Abstract
Physical activity improves glycemic control and reduces the risk of cardiovascular disease (CVD) and mortality in patients with type 2 diabetes (T2D). Moderate to vigorous physical activity is recommended to manage T2D; however, patients with T2D can be physically weak, making it difficult to engage in the recommended levels of physical activity. Daily physical activity includes various activities performed during both occupational and leisure time such as walking, gardening, and housework that type 2 diabetic patients should be able to perform without considerable physical burden. This review focuses on the association between daily physical activity and T2D. Walking was the most common form of daily physical activity, with numerous studies demonstrating its beneficial effects on reducing the risk of T2D, CVD, and mortality. Walking for at least 30 min per day was shown to reduce the risk of T2D by approximately 50%. Additionally, walking was associated with a reduction in mortality. In contrast, evidence was extremely limited regarding other daily physical activities such as gardening and housework in patients with T2D. Recent studies have suggested daily physical activity, including non-exercise activity thermogenesis, to be favorably associated with metabolic risks and mortality. However, well-designed longitudinal studies are warranted to elucidate its effects on overall health.
Keywords: Type 2 diabetes, Daily physical activity, Walking, Non-exercise activity thermogenesis
Core tip: In addition to moderate- to vigorous-intensity exercise, daily physical activity is also important for the prevention and management of type 2 diabetes (T2D). Of note, individuals can engage in daily physical activity without remarkable physical burden, anywhere and at any time. It is well known that exercise improves the outcomes of metabolic diseases and reduces cardiovascular disease risk and mortality. However, the literature pertaining to the effects of specific types of daily physical activity on health is sparse. It is necessary to accumulate evidence on the positive effects of daily physical activity on the management of T2D.
INTRODUCTION
Exercise therapy is essential for the management of diabetes. A sedentary lifestyle is known to be a major risk factor of cardiovascular disease (CVD)[1]. The American College of Sports Medicine and the American Diabetes Association have recommended at least 150 min/wk of moderate (50%-70% of an individual’s maximum heart rate) to vigorous (> 70% of an individual’s maximum heart rate) physical activity for patients with type 2 diabetes (T2D)[2]. As for patients with type 1 diabetes, regular physical activity has also shown beneficial effects on glycemic control and other health-related outcomes, although the evidence is limited[3].
However, the recommended intensity and duration of exercise could present a physical burden to diabetic patients and lead to cessation of exercise therapy because diabetic patients have a lower physical performance threshold than healthy individuals. Patients with T2D show a lower energy expenditure, number of steps, and duration of physical activity compared to subjects without diabetes[4], as well as low cardiorespiratory fitness[5,6]. Moreover, the muscle strength of individuals with T2D is significantly lower than those without[7,8]. In fact, upper and lower extremity muscle strength have been shown to be inversely associated with the degree of diabetic complications[9], suggesting that diabetes progression hinders engagement in physical activity. Indeed, the percentage of patients with diabetes found to engage in exercise therapy was approximately 40%[10], and only 28.2% of diabetic patients in the United States achieved the recommended level of physical activity[11]. In a large-scale cohort study, individuals who performed low-volume physical activity, which was defined as 15 min/d or 90 min/wk, had a 14% reduced risk of all-cause mortality and a life expectancy increase of 3 years[12]. Thus, it is important to note that in addition to moderate- to vigorous-intensity physical activity, light- to moderate-intensity daily physical activity should also be considered an alternative and supportive exercise therapy regimen for diabetic individuals.
The purpose of this review is to highlight the effects of daily physical activity on health in type 2 diabetic patients and to further suggest a strategy for the treatment of T2D by changing the amount of daily physical activity a patient performs. This review will help physical therapists, clinicians, and patients manage T2D.
DAILY PHYSICAL ACTIVITY
Daily physical activity is defined as continuous bodily movements via the contraction of skeletal muscle that results in an increase in energy expenditure in daily life[13]. This includes various activities that are conducted in both occupational and leisure time such as walking, working at a desk, washing, cooking, and sports. On the other hand, exercise is defined as planned, structured, and repetitive physical activity that has the objective of improving physical fitness[13]. Physical activity is usually classified by its intensity and duration. The metabolic equivalent (MET) is a useful measurement for representing the intensity of physical activity and is defined as the amount of oxygen uptake while sitting at rest. An oxygen uptake of 3.5 mL/kg per minute is equal to the basal resting metabolic rate and is considered to be 1 MET[14]. Ainsworth et al[15] listed MET values for 821 specific physical activities that vary in their intensity of daily physical activity according to the situation in which they are performed. For example, walking inside is equal to only 2.0 METs (light intensity) whereas walking with children is the equivalent of 4.0 METs (moderate intensity). Therefore, it should be noted that daily physical activity covers a wide range of intensity that at times are the same as structured exercise (Table 1).
Table 1.
Excerpt of various daily physical activities and their associated metabolic equivalents
| Daily physical activity | METs |
| Walking | |
| Very slow, < 3.2 km/h | 2 |
| Slow, 3.2-4.0 km/h | 2.8-3.0 |
| For pleasure, moderate pace, 4.5-5.1 km/h | 3.5 |
| Brisk, 5.6 km/h | 4.3 |
| Very brisk, 6.4-7.2 km/h | 5.0-7.0 |
| Stair climbing, slow pace | 4 |
| Stair climbing, fast pace | 8.8 |
| Gardening | 3.8 |
| Yard work | 3.0-6.0 |
| Mowing lawn | 5.5 |
| Shoveling | 5.3-7.5 |
| Housework | |
| Washing dishes | 1.8-2.5 |
| Cleaning | 2.3-3.8 |
| Cooking | 2.0-3.0 |
| Child care | 2.0-3.0 |
| Elder care | 2.3-4.0 |
Each activity is quoted from Ainsworth et al[15] (2011 Compendium Physical Activities). METs: Metabolic equivalents.
SEDENTARY LIFESTYLE
Sedentary behavior refers to the tendency to sit during waking hours with low energy expenditures. The mean sitting time is estimated to be approximately 6-7 h/d in developed countries, and a decreased level of physical activity has been shown to be inversely associated with increased sitting time[16]. In today’s world, individuals are more prone to sit on a daily basis.
Whose lifestyle is healthier: A person who engages in the recommended amount of exercise during their leisure time but is extremely sedentary in their spare time, or a person who does not achieve the recommended level of physical activity but is quite physically active in the workplace? The answer: Exercise and sedentary behavior may be mutually contradictory[17]. Sedentary time has been recognized as an independent risk factor for CVD, T2D, and all-cause mortality[17-19]. The American Diabetes Association has recommended that patients with diabetes should be encouraged to reduce their sedentary time and to not sit for more than 90 min[20]. Changing one’s sedentary lifestyle to a more active lifestyle is key to the better management of T2D. In addition, sitting time may not affect all-cause mortality if it is combined with walking time, suggesting that the positive association between sitting time and mortality is only evident in individuals who sit for very long periods of time[21]. This review focuses on clinical studies that have investigated the effects of walking on CVD risk factors and mortality.
WALKING
Walking is one of the most common physical activities of daily life[22,23]. However, 54.6% of patients with T2D have reported engaging in no weekly physical activity through walking[24], demonstrating that patients with T2D should walk more frequently. Epidemiological studies have suggested that walking is associated with a reduced risk of T2D. Hu et al[25] examined the relationship between physical activity, based on the use of a questionnaire, and the incidence of T2D in individuals selected from the Nurses’ Health Study database. A total of 70102 women without diabetes were followed for 8 years. Overall, walking MET scores had a strong negative association with the risk of T2D. More specifically, a normal walking pace (3.2-4.8 km/h) was associated with an approximate 20%-30% reduction in the risk of T2D in women who did not engage in vigorous physical activity, and a faster walking pace was independently associated with a reduced risk of T2D. Moreover, Tanasescu et al[26] examined the relationship of walking with CVD risk and mortality among 3058 men with T2D. Frequent walking (≥ 16.1 MET-hours/week) was associated with a nearly 40% reduced risk of mortality, and walking pace was inversely associated with CVD and total mortality independent of walking hours. Gregg et al[27] also investigated whether walking reduced mortality among 2896 United States adults with diabetes. Patients who walked at least 2 h/wk had a 39% lower all-cause mortality rate and a 34% lower CVD mortality rate compared with sedentary patients. However, it is important to note that these studies were observational studies, and a causal relationship between walking and incidence of T2D and/or CVD cannot therefore be deduced. Moreover, physical activity, including the amount and speed of walking, might have been over or underestimated in these studies because physical activity was evaluated by questionnaires, and such subjective data are known to have poor validity[28], as eliminating bias is quite difficult. The Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research trial[29-31] investigated whether changes in ambulatory activity, assessed objectively through the use of a pedometer, were associated with a reduced risk of cardiovascular events in individuals with impaired glucose tolerance. During a 45211 person-years follow-up, ambulatory activity was found to be inversely associated with the risk of a cardiovascular event. Specifically, an increase of 2000 steps/d at baseline was associated with a 10% reduction in having a cardiovascular event, and a 2000-step increase in daily life from baseline to 12 mo was associated with an 8% lower cardiovascular event rate[32]. On the other hand, the Nakanojo Study[33] suggested that not just walking but the combination of walking (> 8000-10000 steps/d) and physical activity at an intensity > 3 METs was necessary to prevent metabolic syndrome. Daily physical activity that reaches an intensity > 3 METs may need to be emphasized. A list of published articles that focused on the beneficial association of walking with the risk of T2D, CVD, and mortality is shown in Table 2.
Table 2.
Epidemiological studies investigating the associations of walking with the risk of type 2 diabetes, cardiovascular events, and mortality
| Ref. | Study design | Subjects | Physical activity measurement | Outcome, results |
| Hu et al[25] | Prospective cohort study | 70102 female participants without diabetes, CVD, or cancer | MET score and walking pace based on a questionnaire | Risk of type 2 diabetes, normal walking pace (3.2-4.8 km/h): RR = 0.72; 95%CI: 0.62-0.85 |
| Brisk or very brisk walking pace (> 4.8 km/h): RR = 0.41; 95%CI: 0.33-0.52 | ||||
| Tanasescu et al[26] | Prospective cohort study | 3058 men with type 2 diabetes | MET-hour score measured by a questionnaire | Mortality, walking ≥ 16.1 MET-hours/week: RR = 0.60; 95%CI: 0.41-0.88 |
| Very brisk walking pace (≥ 4 mph): RR = 0.42; 95%CI: 0.19-0.97 | ||||
| Gregg et al[27] | Prospective cohort study | 2896 subjects with diabetes | Time spent walking measured by a questionnaire | Mortality, walking ≥ 2 h/wk, all-cause mortality: HRR = 0.61; 95%CI: 0.48-0.78; CVD mortality: HRR = 0.66; 95%CI: 0.45-0.96 |
| Yates et al[32] | Prospective data analysis from the NAVIGATOR trial (a multicenter, randomized, placebo controlled, 2 × 2 factorial trial) | 9306 individuals with impaired glucose tolerance | Number of steps assessed by a pedometer | Cardiovascular events, baseline ambulatory activity (2000 step/d increment): HR = 0.90; 95%CI: 0.84-0.96 Change in ambulatory activity from baseline to 12 mo (2000 step/d difference in change): HR = 0.92; 95%CI: 0.86-0.99 |
MET: Metabolic equivalents; RR: Relative risk; HRR: Hazard rate ratio; CVD: Cardiovascular disease; HR: Hazard ratio; NAVIGATOR: Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research.
The Diabetes Prevention Program randomly assigned 3234 individuals with impaired glucose tolerance to one of three interventions: Standard lifestyle recommendations plus metformin treatment, standard lifestyle recommendations plus placebo, or an intensive program of lifestyle modification. The lifestyle intervention reduced the incidence of diabetes by 58% after a 2.8-year follow-up. Brisk walking for at least 150 min/wk was a very effective way to prevent T2D[34]. The Da Qing IGT and Diabetes Study investigated the effects of walking for at least 30 min/d on individuals with impaired glucose tolerance. A total of 577 subjects were randomized to one of three groups: Diet only, walking only, or diet plus walking. Over a 6-year follow-up, the incidence of diabetes was significantly reduced by 46% in the walking group[35]. Kosaka et al[36] showed that a lifestyle intervention for men with impaired glucose tolerance effectively reduced the risk of diabetes. They recommended the following activities to their subjects to increase their physical activity: Walking for 30-40 min/d, using a staircase instead of an elevator or an escalator, performing 30 min of cycling on weekends, and getting off a bus one stop before their destination. Their findings demonstrated that the physical activity intervention combined with diet therapy reduced the risk of diabetes by 67.4%. A list of published articles with a focus on the effects of walking on the risk of T2D is shown in Table 3.
Table 3.
Clinical trials investigating the effects of walking on the risk of type 2 diabetes
| Ref. | Study design | Subjects, follow-up time | Intervention | Results |
| Knowler et al[34] | Randomized clinical trial | 3234 individuals with impaired glucose tolerance, 2.8 yr | A minimum of 150 min of physical activity similar intensity to brisk walking and 7% weight loss | 58% reduction in the incidence rate of type 2 diabetes |
| Pan et al[35] | Randomized clinical trial | 577 individuals with impaired glucose tolerance, 6 yr | At least 30 min/d of walking | 46% reduction in the risk of developing diabetes |
| Kosaka et al[36] | Randomized clinical trial | 458 men with impaired glucose tolerance, 4 yr | Recommendations for physical activity: walking 30-40 min/d, using staircase instead of an elevator or an escalator, 30-min cycling on weekends and getting off one bus stop before the destination | 67.4% reduction in the risk of developing diabetes |
Several small-scale intervention studies have revealed favorable effects of walking on CVD risk factors. A walking program of 1 h/d for 12 wk was shown to improve physical fitness, body composition, and glycemic control in postmenopausal women with T2D[37]. Similarly, walking at least 10000 steps/d combined with diet therapy in obese patients with T2D improved their insulin sensitivity[38]. Moreover, a meta-analysis of randomized controlled trials summarized the effects of walking on glycemic control and CVD risks; specifically, walking significantly decreased glycated hemoglobin levels by 0.50% (95%CI: -0.78% to -0.21%), body mass index by -0.91 kg/m2 (95%CI: -1.22 to -0.59 kg/m2), and diastolic blood pressure by -1.97 mmHg (95%CI: -3.94 to -0.0 mmHg)[39]. Recently, an interesting study investigating postprandial changes in glucose, insulin, and non-esterified fatty acids in postmenopausal women at high risk of T2D was published. In this study, 22 participants were randomly assigned to one of six groups: Prolonged sitting (7.5 h) plus standing (total of 60 min), standing plus walking (total of 60 min), walking plus sitting, standing plus sitting, or walking plus standing. Both standing and walking significantly reduced postprandial glucose, insulin, and non-esterified fatty acids response compared with prolonged sitting[40]. Thus, diabetic patients may only need to stand to improve their metabolic profile.
Additionally, the way in which one walks may also be of significance. Karstoft et al[41] investigated the effects of interval-walking vs continuous-walking in patients with T2D. Four months of free-living interval-walking improved maximal oxygen consumption (VO2 max), body composition, and glycemic control. VO2 max significantly increased by 16.1%, fat mass and visceral fat significantly decreased by 10.8% and 10.6%, respectively, fasting insulin significantly decreased by 19.5%, and mean glucose concentrations measured by the continuous glucose monitoring system significantly decreased by 8.5% in the interval-walking group, whereas no changes were observed in the continuous-walking group. Their next study focused on determining whether interval-walking improved postprandial glucose tolerance and free-living glycemic control more than continuous-walking. Their findings revealed that a greater reduction in postprandial blood glucose levels was observed with a single interval-walking session than with an oxygen consumption- and time duration-matched continuous-walking session[42]. The optimal exercise therapy for individuals with T2D and existing diabetic complications is still unknown. The amount, duration, and intensity of exercise has been emphasized in diabetes care; however, the mode of physical activity such as the variation in intensity and motion may also need to be emphasized.
GARDENING, HOUSEWORK, AND OTHER ACTIVITIES
Gardening is the most popular daily physical activity in older adults[43,44]; however, the current literature does not provide sufficient evidence of the health benefits of gardening[45]. The Evaluating Long-term Diabetes Self-management Among Elder Rural Adults study, a population-based cross-sectional study, described the types of daily physical activities performed among rural older adults with diabetes. The most common physical activities reported by these individuals were walking (79.7%), housework (68.7%), and gardening (64.8%). Health-related quality of life, as measured by the short form 12-item survey scale, has been found to be positively associated with physical activity, suggesting that participating in a greater amount of daily physical activity such as gardening and housework is beneficial for older adults[46]. As domestic physical activity, which includes gardening and housework, has been analyzed as a part of total daily physical activity in previous studies, it is difficult to determine its independent effects on health. However, it is important to investigate the effects of domestic physical activity on glycemic control, CVD risk, and mortality, as domestic chores are the main contributors to total daily physical activity in patients with T2D[47]. Stamatakis et al[48] examined the independent effects of domestic physical activity (e.g., cleaning windows, sweeping, digging, cycling, dancing) on mortality and CVD events in the Scottish Health Survey. Participation in intense domestic physical activity was associated with lower all-cause mortality (men: RR = 0.68, 95%CI: 0.50-0.91; women: RR = 0.70, 95%CI: 0.52-0.93). A recent study showed that heavy housework was associated with reduced mortality (HR = 0.71; 95%CI: 0.56-0.91) and reduced cancer deaths (HR = 0.52) in older Chinese men[49]. However, the associations between domestic physical activity and mortality or CVD risk in diabetic patients were not investigated in these studies. In contrast, Lawlor et al[50] found that participating in at least 2.5 h of heavy housework was not associated with an improvement in obesity among elderly British women. However, this study also lacked information concerning T2D. There is extremely little evidence to suggest that gardening and/or housework have beneficial effects on mortality, CVD risk, or other metabolic disturbances in patients with T2D. Conversely, an observational study in healthy older adults objectively measured free-living activity energy expenditure by using the doubly labeled water method and demonstrated its association with a lower risk of mortality[51]. There was an approximate 30% lower risk of mortality for every 287 kcal consumed per day by free-living activity energy expenditure; however, self-reported high-intensity exercise did not differ between the activity energy expenditure tertiles, suggesting that light- to moderate-intensity daily physical activity plays a key role in healthy living. Beddhu et al[52] analyzed data from the 2003-2004 National Health and Nutrition Examination Survey and showed that the duration of light-intensity physical activity (e.g., casual walking, light gardening, cleaning), objectively assessed by an accelerometer, was significantly associated with a lower mortality risk (HR = 0.59; 95%CI: 0.35-0.98) in the chronic kidney disease (CKD) population; however, an increase in moderate/vigorous activity duration did not result in a significantly lower hazard ratio in the CKD group. Approximately 20%-30% of patients with CKD had diabetes in this study, which suggests that light-intensity daily physical activity can reduce mortality in diabetic patients with CKD. Thus, these findings suggest that in contrast to moderate- to vigorous-intensity exercise, light-intensity daily physical activity may have beneficial health effects on diabetic patients who have progressed complications. Moreover, Steeves et al[53] conducted a study analyzing data from the 2003-2006 National Health and Nutrition Examination Survey and found that the total physical activity levels of patients with diabetes, as measured by an accelerometer, were significantly lower compared to participants with normal glucose tolerance and prediabetes. The diabetic participants were found to be inactive from 10:00 am to 8:00 pm when compared with participants with normal glucose tolerance, and their physical activity per hour declined rapidly after 12:00 pm, with the greatest difference occurring at 4:00 pm[53]. These findings suggest that clinicians should consider activity patterns in daily life as well as total physical activity to achieve optimal glycemic control in patients with T2D.
NON-EXERCISE ACTIVITY THERMOGENESIS
Daily physical activity, with the exception of volitional sports-like activities, is defined as non-exercise activity thermogenesis (NEAT)[54]. NEAT is the main determinant of variability in total daily energy expenditure[55], which varies substantially from person to person by up to 2000 kcal/d[56]. NEAT is influenced by various factors. For example, NEAT has been shown to increase by 25% seven days after a single bout of high-intensity walking exercise[57]. Moreover, regular exercise, especially moderate- to vigorous-intensity exercise, may increase NEAT; in contrast, living in an urban area populated with individuals who live a sedentary lifestyle will likely result in a decrease in NEAT. Indeed, the ambulation levels of rural Jamaicans was found to be more than 60% of those of urban North Americans, suggesting that urbanization is associated with a decrease in NEAT[58]. However, the intensity, frequency, and duration of physical activity required to increase NEAT is unknown. Furthermore, Levine et al[59] examined whether weight gain was associated with a decrease in walking distance. The consumption of an additional 1000 kcal/d significantly decreased walking distance in both lean and obese individuals, suggesting that weight gain due to overeating may result in decreased walking. Schmidt et al[60] examined whether 23 obesity-prone individuals and 32 obesity-resistant individuals had a different response to 3 d of overeating. They found that the walking time of obesity-prone subjects decreased significantly (-2.0%), whereas obesity-resistant subjects maintained their walking time. Taken together, these studies collectively suggest that daily diet, exercise, and living environment all influence the amount of NEAT. Furthermore, endocrine factors such as thyroid hormones[61], leptin[62], sex steroids[63], and orexin[64] have all been shown to affect changes in NEAT. Several biological studies have also demonstrated that genetic variations exist in the propensity for spontaneous physical activity. For example, polymorphisms in the dopamine D2 receptor gene and melanocortin-4 receptor gene have been associated with physical activity variations in adults, and polymorphisms in the Nhlh2 gene have shown effects on the motivation to exercise[65]. NEAT is thus modulated by endocrine, genetic, and sociological factors (Figure 1).
Figure 1.
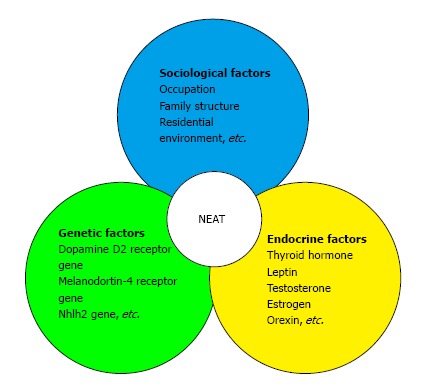
Non-exercise activity thermogenesis is intricately regulated by sociological, endocrinological, and genetic factors. NEAT: Non-exercise activity thermogenesis.
Previous studies have shown that NEAT plays a crucial role in the management of obesity[66]. The Look AHEAD (Action for Health in Diabetes) study did not find a reduction in the rate of cardiovascular events in obese patients with T2D after implementation of an intensive lifestyle intervention that aimed to achieve at least 7% weight loss by a reduction in dietary intake and a minimum of 175 min of moderate-intensity physical activity per week. This finding suggests that this lifestyle intervention should be conducted before an individual becomes obese[67]. Indeed, NEAT is a noteworthy factor in the management of obesity and other metabolic risks. The Shanghai Women’s Health Study, a prospective cohort study, investigated the effects of exercise, walking and cycling for transportation, as well as the effect of non-exercise physical activity, on mortality. A total of 67143 women without a history of heart disease, stroke, or cancer were followed for an average of 5.7 years. That study found that women who reported 10 or more MET-hours per day of non-exercise activity had a 25%-50% lower risk of mortality compared with less active women[68]. Moreover, Hagger-Johnson et al[69] analyzed data from the United Kingdom Women’s Cohort Study (1999-2002) to investigate the association between fidgeting behaviors and all-cause mortality. Data on sitting time and fidgeting behavior of 12778 women were analyzed. Among women in the low fidgeting group, sitting for ≥ 7 h/d (vs < 5 h/d) was associated with a 30% increase in risk of all-cause mortality. Among women in the high fidgeting group, sitting for 5-6 h/d was associated with a decreased mortality risk (HR = 0.63, 95%CI: 0.43-0.91)[69]. Fidgeting as a component of NEAT may reduce all-cause mortality; however, little evidence is available regarding the associations between NEAT and T2D. Hamasaki et al[70,71] previously reported that NEAT was associated with a reduction in waist circumference, improvement in insulin sensitivity and dyslipidemia, and an increase in B-type natriuretic peptide[72] levels in patients with T2D. However, a causal relationship between NEAT and the incidence of T2D could not be deduced, as attempting to conduct a prospective study with a high level of evidence would be quite difficult because, by definition, intervention studies for NEAT would not be practical when the effect of NEAT on metabolic disease is also unknown. As physical activity is no longer an intervention for NEAT, the development of new intervention strategies to target NEAT will be an issue that should be addressed in the future.
CONCLUSION
The current literature provides evidence of the efficacy of walking in preventing T2D and reducing the risk of cardiovascular events and/or mortality. More specifically, previous studies have suggested that brisk walking for at least 30 min/d (e.g., ≥ 15 MET-hours per week) is needed to reduce the risk of T2D. Walking improves insulin sensitivity, glycemic control, and the incidence of obesity. However, there are few studies investigating the independent effects of other daily physical activities such as gardening and housework on health, especially in patients with T2D. The current literature lacks well-conducted controlled longitudinal studies investigating the effects of only daily physical activity on diabetes, dyslipidemia, hypertension, other CVD risks, and mortality. Daily physical activity, including NEAT, may be associated with a reduction in mortality. Although ensuring that patients with T2D engage in daily physical activity may be difficult, well-designed longitudinal studies that focus on daily physical activity independent of structured exercise should be conducted in the future.
Footnotes
Conflict-of-interest statement: No conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 27, 2016
First decision: March 23, 2016
Article in press: May 9, 2016
P- Reviewer: Panchu P, Tamemoto H, Verrotti A S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
References
- 1.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 2.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B; American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yardley JE, Hay J, Abou-Setta AM, Marks SD, McGavock J. A systematic review and meta-analysis of exercise interventions in adults with type 1 diabetes. Diabetes Res Clin Pract. 2014;106:393–400. doi: 10.1016/j.diabres.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Fagour C, Gonzalez C, Pezzino S, Florenty S, Rosette-Narece M, Gin H, Rigalleau V. Low physical activity in patients with type 2 diabetes: the role of obesity. Diabetes Metab. 2013;39:85–87. doi: 10.1016/j.diabet.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 6.Ozdirenç M, Biberoğlu S, Ozcan A. Evaluation of physical fitness in patients with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2003;60:171–176. doi: 10.1016/s0168-8227(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 7.Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28:2541–2542. doi: 10.2337/diacare.28.10.2541. [DOI] [PubMed] [Google Scholar]
- 8.Cetinus E, Buyukbese MA, Uzel M, Ekerbicer H, Karaoguz A. Hand grip strength in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2005;70:278–286. doi: 10.1016/j.diabres.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Balducci S, Sacchetti M, Orlando G, Salvi L, Pugliese L, Salerno G, D’Errico V, Iacobini C, Conti FG, Zanuso S, et al. Correlates of muscle strength in diabetes: The study on the assessment of determinants of muscle and bone strength abnormalities in diabetes (SAMBA) Nutr Metab Cardiovasc Dis. 2014;24:18–26. doi: 10.1016/j.numecd.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22:1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 11.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 12.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 13.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 14.Balducci S, Sacchetti M, Haxhi J, Orlando G, D’Errico V, Fallucca S, Menini S, Pugliese G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res Rev. 2014;30 Suppl 1:13–23. doi: 10.1002/dmrr.2514. [DOI] [PubMed] [Google Scholar]
- 15.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 16.Bauman A, Ainsworth BE, Sallis JF, Hagströmer M, Craig CL, Bull FC, Pratt M, Venugopal K, Chau J, Sjöström M. The descriptive epidemiology of sitting. A 20-country comparison using the International Physical Activity Questionnaire (IPAQ) Am J Prev Med. 2011;41:228–235. doi: 10.1016/j.amepre.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162:123–132. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 18.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of Medical Care in Diabetes-2015. Diabetes Care. 2015;38:S1–S93. doi: 10.2337/dc14-2142. [DOI] [PubMed] [Google Scholar]
- 21.Pulsford RM, Stamatakis E, Britton AR, Brunner EJ, Hillsdon M. Associations of sitting behaviours with all-cause mortality over a 16-year follow-up: the Whitehall II study. Int J Epidemiol. 2015;44:1909–1916. doi: 10.1093/ije/dyv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 23.Rafferty AP, Reeves MJ, McGee HB, Pivarnik JM. Physical activity patterns among walkers and compliance with public health recommendations. Med Sci Sports Exerc. 2002;34:1255–1261. doi: 10.1097/00005768-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Hays LM, Clark DO. Correlates of physical activity in a sample of older adults with type 2 diabetes. Diabetes Care. 1999;22:706–712. doi: 10.2337/diacare.22.5.706. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 26.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107:2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 27.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440–1447. doi: 10.1001/archinte.163.12.1440. [DOI] [PubMed] [Google Scholar]
- 28.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206; discussion 206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Califf RM, Boolell M, Haffner SM, Bethel M, McMurray J, Duggal A, Holman RR; NAVIGATOR Study Group. Prevention of diabetes and cardiovascular disease in patients with impaired glucose tolerance: rationale and design of the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) Trial. Am Heart J. 2008;156:623–632. doi: 10.1016/j.ahj.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 30.NAVIGATOR Study Group, Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–1476. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 31.NAVIGATOR Study Group, McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 32.Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, Califf RM, Holman RR, McMurray JJ, Bethel MA, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383:1059–1066. doi: 10.1016/S0140-6736(13)62061-9. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Park H, Togo F, Watanabe E, Yasunaga A, Yoshiuchi K, Shephard RJ, Aoyagi Y. Year-long physical activity and metabolic syndrome in older Japanese adults: cross-sectional data from the Nakanojo Study. J Gerontol A Biol Sci Med Sci. 2008;63:1119–1123. doi: 10.1093/gerona/63.10.1119. [DOI] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 36.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67:152–162. doi: 10.1016/j.diabres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Walker KZ, Piers LS, Putt RS, Jones JA, O’Dea K. Effects of regular walking on cardiovascular risk factors and body composition in normoglycemic women and women with type 2 diabetes. Diabetes Care. 1999;22:555–561. doi: 10.2337/diacare.22.4.555. [DOI] [PubMed] [Google Scholar]
- 38.Yamanouchi K, Shinozaki T, Chikada K, Nishikawa T, Ito K, Shimizu S, Ozawa N, Suzuki Y, Maeno H, Kato K. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1995;18:775–778. doi: 10.2337/diacare.18.6.775. [DOI] [PubMed] [Google Scholar]
- 39.Qiu S, Cai X, Schumann U, Velders M, Sun Z, Steinacker JM. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta-analysis. PLoS One. 2014;9:e109767. doi: 10.1371/journal.pone.0109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henson J, Davies MJ, Bodicoat DH, Edwardson CL, Gill JM, Stensel DJ, Tolfrey K, Dunstan DW, Khunti K, Yates T. Breaking Up Prolonged Sitting With Standing or Walking Attenuates the Postprandial Metabolic Response in Postmenopausal Women: A Randomized Acute Study. Diabetes Care. 2016;39:130–138. doi: 10.2337/dc15-1240. [DOI] [PubMed] [Google Scholar]
- 41.Karstoft K, Winding K, Knudsen SH, Nielsen JS, Thomsen C, Pedersen BK, Solomon TP. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36:228–236. doi: 10.2337/dc12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karstoft K, Christensen CS, Pedersen BK, Solomon TP. The acute effects of interval- Vs continuous-walking exercise on glycemic control in subjects with type 2 diabetes: a crossover, controlled study. J Clin Endocrinol Metab. 2014;99:3334–3342. doi: 10.1210/jc.2014-1837. [DOI] [PubMed] [Google Scholar]
- 43.Rowinski R, Dabrowski A, Kostka T. Gardening as the dominant leisure time physical activity (LTPA) of older adults from a post-communist country. The results of the population-based PolSenior Project from Poland. Arch Gerontol Geriatr. 2015;60:486–491. doi: 10.1016/j.archger.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Mooney SJ, Joshi S, Cerdá M, Quinn JW, Beard JR, Kennedy GJ, Benjamin EO, Ompad DC, Rundle AG. Patterns of Physical Activity Among Older Adults in New York City: A Latent Class Approach. Am J Prev Med. 2015;49:e13–e22. doi: 10.1016/j.amepre.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicklett EJ, Anderson LA, Yen IH. Gardening Activities and Physical Health Among Older Adults: A Review of the Evidence. J Appl Gerontol. 2014:Dec 16; Epub ahead of print. doi: 10.1177/0733464814563608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arcury TA, Snively BM, Bell RA, Smith SL, Stafford JM, Wetmore-Arkader LK, Quandt SA. Physical activity among rural older adults with diabetes. J Rural Health. 2006;22:164–168. doi: 10.1111/j.1748-0361.2006.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cloix L, Caille A, Helmer C, Bourdel-Marchasson I, Fagot-Campagna A, Fournier C, Lecomte P, Oppert JM, Jacobi D. Physical activity at home, at leisure, during transportation and at work in French adults with type 2 diabetes: the ENTRED physical activity study. Diabetes Metab. 2015;41:37–44. doi: 10.1016/j.diabet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Stamatakis E, Hamer M, Lawlor DA. Physical activity, mortality, and cardiovascular disease: is domestic physical activity beneficial? The Scottish Health Survey -- 1995, 1998, and 2003. Am J Epidemiol. 2009;169:1191–1200. doi: 10.1093/aje/kwp042. [DOI] [PubMed] [Google Scholar]
- 49.Yu R, Leung J, Woo J. Housework reduces all-cause and cancer mortality in Chinese men. PLoS One. 2013;8:e61529. doi: 10.1371/journal.pone.0061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawlor DA, Taylor M, Bedford C, Ebrahim S. Is housework good for health? Levels of physical activity and factors associated with activity in elderly women. Results from the British Women’s Heart and Health Study. J Epidemiol Community Health. 2002;56:473–478. doi: 10.1136/jech.56.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 52.Beddhu S, Wei G, Marcus RL, Chonchol M, Greene T. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol. 2015;10:1145–1153. doi: 10.2215/CJN.08410814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steeves JA, Murphy RA, Crainiceanu CM, Zipunnikov V, Van Domelen DR, Harris TB. Daily Patterns of Physical Activity by Type 2 Diabetes Definition: Comparing Diabetes, Prediabetes, and Participants with Normal Glucose Levels in NHANES 2003-2006. Prev Med Rep. 2015;2:152–157. doi: 10.1016/j.pmedr.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine JA. Non-exercise activity thermogenesis (NEAT) Nutr Rev. 2004;62:S82–S97. doi: 10.1111/j.1753-4887.2004.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 55.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Curr Opin Clin Nutr Metab Care. 2004;7:599–605. doi: 10.1097/00075197-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Levine JA, Vander Weg MW, Hill JO, Klesges RC. Non-exercise activity thermogenesis: the crouching tiger hidden dragon of societal weight gain. Arterioscler Thromb Vasc Biol. 2006;26:729–736. doi: 10.1161/01.ATV.0000205848.83210.73. [DOI] [PubMed] [Google Scholar]
- 57.Alahmadi MA, Hills AP, King NA, Byrne NM. Exercise intensity influences nonexercise activity thermogenesis in overweight and obese adults. Med Sci Sports Exerc. 2011;43:624–631. doi: 10.1249/MSS.0b013e3181f7a0cb. [DOI] [PubMed] [Google Scholar]
- 58.Levine JA, McCrady SK, Boyne S, Smith J, Cargill K, Forrester T. Non-exercise physical activity in agricultural and urban people. Urban Stud. 2011;48:2417–2427. doi: 10.1177/0042098010379273. [DOI] [PubMed] [Google Scholar]
- 59.Levine JA, McCrady SK, Lanningham-Foster LM, Kane PH, Foster RC, Manohar CU. The role of free-living daily walking in human weight gain and obesity. Diabetes. 2008;57:548–554. doi: 10.2337/db07-0815. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity (Silver Spring) 2012;20:2186–2193. doi: 10.1038/oby.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine JA, Nygren J, Short KR, Nair KS. Effect of hyperthyroidism on spontaneous physical activity and energy expenditure in rats. J Appl Physiol (1985) 2003;94:165–170. doi: 10.1152/japplphysiol.00499.2002. [DOI] [PubMed] [Google Scholar]
- 62.Levine JA, Eberhardt NL, Jensen MD. Leptin responses to overfeeding: relationship with body fat and nonexercise activity thermogenesis. J Clin Endocrinol Metab. 1999;84:2751–2754. doi: 10.1210/jcem.84.8.5910. [DOI] [PubMed] [Google Scholar]
- 63.Bowen RS, Knab AM, Hamilton AT, McCall JR, Moore-Harrison TL, Lightfoot JT. Effects of Supraphysiological Doses of Sex Steroids on Wheel Running Activity in Mice. J Steroids Horm Sci. 2012;3:110. doi: 10.4172/2157-7536.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol Behav. 2006;88:294–301. doi: 10.1016/j.physbeh.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 65.Garland T, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villablanca PA, Alegria JR, Mookadam F, Holmes DR, Wright RS, Levine JA. Nonexercise activity thermogenesis in obesity management. Mayo Clin Proc. 2015;90:509–519. doi: 10.1016/j.mayocp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matthews CE, Jurj AL, Shu XO, Li HL, Yang G, Li Q, Gao YT, Zheng W. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165:1343–1350. doi: 10.1093/aje/kwm088. [DOI] [PubMed] [Google Scholar]
- 69.Hagger-Johnson G, Gow AJ, Burley V, Greenwood D, Cade JE. Sitting Time, Fidgeting, and All-Cause Mortality in the UK Women’s Cohort Study. Am J Prev Med. 2016;50:154–160. doi: 10.1016/j.amepre.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 70.Hamasaki H, Yanai H, Mishima S, Mineyama T, Yamamoto-Honda R, Kakei M, Ezaki O, Noda M. Correlations of non-exercise activity thermogenesis to metabolic parameters in Japanese patients with type 2 diabetes. Diabetol Metab Syndr. 2013;5:26. doi: 10.1186/1758-5996-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamasaki H, Noda M, Moriyama S, Yoshikawa R, Katsuyama H, Sako A, Mishima S, Kakei M, Ezaki O, Yanai H. Daily Physical Activity Assessed by a Triaxial Accelerometer Is Beneficially Associated with Waist Circumference, Serum Triglycerides, and Insulin Resistance in Japanese Patients with Prediabetes or Untreated Early Type 2 Diabetes. J Diabetes Res. 2015;2015:526201. doi: 10.1155/2015/526201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamasaki H, Yanai H, Kakei M, Noda M, Ezaki O. The association between daily physical activity and plasma B-type natriuretic peptide in patients with glucose intolerance: a cross-sectional study. BMJ Open. 2015;5:e006276. doi: 10.1136/bmjopen-2014-006276. [DOI] [PMC free article] [PubMed] [Google Scholar]


