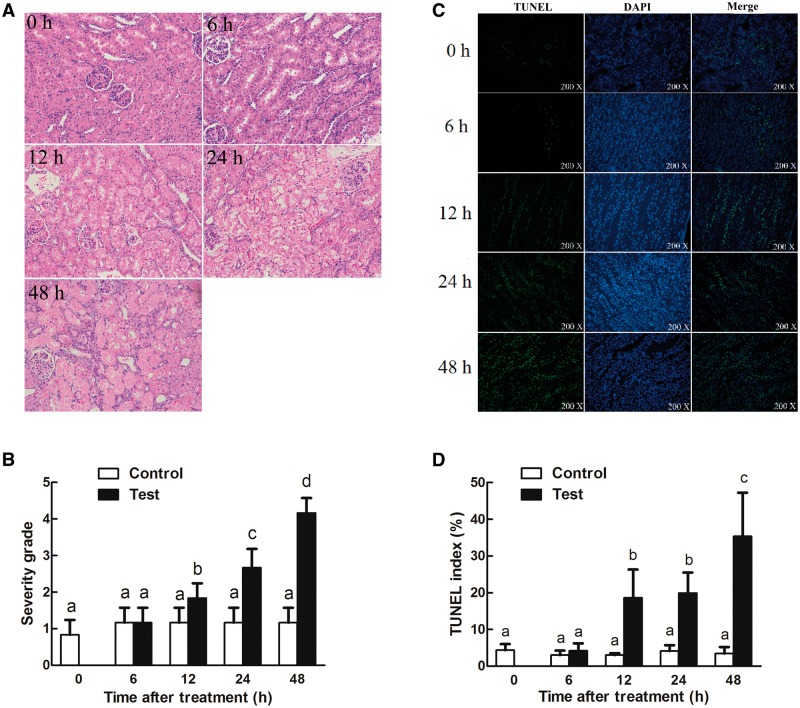
3-MCPD 1-monopalmitic ester (MPE) treatment resulted in the biomarkers of kidney damage. Rat exposed to MPE (1 g/kg BW) had greater absolute and relative kidney weights than those in the control groups in a time-dependent manner (Table 1). Rats in the treatment groups had significant lower EO, RBC, and RDW-CV (Table 2). There was a significant increase of MONO in rats gavaged with MPE at 12 and 24 h, respectively (Table 2), and a smaller increase was noticed for WBC (Table 2). The urine volume, blood, protein, and WBC levels were significantly increased in rats treated with 1 g/kg MPE at indicated time points (Table 3). The urine pH also decreased at 24 and 48 h after MPE treatment (Table 3). In addition, serum creatinine and urea nitrogen levels were significantly increased 24 and 48 h after MPE treatment (Figs. 1A and B). Kim-1, one of the most important biomarkers of kidney injury (Timmeren et al., 2006; Ragab et al., 2014), was also found to be greater in serum and urine 24 h after the treatment (Figs. 1C and D). Histopathological analysis indicated that MPE induced the degeneration, necrosis of tubular cells, and protein casts in the rats in a time-dependent manner (Figs. 2A and B). TUNEL staining (Figs. 2C and D) detected more apoptotic cells in the MPE treated rats than that in the control groups. Together, these results indicated that 3-MCPD 1-monopalmitic ester is nephrotoxic and could induce tubular cell apoptosis in SD rats.
TABLE 1.
Absolute and Relative Kidney Weight of Sprague Dawley Rats Gavaged With 1 g/kg 3-MCPD 1-palmitate
| Absolute Weight/g | Relative weight | |
|---|---|---|
| Blank | 1.8176ab ± 0.0874 | 0.0091a’b’ ± 0.0003 |
| 6 h-control | 1.8482ab ± 0.1340 | 0.0090a’ ± 0.0005 |
| 6 h-test | 2.0677abc ± 0.1777 | 0.0103a’b’ ± 0.0007 |
| 12 h-control | 1.7339a ± 0.0788 | 0.0087a’ ± 0.0003 |
| 12 h-test | 2.1684abc ± 0.1065 | 0.0113b’c’ ± 0.0005 |
| 24 h-control | 1.7550a ± 0.0635 | 0.0089a’ ± 0.0002 |
| 24 h-test | 2.5080c ± 0.0988 | 0.0128c’ ± 0.0004 |
| 48 h-control | 1.7232a ± 0.0385 | 0.0094a’b’ ± 0.0000 |
| 48 h-test | 2.3531bc ± 0.1308 | 0.0129c’ ± 0.0005 |
Different letters represent significant differences (P < .05).
TABLE 2.
Selected Hematology Parameters in Rats Exposed to a Single Dose of 3-MCPD 1-palmitate for 6, 12, 24, and 48 h, Respectively
| Control |
Test |
|||||||
|---|---|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | 6 h | 12 h | 24 h | 48 h | |
| EO# | 0.06 ± 0.01 | 0.053 ± 0.014 | 0.10 ± 0.011 | 0.20 ± 0.029 | 0.039 ± 0.0044 | 0.030 ± 0.0058 | 0.03 ± 0.02* | 0.062 ± 0.020* |
| EO% | 0.74 ± 0.19 | 0.73 ± 0.15 | 1.16 ± 0.12 | 1.38 ± 0.19 | 0.69 ± 0.069* | 0.28 ± 0.080* | 0.20 ± 0.10* | 0.48 ± 0.14* |
| HCT | 46.70 ± 0.79 | 43.97 ± 0.59 | 47.70 ± 0.66 | 58.03 ± 0.94 | 49.06 ± 1.12 | 48.27 ± 0.54 | 45.68 ± 0.83 | 44.82 ± 0.89* |
| HGB | 145.17 ± 2.80 | 142.50 ± 2.70 | 157.40 ± 2.32 | 165.67 ± 2.42 | 152.50 ± 3.40 | 156.86 ± 1.65 | 151.00 ± 3.12 | 154.60 ± 2.52* |
| MCH | 21.53 ± 0.18 | 21.33 ± 0.16 | 21.38 ± 0.22 | 21.02 ± 0.18 | 21.58 ± 0.26 | 21.67 ± 0.23 | 21.72 ± 0.32 | 21.46 ± 0.34 |
| MCHC | 310.67 ± 3.24 | 324.00 ± 3.16 | 330.20 ± 3.01 | 312.50 ± 2.53 | 310.88 ± 1.06 | 325.14 ± 1.58 | 330.57 ± 3.52 | 345.00 ± 3.15 |
| MCV | 69.28 ± 1.05 | 65.85 ± 0.99 | 64.84 ± 0.59 | 67.25 ± 0.58 | 69.41 ± 0.76 | 66.70 ± 0.86 | 65.76 ± 0.90 | 62.26 ± 1.26 |
| MONO# | 0.20 ± 0.03 | 0.12 ± 0.037 | 0.44 ± 0.044 | 0.49 ± 0.034 | 0.25 ± 0.66 | 1.34 ± 0.15* | 1.67 ± 0.22* | 1.14 ± 0.28 |
| MONO% | 2.43 ± 0.38 | 1.83 ± 0.56 | 5.06 ± 0.34 | 3.72 ± 0.16 | 4.36 ± 0.89 | 11.82 ± 1.32* | 12.37 ± 1.40* | 10.92 ± 1.73 |
| MPV | 9.15 ± 0.10 | 8.77 ± 0.17 | 8.10 ± 0.21 | 8.03 ± 0.092 | 9.30 ± 0.16 | 8.61 ± 0.11 | 8.24 ± 0.13 | 8.04 ± 0.12 |
| PCT | 1.16 ± 0.01 | 1.11 ± 0.040 | 1.07 ± 0.042 | 1.15 ± 0.029 | 1.35 ± 0.12 | 1.10 ± 0.04 | 1.12 ± 0.082 | 1.13 ± 0.04 |
| PDW | 10.05 ± 0.15 | 9.55 ± 0.26 | 8.66 ± 0.28 | 8.77 ± 0.14 | 10.59 ± 0.30 | 9.36 ± 0.21 | 8.77 ± 0.17 | 8.56 ± 0.15 |
| PLT | 1273.83 ± 18.16 | 1261.83 ± 35.45 | 1321.20 ± 58.73 | 1435.33 ± 35.18 | 1442.25 ± 97.72 | 1275.43 ± 47.46 | 1355.14 ± 83.05 | 1409.20 ± 48.73 |
| RBC | 6.74 ± 0.11 | 6.68 ± 0.14 | 7.36 ± 0.11 | 7.89 ± 0.16 | 7.08 ± 0.22 | 7.24 ± 0.13 | 6.95 ± 0.11* | 7.20 ± 0.15* |
| RDW-CV | 17.32 ± 0.42 | 17.10 ± 0.48 | 16.86 ± 0.52 | 18.88 ± 0.24 | 18.20 ± 1.03 | 16.67 ± 0.35 | 15.41 ± 0.27* | 16.44 ± 0.60* |
| WBC | 8.45 ± 0.50 | 7.05 ± 0.65 | 8.72 ± 0.50 | 13.30 ± 0.73 | 5.78 ± 0.47 | 10.92 ± 1.03* | 13.59 ± 0.84* | 10.74 ± 1.88 |
*Represents significant differences compared with the control at the same time point (P < .05). EO#, absolute levels of EO counts; MONO#, absolute levels of MONO counts.
TABLE 3.
Urine Chemistry Alterations in Rats Exposed to a Single Dose of 3-MCPD 1-palmitate for 6, 12, 24, and 48 h, Respectively
| Control |
Test |
|||||||
|---|---|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | 6 h | 12 h | 24 h | 48 h | |
| Urine volume (ml) | 3.07 ± 1.09 | 1.75 ± 0.57 | 6.40 ± 1.57 | 6.50 ± 0.72 | 9.19* ± 0.63 | 1.76 ± 0.85 | 3.50 ± 1.10 | 3.49 ± 1.78 |
| pH | 6.25 ± 0.14 | 6.50 ± 0.20 | 6.83 ± 0.21 | 6.80 ± 0.20 | 6.31 ± 0.13 | 6.38 ± 0.24 | 5.80* ± 0.20 | 5.75* ± 0.25 |
| BLD | — | — | — | — | — | — | 0.75* | 2. 5* |
| PRO | — | 0.125 | 0.125 | 0.125 | — | 2. 25* | 2. 2* | 3* |
| WBC | — | — | — | — | 0. 67* | 0.5* | 0.9* | 3* |
*Represents significant differences compared with the control at the same time point (P < .05).
BLD, blood; PRO, protein; WBC, white blood cells.
FIG. 1.
Time course of serum and urine chemistry parameters in Sprague Dawley rats exposed to 1 g/kg of 3-MCPD 1-palmitate. A, Serum urine nitrogen levels; B, serum creatinine levels; C, Kim-1 production in the serum; and D, Kim-1 excretion in the urine were measured at the indicated time points. Different letters represent significant differences at P < .05.
FIG. 2.
Time course of tubular damage and tubular cell apoptosis in Sprague Dawley rats treated with 1 g/kg of 3-MCPD 1-palmitate. Kidney was harvested at the indicated time points and examined with (A and B) hematoxylin and eosin staining and (C and D) TUNEL staining as described in the Material and Methods section. The evaluation of tubular damage and tubular cell apoptosis involved averaging the values from 10 fields per kidney under microscope (×200 magnification). Different letters represent significant differences at P < .05.
Microarray Analysis of mRNA Expression Profile in the Kidney of Sprague Dawley Rats Exposed to MPE
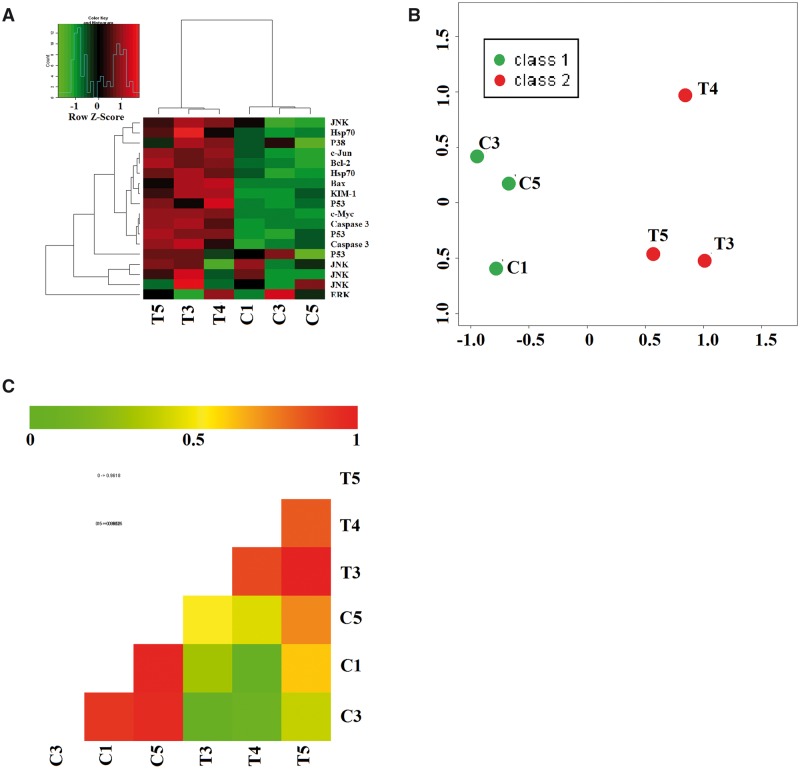
A total of 2566 mRNA were altered by MPE treatment as compared with the control group using microarray (P < .05; data not shown). Analysis with Gene Ontology categories and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways revealed that MPE altered mRNAs related to a large set of genes mainly involved in MAPK and p53 signal transduction pathways. The distribution of group specific signal intensities of mRNAs related to MAPK, p53 and their downstream apoptosis pathways were listed in Figure 3. Heatmap, principal component analysis (PCA) and correlation analysis of the differentially mRNAs specific to the target pathways suggested that there were significant differences in these genes between the control and test groups (Figure 3).
FIG. 3.
mRNA expression profiling in the kidney of Sprague Dawley rats gavaged with 1 g/kg 3-MCPD 1-palmitate for 24 h. A, Heat-map depicts mRNAs that are either upregulated or downregulated between test (T3, T4, T5) and control (C1, C3, C5) groups. Y-axis represents the normalized microarray hybridization signal. (Green): lower expression (red): higher expression. B, Principal component analysis; and C, Correlation analysis.
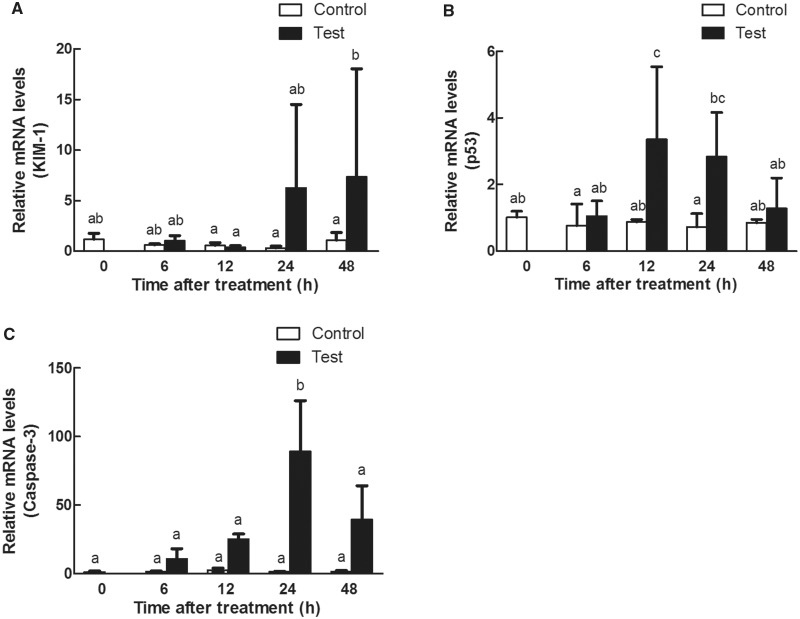
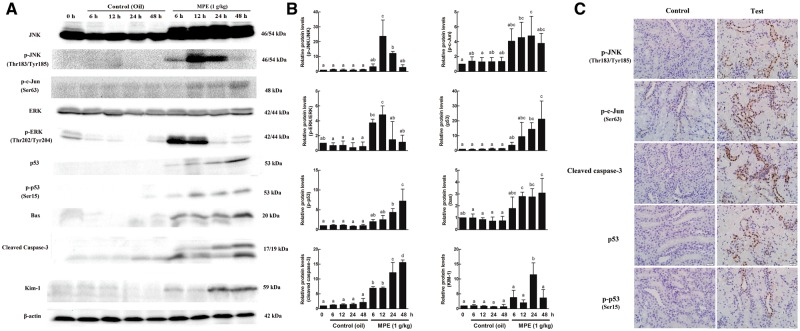
The expression patterns of the mRNAs detected by microarray analysis were confirmed with qRT-PCR gene chip data. Kim-1 expression level was significantly increased (P < .05) in a time-dependent manner (Figure 4A). p53 (Figure 4B) and caspase-3 (Figure 4C) expressions increased, and reached the peak at 24 h (P < .05) and decreased at 48 h.
FIG. 4.
Time response study on the renal mRNA expressions of (A) Kim-1, (B) p53, and (C) caspase-3 in Sprague Dawley rats gavaged with 1 g/kg 3-MCPD 1-palmitate. Different letters represent significant differences at P < .05.
MPE Induced Tubular Cells Apoptosis via MAPK and p53 Signal Transduction Pathways
The effects of MPE on the target proteins related to MAPK and p53 apoptosis pathway were also analyzed by Western blot. The phosphorylation levels of JNK and c-Jun were upregulated in the kidney of the SD rats gavaged with MPE (Figs. 5A and B). ERK was also activated by MPE treatment (Figs. 5A and B) but p38 was not (Supplementary Figure S1). The p53 protein translation level was upregulated, along with an increase in its phosphorylation levels (Figs. 5A and B). In addition, both bax and the cleaved caspase-3 levels were significantly increased (Figs. 5A and B). However, no significant change was found for bcl-2 (Supplementary Figure S1). The protein level of Kim-1was also time-dependently increased and reached the maximum level at 24 h (Figs. 5A and B). IHC assay was performed to confirm the regions in kidney that showing target proteins expressions. The protein levels of p-JNK, p-c-Jun, cleaved caspase-3, p53, and p-p53 were all upregulated in the kidney of rats gavaged with 1 g/kg MPE compared with those in the control rats (Figure 5C). Also, proteins related to MAPK and p53 apoptosis pathways were all expressed in the tubular cells of the kidney cortex (Figure 5C).
FIG. 5.
Effects of 3-MCPD palmitate on the protein levels in the kidney of Sprague Dawley rats using Western blot and immunohistochemistry (IHC) staining. A, Representative Western blot. Western blot was performed using antibodies against JNK, p-JNK, p-c-Jun, ERK, p-ERK, p53, p-p53, bax, cleaved caspase-3, and Kim-1. β-actin was used as a loading control. B, Quantitated Western blot data using Image Lab software. Western blots from 3 animals were quantitated as described in Materials and Methods. Different letters represent significant differences at P < .05. C, Representative IHC of kidney cortex tubular cells for localization of p-JNK, p-c-Jun, cleaved caspase-3, p53, and p-p53 (×200 magnification).
The Possible Role of p53 in Upregulation of Cleaved Caspase-3 and Tubular Cells Apoptosis Induced by MPE
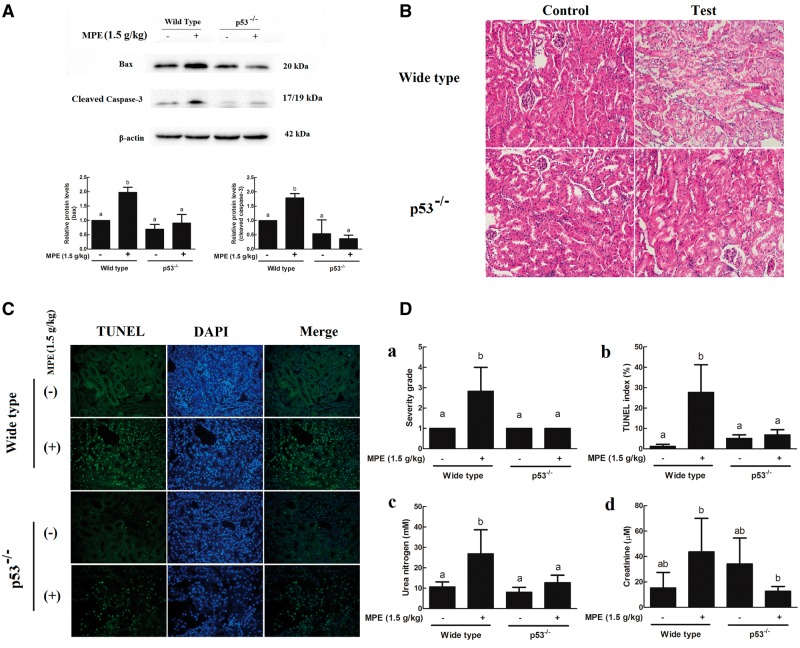
To ascertain whether p53 was the key target for MPE induced tubular cells apoptosis, p53 knockout mice were employed to examine their responses to MPE treatment. Unlike WT mice, protein levels of bax and the cleaved caspase-3 in the kidney of p53−/− mice gavaged with MPE had no detectable change as compared with the control mice but were lower than that in the treated WT mice (P < .05) (Figure 6A).
FIG. 6.
p53 is required for upregulation of proteins, tubular damage, tubular cells apoptosis, and serum chemistry levels induced by 3-MCPD 1-palmitate. A, Western blots analysis of kidney extracts showed increased levels of apoptosis-related proteins bax and cleaved caspase-3 in wild type (WT) C57BL/6 mice but not in the p53−/− mice. Kidney were harvested after 24 h of the treatment and examined with (B, Da) hematoxylin and eosin staining and (C, Db) TUNEL staining as described in the Material and Methods section. The evaluation of tubular damage and tubular cell apoptosis involved averaging the values from 10 fields per kidney under microscope (×200 magnification). (Dc) Serum urea nitrogen and (Dd) creatinine levels were measured in WT and p53−/− mice after 24 h treatment of 3-MCPD 1-palmitate. Different letters represent significant differences at P < .05.
Histopathological examination showed that p53−/− mice were less sensitive than WT mice to MPE induced kidney injury (Figs. 6B and Da). Consistently, there were less apoptotic tubular cells in the kidney of p53−/− mice than that in the WT mice (Figs. 6C and Db). In addition, serum urea nitrogen level was significantly increased in the WT mice but no change was observed in the p53−/− mice treated with the same concentrations of MPE (Figure 6Dc). However, neither WT nor p53−/− mouse had a significantly greater level of serum creatinine (Figure 6Dd).
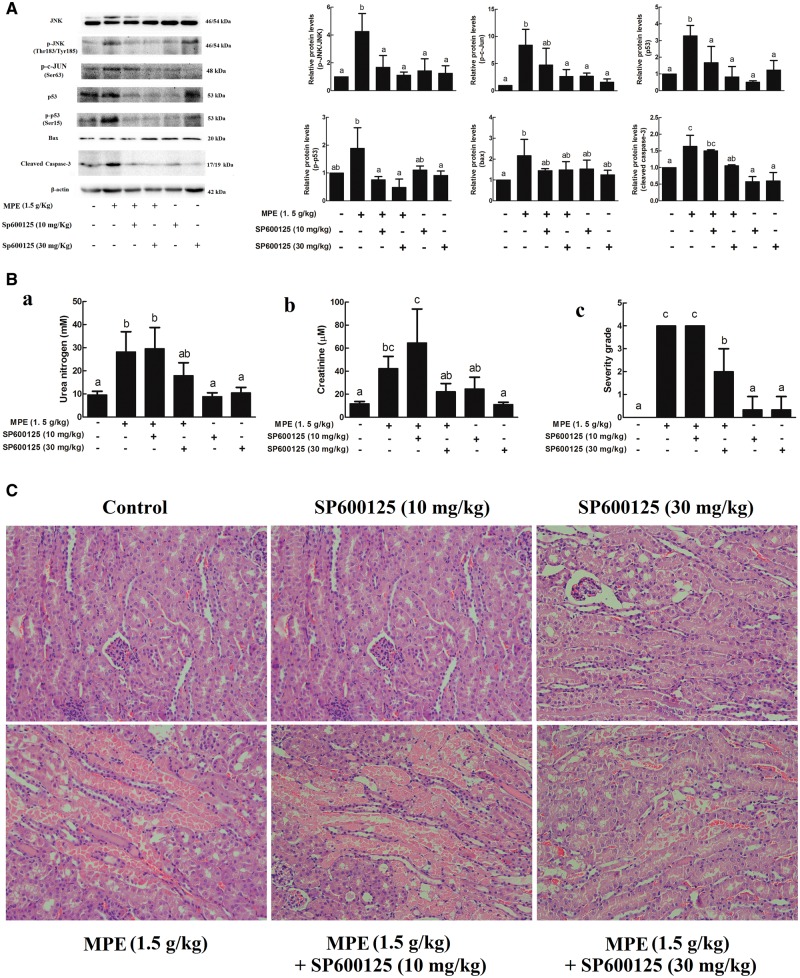
Confirmation of the Critical Role of JNK in MPE-Induced Tubular Cells Apoptosis
To determine whether p-JNK was involved in the MPE induced kidney injury, JNK inhibitor SP600125 was given to mice through intraperitoneal injection at 10 and 30 mg/kg BW doses. Kidney JNK activity, as represented by the amount of phosphorylated JNK and c-Jun in the kidney 24 h after MPE treatment, was significantly induced as compared with that in the control group, and this increase was not observed in the mice given both MPE and SP600125 (Figure 7A). SP600125 also blocked the MPE-induced kidney JNK activity increase in a dose-dependent manner (Figure 7A). Moreover, the protein levels of p53, p-p53, and cleaved caspase-3 were inhibited by SP00125 (Figure 7A). In addition, SP600125 injection didn’t abrogate ERK activation (Supplementary Figure S2) and had no effect on the p-p38 or bcl-2 protein level (Supplementary Figure S2) or serum urea nitrogen and creatinine levels in mice given 30 mg/kg SP600125 (Figure 7Bab). Interestingly, 30 mg/kg JNK inhibitor almost completely eliminated kidney injury induced by MPE (Figs. 7Bc and C).
FIG. 7.
Effects of JNK inhibitor SP600125 on the protein levels, serum chemistry levels and kidney damage induced by 3-MCPD 1-palmitate. SP600125 were administrated to C57BL/6 mice through intraperitoneal injection at doses of 10 and 30 mg/kg body weight 1 h before 3-MCPD 1-palmitate treatment. Twenty-four hours later, kidney was harvested for (A) Western blot analysis. Blood were collected for (Ba) serum urea nitrogen and (Bb) creatinine determination. (Bc, C) Kidney damage were evaluated by using hematoxylin and eosin staining and graded based on the 0–5 Jablonsky grading scale under microscopy (×200 magnification). Different letters represent significant differences at P < .05.
Potential Role of ERK in MPE-Induced Acute Kidney Injury
Whether ERK activation is necessary for MPE-induced tubular cells apoptosis was investigated using ERK inhibitor U0126. As shown in Supplementary Figures S3A and B, ERK phosphorylation was increased after MPE administration, which was significantly alleviated by U0126. Meanwhile, no change in p53, p-p53, or cleaved caspase-3 levels was observed in the kidney of mice administrated U0126 (Supplementary Figs. S3A and B). Serum creatinine and urea nitrogen levels had no change in mice regardless of U0126 administration (Supplementary Figure S3C).
DISCUSSION
Recent researches suggested that kidney was the major target of 3-MCPD fatty acid esters in Wistar and F344 rat and Swiss mouse models (Barocelli et al., 2011; Liu et al., 2012; Onami et al., 2014). This study confirmed that 3-MCPD 1-monopalmitic ester (MPE) induced kidney injury in both experimental Sprague Dawley rats and C57BL/6 mice at 1 and 1.5 g/kg BW, respectively.
In 2003, Padanilam (2003) indicated that tubular cell apoptosis plays a central role in xenobiotic-induced kidney injury. Human clinical studies also suggested a correlation between cell death and acute kidney injury (Price et al, 2009). This study also investigated the hypothesis that tubular cell apoptosis plays a critical role in acute renal damage induced by 3-MCPD fatty acid esters. Western blot assay showed upregulation of bax, and IHC results showed increased cleaved caspase-3 expression in rats treated with MPE in the tubular cells of the kidney cortex area. The results clearly demonstrated that MPE significantly induced tubular cell apoptosis. This finding is supported by observations that free 3-MCPD and its esters induced-apoptosis in several other cell types (Sun et al., 2013; Buhrke et al., 2014). Sun et al. (2013) demonstrated that 3-MCPD significantly induced early apoptosis in R2C rat leydig cells, which was associated with mitochondrial injury. In 2014, Buhrke et al. (2014) observed that 3-MCPD ester induced caspase activity in Caco-2 cells. Taken together, the malfunction of kidney induced by MPE in this study might be attributed to inducing tubular cell apoptosis.
In addition, the results from this study suggested a role of MAPKs/p53 activation in the MPE-induced tubular cell apoptosis under the experimental conditions, for the first time. This conclusion is supported by qRT-PCR (Figure 3), western and IHC data (Figure 5) and being further confirmed using p53 knockout (p53−/−) mice and specific inhibitors altering MAPKs (Figure 6 and Supplementary Figure S3) and p53 (Figure 7) interactions. The MAPKs are well accepted upstream modulators of apoptosis (Chang et al., 2014; Lee et al., 2008), including ERKs, JNKs, and p38 pathways. The phosphorylation levels of JNK and c-Jun were upregulated in the kidney of rats gavaged with MPE and p-JNK and p-c-Jun positive cells were tubular cells in the kidney cortex area. In addition, JNK inhibitor almost completely eliminated kidney injury induced by MPE. Taken together, it was concluded that JNK plays a pivotal role in the tubular cell apoptosis, consistent with its function in nephrotoxicity (Servais et al., 2008). Although the significant activation of ERK induced by MPE was found, the inhibitor of ERK failed to block caspase-3 activation (Supplementary Figure S3), which argues against the possibility of a primary role for this pathway in MPE-induced tubular cell apoptosis. Also, MPE could not affect the phosphorylation levels of p38MAP kinase. These results are inconsistent with common roles of ERK and p38 in cell death regulation (Chang et al., 2014; Lee et al., 2008). p53 is critical for apoptosis induction, which involves interaction with members of the bcl-2 family proteins (Moll et al., 2005; Servais et al., 2008; Peng et al., 2015). Our western and IHC results indicated the increased expression of p53 and p-p53 in the kidney cortex of rats gavaged with MPE. Moreover, much less apoptotic cells by TUNEL assay were observed in the kidney cortex of p53−/− mice than that in WT mice after MPE treatment, indicating that MPE induced tubular cell apoptosis was p53 dependent. Furthermore, the MPE-induced p53 dependent apoptosis was associated with bax expression, which was supported by no detectable change in the protein levels of bax in the kidneys of p53−/− mice gavaged with MPE compared with the control mice but were lower than that in the treated WT mice. Together, these data suggest that MPE induced “p53-mitochondria” cross-talk. In addition, we identified JNK as the upstream targets of p53 activation using JNK inhibitor SP600125, which revealed that JNK/p53 pathway played a critical role in the kidney apoptosis induced by MPE. The similar interaction between JNK and p53 was also found in cardiomyocytes (Sun et al., 2014), prostate cancer cells (Itsumi et al., 2014), and human umbilical vein endothelial cells (Liu and Sun, 2010). In contrast, JNK mediated apoptosis through p53 defect pathway in SNU-16, U937, and 293T cell lines (Bae et al., 2006). The different interaction between JNK and p53 might be partially explained by the cell-type-specific differences and the contexts of experimental conditions. It needs to be pointed out that JNK1, JNK2, and JNK3 may play different roles in cell apoptosis (Qu et al., 2013; Fernandes et al., 2012). The molecular mechanisms of MPE in triggering the activation of JNK1, JNK2, or JNK3-mediated tubular cell apoptosis are still unclear, and should be investigated further. In conclusion, exposure to the common food contaminant MPE may induce kidney toxicity through inducing the tubular cell apoptosis by activating JNK/p53 related apoptosis pathway.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported by the financial support from a Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201203069), the National High Technology Research and Development Program of China (Grant Nos. 2013AA102202, 2013AA102207), a Grant from the Wilmar (Shanghai) Biotechnology Research & Development Center Co Ltd and a Grant from Beijing Advanced Innovation Center for Food Nutrition and Human Health.
Supplementary Material
REFERENCES
- Bae I. H., Kang S. W., Yoon S. H., Um H. D. (2006). Cellular components involved in the cell death induced by cisplatin in the absence of p53 activation. Oncol. Rep. 15, 1175–1180. [PubMed] [Google Scholar]
- Barocelli E., Corradi A., Mutti A, Petronini P. G. (2011). Comparison between 3-MCPD and its palmitic esters in a 90-day toxicological study. Available at: http://www.efsa.europa.eu/en/supporting/pub/187e.htm. Accessed June 2011.
- Buhrke T., Frenzel F., Kuhlmann J., Lampen A. (2014). 2-Chloro-1, 3-propanediol (2-MCPD) and its fatty acid esters: Cytotoxicity, metabolism, and transport by human intestinal Caco-2 cells. Arch. Toxicol. 89, 2243–2251. [DOI] [PubMed] [Google Scholar]
- Chang C. Y., Shen C. Y., Kang C. K., Sher Y. P., Sheu W. H., Chang C. C., Lee T. H. (2014). Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol. Appl. Pharmacol. 279, 351–363. [DOI] [PubMed] [Google Scholar]
- Fernandes K. A., Harder J. M., Fornarola L. B., Freeman R. S., Clark A. F., Pang I. H., John S. W., Libby R. T. (2012). JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol. Dis. 46, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlet C. G., Sadd P. A. (2004). Chloropropanols and their esters in cereal products. Czech J. Food Sci. 22, 259–262. [Google Scholar]
- Hortelano S., Castilla M., Torres A. M., Tejedor A., Boscá L. (2000). Potentiation by nitric oxide of cyclosporin A and FK506-induced apoptosis in renal proximal tubule cells. J. Am. Soc. Nephrol. 11, 2315–2323. [DOI] [PubMed] [Google Scholar]
- Islam C. F., Mathie R. T., Dinneen M. D., Kiely E. A., Peters A. M., Grace P. A. (1997). Ischaemia-reperfusion injury in the rat kidney: The effect of preconditioning. Br. J. Urol. 79, 842–847. [DOI] [PubMed] [Google Scholar]
- Itsumi M., Shiota M., Yokomizo A., Takeuchi A., Kashiwagi E., Dejima T., Inokuchi J., Tatsugami K., Uchiumi T., Naito S. (2014). PMA induces androgen receptor downregulation and cellular apoptosis in prostate cancer cells. J. Mol. Endocrinol. 53, 31–41. [DOI] [PubMed] [Google Scholar]
- Jo S. K., Cho W. Y., Sung S. A., Kim H. K., Won N. H. (2005). MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 67, 458–466. [DOI] [PubMed] [Google Scholar]
- Kaze N., Sato H., Yamamoto H., Watanabe Y. (2011). Bidirectional conversion between 3-monochloro-1, 2-propanediol and glycidol in course of the procedure of DGF standard methods. J. Am. Oil Chem. Soc. 88, 1143–1151. [Google Scholar]
- Larsen J. C. 2009. 3-MCPD esters in food products. Summary report of a workshop held in February 2009 in Brussels, Belgium. Organised by the International Life Sciences Institute (ILSI) Europe process-related compounds and natural toxins task force and risk assessment of chemicals in food task force in association with the European Commission (EC) and the European Food Safety Authority (EFSA). Available at:http://www.ilsi.org/europe/publications/final%20version%203%20mcpd%20esters.pdf. Accessed October 2009.
- Lee K. B., Kim K. R., Huh T. L., Lee Y. M. (2008). Proton induces apoptosis of hypoxic tumor cells by the p53-dependent and p38/JNK MAPK signaling pathways. Int. J. Oncol. 33, 1247–1256. [PubMed] [Google Scholar]
- Liu M., Gao B. Y., Qin F., Wu P. P., Shi H. M., Luo W., Ma A. N., Jiang Y. R., Xu X. B., Yu L. L. (2012). Acute oral toxicity of 3-MCPD mono- and di-palmitic esters in Swiss mice and their cytotoxicity in NRK-52E rat kidney cells. Food Chem. Toxicol. 50, 3785–3791. [DOI] [PubMed] [Google Scholar]
- Liu X., Sun J. (2010). Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-κB pathways. Biomaterials 31, 8198–8209. [DOI] [PubMed] [Google Scholar]
- Mikhailov V., Mikhailova M., Degenhardt K., Venkatachalam M. A., White E., Saikumar P. (2003). Association of bax and bak homo-oligomers in mitochondria: Bax requirement for bak reorganization and cytochrome c release. J. Biol. Chem. 278, 5367–5376. [DOI] [PubMed] [Google Scholar]
- Moll U. M., Wolff S., Speidel D., Deppert W. (2005). Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 17, 631–636. [DOI] [PubMed] [Google Scholar]
- Onami S., Cho Y. M., Toyoda T., Mizuta Y., Yoshida M., Nishikawa A., Ogawa K. (2014). A 13-week repeated dose study of three 3-monochloropropane-1, 2-diol fatty acid esters in F344 rats. Arch. Toxicol. 88, 871–880. [DOI] [PubMed] [Google Scholar]
- Padanilam B. J. (2003). Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am. J. Physiol. Renal Physiol. 284, 608–627. [DOI] [PubMed] [Google Scholar]
- Peng J., Li X., Zhang D., Chen J. K., Su Y., Smith S. B., Dong Z. (2015). Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 87, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. M., Safirstein R. L., Megyesi J. (2009). The cell cycle and acute kidney injury. Kidney Int. 76, 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C., Li W., Shao Q., Dwyer T., Huang H., Yang T., Liu G. (2013). c-Jun N-terminal kinase 1 (JNK1) is required for coordination of netrin signaling in axon guidance. J. Biol. Chem. 288, 1883–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros Y., Sánchez-González P. D., López-Hernández F. J., Morales A. I., López-Novoa J. M. (2013). Cardiotrophin-1 administration prevents the renal toxicity of iodinated contrast media in rats. Toxicol. Sci. 132, 493–501. [DOI] [PubMed] [Google Scholar]
- Ragab D., Abdallah D. M., El-Abhar H. S. (2014). Cilostazol renoprotective effect: Modulation of PPAR-γ, NGAL, Kim-1 and IL-18 underlies its novel effect in a model of ischemia-reperfusion. PLoS One 9, e95313.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais H., Ortiz A., Devuyst O., Denamur S., Tulkens P. M., Mingeot-Leclercq M. P. (2008). Renal cell apoptosis induced by nephrotoxic drugs: Cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 13, 11–32. [DOI] [PubMed] [Google Scholar]
- Shi H., Yang H., Zhang X., Sheng Y., Huang H., Yu L. (2012). Isolation and characterization of five glycerol esters from Wuhan propolis and their potential anti-inflammatory properties. J. Agric. Food Chem. 60, 10041–10047. [DOI] [PubMed] [Google Scholar]
- Sun A., Zou Y., Wang P., Xu D., Gong H., Wang S., Qin Y., Zhang P., Chen Y., Harada M., et al. (2014). Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J. Am. Heart Assoc. 3, e000779.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Bai S., Bai W., Zou F., Zhang L., Su Z., Zhang Q., Ou S., Huang Y. (2013). Toxic mechanisms of 3-monochloropropane-1, 2-diol on progesterone production in R2C rat leydig cells. J. Agric. Food Chem. 61, 9955–9960. [DOI] [PubMed] [Google Scholar]
- Svejkovská B., Novotný O., Divinová V., Réblová Z., Doležal M., Velíšek J. (2004). Esters of 3-chloropropane-1, 2-diol in foodstuffs. Czech J. Food Sci. 22, 190–196. [Google Scholar]
- Timmeren M. M., Bakker S. J., Vaidya V. S., Bailly V., Schuurs T. A., Damman J., Stegeman C. A., Bonventre J. V., Goor H. V. (2006). Tubular kidney injury molecule-1 in protein-overload nephropathy. Am. J. Physiol. Renal Physiol. 291, 456–464. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhu S., Qian L., Gao J., Wu M., Gao J., Zhang Y., Chan G. L., Yu Y., Han W. (2014). IL-1Ra selectively protects intestinal crypt epithelial cells, but not tumor cells, from chemotoxicity via p53-mediated upregulation of p21WAF1 and p27KIP1. Pharmacol. Res. 82, 21–33. [DOI] [PubMed] [Google Scholar]
- Yoshikane Y., Koga M., Imanaka-Yoshida K., Cho T., Yamamoto Y., Yoshida T., Hashimoto J., Hirose S., Yoshimura K. (2015). JNK is critical for the development of Candida albicans-induced vascular lesions in a mouse model of Kawaski disease. Cardiovasc. Pathol. 24, 33–40. [DOI] [PubMed] [Google Scholar]
- Zelinková Z., Svejkovská B., Velíšek J., Doležal M. (2006). Fatty acid esters of 3-chloropropane-1, 2-diol in edible oils. Food Addit. Contam. 23, 1290–1298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.