Abstract
Introduction
Intensity‐modulated radiotherapy (IMRT) has become the standard of care for squamous cell cancer of the head and neck (HNSCC). This report presents early outcomes of IMRT with concomitant chemotherapy in a community setting in New Zealand.
Methods
Forty‐eight patients with stage III and IV advanced HNSCC received definitive treatment with IMRT. A dose of 66 Gy in 30 fractions was delivered over 6 weeks with 3‐weekly concurrent cisplatin after a single induction cycle of cisplatin and 5‐fluorouracil. Acute toxicity, locoregional control (LRC), disease‐free survival and overall survival (OS) outcomes were analysed.
Results
Follow‐up ranged from 2 to 82 months (median 34 months). Acute grade 2 toxicity was observed in 27 patients and grade 3 toxicity in 19 patients. No patients experienced grade 4 toxicity and there were no treatment‐related deaths. Locoregional failures occurred in six patients and distant metastatic disease occurred in five patients. Actuarial estimates of 3‐year LRC, disease‐free survival and OS were 87.3%, 74.4% and 73.7% respectively.
Conclusion
Definitive treatment of stage III and IV cancer of the head and neck with IMRT and concurrent chemotherapy was achievable in the community setting. Acute toxicities were manageable and 3‐year outcomes were comparable to other published series.
Keywords: Chemoradiotherapy; head and neck neoplasms; radiotherapy, intensity‐modulated; treatment outcome
Introduction
Squamous cell cancer of the head and neck (HNSCC) accounts for 2–3% of new cancer diagnoses in New Zealand, with around 520 new cases diagnosed per year.1 Radical radiotherapy (RT) with concurrent platinum‐based chemotherapy is the standard of care for unresectable primary tumours, or in cases where surgery would cause unacceptable morbidity.2, 3 Classical fractionation schedules consist of a tumour dose of 70 Gy, with clinically uninvolved lymph nodes receiving 50 Gy. These schedules deliver 2 Gy per fraction, given as five fractions per week for a total of 7 weeks, with 3‐weekly cisplatin chemotherapy administered in weeks 1, 4 and 7. Three‐dimensional conformal radiotherapy (3D‐CRT) usually involves a two‐phase or ‘shrinking field’ approach where both the low‐risk and high‐risk volumes receive treatment for 5 weeks, but only the high‐risk volumes receive treatment in the final 2 weeks. The control of dose distribution afforded by intensity‐modulated radiotherapy (IMRT) facilitates the delivery of different doses to high‐ and low‐risk volumes within each treatment fraction. This in turn permits treatment to be given in a single phase using a simultaneous integrated boost (SIB) technique where all volumes are treated daily, but receive different daily doses. This approach, combined with altered fractionation, has been shown to produce excellent local control with reduced toxicity in prospective trials.4, 5
IMRT has increasingly become the standard of care in radiotherapy for cancers of the head and neck.6, 7, 8 In the early part of the 21st century, reports in the literature progressed from studies indicating superior dosimetry, to case series and interventional studies indicating superior outcomes in terms of late toxicity.4, 5 More recently, mature data from large institutional series have confirmed excellent disease control and late toxicity outcomes.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Multiple case series have reported encouraging results with IMRT and concomitant chemotherapy.19
The Regional Cancer Treatment Service in Palmerston North Hospital is located in the lower North Island of New Zealand and provides radiotherapy services to a population of around 560,000. IMRT using an SIB technique was introduced for head and neck malignancies in 2005. We undertook a retrospective study to evaluate the effectiveness and safety of this treatment in the setting of a smaller, predominantly rural centre. This report describes the outcomes in terms of locoregional control (LRC), overall survival (OS) and acute toxicity for the first 48 patients treated with this technique when delivered with concurrent chemotherapy.
Methods
Patients and staging evaluation
The ethics advisory committee at Palmerston North Hospital gave approval for this analysis to take place, in accordance with New Zealand guidelines for observational studies.20 The notes of 52 patients diagnosed and referred between March 2005 and November 2010 were reviewed. Only the 48 patients treated with primary radical chemoradiotherapy for biopsy‐confirmed squamous cell cancer of the oropharynx, hypopharynx, larynx or unknown head and neck primary site were included in this analysis. Patients with proven primary tumours in the nasopharynx or paranasal sinuses were excluded, and patients with prior surgical resection or who were treated with radiotherapy alone were also excluded. All patients were assessed in the multidisciplinary head and neck cancer clinic, underwent physical examination including flexible nasoendoscopy, and had blood samples taken for baseline biochemistry and haematology tests. All patients were routinely investigated with computed tomography (CT) scan of the head and neck. Positron emission tomography scanning was carried out in cases where the primary site was not identified, or where there were equivocal lymph nodes on CT. Magnetic resonance imaging was carried out where the soft tissue extent of the primary lesion was unclear. All patients had stage III or IV disease according to the 6th edition of the American Joint Committee on Cancer Staging classification (2002). Assessment of HPV status in biopsy specimens was not part of routine analysis in this time period. All patients receiving bilateral neck radiotherapy had a percutaneous enterogastrostomy (PEG) tube inserted.
Radiotherapy
Planning CT scan
Patients had a custom U‐frame thermoplastic head and neck mask made and underwent planning CT scan with a slice thickness of 3 mm, covering the head and neck area to below the level of the clavicles. Images were exported to contouring workstations (Coherence®; Siemens, Erlangen, Germany) for delineation of target volumes and critical normal structures.
Contouring
Delineation of target volumes was carried out on the planning CT by the treating radiation oncologist. The gross tumour volume (GTV) consisted of the primary tumour and clinically involved lymph nodes as determined by clinical examination and imaging. The CTV66 included the GTV with a 5–10 mm margin for microscopic spread, respecting anatomical boundaries. The CTV60 was designed to treat the high‐risk neck areas and included the CTV66, the ipsilateral neck (levels IB/II–V) and ipsilateral retrostyloid and retropharyngeal nodal areas. For hypopharynx and larynx tumours, the retrostyloid space was not routinely included. Bilateral neck nodes were included in this volume if the patient had N2c disease. Level IA was only included where there was evidence of involved lymph nodes in that area, or if there was considered to be a high likelihood of involvement. In patients without features conferring a high risk of contralateral nodal involvement (large primary tumours, encroachment on midline, base of tongue tumours or supraglottic involvement), the CTV54 represented an elective target volume, and comprised the clinically and radiologically negative contralateral neck without the retrostyloid space. Four patients who had well‐lateralised oropharynx tumours had only ipsilateral irradiation with no CTV54 to the contralateral neck. In cases where there was no identifiable mucosal primary, the CTV60 included ipsilateral tonsillar fossa, soft palate, base of tongue, nasopharynx, supraglottis, pyriform fossa and hypopharynx. Neck node levels were contoured in accordance with published consensus guidelines.21 Planning target volumes were constructed using a symmetrical expansion of 5 mm on each CTV. Organs at risk (OARs) included the parotid glands, constrictor muscles, spinal cord and brain stem, and were contoured in all patients. In cases where provisional plans contained unacceptable high‐dose regions in the oral cavity or around the occiput, planning pseudovolumes were created in those areas as an avoidance structure for the IMRT algorithm.
Treatment plan generation and dose prescription
Plans were generated using an inverse‐planning convolution‐superposition algorithm with tissue inhomogeneity correction (XiO® planning system; Elekta, Stockholm, Sweden). Five to seven gantry angles were employed and IMRT was used to treat the entire length of the treated volume. No beam matching was necessary. Plans were optimised to ensure that 95% of the PTV received 98% of the prescribed dose. D50 was recorded. Point maximum doses to the spinal cord and brainstem OARs were initially kept below 45 Gy, but after 2008, planning organ at risk volumes (PRVs) were constructed by adding 5 mm symmetrical expansions, after which point maximum doses of 45 Gy to the spinal cord PRV and 54 Gy to the brainstem PRV were permitted. Mean dose to parotids was kept below 26 Gy. Mean dose to pharyngeal constrictors was kept below 40 Gy where possible. Mean brain and uninvolved oral cavity doses were kept below 40 Gy. A modestly accelerated hypofractionated schedule was employed, delivering the prescribed dose in 30 daily fractions with five fractions per week over 6 weeks. All PTVs were treated simultaneously. PTV66 received 66 Gy in 2.2 Gy fractions, PTV60 received 60 Gy in 2 Gy fractions and PTV54 received 54 Gy in 1.8 Gy fractions.
Treatment delivery
Treatment was administered with 6 MV photons using a step‐and‐shoot technique on Siemens linear accelerators equipped with 58‐leaf multi‐leaf collimators (MLCs), later upgraded to 160‐leaf MLCs. Verification was carried out with daily MV portal imaging with a 3 mm action cut‐off. MV cone beam CT was carried out prior to the first three fractions and weekly thereafter, with the images used to assess set up accuracy and anatomical changes; significant alterations in patient contour triggering repeat planning CT followed by recontouring and production of a new plan. The dose from the cone beam CTs was accounted for in the treatment plan. Patients were routinely reviewed once‐weekly by medical staff. Acute radiation toxicity scores were prospectively collected according to RTOG criteria and recorded on Lantis® (Siemens) and Mosaiq® (Elekta) oncology information systems. Where a daily fraction was missed, efforts were made to preserve the overall treatment time. For preference, a weekend treatment fraction was administered. Where this was not possible, an additional fraction was given on a treatment day after a minimum 6‐h break. Twice‐daily treatment was only used once per course.
Quality assurance
Initial plans were assessed using an anthropomorphic head and neck phantom with dosimetry based on radiochromic film and thermoluminescent dosimeter measurements. These were submitted for external quality assurance via the MD Anderson Quality Assurance Center (University of Texas, USA). Individual plan verification was carried out for every radiotherapy plan using film and ionisation chamber dosimeters. Measured and calculated dose were compared using the gamma dose distribution comparison method.22
Chemotherapy
All patients received a single induction cycle of chemotherapy 3 weeks prior to commencement of RT, comprising cisplatin 35 mg/m2 per day on days 1–3 and 5‐fluorouracil 1000 mg/m2 per day on days 1–4. This coincided with the time of the radiotherapy planning scan. The two remaining cycles of chemotherapy comprised cisplatin alone given at 35 mg/m2 per day on days 1–3, and were given during weeks 1 and 4 of RT.
Follow‐up
Patients were seen within 2–6 weeks of completion of treatment, 3‐monthly for the first 2 years, then 6‐monthly for a planned total of 5 years, with longer follow‐up at the physicians’ discretion. Contrast‐enhanced CT was carried out at 8–10 weeks following completion of treatment, with PET/CT and biopsy carried out where persistent abnormalities were detected. Flexible nasoendoscopy was carried out 6‐monthly.
Statistical methods
Actuarial estimates of disease‐free survival (DFS) and OS were calculated using the Kaplan–Meier product‐limit method. Times were calculated from the date of completion of RT. Confidence intervals were calculated using the method of Fay et al.23 The estimate of LRC was calculated using methods for competing risks, with death as a competing risk.24, 25 The competing risks analysis was carried out in Stata Release 13 (Statacorp LP, College Station, TX). All other analyses were carried out in R version 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
Patient characteristics are noted in Table 1. A total of 48 patients were included in the analysis. The median age was 57 (range: 29–76). Median length of follow‐up was 34 months (range: 2–82).
Table 1.
Clinical and staging characteristics
| Characteristic | n | % |
|---|---|---|
| Age | ||
| <65 | 38 | 79.2 |
| ≥65 | 10 | 20.8 |
| Sex | ||
| Male | 41 | 85.4 |
| Female | 7 | 14.6 |
| Site | ||
| Oropharynx | 37 | 77.1 |
| Hypopharynx | 1 | 2.1 |
| Larynx | 7 | 14.6 |
| Unknown primary | 3 | 6.3 |
| Smoking status | ||
| Never | 11 | 22.9 |
| Ex | 21 | 43.8 |
| Current | 16 | 33.3 |
| T‐stage | ||
| 0 | 3 | 6.3 |
| 1 | 6 | 12.5 |
| 2 | 13 | 27.1 |
| 3 | 5 | 10.4 |
| 4 | 21 | 43.7 |
| N‐stage | ||
| 0 | 8 | 16.7 |
| 1 | 5 | 10.4 |
| 2a | 5 | 10.4 |
| 2b | 14 | 29.2 |
| 2c | 11 | 22.9 |
| 3 | 5 | 10.4 |
| 0 | 8 | 16.7 |
| AJCC stage group | ||
| III | 6 | 12.5 |
| IVA | 37 | 77.1 |
| IVB | 5 | 10.4 |
Treatment details and compliance
All patients received the prescribed dose of radiotherapy. Seven patients had a break of 1 day during treatment, five patients had a break of 2 days and one patient had a break of 3 days. Four patients (8.3%) were initially considered only fit for two cycles of chemotherapy and received both cycles. Further six patients (12.5%) were considered fit for three cycles, but only completed two. The remaining thirty‐eight (79.2%) received three cycles.
Treatment outcomes
Disease recurrence
There were no deaths during treatment and no deaths from treatment‐related toxicity by the time of analysis. Eight patients in total had recurrence of their disease. Six patients developed locoregional recurrence, all of whom had received bilateral neck radiotherapy. Dosimetric review indicated disease recurrence within the PTV66 for all six patients, suggesting tumour resistance rather than marginal miss. Five patients developed distant metastatic disease; three of these patients also had locoregional recurrence, while the remaining two had distant metastasis only. Characteristics of patients who developed disease recurrence are shown in Table 2. The 3‐year estimate of LRC was 87.3% (95% CI: 76.3–94.9%; Fig. 1).
Table 2.
Characteristics of eight patients with disease recurrence
| Age | Sex | Primary site | Stage | Cycles of chemotherapy received | Smoking status | Site of relapse | Time to failure (months) | Time to death (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | N | Local | Regional | Distant | |||||||
| 63 | Male | Oropharynx | 4 | 3 | 3 | Current | No | No | Yes | 1 | 2 |
| 61 | Male | Larynx | 3 | 0 | 2 | Current | Yes | No | Yes | 4 | 11 |
| 65 | Female | Larynx | 3 | 0 | 3 | Ex | Yes | Yes | No | 5 | 17 |
| 68 | Male | Larynx | 4 | 0 | 3 | Ex | Yes | Yes | Yes | 6 | 15 |
| 58 | Male | Oropharynx | 4 | 3 | 3 | Ex | Yes | No | No | 7 | 24 |
| 67 | Male | Oropharynx | 4 | 3 | 3 | Current | Yes | Yes | Yes | 9 | 12 |
| 62 | Female | Oropharynx | 4 | 0 | 2 | Ex | Yes | No | No | 12 | 17 |
| 66 | Male | Oropharynx | 4 | 2b | 3 | Current | No | No | Yes | 32 | 32 |
Figure 1.
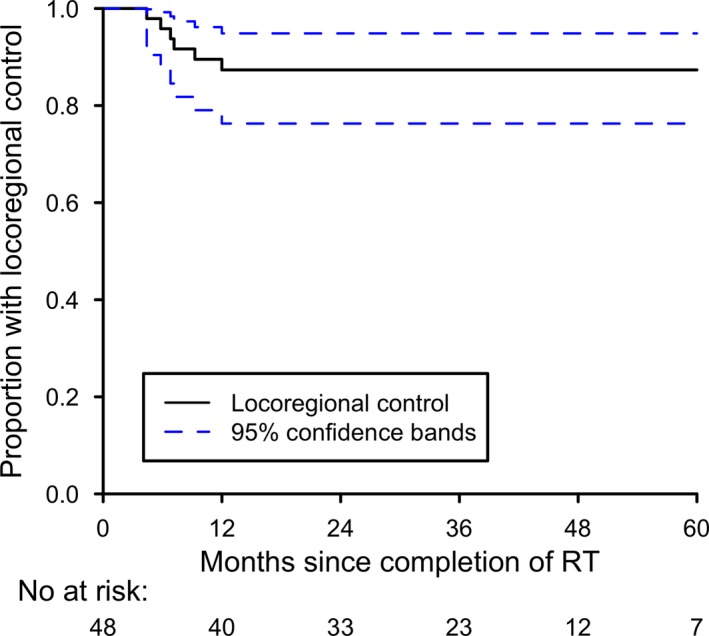
Kaplan–Meier plot of probability of locoregional control.
Survival
Twelve deaths occurred in the patient group. All eight patients who developed disease recurrence later died of their disease, including one who died of metastatic disease 2 months after completion of treatment (Table 2). Of the four patients who did not die of their primary cancer, one died of a second primary (non‐HNSCC) malignancy. Three had non‐cancer deaths: one had a myocardial infarction 12 months after completing treatment, one had a fatal gastrointestinal bleed 20 months after completing treatment and one had a fatal cardiac arrest more than 5 years after completing treatment. Disease‐free survival and OS are shown in Figures 2 and 3. The 3‐year estimates of DFS and OS were 74.4% (95% CI: 58.0–85.2%) and 73.7% (95% CI: 57.1–84.7%).
Figure 2.
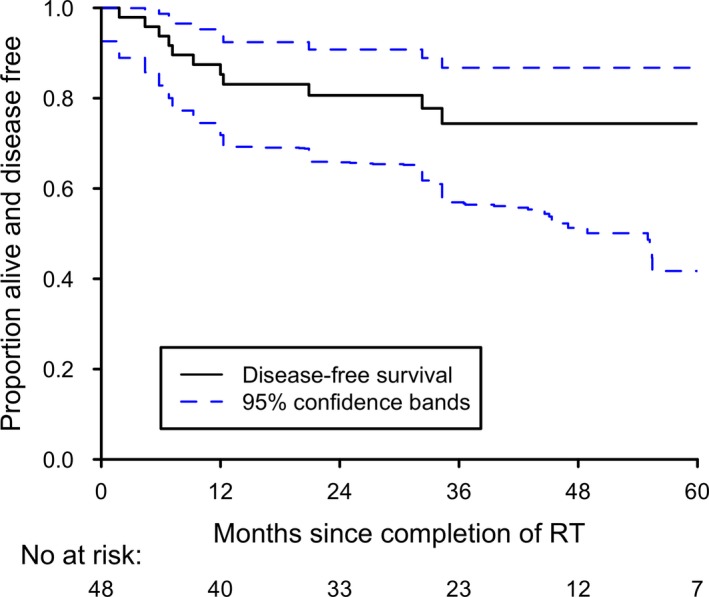
Kaplan–Meier plot of disease‐free survival.
Figure 3.
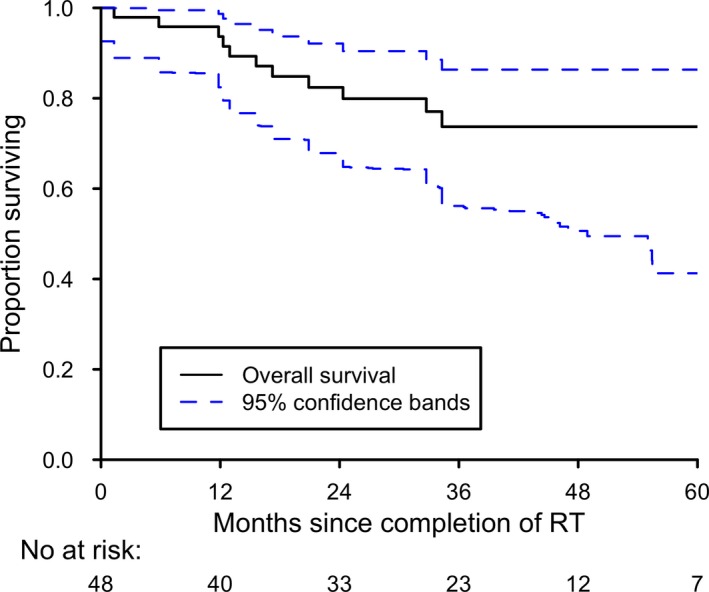
Kaplan–Meier plot of overall survival.
Acute toxicity
Data were available for weight loss, mucositis, skin reaction and odynophagia/dysphagia and are summarised in Table 3. Median weight loss during treatment was 5.2 kg (range: −1 to 15 kg). During the treatment course, a total of 27 patients (56.3%) experienced maximum grade 2 acute radiotherapy toxicity, and a further 19 patients (39.6%) had maximum grade 3 toxicity. No patients developed grade 4 acute radiotherapy toxicity. There were no treatment‐related deaths.
Table 3.
Acute toxicities
| Toxicity | Grade 2 | Grade 3 |
|---|---|---|
| Weight loss | 15 (31.3%) | 0 |
| Skin | 10 (20.8%) | 0 |
| Mucositis | 26 (54.2%) | 16 (38.3%) |
| Dysphagia/odynophagia | 29 (60.4%) | 13 (27.1%) |
Discussion
Minimisation of side effects with highly conformal techniques remains an important goal in radiotherapy practice. Here, we report the first series of patients in New Zealand treated with concurrent chemoradiotherapy with SIB IMRT for head and neck cancer.
We adopted a moderately accelerated hypofractionated schedule delivering 66 Gy in 6 weeks. Assuming a tumour α/β value of 15, the equivalent dose in 2 Gy fractions (EQD2) to the CTV66 would be 69.6 Gy.26 This fractionation was used in the RTOG 00‐22 trial for early‐stage oropharyngeal cancer without chemotherapy and resulted in locoregional failure rates of 9% at 2 years, with low rates of late toxicity, particularly xerostomia.4
A number of single‐institution series have combined moderate acceleration with chemotherapy for advanced HNSCC of multiple subsites with good LRC.10, 11, 12, 13, 15, 16, 18, 27 These studies had median follow‐up periods ranging from 18 to 42 months and are summarised in Table 4. Heterogeneity in patient and treatment characteristics makes comparisons difficult, but if the analysis is confined to only the series from UCSF,13 Utah15 and Chicago18 comprising patients who received definitive concomitant chemoradiotherapy for stages III‐IV head and neck cancer, our control rates and OS are comparable. Similarly, rates of grade ≥3 acute toxicity in our study compare favourably in those domains for which data are available (Table 5). Notably, the proportion of stage IV patients in our series (87.5%) was higher than in any of these studies.
Table 4.
Outcomes of selected accelerated chemoradiotherapy series
| Author (year) | Disease sites | n | Total dose/fraction size (Gy)/treatment time | Percentage of patients receiving concurrent chemotherapy or cetuximab | LC (%) | LRC (%) | DFS (%) | OS (%) | Measurement (years) |
|---|---|---|---|---|---|---|---|---|---|
| Seung11 (2008) | Stage I–IV Nasopharynx, Oropharynx (definitive) | 69 | 66–70/2.12–2.2/6½ weeks | 65 | 98 | – | – | 90 | 2 |
| Huang13(2008) | Stage III–IV Oropharynx (definitive) | 71 | 70/2.12/6½ weeks | 100 | 94 | 90 | 81 | 83 | 3 |
| Studer10 (2010) | Stage I–IV Hypopharynx, Larynx (definitive) | 123 | 66–73.6/2.0–2.2/6–6½ weeks | 86 | 82 | 77 | 75 | 83 | 2 |
| Daly12(2010) | Stage II–IV Oropharynx (79% definitive, 21% postoperative) | 107 |
66/2.2/6 weeks 70/2.0/7 weeks |
98 definitive/50 postoperative | – | 92 | 81 | 83 | 3 |
| Montejo15 (2011) | Stage III–IV Nasopharynx, Oropharynx, Hypopharynx, Larynx (definitive) | 43 | 67.5/2.25/6 weeks | 100 | 85 | 82 | 73 | 65 | 2 |
| Clavel16 (2012) | Stage III–IV Oropharynx (definitive) | 100 | 70/2.12/6½ weeks | 100 | – | 95 | 84 | 92 | 3 |
| Loo27 (2013) | Stage III–IV Oropharynx (definitive following induction chemotherapy) | 52 | 65/2.17/6 weeks | 92 | – | – | 84 | 80 | 3 |
| Spiotto18 (2014) | Stage III–IV Nasopharynx, Oropharynx, Hypopharynx, Larynx, Oral cavity (definitive) | 134 |
70/2.12/6 weeks 66/2.2/6½ weeks |
78 | 69 | – | 55 | 67 | 2 |
LC, local control; LRC, locoregional control; DFS, disease‐free survival; OS, overall survival.
Table 5.
Rates of ≥Grade 3 toxicity in published series
The addition of chemotherapy improves outcomes in HNSCC, with the MACH‐NC meta‐analysis suggesting an absolute improvement of 4.5% in OS at 5 years if chemotherapy was added to locoregional treatment.3 The greatest benefit was observed for concomitant chemotherapy, with no clear benefit from adjuvant chemotherapy. The possible benefits of neoadjuvant (induction) chemotherapy have been the subject of much debate, and its place remains uncertain. The LORHAN analysis indicated that only 16% of HNSCC patients in US centres are treated with induction chemotherapy.6, 7 Two recent prospective trials (DeCIDE and PARADIGM) reported no OS benefit from its addition, although neither trial met its accrual targets.28, 29
Our chemoradiotherapy protocol includes a single neoadjuvant cycle of cisplatin/5‐fluorouracil followed by two concurrent cycles. This was chosen to maintain an overall cisplatin dose comparable to that administered during more traditional 7‐week treatment courses, with the induction cycle given in view of the poor completion rates reported for the conventional chemoradiotherapy approach using three cycles of cisplatin 100 mg/m2 in weeks 1, 4 and 7 of radiotherapy. The high rate of completion (79.2%) of three cycles of chemotherapy in our series suggests that this approach is well tolerated.
Our study has some clear limitations. More than three‐quarters of our patients had an oropharyngeal primary, and HPV status has significant implications in this subsite with superior outcomes reported for HPV‐related tumours.30 Although we were unable to ascertain HPV status for these patients, it is notable that all patients who had disease recurrence were current or former smokers – a poor prognosis feature independent of HPV infection.31
The number of patients in our study and the length of follow‐up were adequate for the purposes of our primary analyses. Nevertheless, further analyses would have been possible if we had had more detailed information on ECOG status, chemotherapy toxicities and late radiotherapy effects. This underscores the value of enrolling such patients in prospective clinical trials where toxicity scoring and follow‐up evaluation can be conducted in line with agreed protocols.
Conclusion
The results of our study confirm that chemoradiotherapy with IMRT can be delivered in a non‐metropolitan setting with manageable toxicity, and that outcomes with respect to LRC, DFS and OS appear to be similar to those reported in published series.
Conflict of Interest
The authors declare no conflict of interest.
J Med Radiat Sci 63 (2016) 96–103
References
- 1. Ministry of Health . Cancer: New registrations and deaths 2010. 2013.
- 2. Denis F, Garaud P, Bardet E, et al. Final results of the 94‐01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced‐stage oropharynx carcinoma. J Clin Oncol 2004; 22: 69–76. [DOI] [PubMed] [Google Scholar]
- 3. Pignon J‐P, le Maître A, Maillard E, Bourhis J. Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4–14. [DOI] [PubMed] [Google Scholar]
- 4. Eisbruch A, Harris J, Garden AS, et al. Multi‐institutional trial of accelerated hypofractionated intensity‐modulated radiation therapy for early‐stage oropharyngeal cancer (RTOG 00‐22). Int J Radiat Oncol Biol Phys 2010; 76: 1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nutting CM, Morden JP, Harrington KJ, et al. Parotid‐sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011; 12: 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong SJ, Harari PM, Garden AS, et al. Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN): analysis of chemoradiation treatment approaches in the United States. Cancer 2011; 117: 1679–86. [DOI] [PubMed] [Google Scholar]
- 7. Ang KK, Chen A, Curran WJ, et al. Head and neck carcinoma in the United States: first comprehensive report of the Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN). Cancer 2012; 118: 5783–92. [DOI] [PubMed] [Google Scholar]
- 8. National Head and Neck Cancer Tumour Standards Working Group . Standards of service provision for head and neck cancer patients in New Zealand ‐ provisional. 2013.
- 9. Garden AS, Kies MS, Morrison WH, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol 2013; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Studer G, Peponi E, Kloeck S, Dossenbach T, Huber G, Glanzmann C. Surviving hypopharynx‐larynx carcinoma in the era of IMRT. Int J Radiat Oncol Biol Phys 2010; 77: 1391–6. [DOI] [PubMed] [Google Scholar]
- 11. Seung S, Bae J, Solhjem M, et al. Intensity‐modulated radiotherapy for head‐and‐neck cancer in the community setting. Int J Radiat Oncol Biol Phys 2008; 72: 1075–81. [DOI] [PubMed] [Google Scholar]
- 12. Daly ME, Le Q‐T, Maxim PG, et al. Intensity‐modulated radiotherapy in the treatment of oropharyngeal cancer: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys 2010; 76: 1339–46. [DOI] [PubMed] [Google Scholar]
- 13. Huang K, Xia P, Chuang C, et al. Intensity‐modulated chemoradiation for treatment of stage III and IV oropharyngeal carcinoma: the University of California‐San Francisco experience. Cancer 2008; 113: 497–507. [DOI] [PubMed] [Google Scholar]
- 14. May JT, Rao N, Sabater RD, et al. Intensity‐modulated radiation therapy as primary treatment for oropharyngeal squamous cell carcinoma. Head Neck 2013; 35: 1796–800. [DOI] [PubMed] [Google Scholar]
- 15. Montejo ME, Shrieve DC, Bentz BG, et al. IMRT with simultaneous integrated boost and concurrent chemotherapy for locoregionally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2011; 81: e845–52. [DOI] [PubMed] [Google Scholar]
- 16. Clavel S, Nguyen DHA, Fortin B, et al. Simultaneous integrated boost using intensity‐modulated radiotherapy compared with conventional radiotherapy in patients treated with concurrent carboplatin and 5‐fluorouracil for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2012; 82: 582–9. [DOI] [PubMed] [Google Scholar]
- 17. Johnston M, Guo L, Back M, et al. Intensity‐modulated radiotherapy using simultaneous‐integrated boost for definitive treatment of locally advanced mucosal head and neck cancer: outcomes from a single‐institution series. J Med Imaging Radiat Oncol 2013; 57: 356–63. [DOI] [PubMed] [Google Scholar]
- 18. Spiotto MT, Weichselbaum RR. Comparison of 3D confromal radiotherapy and intensity modulated radiotherapy with or without simultaneous integrated boost during concurrent chemoradiation for locally advanced head and neck cancers. PLoS ONE 2014; 9: e94456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d'Etude des Tumeurs de la Tête et du Cou (GETTEC). Br J Cancer 2000; 83: 1594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ethical guidelines for observational studies: Observational research, audits and related activities. Revised edn. 2012.
- 21. Grégoire V, Levendag P, Ang KK, et al. CT‐based delineation of lymph node levels and related CTVs in the node‐negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother. Oncol. 2003; 69: 227–36. [DOI] [PubMed] [Google Scholar]
- 22. Low DA, Dempsey JF. Evaluation of the gamma dose distribution comparison method. Med Phys 2003; 30: 2455. [DOI] [PubMed] [Google Scholar]
- 23. Fay MP, Brittain EH, Proschan MA. Pointwise confidence intervals for a survival distribution with small samples or heavy censoring. Biostatistics 2013; 14: 723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706. [DOI] [PubMed] [Google Scholar]
- 25. Choudhury JB. Non‐parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Stat Med 2002; 21: 1129–44. [DOI] [PubMed] [Google Scholar]
- 26. Orlandi E, Palazzi M, Pignoli E, Fallai C, Giostra A, Olmi P. Radiobiological basis and clinical results of the simultaneous integrated boost (SIB) in intensity modulated radiotherapy (IMRT) for head and neck cancer: a review. Crit Rev Oncol Hematol 2010; 73: 111–25. [DOI] [PubMed] [Google Scholar]
- 27. Loo SW, Geropantas K, Wilson P, Martin WMC, Roques TW. Target volume definition for intensity‐modulated radiotherapy after induction chemotherapy and patterns of treatment failure after sequential chemoradiotherapy in locoregionally advanced oropharyngeal squamous cell carcinoma. Clin Oncol (R Coll Radiol) 2013; 25: 162–70. [DOI] [PubMed] [Google Scholar]
- 28. Haddad R, O'Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013; 14: 257–64. [DOI] [PubMed] [Google Scholar]
- 29. Cohen EEW, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 2014; 32: 2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lassen P, Primdahl H, Johansen J, et al. Impact of HPV‐associated p16‐expression on radiotherapy outcome in advanced oropharynx and non‐oropharynx cancer. Radiother Oncol 2014; 113: 310–16. [DOI] [PubMed] [Google Scholar]
- 31. Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16‐positive and p16‐negative oropharyngeal cancer. J Clin Oncol 2012; 30: 2102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


