Abstract
High-throughput screening for potential thyroid-disrupting chemicals requires a system of assays to capture multiple molecular-initiating events (MIEs) that converge on perturbed thyroid hormone (TH) homeostasis. Screening for MIEs specific to TH-disrupting pathways is limited in the U.S. Environmental Protection Agency ToxCast screening assay portfolio. To fill 1 critical screening gap, the Amplex UltraRed-thyroperoxidase (AUR-TPO) assay was developed to identify chemicals that inhibit TPO, as decreased TPO activity reduces TH synthesis. The ToxCast phase I and II chemical libraries, comprised of 1074 unique chemicals, were initially screened using a single, high concentration to identify potential TPO inhibitors. Chemicals positive in the single-concentration screen were retested in concentration-response. Due to high false-positive rates typically observed with loss-of-signal assays such as AUR-TPO, we also employed 2 additional assays in parallel to identify possible sources of nonspecific assay signal loss, enabling stratification of roughly 300 putative TPO inhibitors based upon selective AUR-TPO activity. A cell-free luciferase inhibition assay was used to identify nonspecific enzyme inhibition among the putative TPO inhibitors, and a cytotoxicity assay using a human cell line was used to estimate the cellular tolerance limit. Additionally, the TPO inhibition activities of 150 chemicals were compared between the AUR-TPO and an orthogonal peroxidase oxidation assay using guaiacol as a substrate to confirm the activity profiles of putative TPO inhibitors. This effort represents the most extensive TPO inhibition screening campaign to date and illustrates a tiered screening approach that focuses resources, maximizes assay throughput, and reduces animal use.
Keywords: endocrine, thyroid < endocrine toxicology in vitro and altenatives, predictive toxicology < in vitro and altenatives
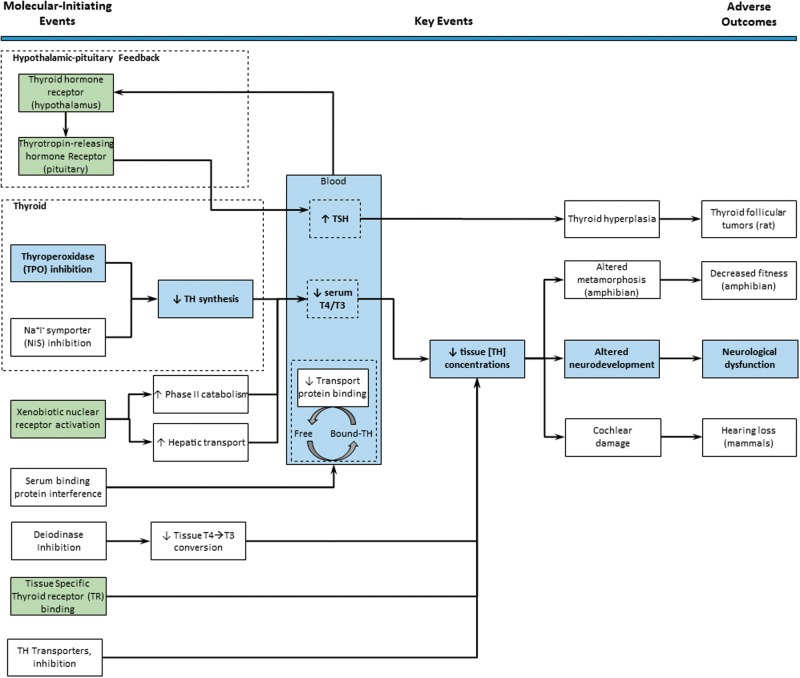
The U.S. Environmental Protection Agency (EPA) developed the Endocrine Disrupter Screening Program (EDSP) to determine the potential for environmentally relevant chemicals to interfere with the estrogen pathway, and additional efforts have been made to consider other endocrine pathways, including androgen and thyroid hormone (TH). TH homeostasis can be disrupted by xenobiotics via a number of postulated molecular-initiating events (MIEs) including: (1) interference with hypothalamic-pituitary feedback mechanisms; (2) inhibition of thyroperoxidase (TPO); (3) inhibition of glandular iodide uptake by the sodium-iodide symporter; (4) nuclear receptor-mediated increases in the metabolic clearance rate of THs; (5) competitive binding to TH serum binding proteins; (6) inhibition of peripheral iodothyronine deiodinases; (7) interference with the TH receptor complex in target tissues; and/or (8) inhibition of TH transporters in target tissues (Figure 1) (Capen, 1994; Crofton et al., 2005; DeVito et al., 1999; Murk et al., 2013). As maintenance of euthyroid status involves a system of regulatory processes across multiple tissues, an integrated high-throughput screening (HTS) approach to identify chemicals that perturb thyroid signaling via any 1 of these MIEs is a critical step toward reducing whole animal testing and focusing additional in silico, in vitro, and in vivo testing resources on chemicals that may perturb early key events in thyroid-related adverse outcome pathways (AOPs) (Miller et al., 2009; Perkins et al., 2013).
FIG. 1.
A network view of known potential molecular-initiating events (MIEs) for thyroid disruption. Several putative adverse outcome pathways (AOPs) for thyroid hormone (TH) disruption are integrated into a combined AOP network. Given significant perturbation at each step, MIEs (left) proceed to key events (middle), and then to adverse outcomes (right). MIEs with related assays available in ToxCast are highlighted in green. Thyroperoxidase (TPO) inhibition, and the key events of the AOP connecting TPO inhibition to adverse neurological outcomes, are highlighted in blue (Zoeller and Crofton, 2005). Other adverse outcomes related to TH perturbation are also shown, including hearing loss, altered amphibian metamorphosis, and rodent thyroid tumors (Crofton and Zoeller, 2005; Pickford, 2010; McClain, 1989).
A well-characterized MIE for adverse thyroid-mediated outcomes is inhibition of TPO. TPO catalyzes iodine oxidation in the presence of hydrogen peroxide, regulates nonspecific iodination of tyrosyl residues of thyroglobulin to form TH precursors, monoiodotyrosine (MIT), and diiodotyrosine (DIT) (Kessler et al., 2008), and modulates coupling of these iodotyrosyl residues (Divi and Doerge, 1994; Ruf and Carayon, 2006; Taurog et al., 1996). Thyroxine (T4, derived from DIT + DIT coupling) and triiodothyronine (T3, derived from MIT + DIT coupling) are subsequently liberated from thyroglobulin by proteolysis in the thyroid follicular cells and released into systemic circulation (Zoeller et al., 2007). Xenobiotics known to inhibit TPO in model systems include the ultraviolet filter benzophenone-2 (BP2), dietary isoflavones, the parasiticide malachite green, and ethylene bisdithiocarbamate pesticides including mancozeb, ziram, and zineb (Brucker-Davis, 1998). The consequence of TPO inhibition in vivo, as demonstrated by anti-hyperthyroidism medications including methimazole (MMI) and 6-propyl-2-thiouracil (PTU), is decreased TH synthesis in humans and across other animal species (Coady et al., 2010; Emiliano et al., 2010; Trepanier, 2006; Van Herck et al., 2013). Moderate, subclinical decreases in thyroxine availability during early gestation alter TH-dependent neurodevelopment (Berbel et al., 2009; Cuevas et al., 2005; Howdeshell, 2002; Morreale de Escobar et al., 2000; Zoeller and Crofton, 2000). Maternal hypothyroxinemia is a risk factor strongly correlated to adverse outcomes in human children, including irreversible neurological dysfunction marked by decreased measures of intelligence, cognition, motor skills, and learning deficits (Haddow et al., 1999; Henrichs et al., 2010; Kooistra et al., 2006; Li et al., 2010; Pop et al., 2003, 1999; Zoeller and Rovet, 2004). It is this link between TH signaling and neurodevelopment which propels the effort to identify chemicals that potentially interfere with TH homeostasis, including chemicals that may inhibit TPO activity.
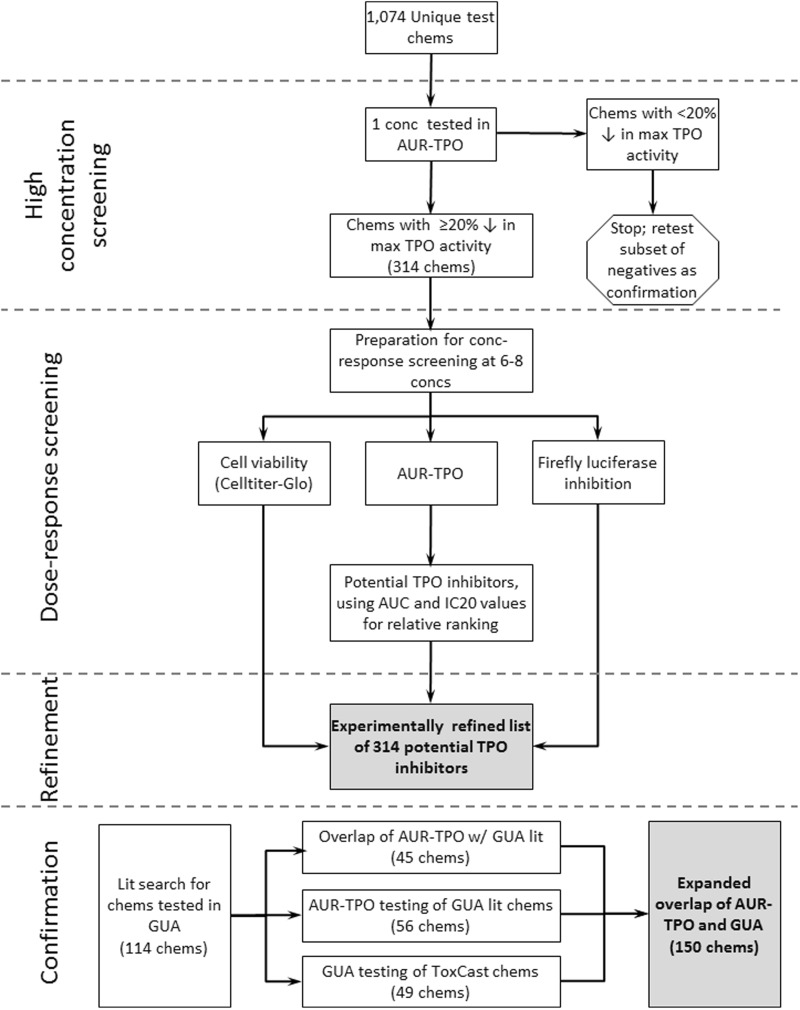
Previously, screening for MIEs specific to TH-disrupting pathways has been limited in the U.S. EPA ToxCast program (Judson et al., 2010; Kavlock et al., 2012; Reif et al., 2010) (green boxes, Figure 1). In support of the EDSP21 workplan (EPA, 2014), efforts are underway to expand the ToxCast assay portfolio to include more assays assessing thyroid-related MIEs. We previously evaluated the performance of a HTS assay, the Amplex UltraRed-TPO (AUR-TPO) assay, using a 21-chemical training set to measure TPO inhibition in vitro (Paul et al., 2014). Herein, we report the use of this new screening assay to identify potential TPO inhibitors within the ToxCast phase I and II chemical libraries, comprised of approximately 1000 chemicals. A tiered screening strategy (diagrammed in Figure 2) was employed to reduce the time and number of animals required to identify putative TPO inhibitors, and then experimental and literature information were used to systematically prioritize in vitro TPO inhibitors for further confirmation. Initially, 1074 unique chemicals were tested at a single, high concentration in the AUR-TPO assay to identify chemicals that elicited a ≥ 20% decrease in maximal TPO activity. Next, positive chemicals from the initial screen were evaluated in concentration-response using the AUR-TPO assay, a cytotoxicity assay to estimate a cellular tolerance limit, and a cell-free firefly luciferase assay to evaluate nonspecific enzyme inhibition. Finally, confirmation with an orthogonal test, the guaiacol oxidation (GUA) assay for TPO inhibition, was conducted using a combination of published GUA assay results, new testing of ToxCast chemicals in the GUA assay, and AUR-TPO testing of additional chemicals from the literature that were not included in the ToxCast test set of chemicals. This tiered screening strategy, used to assess TPO inhibition activity for over 1000 chemicals, represents a novel and significant contribution to the field of endocrine disruptor screening.
FIG. 2.
The tiered screening approach to identity, stratify, and confirm TPO inhibitors. One thousand seventy-four unique ToxCast chemicals were initially screened using a single, high concentration to identify potential TPO inhibitors. Chemicals testing positive in the single-concentration screen were retested in concentration-response for TPO inhibition. A cytotoxicity and luciferase inhibition assay were employed in parallel to identify possible sources of nonspecific assay signal loss, enabling stratification of roughly 300 putative TPO inhibitors based upon selective Amplex UltraRed-TPO (AUR-TPO) activity. The TPO inhibition activities of 150 chemicals were compared across the AUR-TPO and guaiacol oxidation (GUA) assays to confirm the activity profiles of putative TPO inhibitors. Lit refers to publicly available literature as described in the Materials and Methods.
MATERIALS AND METHODS
Animals
Untreated male Long Evans rats (68–72 days old) were obtained from Charles River Laboratories Inc, Raleigh, North Carolina in groups of 60 and acclimated 1–7 days in an American Association for Accreditation of Laboratory Animal Care International approved animal facility. Details of animal management and procedures for obtaining rat thyroids have been reported previously (Paul et al., 2014). All animal procedures were approved in advance by the Institutional Animal Care and Use Committee of the National Health and Environmental Effects Research Laboratory of the U.S. EPA.
Rat thyroid microsomes
Microsomes were made from a pool of 120 rat thyroids, totaling approximately 2 g of tissue. Thyroid microsomes were prepared as described previously (Chang and Doerge, 2000; Paul et al., 2013, 2014). Briefly, frozen thyroid glands were homogenized in 2 ml of ice-cold buffer (5 mM potassium phosphate, 200 mM sucrose, 1 mM EDTA, and 500 U/ml catalase) per 0.01 g of thyroid tissue. This homogenate was processed briefly with a teflon mortar and pestle. This homogenate was then centrifuged at 29.4 × g for 10 min at 4 °C to remove larger debris, and then ultracentrifuged at 151 515 × g for 60 min at 4 °C. The supernatant was discarded, and the pellet was resuspended in homogenization buffer (without catalase), with 0.25 ml of buffer per 0.01 g of thyroid tissue using a teflon-coated mortar and pestle. Glycerol (5%) was added to the final microsomal preparation, and aliquots were stored at −80 °C until thawed 1 time for use. A Bradford protein assay was performed to determine the total protein content of microsomal lots, with a range of 1.03–1.29 mg/l protein.
Porcine thyroid microsomes
Porcine thyroid glands, approximately 1 g each, were procured from male and female 6- to 8-week-old pigs at a veterinary research facility at North Carolina State University in Raleigh, North Carolina, snap frozen on dry ice, and stored at −80 °C. The method of Chang and Doerge (2000) was adapted for preparing microsomes as previously described (Tietge et al., 2013). Two microsome preparations derived from 10 total pig thyroid glands (11.55 g total tissue weight) were pooled and used in this study to perform follow-up GUA assays. The mean of the protein concentrations of these 2 of batches of microsomes was 15.95 mg/ml, as determined by a Bradford protein assay.
Control chemicals and reagents
Chemical controls MMI (positive control in TPO inhibition assays), 2,3-dichloro-1,4-napthoquinione (DCNQ; positive control in cytotoxicity assay), and diluent dimethyl sulfoxide (DMSO), along with potassium phosphate, sucrose, EDTA, catalase, and glycerol used in microsome preparation, as well as guaiacol and hydrogen peroxidase used in TPO inhibition assays, were all purchased from Sigma (St Louis, Missouri). The competitive luciferase inhibitor, 4-(1,3-benzothiazol-2-yl)-N, N-dimethylaniline (LUCINH2), was purchased from EMD Millipore (Billerica, Massachusetts). Coenzyme A and D-luciferin were purchased from Nanolight Technology (Pinetop, Arizona).
Chemical test set
The ToxCast phase I and II chemical libraries were provided as 1123 blinded chemical samples drawn from a set of 1074 unique chemicals, the difference owing to 13 test chemicals represented as 62 internally replicated samples used to measure intra-assay reproducibility. The chemical library, quality control (QC) analysis, and structure data format files are available at http://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data. Stock solutions of all chemicals were provided in 100% DMSO at a target concentration of 20 mM in 384-well plates, with any deviation from this top stock concentration based on maximum solubility in DMSO. Compound plates containing all control and test compound were prepared using 100% DMSO as the diluent and stored at room temperature, away from light, in a desiccator. All experiments were conducted within 4 weeks of compound plate preparation.
Experimental screening strategy for the chemical test set
Screening was conducted using a tiered strategy, starting with a single-concentration screen in the AUR-TPO assay (Figure 2). All ToxCast phase I and II chemicals were screened initially at an 87.5 µM assay concentration, unless otherwise noted. All test chemicals eliciting a mean (n = 3 biological replicates) 20% or greater reduction in maximal TPO activity at this single 87.5 µM concentration were subsequently tested at 6 or 8 concentrations, in the AUR-TPO, Cell Titer Glo (cytotoxicity), and firefly luciferase inhibition assays. The target compound plate and assay concentrations for ToxCast phase I and II libraries are presented in Table 1. All AUR-TPO assays (single- and multi-concentration) were run in 3 separate trials, with freshly prepared reagents at each trial, for 3 biological replicates (n = 3). There were no technical replicates employed for the test chemicals within a biological replicate; therefore, each concentration for any single test chemical was present only in a single well per biological replicate. A titrated series of the positive control, MMI, was included on all assay plates, with 8–12 concentrations, with the lowest concentration ranging from 56 pM to 1.5 nM and a top concentration of 271 µM (final).
TABLE 1. Target Compound Plate and Assay Plate Concentrations for Chemical Libraries
| Compound Plate Stock (mM) | Assay Plate Concentration (µM) | ToxCast Chemical Libraries Tested (I, II) |
|---|---|---|
| 20.0 | 87.5 | I and II |
| 5.00 | 21.9 | I and II |
| 1.25 | 5.47 | I and II |
| 0.313 | 1.37 | I and II |
| 0.0781 | 0.342 | I and II |
| 0.0195 | 0.0854 | I and II |
| 0.00488 | 0.0214 | I only |
| 0.00122 | 0.00534 | I only |
Test chemicals were solubilized in DMSO at a top concentration of 20 mM, unless constrained by solubility in DMSO (adjustments were then made during data analysis).
Delivery of chemicals to assay plates
Test compounds were administered using a BioMek FX Automated Laboratory Workstation (Beckman Coulter; Brea, California) equipped with a high density replicating tool (HDRT) fitted with a 384-pintool with 200 nl slot pins (V&P Scientific; San Diego, California) to deliver a fixed volume of 350 nl using stainless steel pins that dip into a compound plate and then into the assay plate. The HDRT was used to deliver chemicals to the assay plates for the AUR-TPO, Cell Titer Glo, and firefly luciferase inhibition assays, as well as the GUA assay.
AUR-TPO inhibition assay
Amplex ® UltraRed (AUR) (LifeTech, Cat. No. A36006) is sold for the sensitive detection of H2O2 released from biological samples, including cells, in the presence of excess horseradish peroxidase; AUR is a fluorogenic substrate that is converted from AUR to Amplex UltroxRed by horseradish peroxidase in the presence of H2O2. As described previously (Paul et al., 2014), the AUR substrate was repurposed to detect peroxidase (ie, TPO) activity in the presence of excess H2O2 for the AUR-TPO assay. The reaction profile for the 384-well format AUR-TPO assay is scaled to an 80 µl assay volume containing 25 µM AUR, 300 µM H2O2, and 5 µg microsomal protein in 200 mM phosphate buffer. The assay concentration of thyroid microsomal protein was approximately 0.0625 µg/ml. First, rat microsomes containing TPO and diluent 200 mM potassium phosphate buffer were added to opaque, black 384-well plates (Greiner, BioExpress; Kaysville, Utah) using an automated liquid handler (BioTek MicroFlo; Winooski, Vermont) with a plastic 5 µl tubing cassette. Similarly, AUR was added next by automated liquid handling. Test compounds were administered using a HDRT as described earlier to achieve a 0.5% final DMSO concentration. Finally, H2O2 (300 µM, final concentration) was then added by the BioTek MicroFlo to initiate the reaction. Microplates were incubated at 37 °C in the dark for 30 min. Plates were then shaken on a fluorescence plate reader (FLUOStar Optima, BMG LabTech; GmbH, Germany), and endpoint fluorescence (top read) was measured using 10 flashes per well at 544/590 nm excitation/emission. Three biological replicates of technical singles were run for every chemical concentration tested. A titrated range of MMI served as a positive control for TPO inhibition on each plate of the AUR-TPO screening campaign, as described earlier.
Cytotoxicity assay
Human HEK293T cells (GenHunter; Nashville, Tennessee) were seeded overnight in 384-well, solid white assay plates (Greiner, BioExpress) at a density of 10 000 cells per well in 65 µl Dulbecco’s Modified Eagle Medium (Life Technologies; Carlsbad, California) supplemented with 1% fetal bovine serum (HyClone; Logan, Utah). Test compounds were administered using the HDRT as described earlier to achieve a 0.5% final DMSO concentration. Cells were then incubated at 37 °C for 24 hr in a humidified 5% CO2 incubator. Cell Titer Glo (Promega; Madison, WI) reagent (25 µl) was then added by the BioTek MicroFlo and microplates were incubated at room temperature in the dark for 20 min. Plates were read on a plate reader (FLUOStar Optima) using an endpoint luminescence protocol (top read). Three biological replicates of technical singles were run for every chemical concentration tested. A titrated range of DCNQ served as a positive control on each plate of the cytotoxicity screening assay.
Quanti-Lum luciferase inhibition assay (QLI)
Quanti-Lum recombinant luciferase (Promega; Madison, Wisconsin) was diluted to 76.9 ng/ml with 200 mM potassium phosphate buffer supplemented with 1 mg/ml bovine serum albumin and 65 μl (5 ng recombinant luciferase) per well was dispensed into opaque, white 384-well plates (Greiner, BioExpress) using an automated liquid handler (BioTek MicroFlo) with a plastic 5 µl tubing cassette. Test compounds were administered using the HDRT as described earlier to achieve a 0.5% final DMSO concentration. Microplates were incubated at 37 °C in the dark for 30 min to mimic the AUR-TPO assay conditions. Luciferase detection reagent (25 μl per well; 9.2 mM dithiothreitol, 75 μM Coenzyme A, 147 μM dATP, 150 μM D-luciferin, 8.9 mM Tricine, 297 μM (MgCO3)4·Mg(OH)2·5H20, 742 μM MgSO4, 27.8 μM EDTA, pH 7.8, final assay concentrations) was then added using a BMG Clariostar plate reader equipped with syringe injectors (BMG LabTech; GmbH). Plates were read using a kinetic luminescence protocol (top read) whereby each well was individually injected with luciferase detection reagent immediately prior to measurement. Three biological replicates of technical singles were run for every chemical concentration tested. A titrated range of LUCINH2 served as a positive control on each plate of the luciferase inhibition screening assay.
Confirmation using orthogonal GUA assay
The GUA assay was used to retest a subset of chemicals that tested positive and negative in the AUR-TPO assay to orthogonally confirm AUR-TPO assay activity. Forty-seven ToxCast chemicals were selected based on AUR-TPO activity profile, relative AUR-TPO potency (to select chemicals with AUR-TPO assay activity at concentrations lower than those associated with luciferase inhibition and cytotoxicity), and chemical features found to be enriched among AUR-TPO positives (the chemotype analysis method is described in a article currently in preparation). Three additional chemicals with inconclusive GUA assay profiles from literature reports and another 4 chemicals suspected of producing signal artifacts in AUR-TPO screening were also selected. The GUA assay has been described previously for both rat and pig thyroid microsomes (Chang and Doerge, 2000; Paul et al., 2013; Tietge et al., 2013). All reactions were conducted in a final reaction volume of 65 µl. Porcine thyroid microsomes (25 µg) diluted in 45 µl of 200 mM potassium phosphate buffer (per well) was dispensed into clear, 384-well plates. Test compounds were administered using the HDRT as described earlier. Guaiacol (10 µl; 35 mM final assay concentration) was then added using a BMG Clariostar plate reader equipped with syringe injectors and incubated with test chemical exposure at 37 °C for 30 s. H2O2 (10 µl; 1 mM final assay concentration) was then added using syringe injectors to initiate the reaction. The oxidation of guaiacol was monitored during the linear phase (first 80 s of the reaction) at 450 nm, and the change in absorbance over this period was recorded for data analysis. Three biological replicates of technical singles were run for every chemical concentration tested. A titrated range of MMI served as a positive control for TPO inhibition on each plate ranging from 0.01 to 100 µM final concentration.
Literature search for chemicals tested in GUA assay
An open source literature search was conducted to identify chemicals previously tested using TPO or closely related peroxidases in the GUA assay. Search terms included “guaiacol” paired with “thyroid,” “thyroperoxidase,” “tpo,” “lactoperoxidase,” “lpo,” “myeloperoxidase,” “mpo,” “eosinophil peroxidase,” or “epo.” The resulting research reports and book chapters (as of March 10, 2015) were then manually curated for chemicals that met the following criteria: (1) confirmed to have been tested in the GUA assay; (2) resulting GUA activity was clearly reported with null hypothesis significance testing (ie, P value); (3) test concentration(s) used in GUA assay were clearly reported; (4) chemical had a CAS Registry Number (CASRN); and (5) chemical had at least 1 commercial source. This search yielded 86 chemicals listed in Supplementary Table 2. An additional 28 chemicals were identified that were tested in unpublished pilot studies using the GUA assay (Hornung, unpublished data). Of the 114 chemicals previously tested in the GUA assay, only 45 were represented in the ToxCast chemical test set. Twenty-nine of the remaining chemicals were obtained through the ToxCast Inventory (http://www.epa.gov/chemical-research/toxicity-forecasting), and another 32 were procured commercially. Five of the DSSTox Inventory chemicals were insoluble in DMSO. The remaining 56 chemicals were solubilized in DMSO and tested in the AUR-TPO assay as described earlier.
Data analysis
Concentration-response data were analyzed using the ToxCast Analysis Pipeline R software package (tcpl v1.0) and MySQL database (http://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data)
- AUR-TPO assay
- Single-concentration screen. The data were obtained as raw fluorescence units (rval) and normalized to percent inhibition by plate with equation resp = 100 * (rval − bval)/(0 − bval) where bval is the mean of the DMSO vehicle control values. The mean of the n replicates was calculated and reported as the percent inhibition. A 20% maximal activity inhibition was selected as a threshold for a positive assay response in the AUR-TPO assay, as this value was greater than the anticipated coefficient of variation (CV) based on previous work (Paul et al., 2014), and thus likely to be outside the range of nonspecific assay interference around the baseline. This threshold for positivity is also > 3 times the baseline median absolute deviation (MAD) (± 17%), often used as a threshold for distinguishing signal from noise.
- Multiple-concentration screen. The data were obtained as raw fluorescence units (rval) and normalized to percent inhibition by plate with equation resp = 100 * (rval − bval)/(pval − bval), where bval is the median of the DMSO vehicle control values, and pval is the minimum of the blank wells (wells lacking H2O2). Curves were fit to the data using 3 separate models. The constant model has 1 parameter with equation:
The second model used was a constrained Hill model. In this model tp is the top asymptote, ga is the AC50, gw is the Hill coefficient, and the bottom asymptote set to 0, with equation:
Where xi represented the log concentration at the ith observation. The Hill model had the following constraints:- 0 ≤ tp ≤ 1.2 times the maximum response value;
- Minimum log concentration − 2 ≤ ga ≤ maximum log concentration + 0.5; and
- 0.3 ≤ gw ≤ 8.
The gnls model had the following constraints:- 0 ≤ tp ≤ 1.2 times the maximum response value;
- Minimum log concentration − 2 ≤ ga ≤ maximum log concentration;
- 0.3 ≤ gw ≤ 8;
- Minimum log concentration − 2 ≤ la ≤ maximum log concentration + 2;
- 0.3 ≤ gw ≤ 18; and
- la - ga > 0.25.
- Cytotoxicity assay (CTG)The data were obtained as raw luminescence units (rlu), normalized with pval set to zero, and fit using the same method as for AUR-TPO multiple concentration data.
- Luciferase inhibition assay (QLI)The data were obtained as raw luminescence units (rlu), normalized with pval set to zero, and fit using the same method as for AUR-TPO multiple concentration data.
- Area under the fitted curve (AUC)For each sample, a value of the AUC was calculated. For each curve with hitc = 1, the winning model (Hill or gnls) and winning model parameters were selected. The model and parameters were integrated using the integrate function of the stats R package. The upper and lower limits of integration were set to the minimum and maximum concentration measured for that sample. Samples where hitc = 0 were assigned an AUC of 0, such that any chemical that demonstrated less than 20% maximal TPO inhibition was assigned an AUC = 0.
- Using cytotoxicity and luciferase inhibition to calculate selectivity of the AUR-TPO assay responseA selectivity value was calculated for each chemical using equation:Where CTGACC, QLIACC, and AURACC are the log10 µM concentrations at which the winning model has a predicted response value equivalent to the assay response cutoff (modl_acc, equal to a 20% decrease from a maximal response in all 3 assays), and the sample has a hitc = 1. The value cyto_med was calculated as log10 of the median of the set of concentrations corresponding to the modl_acc values for 37 ToxCast cytotoxicity assays (Supplementary Table 3). If the winning model corresponded to a hitc = 0 for a given assay, a value of 5 was used for the modl_acc for that assay.
- GUA assayThese kinetic assay data were obtained as raw absorbance units as a function of time. A response value (rval) was calculated by taking the median of 19 detection measurements between 145 and 147 s (during the signal zenith) and subtracting the median of 2 measurements taken at 64 and at 64.25 s (at the addition of hydrogen peroxide). The data were then normalized to percent inhibition by plate with equation: resp = 100 * (rval − bval)/(0 − bval), where bval is the median of the DMSO vehicle control values. Normalized values were fit using the same methods as AUR-TPO with the same assay cutoff of 20%.
- Assay QCAssay quality was calculated on a plate by plate basis. A robust CV (rCV) was calculated by using equation:Where is the median of the raw fluorescence values for DMSO vehicle control wells on the ith plate, and is the rescaled MAD of the raw fluorescence values of the DMSO wells of the ith plate:The rCV utilizes the rescaled MAD and median of the raw fluorescence from vehicle control wells in place of SD and the mean, respectively, typically used in a CV calculation. The rCV accounts for more of the variability that may be present in the values specific to the ith plate than the CV.A robust Z’-factor (rZ’) was calculated on the normalized data using equation:WhereWhere is the rescaled MAD of the normalized positive control (MMI curve) values at the top 2 concentrations of the positive control on the ith plate, is the rescaled MAD of the normalized DMSO values on the ith plate, is the median of the positive control values at the top 2 concentrations of the control on the ith plate, and is the median of the normalized DMSO values on the ith plate. The rZ’ factor differs from Z’ factor in that the rZ’ factor utilizes the rescaled MAD of top positive control values in place of the SD of the top value, and the median of positive and vehicle control values in place of the mean. The rZ’ approach should account more for variability in the positive control curves. The rZ’ is a measure of assay quality, with a rZ’ of 0.5–1.0 corresponding to an assay with a sufficiently high signal-to-background distinction and a intersample variability low enough to adequately distinguish between positive and negative test chemicals. As the rZ’ approaches 1.0, the confidence that positive and negative behavior can be distinguished from the assay results increases.
- Classification functionsBalanced accuracy, sensitivity, and specificity of the AUR-TPO assay were calculated using the following formulas:Where A is the number of chemicals active in both GUA and AUR-TPO assays, B is the number of chemicals active in the GUA assay and inactive in the AUR-TPO assay, C is the number of chemicals inactive in both GUA and AUR-TPO assays, D is the number of chemicals inactive in the GUA assay and active in the AUR-TPO assay, and n is the total number of chemicals compared across both assays.
RESULTS
Single-Concentration Chemical Screening
The AUR-TPO assay was used to assess test chemicals at a single, maximal concentration of 87.5 µM (solubility permitting). Assay performance was evaluated using DMSO (vehicle control) and a MMI (positive control) concentration-response curve. The AUR-TPO assay performed reliably with a mean rCV ± SD of 3.7% ± 1.0% and a mean rZ’ factor ± SD of 0.83 ± 0.04. MMI produced an AC50 value (ie, concentration calculated to inhibit a one-half maximal response) of 34.7 nM (Supplementary Figure 1A).
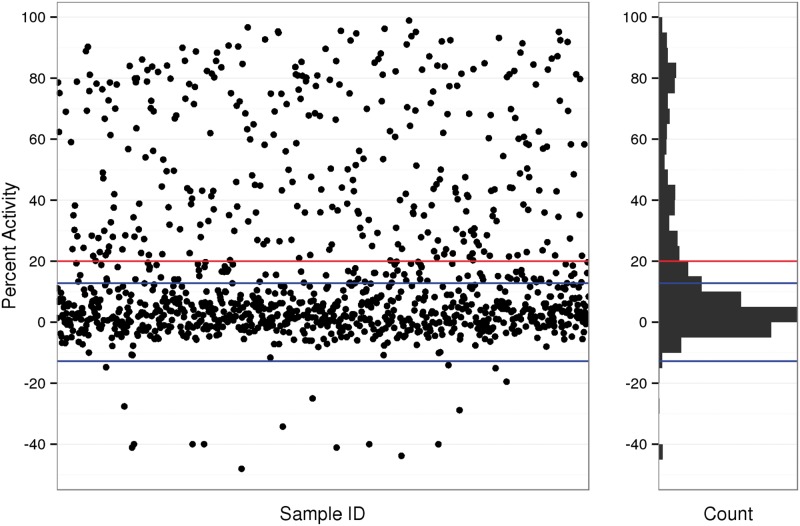
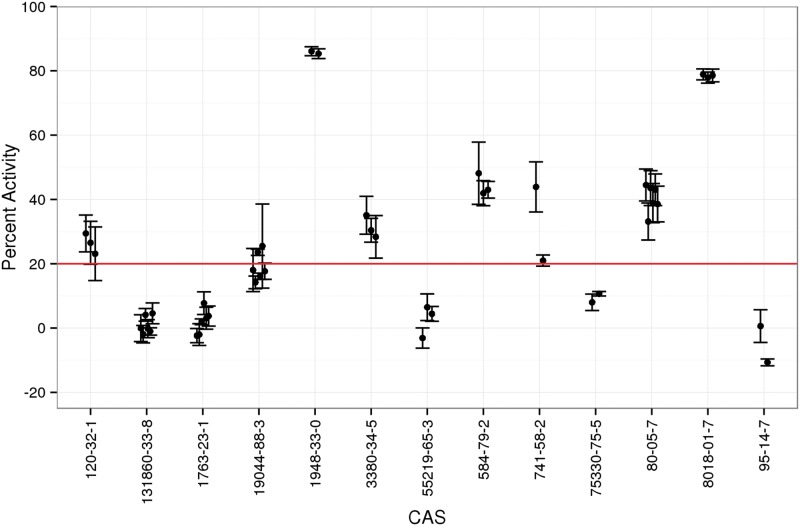
As Figure 3 illustrates, a wide range of responses were observed, with most test chemicals clustering around the vehicle control response value. Of the 1074 unique chemicals tested, 314 (29%) decreased maximal TPO activity by ≥ 20%, the threshold used to define a positive hit response (Figure 3, red line; Supplementary Table 1 for a complete list of test chemicals). This threshold was only slightly higher than 3 times the baseline MAD (± 17%; Figure 3, blue lines), an approach often used to delineate a threshold for a positive assay response. Several chemicals induced an unexpected supramaximal TPO activity response (Figure 3, % activity ≤ −17%), most likely owing to assay interference. The reproducibility of 13 internally replicated chemicals samples, each tested in triplicate experiments in the single-concentration screen, is shown in Figure 4. These results indicate that intra- and inter-assay results are highly replicable for the AUR-TPO assay, as chemicals repeated within plate and in 3 replicate experiments produced very similar responses.
FIG. 3.
Mean AUR-TPO activity for 1074 ToxCast chemicals tested at a single, maximal concentration. Test chemical sample IDs run across the x-axis. The y-axis represents percent activity, inverted such that vehicle control is 0% (no inhibition) and 100% indicates maximal inhibition. Each black dot represents a unique test chemical. The horizontal blue lines indicate 3 times the baseline median absolute deviation (± 17%), and the horizontal red line indicates the threshold for a positive assay response, 20% inhibition. The histogram (right) shows the frequency distribution using 5% activity bins.
FIG. 4.
AUR-TPO activity of internally replicated compounds with ToxCast test library. The results of 13 chemicals internally replicated as separate samples within the library in the single-concentration screen (n = 3) are represented as mean (black dots) ± SD (error bars). The y-axis represents percent activity, inverted such that vehicle control is 0% (no inhibition) and 100% indicates maximal inhibition. The horizontal line indicates the threshold for a positive assay response, 20% inhibition.
Concentration-Response Screening
The 314 chemicals identified as putative TPO inhibitors in the initial single-concentration screen were subsequently tested in the AUR-TPO assay using an 8- or 6-point concentration-response series for ToxCast phase I and II samples, respectively. The AUR-TPO assay performed reliably and similarly to the initial single-concentration screen, with a mean rCV ± SD of 3.6% ± 1.5% and a mean rZ’ factor ± SD of 0.77 ± 0.08. The positive control, MMI, produced an AC50 value of 148 nM (Supplementary Figure 1B). Ninety percent of the retested chemicals (283) tested in concentration-response achieved the 20% inhibition threshold observed in the initial single-concentration screen. Most of the chemicals that failed to reproduce a positive response in confirmation testing barely exceeded the 20% threshold in the initial single-concentration screen.
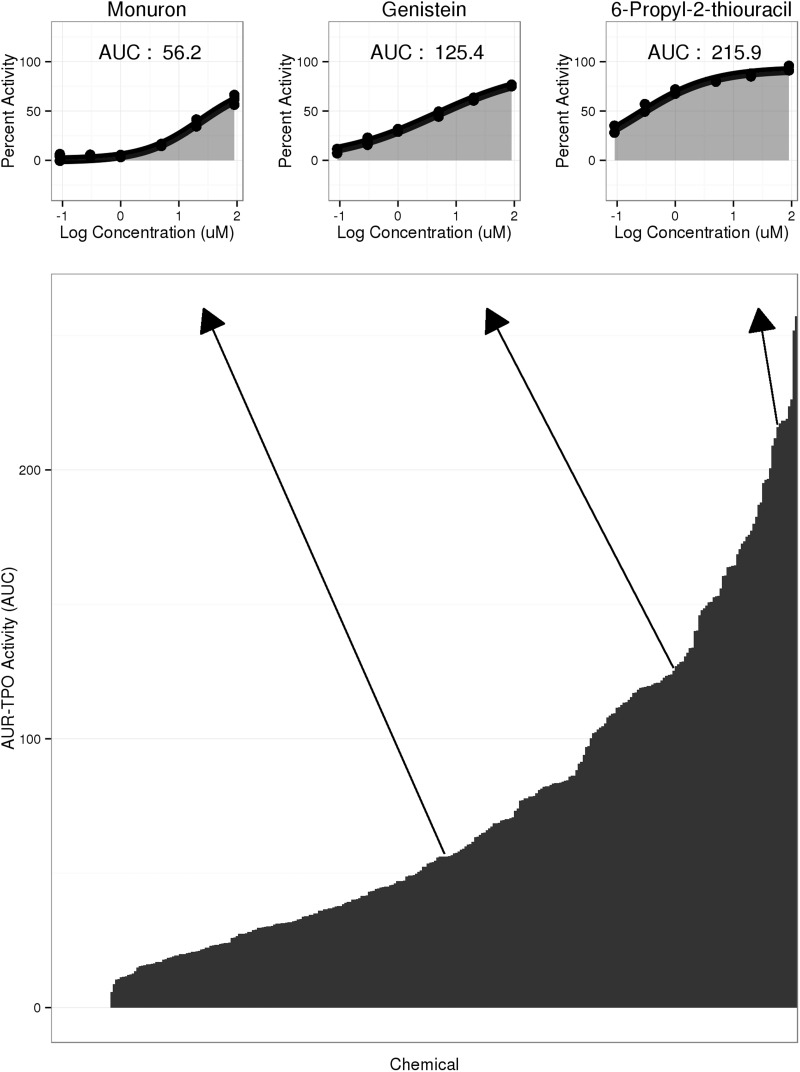
All fitted curves from the concentration-response data are available in Supplementary Figure 2. The 25 most potent inhibitors in the AUR-TPO assay are listed in Table 2. AUC values were calculated to rank all of the putative TPO inhibitors by activity (Figure 5). A diverse range of AUR-TPO assay inhibition activities was observed, with AUC values > 200 for highly potent and efficacious TPO inhibitors, such as anti-hyperthyroidism drugs MMI and PTU, and AUC values approaching zero for chemicals such as thiram (AUC = 6) with modest efficacy and/or only those active at the highest tested concentrations. Though MMI and PTU were among the most active chemicals tested, the AUC values of 3 ToxCast chemicals exceeded that of MMI and 7 ToxCast chemicals had AUC values higher than that for PTU. Generally the potency of AUR-TPO inhibition correlated with AUC values although there were some exceptions. For example, sodium azide (Table 2) was relatively potent but had a low AUC value (45) because of its low Emax (41.8%). The cause of reduced efficacies in the AUR-TPO assay for these chemicals is not understood.
TABLE 2. Top 25 TPO Inhibitors Ranked by Potency in AUR-TPO Assay
| Chemical Name | CASRN | IC20a (µM) | % Emaxb | AUC |
|---|---|---|---|---|
| 4-Hexylresorcinol | 136-77-6 | 0.000052 | 96.9 | 257 |
| Resorcinol | 108-46-3 | 0.006 | 81.8 | 226 |
| MMI | 60-56-0 | 0.013 | 84.7 | 224 |
| 4,4′-Methylenedianiline | 101-77-9 | 0.018 | 86.3 | 219 |
| 4-Pentylaniline | 33228-44-3 | 0.020 | 83.4 | 218 |
| 6-Propyl-2-thiouracil | 51-52-5 | 0.036 | 95.7 | 216 |
| 4-Methylaniline | 106-49-0 | 0.037 | 73.2 | 201 |
| Salicylhydroxamic acid | 89-73-6 | 0.039 | 94.2 | 212 |
| 6-Methyl-2-thiouracil | 56-04-2 | 0.042 | 90.1 | 195 |
| 2,2′,4,4′-Tetrahydroxybenzophenone | 131-55-5 | 0.042 | 87.3 | 209 |
| 2-Mercaptobenzothiazole | 149-30-4 | 0.043 | 97.0 | 217 |
| Quercetin | 117-39-5 | 0.057 | 98.9 | 252 |
| Sodium azide | 26628-22-8 | 0.071 | 41.8 | 45 |
| 3-Methylaniline | 108-44-1 | 0.079 | 81.9 | 197 |
| 4-Chloroaniline | 106-47-8 | 0.087 | 78.4 | 187 |
| Tannic acid | 1401-55-4 | 0.088 | 87.3 | 140 |
| 6-Thioguanine | 154-42-7 | 0.096 | 92.9 | 212 |
| Catechol | 120-80-9 | 0.100 | 81.5 | 164 |
| Acibenzolar-S-methyl | 135158-54-2 | 0.104 | 87.5 | 218 |
| CI-1029 | 207736-05-8 | 0.108 | 95.0 | 196 |
| 2-Naphthylamine | 91-59-8 | 0.115 | 74.1 | 180 |
| 4,4′-Oxydianiline | 101-80-4 | 0.117 | 86.7 | 177 |
| 2-Anisidine | 90-04-0 | 0.122 | 74.5 | 173 |
| Isoproterenol hydrochloride | 51-30-9 | 0.154 | 70.4 | 153 |
| 4,4′-Methylenebis(2-methylaniline) | 838-88-0 | 0.171 | 77.0 | 161 |
aIC20 represents the concentration at which maximal activity was inhibited by 20% in the AUR-TPO assay.
b% Emax refers to the percent maximum inhibition observed in concentration-response.
FIG. 5.
Ranked area under the fitted curves (AUCs) for 314 putative TPO inhibitors confirmed in dose-response in the AUR-TPO assay. Three hundred fourteen AUR-TPO actives retested in multiple-concentration screening were ranked by AUC. AUC (y-axis) compresses potency and efficacy into a single value to reflect the relative overall activity in the AUR-TPO assay. Thirty-one chemicals characterized as active in the initial single-concentration screen tested inactive in multiple-concentration screening (AUC = 0). Concentration-response curves with shaded AUCs are shown earlier for monuron, genistein, and 6-propyl-2-thiouracil.
To ensure assay reproducibility in scaling from a small chemical training set (Paul et al., 2014) to the larger test set of ToxCast chemicals evaluated in this report, a comparison of the AC50 values for 14 reference chemicals common to both the training set and this test set is presented in Table 3. Qualitative results were consistent across studies, as each of the 10 training set positives retested as active in this study and each of the 4 training set negatives retested as inactive. Minor quantitative differences between the training set and test set were observed for most chemicals but with AC50 values within a range of 0.5 log units between studies. For 2 chemicals, resorcinol and ethylene thiourea (ETU), a larger quantitative difference in AC50 responses was observed between studies.
TABLE 3. Comparative Potency and AUC Values for Chemicals Common Between the AUR-TPO Training Study and This Study
| Chemical Name | CASRN | AC50 Paul et al. (2014) (µM) | AC50 Current Study (µM) | AUC Current Study |
|---|---|---|---|---|
| MMI | 60-56-0 | 0.025 | 0.06 | 224 |
| ETU | 96-45-7 | 0.034 | 7.8 | 123 |
| 6-Propyl-2-thiouracil | 51-52-5 | 0.12 | 0.23 | 216 |
| 2,2′,4,4′-Tetrahydroxy-benzophenone | 131-55-5 | 0.16 | 0.17 | 209 |
| 2-Mercaptobenzothiazole | 149-30-4 | 0.45 | 0.36 | 217 |
| Genistein | 446-72-0 | 4.5 | 3.5 | 144 |
| Daidzein | 486-66-8 | 23 | 10 | 115 |
| 4-Nonylphenol | 104-40-5 | 44 | 91 | 53 |
| Triclosan | 3380-34-5 | 142 | 48 | 39 |
| Resorcinol | 108-46-3 | 253 | 0.025 | 226 |
| 2-Hydroxy-4-methoxybenzophenone | 131-57-7 | inactive | inactive | 0 |
| Dibutylphthalate | 84-74-2 | inactive | inactive | 0 |
| Diethylhexylphthalate | 117-81-7 | inactive | inactive | 0 |
| Diethylphthalate | 84-66-2 | inactive | inactive | 0 |
Stratification of TPO Inhibitors Based on Selective Activity
To identify chemicals with the most selective AUR-TPO assay response, 2 assays conducted in parallel were used to identify chemicals active in the AUR-TPO assay that may act through nonspecific mechanisms, ie, nonspecific enzyme inhibition and/or promiscuous reactivity. A firefly luciferase inhibition assay was used to evaluate nonspecific enzyme inhibition in a cell-free model, and a cytotoxicity assay using a human cell line was employed to estimate the cellular concentration limit to identify reactive compounds. The results from both assays exceeded acceptable performance criteria. The cytotoxicity assay performed well, with a rCV ± SD of 3.2% ± 2.2% and a rZ’ factor ± SD of 0.93 ± 0.02. The AC50 value of the positive control, DCNQ, was 4.49 µM (Supplementary Figure 1C). The firefly luciferase inhibition assay demonstrated a rCV ± SD of 8.5% ± 3.5% and a rZ’ factor of 0.58 ± 0.12. The AC50 value of the positive control, LUCINH2, was 1.33 nM (Supplementary Figure 1D).
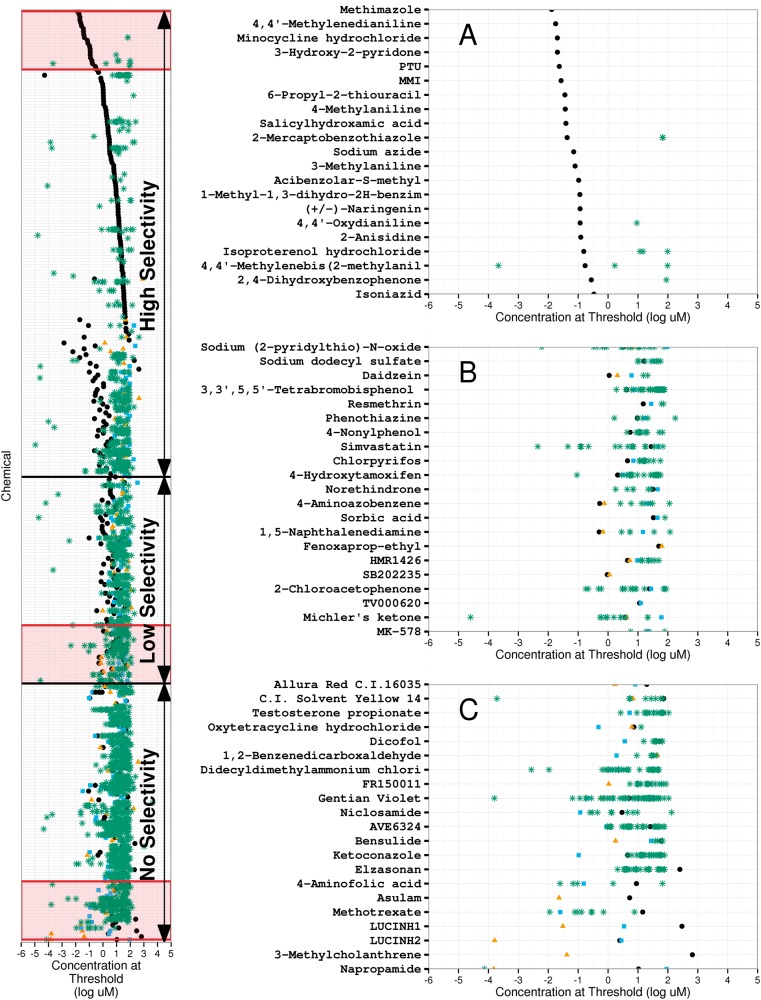
The concentration calculated to inhibit 20% of the maximal response (IC20) was determined for each chemical across all 3 assays, the AUR-TPO inhibition assay, the firefly luciferase inhibition assay, and the cytotoxicity assay. AUR-TPO selectivity was calculated using the difference between the log IC20 for the AUR-TPO assay and either (1) the lower log IC20 value of the luciferase inhibition and cytotoxicity assay or (2) the median log modl_acc value of 37 cytotoxicity and proliferation assays from ToxCast (Supplementary Table 3). The differential log IC20 values (selectivity) were used to stratify the 314 putative TPO inhibitors confirmed in the concentration-response screen (Figure 6; values listed in Supplementary Table 4), with the most selective chemicals demarcated by increased separation of the AUR-TPO assay log IC20 value from possible confounding activities identified in the other assays. Arbitrary selectivity cutoffs of 1 and 0 trisected the list into 141 highly selective chemicals (44.9%), 59 chemicals with low AUR-TPO selectivity (18.8%) and 114 nonselective chemicals (36.3%). Figure 6 also illustrates magnified insets representative of the top (A), middle (B), and bottom (C) regions of the stratified list (full stratified list of 314 chemicals available as Supplementary Figure 3). In panel A, known TPO inhibitors MMI, PTU, and mercaptobenzothiazole (Tietge et al., 2013), and several aniline compounds, among others, have AUR-TPO inhibition activity at concentrations several orders of magnitude lower than the calculated IC20 values of the luciferase or cytotoxicity assays. Panel B illustrates chemicals with reduced selectivity for the AUR-TPO assay, indicating activity in the confounder assays at concentrations similar to the concentrations required for observed activity in the AUR-TPO assay, including chemicals such as the detergent sodium dodecyl sulfate, the statin simvastatin, and the organophosphate pesticide chlorpyrifos. Panel C highlights examples of chemicals devoid of selective AUR-TPO activity, such as the bacteriostat triclosan, the antibiotic oxytetracycline, and the antifungal ketoconazole.
FIG. 6.
Putative TPO inhibitors, ranked by selective activity in the AUR-TPO assay. Insets (A–C) magnify representative regions of the stratified list of putative TPO inhibitors ordered from most selective (top) to least selective (bottom) for AUR-TPO activity. Selectivity was calculated using the difference between the log IC20 value for the AUR-TPO assay (black dots) and minimum log IC20 value for the cytotoxicity assay (blue square), luciferase assay (yellow triangle), or median modl_acc from 37 ToxCast cytotoxicity assays (all shown as green asterisks). Black horizontal bars demarcate the 1-log (top bar) and 0-log (bottom bar) thresholds that separate chemicals highly selective for AUR-TPO activity from those chemicals with low or no selective AUR-TPO activity. An absence of symbols for cytotoxicity or luciferase assay data for a given chemical indicates it was negative within the concentration range tested (the x-axis extends several orders of magnitude beyond the full tested concentration range).
Comparison of AUR-TPO Assay Activity to GUA Assay Activity
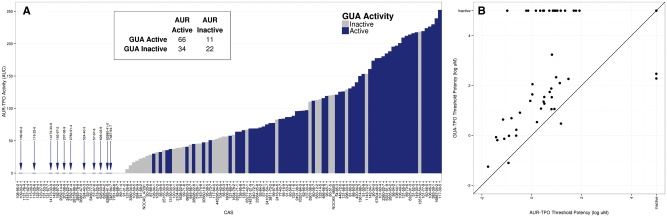
A literature search for reports of commercially-available chemicals tested using the GUA assay identified 114 chemicals for which both the activity and concentration(s) tested were listed (Supplementary Table 2). Only 45 of the GUA-tested chemicals were already included in the ToxCast chemical libraries tested in the AUR-TPO assay, ie, 39% of GUA-tested chemicals could be compared with 4% of chemicals tested in AUR-TPO for activity across both assays. To increase the number of chemicals tested across both assays, 49 ToxCast test chemicals screened in the AUR-TPO assay screen were also tested in the GUA assay (Table 4) using porcine thyroid microsomes, the predominant TPO source used in previously published reports. The GUA assay concentration-response curves for these 49 chemicals can be found in Supplementary Figure 2. Conversely, 56 GUA-tested chemicals identified in the literature search (but not tested in the ToxCast AUR-TPO screen) were procured and tested in the AUR-TPO assay (Table 5). The AUR-TPO assay concentration-response curves for these 56 chemicals can be found in Supplementary Figure 2. Together, these data enabled a comparison of the GUA and AUR-TPO assay data for 150 chemicals, representing 92% of the GUA-tested chemicals and 13% of the tested ToxCast chemical library. These 150 chemicals were ranked by AUC value, derived from the AUR-TPO assay data, and then labeled as “active” or “inactive” based on GUA activity (Figure 7A). GUA activity reported previously in the literature was used to score 97 of the chemicals. Pyrene, caffeine, and perfluorooctanoic acid (PFOA) were reported as active in a single study but produced highly uncharacteristic inhibition responses (Song et al., 2012); therefore, these chemicals were procured and retested in the GUA assay in this work. All 3 tested negative and are labeled as inactive in Figure 7A. Mercury chloride was retested in this study because of conflicting published reports. Mercury chloride was shown to be positive upon retest in the GUA assay and is labeled as active in Figure 7A. The GUA assay activity reported in the literature was used to score 97 of the 101 chemicals previously tested in the GUA assay; data collected using the GUA assay in this study were used to score the remaining 4 chemicals identified in the literature and the 49 ToxCast chemicals that had not been previously tested in the GUA assay. The assay overlap confirms that both the AUR-TPO assay and the GUA assay identify highly active TPO inhibitors. A majority (71%) of chemicals active in the AUR-TPO assay (AUC > 0) were also reported as or tested positive in the GUA assay. Some disagreement between the AUR-TPO and GUA assay results was observed. The AUR-TPO assay identified 34 chemicals as positive that were previously reported as or tested negative in the GUA assay; these chemicals have been labeled “inactive” in Figure 7A. Conversely, 11 chemicals either tested or reported as positive in the GUA assay failed to achieve the 20% activity threshold in the AUR-TPO assay (Figure 7A, blue CAS numbers). Using the GUA assay activity determinations (active or inactive) as the standard of in vitro TPO inhibition for these 150 chemicals, the AUR-TPO assay has a balanced accuracy of 70% with greater sensitivity (88.3%) than specificity (39.3%). The low specificity of the AUR-TPO assay resulted from 34 chemicals categorized as “inactive” using GUA assay results that tested positive in the AUR-TPO assay. A comparison of the GUA and AUR-TPO assay IC20 values for the 49 chemicals tested in the GUA assay in this study revealed that for nearly every chemical active in both assays, the AUR-TPO assay IC20 was lower than the GUA assay IC20 (Figure 7B). Within this set of 49 chemicals, only 2 chemicals were positive in the GUA assay and negative in the AUR-TPO assay, and in both cases the concentration required to elicit a positive response in the GUA assay exceeded 87.5 µM, the maximum concentration tested in the AUR-TPO assay.
TABLE 4. GUA Assay Results for ToxCast Chemicals
| Chemical Name | CASRN | AUR-TPO AUC | Selectivity | GUA Activity Call |
|---|---|---|---|---|
| 4,4′-Methylenedianiline | 101-77-9 | 219 | 6.75 | active |
| Acibenzolar-S-methyl | 135158-54-2 | 218 | 5.98 | inactive |
| 4-Methylaniline | 106-49-0 | 201 | 6.43 | active |
| 3-Methylaniline | 108-44-1 | 197 | 6.10 | active |
| 6-Methyl-2-thiouracil | 56-04-2 | 195 | 2.78 | active |
| 5-Amino-2-methylphenol | 2835-95-2 | 188 | 1.43 | active |
| 4,4′-Oxydianiline | 101-80-4 | 177 | 5.93 | active |
| 2-Anisidine | 90-04-0 | 173 | 5.91 | active |
| Aniline hydrochloride | 142-04-1 | 161 | 5.62 | active |
| Isoproterenol hydrochloride | 51-30-9 | 153 | 5.81 | active |
| Azinphos-methyl | 86-50-0 | 134 | 1.51 | active |
| Phenolphthalin | 81-90-3 | 131 | 0.76 | active |
| Methyl parathion | 298-00-0 | 129 | 4.83 | inactive |
| Isoniazid | 54-85-3 | 121 | 5.47 | active |
| PharmaGSID_48505 | NOCAS_48505 | 121 | 1.02 | inactive |
| Dimethoate | 60-51-5 | 120 | 1.06 | inactive |
| Fenitrothion | 122-14-5 | 114 | 4.66 | inactive |
| Ethion | 563-12-2 | 112 | 1.72 | inactive |
| Phosmet | 732-11-6 | 88 | 0.64 | active |
| Tetracycline | 60-54-8 | 86 | 4.50 | active |
| Ethoxyquin | 91-53-2 | 86 | 1.31 | active |
| Bisphenol B | 77-40-7 | 83 | 1.29 | active |
| 2,3,6-Trimethylphenol | 2416-94-6 | 82 | 4.51 | inactive |
| N,N,4-Trimethylaniline | 99-97-8 | 77 | 4.52 | active |
| Sodium (2-pyridylthio)-N-oxide | 3811-73-2 | 77 | 0.31 | active |
| Phenothiazine | 92-84-2 | 70 | 0.26 | active |
| Thiophanate-methyl | 23564-05-8 | 69 | 4.22 | active |
| Diclosulam | 145701-21-9 | 69 | 4.21 | active |
| 2-Naphthalenol | 135-19-3 | 66 | 4.63 | active |
| Asulam | 3337-71-1 | 64 | −2.15 | active |
| Malaoxon | 1634-78-2 | 59 | 4.04 | inactive |
| N,N-Dimethylaniline | 121-69-7 | 57 | 4.20 | active |
| CP-671305 | 445295-04-5 | 52 | 4.09 | inactive |
| Dioctyl phthalate | 117-84-0 | 51 | 4.15 | inactive |
| Acephate | 30560-19-1 | 47 | 3.87 | active |
| 2,6-Dimethylphenol | 576-26-1 | 45 | 3.97 | inactive |
| Sodium azide | 26628-22-8 | 45 | 6.15 | active |
| 3,5,3′-Triiodothyronine | 6893-02-3 | 40 | 0.83 | active |
| 4-(Butan-2-yl)phenol | 99-71-8 | 40 | 0.62 | inactive |
| EPN | 2104-64-5 | 38 | 3.66 | inactive |
| Folic acid | 59-30-3 | 35 | 3.83 | active |
| N-Ethyl-3-methylaniline | 102-27-2 | 32 | 3.84 | inactive |
| Bensulide | 741-58-2 | 30 | 0.04 | inactive |
| Sodium dimethyldithiocarbamate | 128-04-1 | 30 | −0.87 | active |
| CI-1044 | NOCAS_47291 | 23 | 3.31 | inactive |
| N-Ethylaniline | 103-69-5 | 16 | 3.08 | inactive |
| 7,12-Dimethylbenz(a)anthracene | 57-97-6 | 12 | 3.20 | active |
| Caffeine | 58-08-2 | 0 | NA | inactive |
| Diphenylamine | 122-39-4 | 0 | NA | inactive |
| Disulfoton | 298-04-4 | 0 | NA | inactive |
| Mercuric chloride | 7487-94-7 | 0 | NA | active |
| PFOA | 335-67-1 | 0 | NA | inactive |
| Pyrene | 129-00-0 | 0 | NA | inactive |
TABLE 5. AUR-TPO Assay Results for Chemicals Reported With GUA Assay Results in the Literature
| Chemical Name | CASRN | Source | GUA Lit Activity Call | AUR-TPO AUC | Selectivity |
|---|---|---|---|---|---|
| (±)-Naringenin | 67604-48-2 | Sigma | active | 177 | 5.93 |
| 1-Methyl-1,3-dihydro-2H-benzimidazole-2-thione | 2360-22-7 | Sigma | active | 180 | 5.94 |
| 2,4-Dihydroxybenzophenone | 131-56-6 | DSSTox inventory | active | 126 | 5.56 |
| 2-Aminobenzothiazole | 136-95-8 | Sigma | active | 64 | 0.89 |
| 2-Bromo-4-hydroxyacetophenone | 2491-38-5 | DSSTox inventory | active | 95 | 4.74 |
| 2-Mercaptobenzimidazole | 583-39-1 | DSSTox inventory | active | 230 | 2.99 |
| 3-Hydroxy-2(hydroxymethyl)pyridine | 14173-30-9 | Sigma | active | 0 | NA |
| 3-Hydroxy-2-pyridone | 16867-04-2 | DSSTox inventory | active | 239 | 6.69 |
| 3-Hydroxypyridine | 109-00-2 | Sigma | active | 0 | NA |
| 3-Methylcholanthrene | 56-49-5 | Sigma | active | 32 | −4.20 |
| 5,5-Dimethyl-1-Hydroxymethylhydantoin | 116-25-6 | DSSTox inventory | active | 0 | NA |
| 5-Chloro-2-mercaptobenzothiazole | 5331-91-9 | Sigma | active | 185 | 2.94 |
| 5-Nitro-2-mercaptobenzimidazole | 6325-91-3 | Sigma | active | 214 | 3.37 |
| 6-Hydroxy-2-oxopyridinium chloride | 10357-84-3 | Sigma | active | 96 | 4.62 |
| Benzo(e)pyrene | 192-97-2 | DSSTox inventory | active | 0 | NA |
| Benzo(k)fluoranthene | 207-08-9 | Sigma | active | 0 | NA |
| Benzothiazole | 95-16-9 | DSSTox inventory | active | 84 | 1.36 |
| DL-Goitrin | 13190-34-6 | Santa Cruz | active | 37 | 3.64 |
| Leucomalachite green | 129-73-7 | DSSTox inventory | active | 51 | 0.66 |
| Minocycline hydrochloride | 13614-98-7 | DSSTox inventory | active | 150 | 6.69 |
| N,N,N′,N′-Tetramethylthiourea | 2782-91-4 | TCI | active | 0 | NA |
| Ricinine | 524-40-3 | Sigma | active | 0 | NA |
| Salicylhydroxamic acid | 89-73-6 | Sigma | active | 212 | 6.41 |
| Sodium thiocyanate | 540-72-7 | DSSTox inventory | active | 69 | 4.53 |
| Sulfamethazine | 57-68-1 | DSSTox inventory | active | 72 | 4.57 |
| Sunitinib malate | 341031-54-7 | Sigma | active | 75 | 0.87 |
| Thiourea | 62-56-6 | DSSTox inventory | active | 96 | 0.69 |
| 2-(2-Hydroxy-5-methylphenyl)benzotriazole | 2440-22-4 | DSSTox inventory | inactive | 0 | NA |
| 2(3H)-Benzothiazolone | 934-34-9 | Sigma | inactive | 19 | 3.39 |
| 2-(8-Heptadecenyl)-2-imidazoline-1-ethanol | 95-38-5 | DSSTox inventory | inactive | 48 | −0.13 |
| 2,2′-Dihydroxy 4-methoxybenzophenone | 131-53-3 | Sigma | inactive | 0 | NA |
| 2,3,4-Trihydroxbenzophenone | 1143-72-2 | DSSTox inventory | inactive | 153 | 2.45 |
| 2,4-Dihydroxypyridine | 626-03-9 | Sigma | inactive | 0 | NA |
| 2-Hydroxy 4-methoxybenzophenone | 131-57-7 | DSSTox inventory | inactive | 0 | NA |
| 2-Hydroxypyridine | 142-08-5 | DSSTox inventory | inactive | 0 | NA |
| 3-(4-Methylbenzylidene)camphor | 36861-47-9 | Sigma | inactive | 0 | NA |
| 3-Hydroxy-6-methylpyridine | 1121-78-4 | DSSTox inventory | inactive | 0 | NA |
| 3-Methoxy-4-hydroxypyridine | 62885-41-0 | Frontier | inactive | 0 | NA |
| 4-Butylphenol | 1638-22-8 | DSSTox inventory | inactive | 39 | 0.90 |
| 4-Ethylphenol | 123-07-9 | DSSTox inventory | inactive | 0 | NA |
| 4-Hydroxybenzophenone | 1137-42-4 | Sigma | inactive | 0 | NA |
| 4-Hydroxypyridine | 626-64-2 | DSSTox inventory | inactive | 0 | NA |
| 4-Propylphenol | 645-56-7 | DSSTox inventory | inactive | 44 | 0.68 |
| Benzhydrol | 91-01-0 | Sigma | inactive | 0 | NA |
| Butyl salicylate | 2052-14-4 | Sigma | inactive | 0 | NA |
| Dibenzo(a,h)anthracene | 53-70-3 | DSSTox inventory | inactive | 0 | NA |
| Dipyrone monohydrate | 5907-38-0 | Sigma | inactive | 110 | 5.16 |
| Ethyl-3-hydroxybenzoate | 7781-98-8 | Sigma | inactive | 0 | NA |
| Methyl methylbenzoate | 89-71-4 | DSSTox inventory | inactive | 0 | NA |
| Methylmercury chloride | 115-09-3 | Sigma | inactive | 0 | NA |
| Methylthiobenzothiazole | 615-22-5 | Sigma | inactive | 0 | NA |
| N′-tert-butyl-n-cyclopropyl-6-(methylthio)-1,3,5-triazine-2,4-diamine | 28159-98-0 | DSSTox inventory | inactive | 0 | NA |
| Octinoxate | 5466-77-3 | Sigma | inactive | 0 | NA |
| Phenol red | 143-74-8 | DSSTox inventory | inactive | 45 | −0.06 |
| Phenylbutazone | 50-33-9 | DSSTox inventory | inactive | 26 | 3.61 |
Lit = literature; NA = not applicable.
FIG. 7.
One hundred and fifty guaiacol-tested chemicals ranked by AUC in the AUR-TPO assay. A, 150 chemicals (x-axis) tested across the AUR-TPO and GUA assays were ranked by AUC value (y-axis) derived from the AUR-TPO assay and then scored by GUA activity (dark blue and light gray bars). The reported literature GUA activity was used to score 97 of the 101 chemicals previously tested in the GUA assay, while GUA collected in this study was used to score the remaining 4 literature chemicals and 49 ToxCast chemicals with no previous GUA testing. Blue arrow denotes the 11 chemicals tested or reported as GUA-active that tested inactive in AUR-TPO. B, Comparison of AUR-TPO (x-axis) and GUA (y-axis) threshold potencies for 49 ToxCast chemicals tested in both assays. Potency values are reported as log μM. Chemicals tested as inactive in GUA are plotted across the top and those tested as inactive in AUR-TPO are plotted along the right. The black diagonal line denotes equal potency.
DISCUSSION
The work presented herein describes an extensive screening effort to identify putative inhibitors of TPO using a new high-throughput assay and a tiered screening strategy to evaluate 1074 unique chemicals in the ToxCast chemical libraries. A single-concentration screen initially identified 314 putative TPO inhibitors. Concentration-response data confirmed 90% of these putative in vitro TPO inhibitors. Assays conducted in parallel to distinguish AUR-TPO activity possibly confounded by nonspecific enzyme inhibition and/or chemical reactivity facilitated the distinction of selective AUR-TPO inhibitors from those chemicals with more promiscuous activities. Finally, the results from the recently developed AUR-TPO assay were compared with results from the GUA assay, an assay commonly used to identify in vitro TPO inhibitors, and confirmed that the AUR-TPO assay is a dependable, high-throughput method to identify TPO inhibitors in vitro.
This first implementation of the AUR-TPO inhibition assay with a large chemical test set proved its reproducibility and fitness for use in a HTS effort, as evidenced by several metrics. Critical for HTS is reliable assay performance; the AUR-TPO inhibition assay demonstrated optimal performance and intra-laboratory reproducibility with assay rZ’ values that ranged from 0.77 to 0.83 and rCV values that ranged 3–4%. Additionally, replicated chemical samples embedded within the ToxCast chemical library for QC showed good reproducibility within and across replicated experiments. The AC50 values for 14 chemicals common to the ToxCast test chemical set and the training set previously used (Paul et al., 2014) were very similar, even though those studies were separated by more than 1 year. Finally, a key to screening utility is resource-efficiency, and the AUR-TPO assay enabled the rapid evaluation of more than 1000 chemicals (n = 3) in just days compared with the 5–6 weeks that would be needed to evaluate these chemicals with the GUA assay. AUR-TPO assay miniaturization (approximately 90% reduction in total protein used) coupled with a tiered screening approach (approximately 50% reduction in total protein used) reduced the number of animals required for a comparable quantitative HTS screen using the GUA assay by more than 95%.
MMI was the positive control used in this study, and was confirmed as expected as a potent TPO inhibitor, with AC50 values (0.035–0.148 µM) comparable to the AC50 derived for MMI during initial assay development (Paul et al., 2014). One previous report of an in vitro, AUR-based TPO inhibition assay using rat thyroid extracts and human thyroid slices demonstrated an IC50 of approximately 5–6 µM at 24 h of MMI exposure (Vickers et al., 2012). The AC50 values obtained in the AUR-TPO HTS assay employed herein are generally lower than those reported previously in the literature using other methodologies including the GUA assay, perhaps due to AUR-TPO assay miniaturization and reduced total protein in each assay well (Paul et al., 2014). Several chemicals with well-characterized TPO inhibition profiles, including active and inactive chemicals, were included in the ToxCast chemical library (Table 3). Known TPO inhibitors including PTU, 2,2′,4,4′-tetrahydroxy-benzophenone, and 2-mercaptobenzothiazole were confirmed as TPO inhibitors in this study with similar potency as observed during AUR-TPO assay development (Paul et al., 2014). ETU and resorcinol were also identified as active in this study and during assay development; however, in this study ETU generated an AC50 value of 7.8 µM compared with 0.034 µM in previous assay development (Paul et al., 2014). Conversely, resorcinol produced an AC50 value of 0.025 µM in this study and an AC50 value of 253 µM during assay development (Paul et al., 2014). The marked potency differences observed for ETU and resorcinol between these 2 studies likely resulted from differences in test chemical quality or purity, as: (1) the other 8 active chemicals common to both studies produced very similar AC50 values; (2) the training set chemicals were procured independently from the ToxCast chemicals; and (3) there was no discernable pattern to the potency differences, ie, ETU was less potent and resorcinol more potent in this study compared with the previous study. Overall, this implementation of the AUR-TPO assay was reproducible and successfully classified known TPO inhibitors.
Though use of the AUR-TPO assay reduced the resources necessary for in vitro TPO screening, there were also limitations inherent to the irreversible, loss-of-signal assay design. A tiered screening approach was feasible because only a single, high concentration was required to identify positive responses, resulting in resource savings. However, such loss-of-signal assays are prone to high false-positive rates due to nonspecific loss of assay signal (Judson et al., 2013; Thorne et al., 2010). Reactive chemicals tested at concentrations exceeding cellular toxicity limits and nonspecific enzyme inhibition caused by detergents, salts, or cross-reactive compounds can potentially confound the interpretation of activity in the AUR-TPO assay. Thus assays for both cytotoxicity and firefly luciferase enzyme inhibition were conducted during the concentration-response screen. This approach facilitated the stratification of putative TPO inhibitors by selectivity of the AUR-TPO assay response and thereby increased the potential utility of the AUR-TPO assay data. The inclusion of the luciferase inhibition and cytotoxicity assays clearly enriched the quality of the stratified list, as evidenced by MMI ranking as the top priority TPO inhibitor based on selectivity. This approach distinguished those chemicals that were highly active in the AUR-TPO assay and failed to demonstrate confounding activities in the luciferase inhibition or cytotoxicity assays from those chemicals that were positive in the AUR-TPO assay but were confounded either by cytotoxicity or nonspecific enzyme inhibition.
Though stratification of putative in vitro TPO inhibitors using other experimental data improved interpretation of the AUR-TPO assay data, there are additional uncertainties in the use and understanding of these data. First, multiple and perhaps unforeseen mechanisms can cause assay signal loss, and only 2 potential sources of confounded positive results were examined (cellular toxicity limit as a measure of chemical reactivity and nonspecific enzyme inhibition). Another barrier to complete interpretation of the AUR-TPO assay data is the screening assay chemistry. As the reaction chemistry and oxidation product of AUR are proprietary, the AUR-TPO inhibition assay has been verified for use on an empirical basis, using training and test set information. As TPO is a multi-functional enzyme that catalyzes iodine oxidation in the presence of hydrogen peroxide, nonspecific iodination of tyrosyl residues, and coupling of iodotyrosyl residues (Divi and Doerge, 1994; Ruf and Carayon, 2006; Taurog et al., 1996), it is not known from the AUR-TPO inhibition assay result which reaction(s) would be inhibited, and whether this inhibition would be reversible or irreversible. An additional possible confounding mechanism left unexplored in this study is fluorescent quenching. A compound capable of quenching red fluorescent light at approximately 590 nm would be misclassified as positive in the AUR-TPO assay, absent any corrective measures. Other confounding factors may have produced false negative AUR-TPO inhibition assay results, including TPO-independent oxidation of AUR in the presence of hydrogen peroxide and compound autofluorescence; as such, verification of the capability of a chemical to autofluoresce and interfere with the signal (Simeonov et al., 2008) of the AUR-TPO assay should be investigated before moving to more biologically complex confirmatory models. Stratification can, in the near term, help focus lower-throughput and more resource intensive testing on those chemicals most likely to disrupt TH synthesis in vivo, though promiscuous bioactivity does not preclude a compound from inhibiting TPO in more complex biological models.
The recent development of the AUR-TPO assay, and its reliance on a proprietary substrate, prompted additional confirmation of AUR-TPO screening results using the GUA assay. The GUA assay was first developed over 60 years ago and has been used to generate the majority of the published TPO inhibition data (Murk et al., 2013). A literature search identified 114 chemicals with published reports describing GUA assay results. Thirteen of these chemicals were unavailable commercially or were insoluble in DMSO; the remaining 101 chemicals were tested in the AUR-TPO assay. An additional 49 ToxCast library chemicals were tested in the GUA assay. These efforts enabled the comparison of 150 total chemicals tested in both assays. Several key factors complicated any comparison of reported GUA results, notwithstanding comparisons to AUR-TPO. First, all of the AUR-TPO retesting was conducted using a concentration-response design; therefore, AUR-TPO data could be ranked continuously by activity using the AUC values. GUA activity was scored using a binary “active” or “inactive” function because of differences in testing methodology across laboratories and a lack of access to numerical concentration-response data from literature reports. Though most published studies used porcine thyroid microsomes as the TPO source, others used rat thyroid microsomes, and 1 study used microsomes derived from a recombinant human cell line. Other important variations included test chemical concentrations (and ranges), solvents, and criteria for determining activity in the assay. These differences complicated comparisons using the literature-derived GUA assay data. Despite these limitations, the AUR-TPO assay proved to be a reliable predictor of GUA activity with a balanced accuracy of 70%. In this case, sensitivity is valued over specificity because toxicity screening assays attempt to minimize false negatives. AUR-TPO sensitivity (88.3%) was good but somewhat diminished by 11 chemicals that were inactive in the AUR-TPO assay and active in the GUA assay. Two of these compounds, benzo(e)pyrene and benzo(k)fluoranthene, were published in the same study that reported pyrene, caffeine, and PFOA as positive in the GUA assay (Song et al., 2012); however, on retesting, pyrene, caffeine, and PFOA tested negative in the GUA assay in this study. Another polyaromatic hydrocarbon, 7,12-dimethylbenz(a)anthracene, tested positive in the GUA assay in this study. Four of the remaining compounds are pyridines published in a single report (Gaitan, 1989); 3 of these (3-hydroxypyridine, 2,4-dihydroxypyridine, and 3-methoxy-4-hydroxypyridine) were modestly active but did not exceed the 20% inhibition threshold. The specificity of AUR-TPO was affected by 34 chemicals active in the AUR-TPO assay and inactive in the GUA assay; this was not unexpected given that 2 of the 11 training set chemicals thought to be negative based on GUA assay data tested as positives with low potencies and efficacies in AUR-TPO assay development (Paul et al., 2014), and that chemicals active in both assays typically have greater potencies (lower IC20 values) in the AUR-TPO assay than in the GUA assay (Figure 7B). The computed sensitivity (88.3%) and specificity (39.3%) for the AUR-TPO assay when compared with the GUA assay results suggest that the AUR-TPO assay may be more sensitive for identification of putative TPO inhibitors, diminishing the likelihood of false negatives. These data also highlight the importance of using additional experimental information to further prioritize putative in vitro TPO inhibitors due to the possibility of false or low priority positive responses in the AUR-TPO assay.
In demonstrating the fit-for-purpose utility of the AUR-TPO assay for thyroid-related HTS efforts, there are further considerations beyond reproducibility and assay performance metrics. As discussed earlier, training set data and test set data are now available for this HTS AUR-TPO assay. Previously published guidance on the development of alternative screening methods suggests consideration of 15 methodological elements: key event, endpoint measurement, dynamic range, parametric controls, response characterization, concentration, endpoint-selectivity, endpoint-selective controls, training set chemicals, test set chemicals, specificity and sensitivity, high-throughput capacity, documentation, transferability, and data sharing (Crofton et al., 2011). A rating of the AUR-TPO inhibition assay using these criteria is found in Table 6. In addition to superior assay performance metrics and the high-throughput nature of the AUR-TPO assay, the large amount of concentration-response data now available from the ToxCast chemical library test set and the availability of the method and data all demonstrate key strengths for the future use of the AUR-TPO assay. In qualifying the assay, it is important to note that though the key event modeled by this assay, TPO inhibition, is well defined in an AOP for TH-related adverse outcomes, the endpoint measured in the assay itself is loss of a fluorescence signal; as such, this assay is well suited to HTS and prioritization but not necessarily for mechanistic evaluation of TPO inhibition. In this work, response characterization has been improved by adding additional data from assays intended to assess potential contributions of confounding activity in vitro. The primary throughput and transferability limitation of the AUR-TPO inhibition assay in its current design is the use of animals as the source of thyroidal TPO. Ideally future use of this assay would substitute human recombinant TPO enzyme. Another more immediate solution could be use of lactoperoxidase instead of TPO, which has been used previously as a surrogate for TPO, although the specificity of this approach would require further evaluation (Divi and Doerge, 1994; Doerge and Niemczura, 1989; Kirthana et al., 2013; Taurog et al., 1996). In addition to addressing ethical concerns regarding animal use for toxicology studies, eliminating reliance on animal thyroid tissue might also improve assay transferability to laboratories without access to a vivarium and trained necropsy technicians. Though it is important to qualify the limitations of the AUR-TPO assay, it is clear that the AUR-TPO inhibition assay meets, and by some elements exceeds, the criteria suggested for alternative screening assay development, further demonstrating the utility of this assay as part of an integrated HTS program to identify thyroid-disrupting chemicals.
TABLE 6. Consideration of the AUR-TPO Inhibition Assay Using Criteria for Alternative Screening Assay Development
| No. | Criteria for Alternative Screening Assay Development | Rating of the AUR-TPO Inhibition Assay |
|---|---|---|
| 1 | Key event | TPO inhibition is clearly linked with decreased TH synthesis, with clinical and animal model information to support the connections between TPO inhibition, decreased TH synthesis, and aberrant neurodevelopment. |
| 2 | Endpoint measurement | The endpoint measured in this assay is a loss of fluorescence, indicating decreased TPO activity. This endpoint is not measured directly, and as such assay confounders may include autofluorescent chemicals, detergents, salts, denaturing agents, pan-active enzyme inhibitors, and highly reactive chemicals. |
| 3 | Dynamic range | The AUR-TPO inhibition demonstrates excellent dynamic range and sufficiently low intersample variability to discriminate clearly between positive and negative test chemicals with a rZ’ of 0.77–0.83. A rZ′ of 0.5 − 1.0 corresponds to an assay with a suitably high signal-to-background difference and low variability to be amenable for HTS applications. |
| 4 | Parametric controls | Conduct of the AUR-TPO assay without H2O2 to initiate the reaction or without thyroid microsomal protein will result in a lack of response. The vehicle control (DMSO) was used in place of a chemical to demonstrate maximal TPO activity. |
| 5 | Response characterization | A response threshold has been set at 20% inhibition of maximal TPO activity, and a link between AUR-TPO activity in vitro and effects on TH homeostasis in vivo is not fully characterized. However, subsequent refinement using assays to indicate potential confounding factors may add relevance to the response characterization. |
| 6 | Concentration | Concentration-response curves are measured and replicable in the AUR-TPO inhibition assay. |
| 7 | Endpoint-selectivity | The AUR-TPO inhibition assay is subject to confounders as previously described that may result in false-positive outcomes. Stratification of AUR-TPO inhibition results as demonstrated herein provides a pragmatic approach for increasing endpoint-selectivity in the AUR-TPO assay. |
| 8 | Endpoint-selective controls | MMI was included on every assay plate and demonstrated predictable performance within and across studies (Paul et al., 2014). |
| 9 | Training set | A training set of chemicals (Paul et al., 2014) with both positive and negative chemicals was developed previously and verified in this study, with qualitative concordance and a good degree of quantitative concordance (Table 4). |
| 10 | Testing set | The ToxCast phase I and II chemical libraries, along with chemicals tested due to positive findings in the GUA assay as reported in the literature, comprise a broad test set for the AUR-TPO assay, including 17 known TPO inhibitors and 32 known negatives, but with the vast majority of these chemicals either never tested in vitro or in vivo for TPO inhibition. |
| 11 | Specificity and sensitivity | Using the 150 total GUA activity calls as the standard of in vitro TPO inhibition, the AUR-TPO assay has a balanced accuracy of 70% with greater sensitivity (88.3%) than specificity (39.3%). The relatively low specificity of AUR-TPO results from a large number of AUR-TPO actives that tested or were reported as inactive in GUA; our studies found that chemicals tested in GUA required higher test concentrations (compared with AUR-TPO) to exceed the activity threshold and this may explain the discordance between the assays. |
| 12 | High-throughput | The AUR-TPO inhibition assay was conducted in a semiautomated 384-well format, enabling hundreds to thousands of chemicals to be tested in 1 day. A major limitation on throughput is the preparation of rat thyroid microsomes. Approximately 87 test wells can be run with a single rat thyroid (approximately 15–20 mg of tissue). |
| 13 | Documentation | This assay is fully documented with all assay details available in this work and in a previous work (Paul et al., 2014). |
| 14 | Transferability | The assay itself is highly transferrable using a commercially available reagent (AUR, Life Technologies) and other commonly available assay reagents. Rat thyroid microsomal tissue remains a limitation on transferability. |
| 15 | Data sharing | All of the data files for this evaluation are available as Supplementary Material and on a public website. |
Stratification of AUR-TPO results for nearly 1100 chemicals also highlighted potential structure-activity relationships among the highly selective TPO inhibitors. Among the most selective AUR-TPO actives were a number of aromatic amine chemicals such as 4,4′-methylenedianiline, 2-anisidine, and 4,4′-oxydianiline. Aromatic amines comprised 10 of the 25 most selective AUR-TPO actives. This suggests that the aniline pharmacophore may be a driver of TPO inhibition. One of the known mechanisms of TPO inhibition is direct interaction and covalent binding with the TPO heme group, which is indispensable to peroxidase function (Divi and Doerge, 1994). The pi electrons of aromatic amines have been shown to interact directly with the tetrapyrrole ring of hemin (Adams et al., 1984). Aromatic amines were also shown to have an affinity for lactoperoxidase that was nearly 5000 times higher than that of horseradish peroxidase (Hosoya et al., 1989). There are no available study data to suggest that aromatic amines disrupt TH signaling in animals; however, anilines produce hemotoxicity in vivo, inducing anemia through a direct interaction with hemoglobin (Adams et al., 1984; Kafferlein et al., 2014; Pizon et al., 2009; Purnell and Singh, 2005). This aniline-hemoglobin interaction suggests that aromatic amines may inhibit TPO in vitro by directly interacting with the heme group in TPO. Moreover, it suggests that the AUR-TPO assay may also detect chemicals that interact with other heme proteins.
As part of the ToxCast program, the AUR-TPO assay was used to screen the largest chemical library to date for TPO inhibition activity to identify potential thyroid-disrupting chemicals. However, the use of the high-throughput assays like AUR-TPO, coupled with assays to detect confounding bioactivities, as demonstrated herein, is likely to be insufficient to adequately predict TH disruption in vivo due to the number of MIEs relevant to TH homeostasis. As suggested in the EDSP21 workplan, and in recent efforts for estrogen receptor pathway screening (Browne et al., 2015), it is evident that a systems biology approach involving multiple models that evaluate different MIEs and key events (Figure 1) will be needed for the thyroid. A particular challenge will be the development of models that include assessment of the interaction of multiple tissue types that comprise the hypothalamic-pituitary axis; clearly computational models and a series of in vitro models will be required to build such an understanding, and this work is ongoing. More immediately, the AUR-TPO assay, along with other information on potential confounding activity, toxicokinetics, and exposure potential, could be used to prioritize chemicals for further assessment in the thyroid-related EDSP Tier I Amphibian Metamorphosis Assay (OCSPP 890.1100), as well as nonguideline, mechanistic studies. Selection of models of increasing biological complexity to confirm the potential hazard of these prioritized chemicals should use a mode-of-action (Zoeller and Crofton, 2005) or AOP-based approach (Boobis et al., 2008; Perkins et al., 2013), selecting the appropriate study design based on data gaps and/or knowledge of the potential MIEs involved. Models of greater biological complexity include the cultured amphibian thyroid explant system and amphibian short-term assays, which have demonstrated the efficacy of reference chemicals including MMI, PTU, and sodium perchlorate to disrupt TH synthesis (Hornung et al., 2010). Short-term intact animal models, including the 7-day amphibian prometamorphic larvae screen and/or a 4-day rat TH screen (DeVito et al., 1999; Hornung et al., 2015; Paul et al., 2010; Tietge et al., 2013) may indicate whether a chemical poses a thyroid-related hazard that could then be further investigated for links to developmental hazard as needed for decision-making. More fit-for-purpose HTS assays like the AUR-TPO inhibition assay for other key MIEs, including inhibition of the sodium-iodide symporter and peripheral deiodinases, better experimental and computational models of hepatic catabolism of THs, and models to demonstrate the relevance of in vitro or in vivo model data to human TH homeostasis, are vital to further development of thyroid screening as part of the ToxCast and EDSP21 programs.
FUNDING
K.P.F. and E.D.W. were supported by appointments to the Research Participation Program of the U.S. Environmental Protection Agency, Office of Research and Development, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. EPA.
Supplementary Material
ACKNOWLEDGMENTS
Dr William Flowers at North Carolina State University for generously allowing us to collect porcine thyroid glands and Kateland Antonazzo for help in collection of these porcine thyroid glands. Dr Mary Gilbert, Dr Andrew Johnstone, Wendy Oshiro, and Stephanie Spring collected additional rat thyroid glands. Angela Buckalew assisted with microsome preparation. Dr William Mundy kindly provided access to plate reader equipment. Mr. Scott Klayner at BMG LabTech assisted with assay protocol development. Dr Brian Chorley and Dr William Mundy for their detailed comments in review of previous versions of this article. When this work was completed K.P.F. was an ORISE/EPA postdoctoral fellow; K.P.F. acknowledges that she is currently employed by Bayer CropScience, which has financial interests in some compounds that appear in the article. This affiliation did not alter the content of this article.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Adams P. A., Adams C., Berman M. C., Lawrence M. C. (1984). The nature of heme-aniline interactions during hemin-mediated oxygen activation and insertion reactions. J. Inorg. Biochem. 20, 291–297. [DOI] [PubMed] [Google Scholar]
- Berbel P., Mestre J. L., Santamaria A., Palazon I., Franco A., Graells M., Gonzalez-Torga A., de Escobar G. M. (2009). Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid 19, 511–519. [DOI] [PubMed] [Google Scholar]
- Boobis A. R., Doe J. E., Heinrich-Hirsch B., Meek M. E., Munn S., Ruchirawat M., Schlatter J., Seed J., Vickers C. (2008). IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit. Rev. Toxicol. 38, 87–96. [DOI] [PubMed] [Google Scholar]
- Browne P., Judson R. S., Casey W. M., Kleinstreuer N. C., Thomas R. S. (2015). Screening chemicals for estrogen receptor bioactivity using a computational model. Environ. Sci. Technol. 49, 8804–8814. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F. (1998). Effects of environmental synthetic chemicals on thyroid function. Thyroid 8, 827–856. [DOI] [PubMed] [Google Scholar]
- Capen C. C. (1994). Mechanisms of chemical injury of thyroid gland. Prog. Clin. Biol. Res. 387, 173–191. [PubMed] [Google Scholar]
- Chang H. C., Doerge D. R. (2000). Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol. Appl. Pharmacol. 168, 244–252. [DOI] [PubMed] [Google Scholar]
- Coady K., Marino T., Thomas J., Currie R., Hancock G., Crofoot J., McNalley L., McFadden L., Geter D., Klecka G. (2010). Evaluation of the amphibian metamorphosis assay: Exposure to the goitrogen methimazole and the endogenous thyroid hormone L-thyroxine. Environ. Toxicol. Chem. 29, 869–880. [DOI] [PubMed] [Google Scholar]
- Crofton K. M., Zoeller R. T. (2005). Mode of action: neurotoxicity induced by thyroid hormone disruption during development—hearing loss resulting from exposure to PHAHs. Crit. Rev. Toxicol. 35, 757–769. [DOI] [PubMed] [Google Scholar]
- Crofton K. M., Craft E. S., Hedge J. M., Gennings C., Simmons J. E., Carchman R. A., Carter W. H, Jr., DeVito M. J. (2005). Thyroid-hormone-disrupting chemicals: Evidence for dose-dependent additivity or synergism. Environ. Health Perspect. 113, 1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton K. M., Mundy W. R., Lein P. J., Bal-Price A., Coecke S., Seiler A. E., Knaut H., Buzanska L., Goldberg A. (2011). Developmental neurotoxicity testing: Recommendations for developing alternative methods for the screening and prioritization of chemicals. ALTEX 28, 9–15. [PubMed] [Google Scholar]
- Cuevas E., Auso E., Telefont M., Morreale de Escobar G., Sotelo C., Berbel P. (2005). Transient maternal hypothyroxinemia at onset of corticogenesis alters tangential migration of medial ganglionic eminence-derived neurons. Eur. J. Neurosci. 22, 541–551. [DOI] [PubMed] [Google Scholar]
- DeVito M., Biegel L., Brouwer A., Brown S., Brucker-Davis F., Cheek A. O., Christensen R., Colborn T., Cooke P., Crissman J., et al. (1999). Screening methods for thyroid hormone disruptors. Environ. Health Perspect. 107, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divi R. L., Doerge D. R. (1994). Mechanism-based inactivation of lactoperoxidase and thyroid peroxidase by resorcinol derivatives. Biochemistry 33, 9668–9674. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Niemczura W. P. (1989). Suicide inactivation of lactoperoxidase by 3-amino-1,2,4-triazole. Chem. Res. Toxicol. 2, 100–103. [DOI] [PubMed] [Google Scholar]
- Emiliano A. B., Governale L., Parks M., Cooper D. S. (2010). Shifts in propylthiouracil and methimazole prescribing practices: Antithyroid drug use in the United States from 1991 to 2008. J. Clin. Endocrinol. Metab. 95, 2227–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitan E. (1989). Environmental Goitrogenesis: CRC Press Taylor and Francis Group. Boca Raton, FL; ISBN 084936728X. [Google Scholar]
- Haddow J. E., Palomaki G. E., Allan W. C., Williams J. R., Knight G. J., Gagnon J., O’Heir C. E., Mitchell M. L., Hermos R. J., Waisbren S. E., et al. (1999). Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 341, 549–555. [DOI] [PubMed] [Google Scholar]
- Henrichs J., Bongers-Schokking J. J., Schenk J. J., Ghassabian A., Schmidt H. G., Visser T. J., Hooijkaas H., de Muinck Keizer-Schrama S. M., Hofman A., Jaddoe V. V., et al. (2010). Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: The generation R study. J. Clin. Endocrinol. Metab. 95, 4227–4234. [DOI] [PubMed] [Google Scholar]
- Hornung M. W., Degitz S. J., Korte L. M., Olson J. M., Kosian P. A., Linnum A. L., Tietge J. E. (2010). Inhibition of thyroid hormone release from cultured amphibian thyroid glands by methimazole, 6-propylthiouracil, and perchlorate. Toxicol. Sci. 118, 42–51. [DOI] [PubMed] [Google Scholar]
- Hornung M. W., Kosian P. A., Haselman J. T., Korte J. J., Challis K., Macherla C., Nevalainen E., Degitz S. J. (2015). In vitro, ex vivo, and in vivo determination of thyroid hormone modulating activity of benzothiazoles. Toxicol. Sci. 146, 254–264. [DOI] [PubMed] [Google Scholar]
- Hosoya T., Sakurada J., Kurokawa C., Toyoda R., Nakamura S. (1989). Interaction of aromatic donor molecules with lactoperoxidase probed by optical difference spectra. Biochemistry 28, 2639–2644. [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L. (2002). A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect. 110(Suppl. 3), 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson R., Kavlock R., Martin M., Reif D., Houck K., Knudsen T., Richard A., Tice R. R., Whelan M., Xia M., et al. (2013). Perspectives on validation of high-throughput assays supporting 21st century toxicity testing. ALTEX 30, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson R. S., Houck K. A., Kavlock R. J., Knudsen T. B., Martin M. T., Mortensen H. M., Reif D. M., Rotroff D. M., Shah I., Richard A. M., et al. (2010). In vitro screening of environmental chemicals for targeted testing prioritization: The ToxCast project. Environ. Health Perspect. 118, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafferlein H. U., Broding H. C., Bunger J., Jettkant B., Koslitz S., Lehnert M., Marek E. M., Blaszkewicz M., Monse C., Weiss T., et al. (2014). Human exposure to airborne aniline and formation of methemoglobin: A contribution to occupational exposure limits. Arch. Toxicol. 88, 1419–1426. [DOI] [PubMed] [Google Scholar]
- Kavlock R., Chandler K., Houck K., Hunter S., Judson R., Kleinstreuer N., Knudsen T., Martin M., Padilla S., Reif D., et al. (2012). Update on EPA’s ToxCast program: Providing high throughput decision support tools for chemical risk management. Chem. Res. Toxicol. 25, 1287–1302. [DOI] [PubMed] [Google Scholar]
- Kessler J., Obinger C., Eales G. (2008). Factors influencing the study of peroxidase-generated iodine species and implications for thyroglobulin synthesis. Thyroid 18, 769–774. [DOI] [PubMed] [Google Scholar]
- Kirthana M. V., Nawaz Khan F., Sivakumar P. M., Doble M., Manivel P., Prabakaran K., Krishnakumar V. (2013). Antithyroid agents and QSAR studies: Inhibition of lactoperoxidase-catalyzed iodination reaction by isochromene-1-thiones. Med. Chem. Res. 22, 4810–4817. [Google Scholar]
- Kooistra L., Crawford S., van Baar A. L., Brouwers E. P., Pop V. J. (2006). Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics 117, 161–167. [DOI] [PubMed] [Google Scholar]
- Li Y., Shan Z., Teng W., Yu X., Li Y., Fan C., Teng X., Guo R., Wang H., Li J., et al. (2010). Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin. Endocrinol. 72, 825–829. [DOI] [PubMed] [Google Scholar]
- McClain R. M. (1989). The significance of hepatic microsomal enzyme induction and altered thyroid function in rats: implications for thyroid gland neoplasia. Toxicol. pathol. 17, 294–306. [DOI] [PubMed] [Google Scholar]
- Miller M. D., Crofton K. M., Rice D. C., Zoeller R. T. (2009). Thyroid-disrupting chemicals: Interpreting upstream biomarkers of adverse outcomes. Environ. Health Perspect. 117, 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G., Obregon M. J., Escobar del Rey F. (2000). Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J. Clin. Endocrinol. Metab. 85, 3975–3987. [DOI] [PubMed] [Google Scholar]
- Murk A. J., Rijntjes E., Blaauboer B. J., Clewell R., Crofton K. M., Dingemans M. M., Furlow J. D., Kavlock R., Kohrle J., Opitz R., et al. (2013). Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol. In Vitro 27, 1320–1346. [DOI] [PubMed] [Google Scholar]
- Paul K. B., Hedge J. M., DeVito M. J., Crofton K. M. (2010). Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in Young Long-Evans rats. Toxicol. Sci. 113, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K. B., Hedge J. M., Macherla C., Filer D. L., Burgess E., Simmons S. O., Crofton K. M., Hornung M. W. (2013). Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology 312, 97–107. [DOI] [PubMed] [Google Scholar]
- Paul K. B., Hedge J. M., Rotroff D. M., Hornung M. W., Crofton K. M., Simmons S. O. (2014). Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem. Res. Toxicol. 27, 387–399. [DOI] [PubMed] [Google Scholar]
- Perkins E. J., Ankley G. T., Crofton K. M., Garcia-Reyero N., LaLone C. A., Johnson M. S., Tietge J. E., Villeneuve D. L. (2013). Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ. Health Perspect. 121, 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford D. B. (2010). Screening chemicals for thyroid-disrupting activity: A critical comparison of mammalian and amphibian models. Crit. Rev. Toxicol. 40, 845–892. [DOI] [PubMed] [Google Scholar]
- Pizon A. F., Schwartz A. R., Shum L. M., Rittenberger J. C., Lower D. R., Giannoutsos S., Virji M. A., Krasowski M. D. (2009). Toxicology laboratory analysis and human exposure to p-chloroaniline. Clin. Toxicol. 47, 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V. J., Brouwers E. P., Vader H. L., Vulsma T., van Baar A. L., de Vijlder J. J. (2003). Maternal hypothyroxinaemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin. Endocrinol. 59, 282–288. [DOI] [PubMed] [Google Scholar]
- Pop V. J., Kuijpens J. L., van Baar A. L., Verkerk G., van Son M. M., de Vijlder J. J., Vulsma T., Wiersinga W. M., Drexhage H. A., Vader H. L. (1999). Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin. Endocrinol. 50, 149–155. [DOI] [PubMed] [Google Scholar]
- Purnell E. T., Singh H. (2005). The hemotoxicity of para-substituted aniline analogs in dog and rat erythrocytes: A species comparison. Ethn. Dis. 15(4 Suppl. 5), S5–81-7. [PubMed] [Google Scholar]
- Reif D. M., Martin M. T., Tan S. W., Houck K. A., Judson R. S., Richard A. M., Knudsen T. B., Dix D. J., Kavlock R. J. (2010). Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ. Health Perspect. 118, 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf J., Carayon P. (2006). Structural and functional aspects of thyroid peroxidase. Arch. Biochem. Biophys. 445, 269–277. [DOI] [PubMed] [Google Scholar]
- Simeonov A., Jadhav A., Thomas C. J., Wang Y., Huang R., Southall N. T., Shinn P., Smith J., Austin C. P., Auld D. S., et al. (2008). Fluorescence spectroscopic profiling of compound libraries. J. Med. Chem. 51, 2363–2371. [DOI] [PubMed] [Google Scholar]
- Song M., Kim Y. J., Park Y. K., Ryu J. C. (2012). Changes in thyroid peroxidase activity in response to various chemicals. J. Environ. Monit. 14, 2121–2126. [DOI] [PubMed] [Google Scholar]
- Taurog A., Dorris M. L., Doerge D. R. (1996). Mechanism of simultaneous iodination and coupling catalyzed by thyroid peroxidase. Arch. Biochem. Biophys. 330, 24–32. [DOI] [PubMed] [Google Scholar]
- Thorne N., Auld D. S., Inglese J. (2010). Apparent activity in high-throughput screening: Origins of compound-dependent assay interference. Curr. Opin. Chem. Biol. 14, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietge J. E., Degitz S. J., Haselman J. T., Butterworth B. C., Korte J. J., Kosian P. A., Lindberg-Livingston A. J., Burgess E. M., Blackshear P. E., Hornung M. W. (2013). Inhibition of the thyroid hormone pathway in Xenopus laevis by 2-mercaptobenzothiazole. Aquat. Toxicol. 126, 128–136. [DOI] [PubMed] [Google Scholar]
- Trepanier L. A. (2006). Medical management of hyperthyroidism. Clin. Tech. Small Animal Pract. 21, 22–28. [DOI] [PubMed] [Google Scholar]
- US EPA, (2014). Endocrine Disruptor Screening Program for the 21st Century: EDSP21 Work Plan. EDSP Comprehensive Management Plan. February 14, 2014. Available at: http://www.epa.gov/sites/production/files/2015-08/documents/edsp_comprehesive_management_plan_021414_f.pdf. Accessed January 20, 2016. [Google Scholar]
- Van Herck S. L., Geysens S., Bald E., Chwatko G., Delezie E., Dianati E., Ahmed R. G., Darras V. M. (2013). Maternal transfer of methimazole and effects on thyroid hormone availability in embryonic tissues. J. Endocrinol. 218, 105-15. [DOI] [PubMed] [Google Scholar]
- Vickers A. E., Heale J., Sinclair J. R., Morris S., Rowe J. M., Fisher R. L. (2012). Thyroid organotypic rat and human cultures used to investigate drug effects on thyroid function, hormone synthesis and release pathways. Toxicol. Appl. Pharmacol. 260, 81–88. [DOI] [PubMed] [Google Scholar]
- Zoeller R. T., Crofton K. M. (2000). Thyroid hormone action in fetal brain development and potential for disruption by environmental chemicals. Neurotoxicology 21, 935–945. [PubMed] [Google Scholar]
- Zoeller R. T., Crofton K. M. (2005). Mode of action: Developmental thyroid hormone insufficiency–neurological abnormalities resulting from exposure to propylthiouracil. Crit. Rev. Toxicol. 35, 771–781. [DOI] [PubMed] [Google Scholar]
- Zoeller R. T., Rovet J. (2004). Timing of thyroid hormone action in the developing brain: Clinical observations and experimental findings. J. Euroendocrinol. 16, 809–818. [DOI] [PubMed] [Google Scholar]
- Zoeller R. T., Tan S. W., Tyl R. W. (2007). General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit. Rev. Toxicol. 37, 11–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.