Abstract
Nasopharyngeal carcinoma (NPC) is endemic in southern China, and its incidence in Hong Kong is relatively high. Radiotherapy is the mainstay treatment for NPC due to its relatively high radiosensitivity and deep‐seated anatomical position, which is not readily accessible by surgery. Although the technique of radiotherapy in NPC has been advancing and offers promising treatment outcome, complications around the irradiation areas are inevitable and the quality of life of the post‐radiotherapy patients is often compromised. Trismus, which is defined as the restricted mouth opening or jaw movement due to the disorder of temporo‐mandibular joint (TMJ), is one of the possible late complications for radiotherapy of NPC and is found in 5–17% of the post‐radiotherapy (post‐RT) patients. Trismus at early stage may only affect the speech, but in severe cases nutritional intake and oral hygiene condition may deteriorate seriously. This article reviewed the possible causes of radiation‐induced TMJ damage, the various assessments including imaging modalities and possible treatments. The conclusion is that the availability of simple, yet effective examinations for trismus is essential for delaying the progression and restoring TMJ functions. Although there is no absolutely effective treatment for trismus, many supportive, restorative and palliative management are possible under different clinical situations.
Keywords: Imaging modalities, nasopharyngeal carcinoma, radiation‐induced trismus, radiotherapy, temporo‐mandibular joint
Introduction
Nasopharyngeal carcinoma (NPC) is endemic in southern China including Hong Kong.1 According to the Hong Kong Cancer Registry,2 overall NPC was the eighth most common cancer in 2010 and it contributed 3.5% of all cancer new cases. The age group with highest incidence was 45–64 years old. At present, radiotherapy is the mainstay of NPC treatment due to its relatively high radiosensitivity and deep‐seated anatomical position, which make surgical resection unfavourable.3 Although radiotherapy in NPC enables promising treatment outcome, complications of the organs around the irradiation areas are inevitable and the quality of life of the patients after treatment is often compromised. Trismus, which is referred as the restricted mouth opening or jaw movement,4 is one of the possible late complications in radiotherapy of NPC due to damage of the temporo‐mandibular joint (TMJ), and is found in 5–17% of the patients.5, 6 Apart from the dose factor, it was found that patients with TGF β1 genotype were prone to develop radiation‐induced trismus.7
Trismus at early stage may only affect the speech, but in severe cases nutritional intake and oral hygiene condition may deteriorate seriously. The oral problem can be compounded with the presence of other radiation‐induced complications such as xerostomia and oral mucositis, which are common in NPC patients. Xerostomia, which is caused by damage to the parotid and submandibular glands, often leads to difficulty in swallowing and degradation of oral hygiene.8 Oral mucositis is a painful condition that is associated with dysphagia and loss of sense of taste and subsequently leads to poor nutritional status.9 Therefore, early detection is important for patient management which allows possible function restoration and prevention of trismus progression.
Trismus can be one of symptoms of primary NPC, however its incidence is very low and only present in extensive tumour involving the TM joint and/or related muscles. Furthermore, this symptom can be easily differentiated from the radiation‐induced trismus as the latter is a late complication and usually occurred at 2–3 years after completion of radiotherapy.
Questionnaires such as Mandibular Function Impairment Questionnaire (MFIQ) and Helkimo Masticatory Dysfunction Index (HMDI) are often used for screening of TMJ disorders, including trismus. Various imaging modalities, such as general x‐ray, computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (US), etc., are used for further diagnosis. With the recent technological advancement and the soft tissue nature of the joint, MRI is now treated as the gold standard for diagnosis for TMJ disorder. However, since MRI examination is more time‐consuming and not very cost‐effective, other imaging modalities may be used as alternatives.
Management of radiation‐induced trismus is challenging due to poor understanding of trismus aetiology and its irreversibility. Although several modalities are found to be possible for treatment, the results are not satisfactory. This review aims to discuss radiation‐induced damage to TMJ, assessment of TMJ by various imaging modalities and the possible treatments of trismus.
Radiotherapy of NPC
In radical treatment for NPC using two‐dimensional (2D) conventional radiotherapy, a tumouricidal radiation dose of 66–70 Gy10 is delivered to the primary tumour using 4–6 MV photons and the associated areas, with organ at risks including spinal cord, brain stem, lens, optic nerve/chiasm, parotid gland, and pituitary gland delineated11 for retaining reasonable quality of life in patients after treatment. With recent technological advancements, apart from 2D conventional radiotherapy, intensity modulated radiotherapy (IMRT) and volumetric‐modulated arc therapy (VMAT) are available. IMRT and VMAT offer much better dose conformity compared to conventional techniques and a high dose of 76 Gy can be delivered to the gross tumour volume without compromising the doses to the organs at risk.12, 13, 14 With such dosimetric superiority over the conventional techniques, it is expected that these new treatment techniques can offer better treatment outcome and therefore are currently the mainstay for NPC treatment in Hong Kong.
Radiation‐Induced Trismus in NPC
Radiation‐induced trimus has been frequently reported in post‐RT head and neck cancer patients.15 Since recurrent tumour involving the TMJ or its related muscles may also lead to trismus, and it is important to differentiate this from post‐RT changes. The situation is relatively common in NPC due to close proximity of TMJ to the nasopharynx and considerable dose is delivered to TMJ during radiotherapy, regardless of the techniques used. Therefore, radiation damage to TMJ, which is clinically presented as trismus, has been commonly encountered in post‐RT NPC patients.
For patients having no history of trismus before radiotherapy, trismus progression was found to be insignificant during radiotherapy, with only 1.3% decrease in maximum incisal distance (MID) per month. But the symptoms greatly progress within the first 9 months after radiotherapy followed by condition stabilisation afterwards, with an average MID decrease of 32% after 4 years of radiotherapy.4
Although precise mechanism for radiation‐induced trismus is not fully understood, it is believed that such incidence can be explained by radiation fibrosis, gradual decrease in vascularity and denervation atrophy of the joint muscles, as well as the injury to the mandible and the TMJ.5, 16 It is also found that the severity of radiation‐induced trismus can be correlated to the radiation field and dose.
In radiation‐induced fibrosis, the abnormal proliferation of fibroblasts is found to be the major change and infiltrating inflammatory cells, atypical fibroblasts and large amount of extracellular matrix are present in the affected areas.17 Fibrosis can be clinically presented as contractures in masticatory muscles, hence reduce the degree of movement of TMJ. In addition, fibrosis in soft tissues and salivary glands which are included in the radiation field may lead to hyposalivation and thus reduced lubrications, together with muscle dysfunction, resulting in oral pain. This may further defer patients from active jaw movements, hence promoting the progression of trismus.18
Trismus can also be caused by osteoradionecrosis in mandible and skull base, though these complications are relatively rare. Necrosis occurs as radiation leads to thrombosis of small blood vessels, fibrosis of the periosteum and damage to osteocytes, osteoblasts and fibroblasts. Damaged bone cells survive until mitotic death occurs and results in slow and protracted loss of bone cells after radiotherapy Therefore, possible bone repair is hindered. In the case of mandibular necrosis, thinning and reduced strength of the bone finally affect the joint movement. Mandible is at a relatively higher risk of osteonecrosis since it composes of a greater proportion of compact bone, and thus probably absorbs more radiation compared with other bony structures within the irradiation field, for example maxilla. In addition, blood supply to mandible is poor after development of cancer, as well as the decreased vascularisation in periosteum.19 Such injury in both mandible and skull base may also cause inflammatory changes of the insertion of the masticator muscles and other adjacent soft tissues, contributing to the development of trismus.
Trismus is quantified by the MID, which is defined as the maximum distance between upper and lower incisors when the subject has fully opened his or her mouth. The severity of trimus can be classified into three grades measured by the maximum inter‐incisal distance (MID), in which the MID of Grade 1–3 are 4.0–2.5 cm, 2.5–1.0 cm and <1.0 cm in adults respectively.20 Dijkstra et al. defined 3.5 cm as the functional cut‐off point for trismus in head and neck cancer patients on the basis of significant mandibular function impairment and perceived restriction in mouth opening.21 A later study conducted by Scott et al. also supported this cut‐off level.22
Assessment of TMJ by Various Imaging Modalities
Questionnaires such as MFIQ are used as primary screening of TMJ disorders. Imaging of TMJ would be implemented when the needs are reflected by the patients' history and clinical examination, for diagnostic purposes. The imaging techniques include plain and panoramic radiography, CT scan, ultrasonography and MRI.23, 24
Plain and panoramic radiography
Plain x‐ray was referred as the pioneer for diagnostic imaging of TMJ. It is suggested that the oblique transcranial, the transmaxillary and the submental‐vertex views, with each one approximate to be the orthogonal projection of the two other views, to be more useful in terms of TMJ visualisation, due to overlapping the skull and zygomatic arch around the joint. However, such examinations are limited to the bony structures, while the non‐mineralised cartilage, soft tissues and the disc positions cannot be demonstrated. Panoramic radiography was once prevalent in TMJ imaging since information about the teeth and other parts of the joint could be obtained (Fig. 1). However, in general, inadequate information about articulation eminence and fossa is provided as only lateral slope and central parts of the mandibular condyles can be seen in the standard views, as well as the overlapping of the anatomical structures. Only obvious erosions, sclerosis and osteophytes of the condyle can be depicted. In a study by Ahmad et al.,25 the sensitivity and specificity of panoramic radiography were 26.2% and 99.3% when compared with CT in the imaging osteoarthritis of TMJ respectively.
Figure 1.

Panoramic radiography showing the bony outline of both left and right TMJs (Courtesy of Implants and Cosmetic Dentistry). MD, mandibular condyle.
Computed tomography
CT provides cross‐sectional images across the region of interest with 3D reconstruction, eliminating the problem of superimposition of anatomical structures near TMJ, hence allows the examination of the osseous component of TMJ (Figs. 2 and 3). In addition, tomography is capable of providing a more accurate assessment of condylar position within the fossa as compared with general radiography by eliminating distortion of the structures due to the use of oblique rays. Same as that for general radiography, the soft tissues particularly for the disc position cannot be accurately determined. In a study by Hayashi et al.26 in the detection of anterior displacement of the articular disc in TMJ, the sensitivity, specificity and accuracy by CT were 91%, 100% and 97%, respectively, in the closed mouth position, and 96%, 99% and 98%, respectively, in the open mouth position. A more recent report from Boeddinghaus et al. commented that CT was able to detect internal disc derangement, arthritis and neoplasms at relatively low radiation dose.27
Figure 2.

Transverse CT views (A–C) arranged from superior to inferior showing the left TMJ (Courtesy of Cancer Hospital, Sun yat‐sen University). JF, joint fossa; MD, mandibular condyle; M, mandible.
Figure 3.
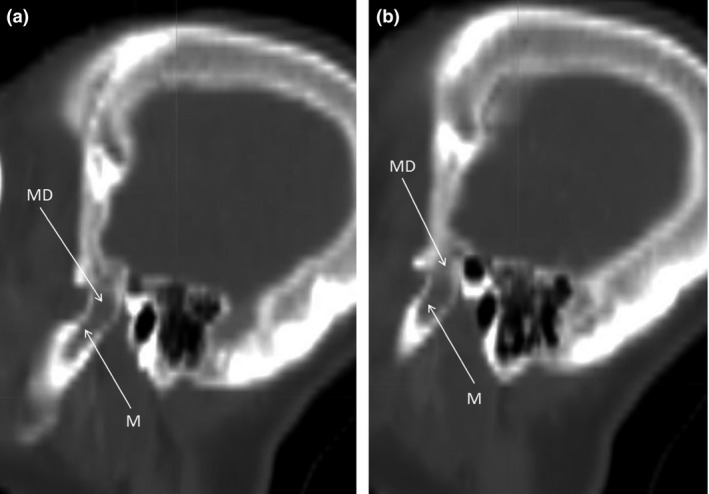
Sagittal CT views (A and B) arranged from medial to lateral showing the left TMJ (Courtesy of Cancer Hospital, Sun yat‐sen University). JF, joint fossa; MD, mandibular condyle; M, mandible.
Magnetic resonance imaging
MRI is commonly used for evaluation of TMJ because it provides superior contrast resolution and is able to acquire dynamic imaging for demonstrating the functionality of the joint.23 Information about the disc location in TMJ in open and closed mouth positions at multiple levels within the region are readily provided from the MRI images (Fig. 4), with the demonstration of disc displacement and perforation, as well as capsular tears. Although MRI is not able to show the bony details as comprehensive as CT, the contour of the osseous structures concerned is available. A study by Bag et al.23 reported that the sensitivity and specificity for detecting avascular necrosis were 78% and 84%, respectively, while another study by Alkhader et al.28 reported 30–82% and 84–98%, respectively, for detecting osseous abnormalities of TMJ. With the high‐contrast sensitivity to tissue differences including TMJ, MRI now has replaced CT and acts as the gold standard for diagnosis of TMJ disorder. Although CT and MRI have high efficacy in differential diagnosis of TMJ disorders, they are relatively more expensive.
Figure 4.

Transverse T1‐weighted MRI images (A–C) arranged from superior to inferior showing the left TMJ (Courtesy of Cancer Hospital, Sun yat‐sen University). MD, mandibular condyle; M, mandible.
Ultrasonography
The principle of ultrasonography is explained by the fact of differential transmission and reflection of ultrasound waves emitted by transducer, as they penetrate through various anatomical structures of different physical densities. Bony structures are described as hypoechoic having low reflection of sound waves, and are shown in black in the US images, whereas for the bone margin, as well as the surface of the joint capsule and muscles, are described as hyperechoic that has high reflection of the sound waves, and are shown in white in the images (Fig. 5). On the other hand, connective tissues and muscles are described as isoechoic and are shown heterogeneously grey in the images. Therefore, based on these facts, the head of the mandibular condyle and the articulation eminence are seen in white in the ultrasound images, while the joint capsule and the masticatory muscles (lateral ptergyoid and masseter muscles) are seen in black in the images. Ultrasonography is found to be more reliable for detecting disc displacement and joint effusion, but relatively less accurate for condylar erosion, particularly in the cases with co‐existence of osteoarthritis in terms of diagnosis of temporo‐mandibular disorders. A study by Hayashi et al.29 reported that sonography's sensitivity, specificity and accuracy for the diagnosis of disc displacement, relative to MR and/or CT as the standard of reference were 83%, 96% and 92% respectively.
Figure 5.
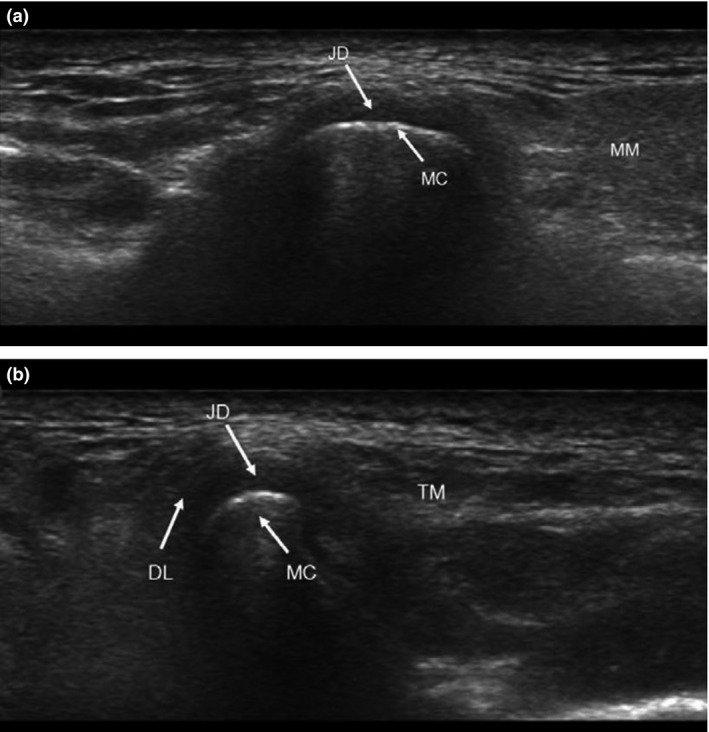
Ultrasound image of TMJ in (A) longitudinal and (B) transverse sections. JD, joint disc; DL, disc ligament; MM, masseter muscle.
Accuracy of US scan in TMJ disorder diagnosis is affected by (1) the limited accessibility to the medial part of the TMJ due to significant absorption of ultrasound waves in mandible and temporal bone, (2) degree of synchronisation of different operators, particularly in terms of degree of inclination of the transducer while scanning for visualisation and (3) the difficulty in localisation of articulation disc and interpretation of images. Limitations in the judgement for static US may be solved with the use of dynamic views and 3D US. Dynamic views allow higher specificity, but with a lower sensitivity in diagnosis when compared with static images, therefore uses of both dynamic and static views are suggested to achieve more accurate diagnosis.
Assessment of Trismus
For assessment of trismus, TMJ function in terms of range of jaw movement and pain on mandibular movement are the main considerations.30 Palpitation of the masticatory muscles and TMJ acts as the first‐line clinical examinations. Besides, mouth opening (MID) measurement is found to be a relatively simple examination for investigating the functional mouth opening (maximum vertical distance for oral cavity assess) and TMJ mobility, though the latter is better to be assessed by angle of opening at joint as suggested in other literatures.31
The validity of MID measurement is affected by various factors: mode of mouth opening in the examination, measuring devices and the habitus of the patients. Active mouth opening and the use of ruler for measurement are adopted in majority of the investigations to obtain more accurate results (greater MID values) since passive mouth opening can be affected manually by variable forces applied, whereas the use of more sophisticated devices, for example callipers or dynasplint for measurement requires much longer time and therefore there is a greater intention for patients to close mouth or relax.31 MID values can be affected by subjects' habitus since comparatively, subject with greater mandibular length or angle tends to show greater MID value, while MID values for subjects with malocclusion are often mis‐estimated.31
Besides MID measurement, which only presents the longitudinal joint movement, Bertrand et al. suggested that capability of the jaw movement should be taken into account in trismus screening, with difference in movements of both joints greater than 25% was defined as trismus.32
Management of Trismus
Prevention is better than cure. Recent studies on NPC patients have reported that significant reduction in occurrence and severity of trismus was achieved by reducing the dose delivered to the TMJ. This has been made possible by using the more advanced IMRT techniques.6, 33, 34 However, when post‐RT patients have developed trismus, prompt treatment should be given to prevent progression of the condition.
Management of trismus is often discouraging mainly due to the absence of specific treatment and irreversibility of the condition. The lack of effective therapy may also be explained by human mechanics that the average biting force (600–1300N) is much greater than that of mouth opening force (around 120N),21 while pain control is an important footstep for prevention of progression and function restoration.35
Pain control with first‐line drugs, such as non‐steroid anti‐inflammatory drugs, encourages patients to initiate jaw movements and therefore starts physiotherapy. The use of more invasive second‐line medication such as voltage‐dependent sodium channel blockers, for example oxcarbazepine or lamotrigene, at trigeminal or other nerves and pterygoid muscles will be considered if no improvement is obtained.18 Alternatively, the use of botulinum toxin (botox) in pain control was suggested in a few cases. However, such injection to TMJ region was only effective in alleviating radiation‐induced pain due to radiation fibrosis syndrome, and subsequent injection was needed to relieve recurrent masseter muscle pain. In addition, no significant improvement regarding the severity of trismus was demonstrated.35
There are several management options for trismus, they include physiotherapy, use of pharmaceuticals, micro‐current therapy and oxygen therapy.17 Surgical intervention, such as coronoidectomy and forced mouth opening under general anaesthesia, would be considered if the above mentioned non‐invasive strategies are ineffective.
Rehabilitation training for patients with trismus can slow down the progress and improve swallowing function.36 This mainly focuses on the use of jaw stretchers and mouth‐opening exercises. Such therapy allows muscle strengthening and increase in joint flexibility. Jaw movement exercises can be done in an active or passive manner with the help of mechanical devices such as Therabite System (ATOS Medical, Horby, Sweden) or conventional tools like stacked tongue depressor. The exercises includes repetitive mouth opening and closing, as well as protraction, retraction and lateral jaw movements under prescribed protocol. A related study showed that the rate of improvement (increase) in MID for patients using TheraBite System and unassisted exercise for mechanically assisted mandibular mobilisation was much better than patients having unassisted exercise only, and using stacked tongue depressor with unassisted exercise.37 However, some reports revealed that the use of mechanical aids for trismus therapy is ineffective due to instability of oral cavity, particularly for patients with dentures during exercise.21 The efficacy of jaw movement exercises was found to be affected by chemotherapy, time from oncological treatment and start exercise, as well as the exercise protocol and patient compliance.38
Medications, like superoxide dismutase (SOD) and pentoxifylline (PTX), mainly slow down trismus progression or even allow regression by reducing free radicals and improve vasculature in soft tissues, hence promoting the repair processes in injured tissues. SOD sweeps up the oxygen‐free radicals and converts them to less active species to slower down the radiation‐induced damage in normal tissues. PTX with immunomodulatory properties can delay the progression of trismus by down‐regulating the production of cytokines, which account for the pathogenesis of radiation‐induced fibrosis. In addition, PTX can increase erythrocyte deformability for decreasing blood viscosity and increasing oxygen release from erythrocytes, hence improve microcirculation and tissue oxygenation, favouring the repair processes. A mean increase of at least 4 mm in MID and symptomatic relieve after treatment for post‐radiotherapy patients suffering from severe trismus have been reported.39, 40
Impedance‐controlled micro‐current therapy and oxygen therapy were once suggested as the restorative management for trismus. The underlying principle for micro‐current therapy is not well understood. However, one possible mechanism was postulated on experimental basis: microvoltage applied induces the migration of extracellular calcium ions into the cells and the elevation of intracellular calcium ion level encourages increased adenosine triphosphate (ATP), followed by protein synthesis, thus promoting cellular repair and replication.40 On the other hand, hyperbaric oxygen therapy serves similar function as PTX by stimulating angiogenesis, proliferation of fibroblasts and osteoblasts and formation of collagen in irradiated area for the repair of osteoradionecrosis.17 Studies showed that micro‐current therapy and oxygen therapy had no prominent effects on trismus in NPC patients,30 which explains their low priority in management.
Surgical interventions are usually considered as a last resort in trismus management because of the invasive nature and the unpredictable side effects. A report suggested coronoidectomy as an alternative management if both pain control and physiotherapy are found to be ineffective, as supported by the significant increase in MID of 22.5 ± 3.5 mm found in head and neck cancer patients after surgery. However, efficacy of the surgery is largely affected by the aggressive post‐operative stretching.41 Trismus was also found to be improved by having forced mouth opening under general anaesthesia, but the effect was short‐lived and could be potentially complicated by alveolus fracture and adjacent soft tissue rupture.42
Conclusion
Radiation‐induced TMJ damage such as trismus in post‐RT is commonly observed in NPC patients. Trismus itself is asymptomatic until moderate, or even severe stage is reached that degrades patient's quality of life. Therefore, availability of simple, yet effective examinations for trismus detection is essential for delaying the progression and restoring TMJ functions. Although there is no absolutely effective treatment for trismus, several supportive, restorative and palliative managements are possible under different clinical situations.
Conflict of Interest
The authors declare no conflict of interest.
J Med Radiat Sci 63 (2016) 124–132
References
- 1. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, (eds). Cancer Incidence in Five Continents, Vol. VIII. IARC scientific publications No. 155. IARC, Lyon, 2002. [Google Scholar]
- 2. Hong Kong Cancer Stat . Hong Kong Cancer Registry, Hospital Authority 2012.
- 3. Brennan B. Nasopharyngeal carcinoma. Orphanet J Rare Dis 2006; 1: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang CJ, Huang EY, Hsu HC, Fang FM, Hsiung CY. The degree and time course assessment of radiation induced trismus occurring after RT for NPC. Laryngoscope 2005; 115: 1458–60. [DOI] [PubMed] [Google Scholar]
- 5. Bhatia K, King A, Abrigo J, Leung S, Ahuja A. MRI finding in patients with severe trismus following radiotherapy for nasopharyngeal carcinoma. Eur Radiol 2009; 19: 2586–93. [DOI] [PubMed] [Google Scholar]
- 6. Zheng Y, Han F, Xiao W, et al. Analysis of late toxicity in nasopharyngeal carcinoma patients treated with intensity modulated radiation therapy. Radiat Oncol 2015; 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyons AJ, Crichton S, Pezier T. Trismus following radiotherapy to the head and neck is likely to have distinct genotype dependent cause. Oral Oncol 2013; 49: 932–6. [DOI] [PubMed] [Google Scholar]
- 8. Jellema AP, Slotman BT, Doomaert P, Leemans CR, Langendijk JA. Impact of radiation‐induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 69: 751–60. [DOI] [PubMed] [Google Scholar]
- 9. Vera‐Llonch M, Oster G, Hagiwara M, et al. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 2006; 106: 329–36. [DOI] [PubMed] [Google Scholar]
- 10. Chan ATC, Teo PML, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol 2002; 13: 1007–15. [DOI] [PubMed] [Google Scholar]
- 11. Wu WC, Yang ZM, Zhang WZ, Lin ZX. Effect of beam arrangement on oral cavity dose in external beam radiotherapy of nasopharyngeal carcinoma. Med Dosim 2012; 37: 122–6. [DOI] [PubMed] [Google Scholar]
- 12. Kam KM, Teo ML, Chau MC, et al. Treatment of nasopharyngeal carcinoma with intensity‐modulated radiotherapy: The Hong Kong experience. Int J Radiat Oncol Biol Phys 2004; 60:1440–50. [DOI] [PubMed] [Google Scholar]
- 13. Huang TL, Chien CY, Tsai WL, et al. Long‐term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated by intensity modulated radiotherapy (IMRT) versus non‐IMRT. Head Neck 2015; doi:10.1002/hed.24150. [DOI] [PubMed] [Google Scholar]
- 14. White P, Chan KC, Cheng KW, Chan KY, Chau MC. Volumetric intensity‐modulated arc therapy vs conventional intensity‐modulated radiation therapy in nasopharyngeal carcinoma: A dosimetric study. J Radiat Res 2013; 54: 532–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rapidis AD, Dijkstra PU, Roodenburg JL, et al. Trismus in patients with head and neck cancer. Etiopathogenesis, diagnosis and management. Clin Otolaryngol 2015; doi:10.1111/coa.12488. [DOI] [PubMed] [Google Scholar]
- 16. Werning JW. Oral Cancer: Diagnosis, Management, and Rehabilitation. Thieme Medical Publishers, Inc, New York, USA, 2007. [Google Scholar]
- 17. Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol 2006; 7: 175–83. [DOI] [PubMed] [Google Scholar]
- 18. Wranicz P, Herlofson BB, Evensen JF, Konsgaard UE. Prevention and treatment of trismus in head and neck cancer: A case report and a systematic review of the literature. Scand J Pain 2010; 1: 84–8. [DOI] [PubMed] [Google Scholar]
- 19. Singh N, Scully C, Joyston‐Bechal S. Oral Complications of cancer therapies: Prevention and management. Clin Oncol (R Coll Radiol) 1996; 8: 15–24. [DOI] [PubMed] [Google Scholar]
- 20. Becker W, Naumann HH, Pfaltz CR, Buckingham RA. Ear, Nose and Throat Diseases: A Pocket Reference. Thieme Medical Publishers, New York, 1989; 320–71. [Google Scholar]
- 21. Dijkstra PU, Huisman PM, Roodenburg JLN. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg 2006; 35: 337–42. [DOI] [PubMed] [Google Scholar]
- 22. Scott B, Butterworth C, Lowe D, Rogers SN. Factors associated with restricted mouth opening and its relationship to health‐related quality of life in patients attending a Maxillofacial Oncology clinic. Oral Oncol 2008; 44: 430–8. [DOI] [PubMed] [Google Scholar]
- 23. Bag AK, Gaddikeri S, Singhal A, et al. Imaging of the temporomandibular joint: An update. World J Radiol 2014; 6: 567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melis M, Secci S, Ceneviz C. Use of ultrasonography for the diagnosis of temporomandibular joint disorders: A review. Am J Dent 2007; 20: 73–8. [PubMed] [Google Scholar]
- 25. Ahmad M, Hollender L, Anderson Q, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): Development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 844–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayashi T, Ito J, Koyama JI, et al. Detectability of anterior displacement of the articular disk in the temporomandibular joint on helical computed tomography: The value of open mouth position. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 88: 106–11. [DOI] [PubMed] [Google Scholar]
- 27. Boeddinghaus R, Whyte A. Computed tomography of the temporomandibular joint. J Med Imaging Radiat Oncol 2013; 57: 448–54. [DOI] [PubMed] [Google Scholar]
- 28. Alkhader M, Ohbayashi N, Tetsumura A, et al. Diagnostic performance of magnetic resonance imaging for detecting osseous abnormalities of the temporomandibular joint and its correlation with cone beam computed tomography. Dentomaxillofac Radiol 2010; 39: 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayashi T, Ito J, Koyama J, Yamada K. The accuracy of sonography for evaluation of internal derangement of the temporomandibular joint in asymptomatic elementary school children: Comparison with MR and CT. AJNR Am J Neuroradiol 2001; 22: 728–34. [PMC free article] [PubMed] [Google Scholar]
- 30. Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: A prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 88: 365–73. [DOI] [PubMed] [Google Scholar]
- 31. Gallagher C, Gallagher V, Whelton H, Cronin M. The normal range of mouth opening in an Irish population. J Oral Rehabil 2004; 31: 110–16. [DOI] [PubMed] [Google Scholar]
- 32. Bertrand J, Luc B, Phillipe M, Phillipe P. Anterior mandibular osteotomy for tumor extirpation: A critical evaluation. Head Neck 2000; 22: 323–7. [DOI] [PubMed] [Google Scholar]
- 33. Hsuing CY, Huang EY, Ting HM, Huang HY. Intensity‐modulated radiotherapy for nasopharyngeal carcinoma: The reduction of radiation‐induced trismus. Br J Radiol 2008; 81: 809–14. [DOI] [PubMed] [Google Scholar]
- 34. Chen YY, Zhao C, Wang J, et al. Intensity‐modulated radiation therapy reduces radiation‐induced trismus in patients with nasopharyngeal carcinoma: A prospective study with >5 years of follow‐up. Cancer 2011; 117: 2910–16. [DOI] [PubMed] [Google Scholar]
- 35. Hartl DM, Cohen M, Julieron M, Marandas P, Janot F, Bourhis J. Botulinum toxin for radiation‐induced facial pain and trismus. Otolaryngol Head Neck Surg 2008; 138: 459–63. [DOI] [PubMed] [Google Scholar]
- 36. Tang Y, Shen Q, Wang Y, Lu K, Wang Y, Peng Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation‐induced dysphagia and trismus. Strahlenther Onkol 2011; 187: 39–44. [DOI] [PubMed] [Google Scholar]
- 37. Buchibbinder D, Currivan RB, Kaplan AJ, Urken ML. Mobilization regimens for the prevention of jaw hypomobility in the radiated patient: A comparison of three techniques. J Oral Maxillofac Surg 1993; 51: 863–7. [DOI] [PubMed] [Google Scholar]
- 38. Kamstra JI, Roodenburg JLN, Beurskens CHG, Reintsema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer 2013; 21: 951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chua DT, Lo C, Yuen J, Foo YC. A pilot study of pentoxifylline in the treatment of radiation‐induced trismus. Am J Clin Oncol 2001; 24: 366–9. [DOI] [PubMed] [Google Scholar]
- 40. Sullivan BO, Levin W. Late Radiation‐related fibrosis: Pathogenesis, manifestations, and current management. Semin Radiat Oncol 2003; 13: 274–89. [DOI] [PubMed] [Google Scholar]
- 41. Bhrany AD, Izzard M, Wood AJ, Futran ND. Coronoidectomy for the treatment of trismus in head and neck cancer patients. Laryngoscope 2007; 117: 1952–6. [DOI] [PubMed] [Google Scholar]
- 42. Stubblefield MD, Manfield L, Riedel ER. A preliminary report on the efficacy of a dynamic jaw opening device as part of the multimodal treatment of trismus in patients with head and neck cancer. Arch Phys Med Rehabil 2010; 91: 1278–82. [DOI] [PubMed] [Google Scholar]


