Abstract
Short bowel syndrome (SBS) patients developing hyperphagia have a better outcome. Gastrointestinal endocrine adaptations help to improve intestinal functions and food behaviour. We investigated neuroendocrine adaptations in SBS patients and rat models with jejuno-ileal (IR-JI) or jejuno-colonic (IR-JC) anastomosis with and without parenteral nutrition. Circulating levels of ghrelin, PYY, GLP-1, and GLP-2 were determined in SBS rat models and patients. Levels of mRNA for proglucagon, PYY and for hypothalamic neuropeptides were quantified by qRT-PCR in SBS rat models. Histology and immunostaining for Ki67, GLP-1 and PYY were performed in SBS rats. IR-JC rats, but not IR-JI, exhibited significantly higher crypt depths and number of Ki67-positive cells than sham. Fasting and/or postprandial plasma ghrelin and PYY concentrations were higher, or tend to be higher, in IR-JC rats and SBS-JC patients than in controls. Proglucagon and Pyy mRNA levels were significantly enhanced in IR-JC rats. Levels of mRNA coding hypothalamic orexigenic NPY and AgRP peptides were significantly higher in IR-JC than in sham rats. We demonstrate an increase of plasma ghrelin concentrations, major changes in hypothalamic neuropeptides levels and greater induction of PYY in SBS-JC rats and patients suggesting that jejuno-colonic continuity creates a peculiar environment promoting further gut-brain adaptations.
Short bowel syndrome (SBS) results from extensive resection of the small intestine. It is the leading cause of chronic intestinal failure and is treated principally by parenteral nutrition (PN). The degree of malabsorption depends on both the extent of the resection and the anatomy of the remnant bowel. Adaptive phenomena occur during the two to three years following the surgery and are characterized by improvements in intestinal absorption1,2, increase of hormonal secretion3,4, development of an hyperphagia2,5 and dysbiosis of the gut microbiota6,7,8. These changes, which are highly variable and unique to each patient, may facilitate weaning off of PN. Oral intake plays a major role in these adaptations9 as does the presence of the terminal ileum and/or colon.
Gut hormones are key factors in this spontaneous intestinal adaptation10. Increased secretions of glucagon-like peptide-1 (GLP-1) and glucagon-like peptide-2 (GLP-2) have been reported in preclinical models11,12 and in SBS patients with the colon in continuity3, but not in SBS patients with jejunostomy (i.e. with no colon and no ileum)4,13 These hormones are secreted, together with Peptide YY (PYY), by the enteroendocrine L cells of the ileum and colon, in response to nutrient stimulation14. They play key roles in orchestrating gastrointestinal functions, such as intestinal trophicity12, expression of intestinal nutrient transporters10,15,16 and gastro-intestinal motility4,17. The role of GLP-2 in these intestinal functions has been largely documented18 in SBS patients and its therapeutic use (in the recombinant form of Teduglutide) has now been approved in adults with SBS dependent on PN. At last, PYY and GLP-1 are considered to be molecular mediators of the ileal brake19. They inhibit gastric emptying and intestinal transit, thereby increasing the time for the nutrients to be absorbed by the intestinal mucosa.
The development of hyperphagia, which increases over time in SBS patients after reestablishment of colonic continuity2, makes it possible to decrease dependence on PN by increasing net nutrient absorption2. It is associated with a good prognosis for the weaning off of PN2,20. The molecular and physiological mechanisms underlying the development of this compensatory overeating in SBS patients remain unknown. In addition to their gastro-intestinal effects, gut hormones (such as CCK, PYY, GLP-1 and ghrelin) participate in the regulation of appetite and food intake14. Ghrelin, is the unique orexigenic gut hormone21,22. Plasma ghrelin levels increase before meal, stimulating hunger and meal initiation, and then decrease after the meal21. These peripheral gastrointestinal signals are integrated into the hypothalamus, which contains two populations of neurons: orexigenic neurons co-expressing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and anorexigenic neurons co-expressing pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART)14,23 Ghrelin increases food intake through the activation of hypothalamic orexigenic neurons24. Little, if anything, is known about the expression of these neuropeptides in preclinical models of intestinal resection. Only one study has reported data for the fasting concentrations of ghrelin and PYY in SBS patients with adaptive hyperphagia but their postprandial levels were not evaluated25. Finally, the measure of circulating gut hormones is recognized of great interest for the follow-up of patients with gastrointestinal pathology and/or surgery. However their baseline and postprandial concentrations are rarely evaluated in combined panels in SBS patients with or without ileum nor compared with control subjects and little is known about what the colon or the ileum specifically brings to intestinal endocrine adaptation in SBS.
To address the colonic cellular and molecular mechanisms of adaptive hormonal secretions, we set up two rat models of extensive intestinal resection with colon in continuity and with or without a resected ileum. We use these models to analyze food intake, hypothalamic neuropeptides expression and to evaluate gastro-intestinal production and secretion of gut hormones (ghrelin, PYY, GLP-1 and GLP-2) in comparison with sham-operated animals. We further analyzed the clinical relevance of hormonal adaptation in relation to food intake (fasting and post-prandial gut hormone secretion) in jejuno-colonic and jejuno-ileal SBS patients.
Results
Preclinical models
Characteristics of rat models
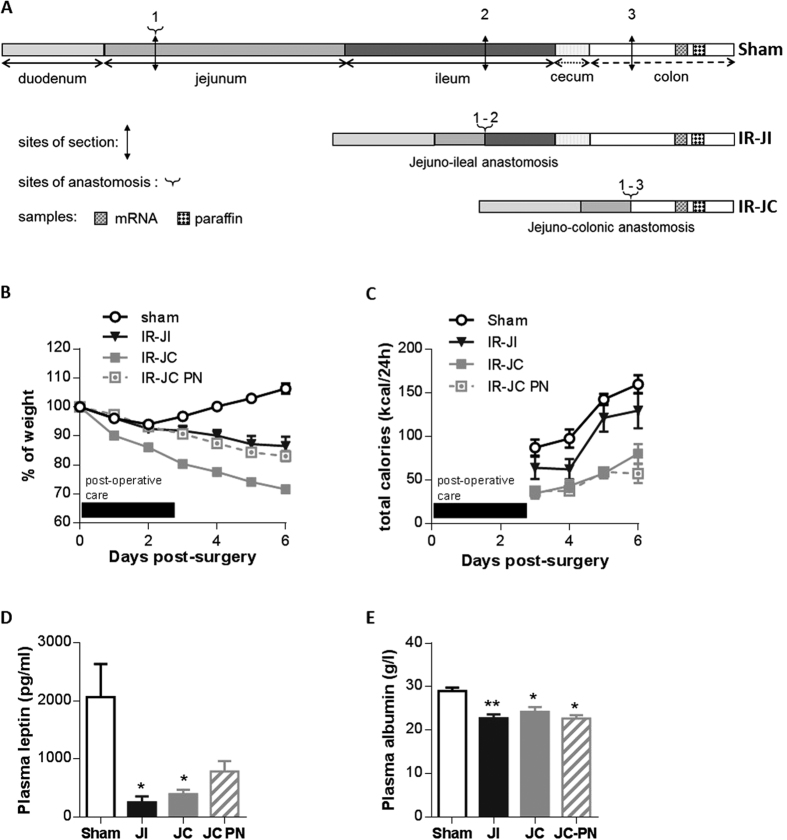
All rats were on oral nutrition with free access to food, but a group of IR-JC rats received also PN to partially compensate for their malabsorption. Seven days after surgery, sham rats had gained 6% of their pre-operative weight, whereas IR jejuno-ileal (IR-JI) and jejuno-colonic (IR-JC) rats had lost 14% and 29% of their pre-operative weight, respectively (Fig. 1B). IR jejuno-colonic rats under enteral and parenteral nutrition (IR-JC PN) had lost 17% of their pre-operative weight without increasing their food intake suggesting that PN (12 kcal/day) was beneficial for the nutritional status of the IR-JC rats. Total calorie intake (oral intake and parenteral nutrition) (Fig. 1C) increased in all groups during the experiment although it remained lower in IR-JC rats. Consistent with their weight loss, plasma leptin and albumin levels were significantly lower in IR-JC and IR-JI groups than in the sham group (P < 0.05) (Fig. 1D,E),and tended to be lower for leptin in IR-JC PN group.
Figure 1. Body weight loss, plasma leptin levels and histological adaptation of the remaining colon mucosa after intestinal resection in rats.
(A) Schematic representation of the surgeries and colonic tissue collections at day 7 post-surgery. Length of resected or remnant part of intestinal segments are not proportional. (B) Comparative time-dependent body weight loss represented as percent of the preoperative weight and (C) total calorie intake (oral intake and parenteral nutrition) in IR jejuno-ileal (IR-JI), IR jejuno-colonic (IR-JC), IR jejuno-colonic PN (IR-JC PN) and sham-operated (Sham) rats during 7 days after surgery. Black box corresponds to the period of the postoperative care. (D,E) Plasma levels of leptin (D) and albumin (E) in the same groups. Data are represented as mean ± SEM of n = 6 for sham, n = 4 to 6 for IR jejuno-ileal, n = 10 for IR jejuno-colonic, n = 5 to 6 for IR jejuno-colonic with PN. *P < 0.05, **P < 0.01, vs sham-operated rats based on non-parametric Kruskal-Wallis test followed by Dunn’s adjusted multiple comparisons.
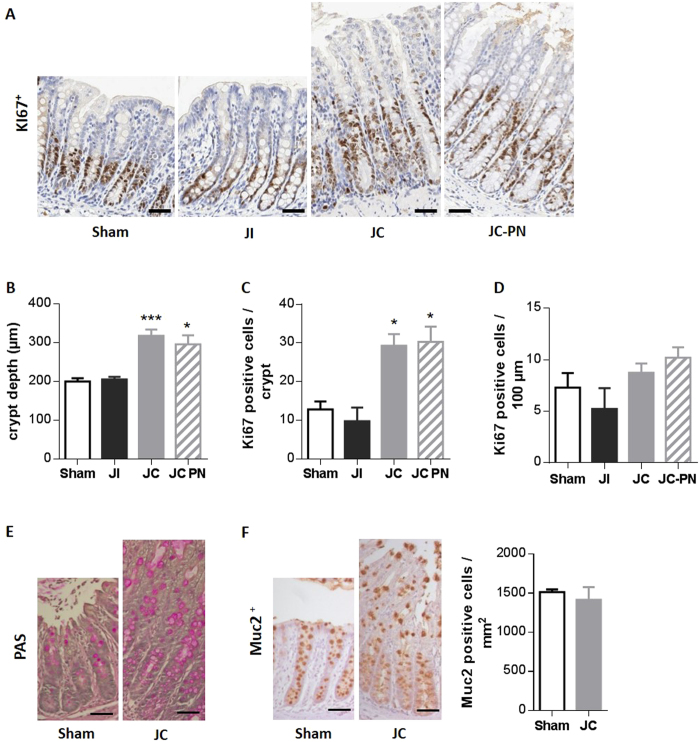
Histological examination of the colon mucosa (Fig. 2A) showed that IR-JC rats had significantly deeper colonic crypts compared to sham rats (P < 0.001 IR JC vs sham and P < 0.05 IR JC-PN vs sham) (Fig. 2B), reflecting morphological adaptation. In addition they displayed higher number of Ki67-positive cells per crypt (Fig. 2A,C) (P < 0.05) than sham rats. On the contrary IR-JI rats were not significantly different from sham rats. The proliferative index was not significantly different between groups suggesting that the epithelial homeostasis was recovered at that post-operative time (Fig. 2D)
Figure 2. histological adaptation of the remaining colon mucosa after intestinal resection in rats.
(A) Representative photomicrographs of colon mucosa of Sham, IR jejuno-ileal (JI), IR jejuno-colonic (JC) and IR jejuno-colonic with PN (JC-PN) rats immunostained with an anti-Ki67 antibody. (B) Measurement of colon mucosa crypt depth in μm (at least 5 crypts analyzed by rat). (C) Quantification of Ki67 positive cells per crypt (at least 5 crypts analyzed by rat), (D) density of Ki67 positive cells expressed as number of Ki67 positive cells per 100 μm of crypt. (E,F) Representative photomicrographs of colon mucosa of sham and IR-JC rats stained with Periodic Acid Schiff (PAS) (E) and Representative photomicrographs of colon mucosa of sham and IR-JC rats immunostained with anti-Muc2 antibody (F) with quantification of Muc2 positive cells per area in mm2. Data are represented as mean ± SEM of n = 6 for sham, n = 4 to 6 for IR jejuno-ileal, n = 10 for IR jejuno-colonic, n = 5 to 6 for for IR jejuno-colonic with PN. *P < 0.05, **P < 0.01, vs sham-operated rats based on non-parametric Kruskal-Wallis test followed by Dunn’s adjusted multiple comparisons. Scale bar: 50 μm.
PAS mucin staining and Muc2 immunostaining of the residual colon mucosa of IR-JC rat, revealed an increase of mucus production compared to sham rat (Fig. 2E,F), as previously described for other preclinical models of large intestinal resection26. This greater number of goblet cells is probably a consequence of the increase of the crypt depth observed in IR-JC rats. The density of these cells was not modified in IR-JC compared to sham (Fig. 2F).
Higher plasma concentrations of gut hormones in rat models of SBS
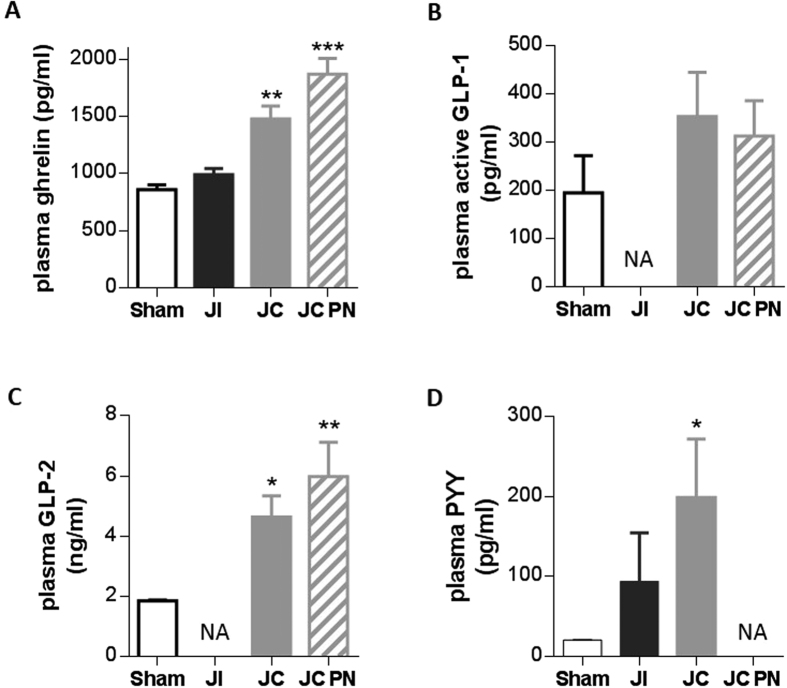
Fasting plasma ghrelin concentration was significantly higher in IR-JC rats with and without PN than in sham rats (P < 0.01) (Fig. 3A), whereas no such difference was observed in IR-JI rats. Fasting plasma GLP-1 and GLP-2 concentrations (Fig. 3B,C) were also higher in IR-JC rats with and without PN (P < 0.05 and < 0.01, respectively versus sham, for GLP-2). Fasting plasma PYY concentration, which was barely detectable in sham rats, was increased in IR-JI rats and was further enhanced in IR-JC rats (P < 0.05) (Fig. 3D). Unfortunately we were not able to determine plasma PYY in IR-JC PN rats and plasma GLP-1 and GLP-2 in IR-JI rats due to technical problems. However the higher plasma GLP-1, GLP-2 and PYY levels observed in overnight fasted rats were also found in non-fasted IR-JC rats (P < 0.01 or P < 0.05 vs sham) (Supplemental Figure S1).
Figure 3. Increased levels of plasma gut hormones after intestinal resection in rats.
Fasting plasma levels of ghrelin (A), GLP-1 (B) GLP-2 (C) and PYY (D) in IR jejuno-ileal (JI), IR jejuno-colonic (JC), IR jejuno-colonic with PN (JC-PN) and sham-operated (Sham) overnight fasted rats 7 days after surgery. Data are represented as mean ± SEM of n = 6 for sham, n = 4 or not available (NA) for IR-JI, n = 4 to 10 for IR JC, n = 4 to 6 or not available (NA) for IR-JC PN. *P < 0.05, **P < 0.01, vs sham-operated rats based on non-parametric Kruskal-Wallis test followed by Dunn’s adjusted multiple comparisons.
No change in the densities of PYY and GLP-1 positive cells but higher levels of mRNA coding for proglucagon and PYY
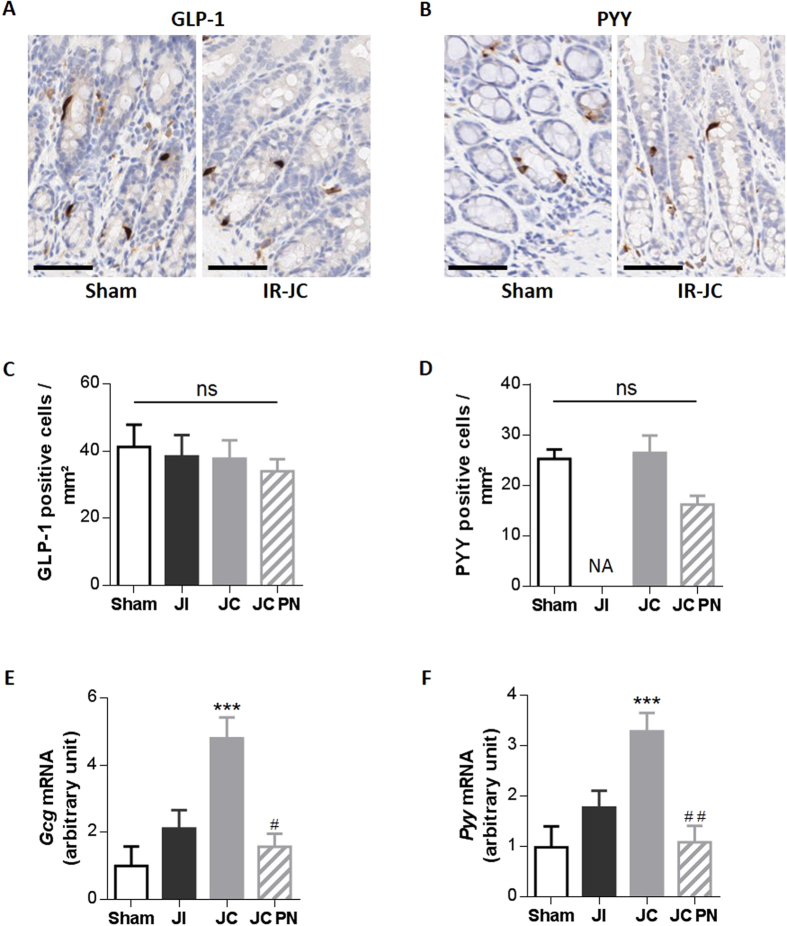
We investigated whether the elevation of these hormone levels was due to an increase in the number of enteroendocrine cells producing them and/or an increase in the hormone production by these cells. The density of GLP-1 positive cells was similar in the 3 groups of IR rats and in sham rats (Fig. 4A,C). The density of PYY-positive cells was unchanged in IR-JC rats without PN, and slightly lower in the colon mucosa of IR-JC rats with PN (Fig. 4B,D). Nevertheless, since the crypt depth is enhanced by 50% (Fig. 2A,B), this means that the total number of these cells will be increased similarly. The levels of mRNA coding for Gcg and PYY were 4 and 3-fold higher, respectively, in the colon mucosa of IR-JC rats than sham rats (P < 0.001) (Fig. 4E,F). IR-JC rats with PN had mRNA levels coding for Gcg and PYY similar to those observed in sham and IR-JI rats, but lower than those observed in IR-JC rats (P < 0.05).
Figure 4. Unchanged number of PYY and GLP-1 positive cells but enhanced levels of proglucagon and Pyy mRNA after intestinal resection in rats.
(A,B) Representative photomicrographs of GLP-1 (A) and PYY (B) immunostaining on colon mucosa sections from sham-operated and IR-JC rats (cytoplasm of GLP-1 or PYY cells are stained in brown), scale bar:50 μm. (C,D) Quantification of GLP-1 (C) and PYY (D) positive cells per area in mm2. Data are represented as mean ± SEM of n = 4 for sham, n = 4 or not available (NA) for IR jejuno-ileal (JI), n = 5 for IR jejuno-colonic (JC), n = 6 for for IR jejuno-colonic with PN (JC-PN). (E–F) Colonic mucosa mRNA levels of proglucagon (Gcg) (E) and Pyy (F) normalized to L19, 7 days after surgery. Data are represented as mean ± SEM of n = 6 for sham, n = 5 for IR-JI, n = 9 for IR-JC, n = 6 for IR-JC with PN. *P < 0.05, **P < 0.01, ***P < 0.001 vs sham-operated rats and #P < 0.05 ##P < 0.01 vs IR-JC based on non-parametric Kruskal-Wallis test followed by Dunn’s adjusted multiple comparisons.
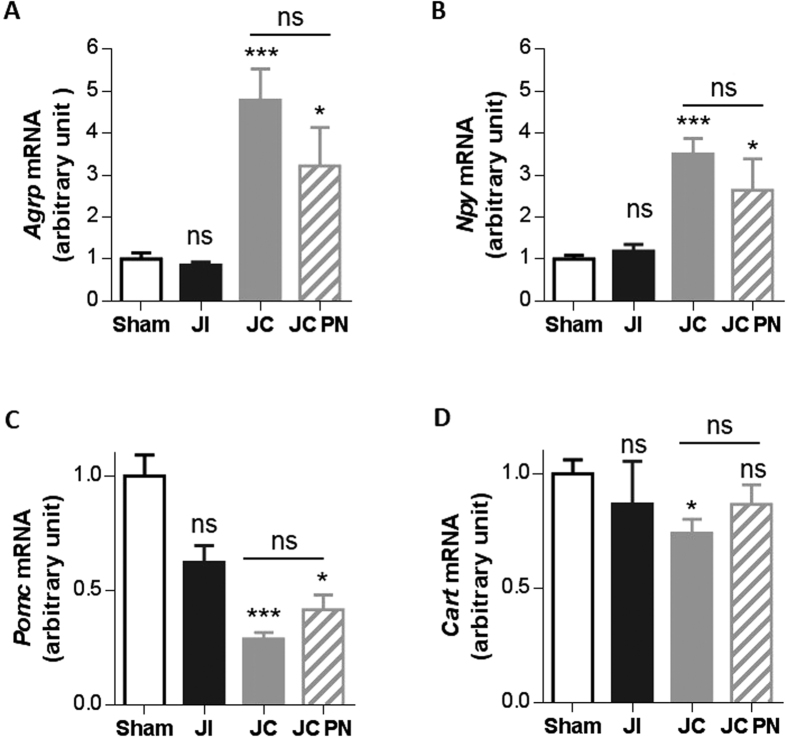
Higher levels of mRNA coding for hypothalamic orexigenic neuropeptides (AgRP, NPY) and lower levels of mRNA coding for anorexigenic neuropeptides (POMC, CART) in rat models of SBS
We investigated changes in the levels of hypothalamic neuropeptides after intestinal resection by analyzing mRNA levels for orexigenic (AgRP and NPY) and anorexigenic (CART and POMC) neuropeptides within the hypothalamus (Fig. 5).
Figure 5. Expression of mRNA coding for hypothalamic orexigenic (AgRP, NPY) and anorexigenic (POMC, CART) neuropeptides after intestinal resection in rats.
Expression of (A) Agrp, (B) Npy, (C) Pomc and (D) Cart mRNA normalized to Hprt mRNA in hypothalamus in IR jejuno-ileal (JI), IR jejuno-colonic (JC), IR jejuno-colonic with PN (JC-PN) and sham-operated (Sham) rats 7 days after surgery. Data are represented as mean ± SEM of n = 6 for sham, n = 5 for IR-JI, n = 10 for IR-JC, n = 6 for for IR-JC with PN.*P < 0.05, ***P < 0.001 vs sham-operated rats based on non-parametric Kruskal-Wallis test followed by Dunn’s adjusted multiple comparisons.
IR-JC rats had significantly higher mRNA levels of AgRP and NPY than the sham rats (P < 0.001) (Fig. 5A,B). These levels were also significantly higher in IR-JC rats with PN than in sham rats (P < 0.05). Actually, mRNA levels of hypothalamic neuropeptides were not statistically different in IR-JC rats with PN or without PN.
Conversely, IR-JC rats (with or without PN) had significantly lower mRNA levels of POMC than the animals of the sham group (P < 0.001 and P < 0.05 respectively) (Fig. 5C,D). The decrease of mRNA levels coding CART was more subtle (P < 0.05). These results show that jejuno-colonic continuity induces substantial changes in the expression of hypothalamic neuropeptides controlling food intake and PN only slightly compensates this effect.
Clinical study
Characteristics of SBS patients and control subjects
We studied SBS patients (medians for clinical characteristics are in Table 1) with jejuno-ileal (SBS-JI, N = 5) or jejuno-colonic (SBS-JC, N = 4) anastomosis. All SBS patients were considered to be in a steady state and had a remnant small bowel of less than 80 cm in length. All had normal albumin and prealbumin levels. Their concentration of plasma leptin was normal or low (Table 1). The average of total oral intake was 2271 (±288) kcal/day equivalent to 1.8 (±0.73) times their resting energy expenditure (REE). Six SBS patients were considered to have a high level of oral intake or hyperphagia2, i.e. with oral intake >1.5 time their REE (Table 1). Six of the SBS patients were dependent on PN (average of 1181 ± 787 kcal/perfusion). Patient’s gut hormone concentrations were compared with those of healthy subjects (C, N = 5) that were disease-free with normal eating behaviour.
Table 1. Clinical and nutritional characteristics of SBS patients.
| SBS patients (sex) | Age | BMI | Remnant jejunum/ ileum length (cm) | Remnant colon (%) | Delay since continuity restablishment (months) | Total oral intake (kcal/day) | PN/Week (n) | PN support (kcal/perfusion) | REE (kcal/day) | Oral intake /REE | Leptin (ng/ml) | Prealbumin (g/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 (M) | 50 | 22 | 10/40 (SBS-JI) | 100 | 12 | 3000 | 2 | 1612 | 1442 | 2,1 | 2,2 | 0,305 |
| P2 (F) | 59 | 16 | 20/0 (SBS-JC) | 75 | 132 | 2200 | 4 | 2450 | 1070 | 2,1 | 2,2 | 0,194 |
| P3 (F) | 52 | 20 | 20/50 (SBS-JI) | 100 | 24 | 2200 | 0 | 0 | 1188 | 1,9 | 1,99 | 0,163 |
| P4 (F) | 49 | 19 | 12/10 (SBS-JI) | 100 | 96 | 3600 | 3 | 1498 | 1192 | 3,0 | 3,09 | 0,221 |
| P5 (F) | 39 | 23 | 70/5 (SBS-JI) | 100 | 60 | 1500 | 0 | 0 | 1419 | 1,1 | 14,5 | 0,187 |
| P6 (F) | 32 | 19 | 0/0 (SBS-JC) | 50 | 12 | 840 | 7 | 1910 | 1348 | 0,6 | 2,71 | 0,313 |
| P7 (M) | 73 | 20 | 10/40 (SBS-JI) | 100 | 96 | 3000 | 0 | 0 | 1214 | 2,5 | 1,99 | 0,263 |
| P8 (M) | 57 | 19 | 15/0 (SBS-JC) | 50 | 120 | 1600 | 5 | 1662 | 1195 | 1,3 | 4,56 | 0,289 |
| P9 (F) | 63 | 25 | 80/0 (SBS-JC) | 50 | 12 | 2500 | 2 | 1498 | 1307 | 1,9 | 43,18 | 0,288 |
| Median [25–75] | 52 [49–59] | 20 [19–22] | 15 [10–45]/5 [0–40] | 100 [50–100] | 60 [12–96] | 2200 [1600–3000] | 2 [0–4] | 1498 [0–1662] | 1214 [1192–1348] | 1,9 [1,1] | 2,71 [2,2–4.56] | 0,276 [0,194–0,289] |
BMI: body mass index (Kg/m2). SBS-JI = SBS patients with jejuno-ileal anastomosis, SBS-JC = SBS patients with jejuno-colonic anastomosis. Remnant jejunum and ileum length and colon in continuity were expressed in cm and in % of common colon length, respectively. Delay was in months from the last surgery establishing an anastomosis between the jejunum and the remaining colon. Total oral was expressed in Kcal/day. Parenteral nutrition (PN) support was expressed in number (n) of perfusion per week (PN/week) and kcal/perfusion. Resting energy expenditure (REE) was expressed in kcal/day. Hyperphagia is defined as oral intake/REE > 1.5. Plasma levels of leptin are in ng/mL and prealbumin are in g/L. Values are median and 25th and 75th percentile. Etiology of SBS were arterial mesenteric infraction (n = 5), volvulus (n = 1), intestinal occlusion (n = 1) and surgical complications (n = 3) with no residual lesions of the remnant intestine.
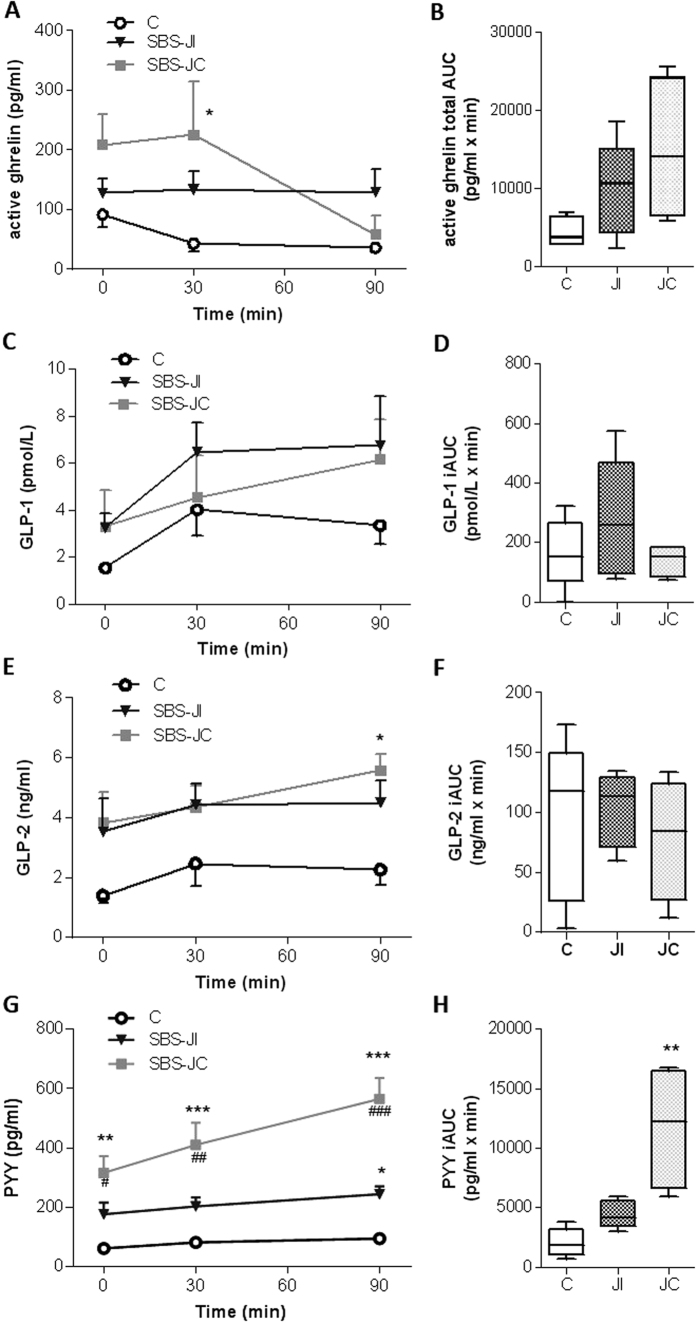
Higher plasma concentrations of active ghrelin, GLP-1, GLP2 and PYY in SBS patients
As expected, active ghrelin levels decreased 30 minutes after eating the calibrated meal in control subjects, remaining low for at least 90 minutes (Supplemental Figure S2A). Fasting active ghrelin levels tended to be higher in SBS patients than in healthy controls (Supplemental Figure S2A),were significantly higher at 30 minutes in SBS patients (P < 0.05 vs controls) and decreased only at 90 minutes. The calculated total area under the curve (AUC), reflecting the absolute levels of ghrelin and the post-prandial response, was significantly greater (by a factor of two, P < 0.05 vs controls) in SBS patients (Supplemental Figure S2B). Distinguishing jejuno-colonic from jejuno-ileal SBS patients revealed that fasting active ghrelin elevation tended to be higher for jejuno-colonic SBS patients than for jejuno-ileal SBS patients and controls (Fig. 6A). Jejuno-colonic SBS patients exhibited a delayed meal-induced inhibition of plasma ghrelin concentration whereas no meal-induced inhibition of ghrelin concentration was observed in jejuno-ileal SBS patients (Fig. 6A).
Figure 6. Increased fasting and post-prandial levels of plasma active Ghrelin, GLP-1, GLP2 and PYY in SBS patients.
Plasma concentrations and secretory response to a calibrated meal (750 kcal) in SBS jejuno-ileal (JI) and SBS jejuno-colonic (JC) patients compared to healthy subjects (C) of: (A,B) active ghrelin (pg/mL) before (T0) and after (T30, T90) the meal (A) and ghrelin secretory response expressed as the total area under the curve (AUC) (B) GLP-1 (pg/mL) before (T0) and after (T30, T90) the meal (C) and GLP-1 secretory response expressed as the incremental area under the curve (iAUC) (D); (E,F) GLP-2 (pg/mL) before (T0) and after (T30, T90) the meal (E) and secretory response expressed as the iAUC (F PYY (pg/mL) before (T0) and after (T30, T90) the meal (G), and secretory response expressed as the iAUC (H). All values are expressed as the mean ± S.E.M of n = 5 for healthy subjects, n = 5 for jejuno-ileal SBS patients and n = 4 for jejuno-colonic SBS patients. *P < 0.05, **P < 0.01, ***P < 0.001 vs healthy subjects and #P < 0.05, ##P < 0.01 and ###P < 0.001 vs jejuno-ileal SBS patients based for A, C, E, G, on Bonferroni’s multiple comparisons test and for B, D, F, H on non-parametric Kruskal-Wallis test followed by Dunn’s adjusted multiple comparisons.
Fasting plasma GLP-1 (3.286 ± 0.706 for SBS patients vs 1.553 ± 0.063 for controls, P < 0.05 in Mann-Whitney test) and GLP-2 (3.664 ± 0.716 for SBS patients vs 1.394 ± 0.252 for controls P < 0.05 in Mann-Whitney test) concentrations were both higher in SBS patients than in controls subjects (see also Supplemental Figure S2C,E). These increases were similar in jejuno-colonic and jejuno-ileal SBS patients (Fig. 6C,E). After the calibrated meal, the average plasma levels of these two hormones remained higher in SBS patients than in control subjects (Fig. 6C,E and supplemental Figure S2C,E), but the calculated incremental AUC (iAUC), reflecting the postprandial secretion response, did not differ significantly between patients and controls (Fig. 6D,F and Supplemental Figure S2D,F).
Fasting plasma PYY concentration was three times higher in SBS patients than in controls (P < 0.01 in Mann-Whitney tests) (Supplemental Figure S2G). This concentration increased significantly after a calibrated meal remaining higher in SBS patients than in controls. Accordingly, the calculated iAUC was significantly larger than that for controls (P < 0.01) (Supplemental Figure S2H). The difference in PYY levels (Fig. 6G) and PYY iAUC (P < 0.01) (Fig. 6H) with respect to controls was significantly greater in jejuno-colonic SBS patients than in those with jejuno-ileal anastomosis. Consistent with these observations, PYY levels were inversely correlated with the length of the remaining small bowel in SBS patients (Spearman coefficient: −0.76; P < 0.05). However, no correlations were found between circulating PYY, GLP-1 or ghrelin levels and daily oral intake or hyperphagic status in SBS patients.
Discussion
This study is the first to demonstrate an increase of plasma ghrelin levels, major changes in the mRNA levels of hypothalamic orexigenic AgRP and NPY neuropeptides, and the elevation of PYY, together with GLP-2 and GLP-1, in rats with large intestinal resection including ileum, that are preclinical models of SBS. We also found that SBS patients had higher fasting and postprandial circulating ghrelin and PYY levels than controls, in particular patients with jejuno-colonic anastomosis, further emphazing the relevance of our preclinical model.
Since morphological and hormonal secretion adaptations have been reported to be maximal in different rat models of resection between day 4 and day 12 after intestinal resection11,27,28, the experimental period for the rat model was set up at 7 days. The colonic morphological adaptation observed in our IR-JC model are in agreement with previous reports for patient with jejuno-colonic anastomosis, i.e increase of crypt depth and of proliferating cell number with a controlled hyperplasia29 and increased GLP-2 secretions3.
Ghrelin is a well-known physiological hunger signal30,31 playing a major role in meal initiation21. Ghrelin-induced hunger involves the activation of central orexigenic pathways32 and stimulation of the expression of AgRP and NPY within the hypothalamus24,33,34. Higher levels of fasting ghrelin and of mRNA coding for orexigenic neuropeptides (AgRP and NPY) suggests a role for ghrelin in the activation of central orexigenic signals in IR jejuno-colonic rats. The lower levels of circulating leptin (an anorexigenic hormone) may also have contributed to the higher expression of the orexigenic neuropeptide23 in these IR rats. Of note, in a previous report36, and in our study, leptin concentrations in SBS patients was low but not different from controls. Furthermore, Molina et al. demonstrated that leptin concentrations did not correlate with hyperphagia in short bowel-patients36.
The central and peripheral variations of mRNA or peptide levels may also reflect the nutritional status of the rats. Ghrelin plays a role in long-term energy balance regulation, protecting against prolonged energy deficiency32. Indeed, prolonged starvation increases plasma ghrelin concentration in mice37 and may also influence NPY and AgRP mRNA levels38. We observed no hyperphagia in the IR rats during these 7 days of adaptation and all IR rats lost weight. Furthermore, the IR jejuno-colonic rats without PN, which are the leanest rats, had the highest levels of orexigenic signals suggesting that this may be an undernutrition response to promote food intake. IR jejuno-colonic rats with PN were heavier than jejuno-colonic rats without PN. Parenteral nutrition may ensure better hydration and improve energy balance by partially compensating for malabsorption, despite providing a moderate amount of calories. But, IR-JC rats with PN also exhibited significant increase in their orexigenic signals compared to IR jejuno-ileal rats even if these two groups had similar body weight. Overall, these observations suggest that the specific environment created by connecting the jejunum to the colon (including modifications of the lumen contents of nutrients, biliopancreatic secretions and microbiota metabolites) may trigger a specific activation of peripheral and central orexigenic cues beyond the known effect of undernutrition/starvation.
Evidence for the clinical relevance of these findings was provided by the study in human SBS patients. Indeed, these patients, who were not undernourished, had similar levels of leptin than healthy subjects and had high plasma concentrations of active ghrelin, with fasting and postprandial active ghrelin concentrations higher in jejuno-colonic SBS patients than in control individuals. In SBS patients, the postprandial inhibition of ghrelin levels was delayed, suggesting that this hunger signal persisted after the initiation of the meal in these patients. It remains unclear whether these persistently high ghrelin levels could induce hyperphagia in the long-term. Despite these differences, no relationships were found between ghrelin concentrations and the amount of ingested calories or the presence of hyperphagia, possibly because of the small number of SBS patients in this study. Further evaluation in a larger cohort of SBS patients would be required to unveil such relations.
The average concentrations of GLP-2, GLP-1 and PYY were systematically increased in IR rats, consistent with data reported for SBS patients (this study and3). Signals of hunger and satiety would be expected to operate in opposite ways. However, this does not appear to be the case here, given the concomitantly high concentrations of anorexigenic14 PYY and GLP-1 and orexigenic ghrelin found in IR rats and SBS patients. As suggested by others for GLP-139,40, the high circulating concentrations of PYY, GLP-1 and GLP-2 may affect principally gastrointestinal tract functions rather than the central nervous system. In particular, these hormones decrease intestinal motility and transit time18, increasing the time for which nutrients are in contact with the remaining epithelium and thereby optimizing their distribution and absorption along the gastrointestinal tract. GLP-2 and PYY can also improve water and electrolyte absorption within the colon41,42. More specifically GLP-2 is also well known for its intestinotrophic effect12,18, and its increase could support, at least partially, the elevation of the colonic surface area we observed. Thus, this enhanced secretion of gut hormone reflects adaptive intestinal response, to improve overall digestion, absorption of water and nutrients and the epithelial surface available for this absorption.
The increase of fasting and postprandial secretion of PYY in SBS patients (this study and ref. 4) was larger in jejuno-colonic than in jejuno-ileal SBS patients and rats compared to control. No such differences were observed for GLP-1 and GLP-2 levels. Enteroendocrine cells are distributed from the duodenum to the colon but cells producing PYY are mostly restricted to the ileum and colon, and recently, it has been shown in rat that the highest concentrations of PYY along the gastrointestinal tract are found in the distal ileum and proximal and distal colon43. The higher PYY levels could result from an increase in the number of cells producing PYY due to morphological adaptation of the residual colon mucosa. The densities of PYY (and GLP-1) producing cells in colon were not affected in IR rats, suggesting that the homeostasis of epithelial cell differentiation was maintained in the colon of resected rats. But, the remaining colon mucosa in jejuno-colonic IR rats was hyperplasic with an enlarged number of proliferating cells and such changes were not observed in the colon mucosa when part of the ileum was preserved. A direct consequence of this colonic overgrowth would be a local increase in the number of enteroendocrine cells that in turn may contribute to the higher plasma concentrations of gut hormones. Alternatively, the colonic hormones-producing cells, and especially the PYY producing cells, may also have become highly responsive to the new environment created by the direct connection of the jejunum to the residual colon. The lumen of the residual colon typically contains large amounts of undigested nutrients5 and a dysbiotic microbiota6,8,44, and is thought to display changes in the fermentative activity generating short-chain fatty acids (SCFAs). SCFAs can modulate the function of enteroendocrine cells secreting GLP-1/GLP-2/PYY in vitro45,46 and possibly in vivo46,47. Bile acids could also participate to the increased colonic production of GLPs and PYY48,49, as well as to the increase number of proliferating cells50, observed in jejuno-colonic individuals. Whether the bile acids are present in different amount and composition in the colonic lumen content of the jejuno-ileal, jejuno-colonic and sham operated rat remain to be evaluated.
In conclusion, we demonstrate that SBS is associated with an increase in fasting plasma ghrelin concentration and an induction of orexigenic signals in the hypothalamus, in a rat model with jejuno-colonic anastomosis and that post-prandial ghrelin inhibition is impaired in SBS patients. The induction of peripheral gut hormones, such as PYY, is stronger in the absence of the ileum. Intestinal resection with jejuno-colonic anastomosis creates a specific environment promoting increase hunger signals and improving gastrointestinal functions associated with nutrient absorption. Further studies are required to determine whether these changes are correlated with the development of hyperphagia, a good prognosis for weaning off of PN.
Materials and Methods
This study followed accepted ethical, scientific and medical standards and was conducted in accordance with recognized international standards, including the principles of the Declaration of Helsinki.
Animal studies
All experimental procedures were performed in accordance with the European Community guidelines and were approved by and the Paris Nord local ethics committee and the Ministry of Education and Research (N°02285.01).
6-week-old male Wistar rats (n = 28) weighing 250–300 g (Elevage Janvier, Le Genest-St-Isle, France) were used. The animals were housed in cage with free access to standard rat chow diet (C1314 Altromin, Genestil, Royaucourt, France) and water, and maintained under standardized temperature (23 °C ± 1 °C) and 12 h light-dark cycles (lights on at 7:00 a.m.). Animals were allowed to acclimatize to their environment for 7 days before experimentation.
Surgical and experimental procedure
On the day of surgery, rats were randomly assigned to undergo resection of 70% of the small bowel with jejuno-ileal anastomosis (IR-JI), 80% of small bowel including the ileum, ileocecal valve and 20% of colon with jejuno-colonic anastomosis (for IR-JC), or a jejuno-jejunal transection with re-anastomosis (Sham) (Fig. 1A). Twelve hours before surgery, animals were fasted but allowed ad libitum access to water. They were anesthetized by intraperitoneal injection of pentobarbital sodium (Ceva, Libourne, France). Standard aseptic procedures were used throughout the surgery. Intestinal length was measured in a standardized method. The bowel was resected 15 cm distal to the ligament of Treitz to either 3 cm distal to the cecum for IR-JC rat groups or to 10 cm proximal to the cecum for IR JI rat groups and followed respectively by jejuno-colonic or jejuno-ileal anastomosis with 6–0 Prolene running sutures. Sham-operated rats underwent a jejuno-jejunal transection at 15 cm below the ligament of Treitz and re-anastomosed. Animals received subcutaneously Xylocaïne 1% (100 μl/100 g) (Astra, France) and Penicillin G (20,000 units/kg) (Panpharma, Luitre, France) to prevent postoperative pain and infections. Subcutaneous injection of 10 ml Bionolyte G5 (Sodium chloride [0.4%], Glucose [5.5%], Potassium Chloride [0.2%]) (Baxter, Maurepas, France) was given to prevent postoperative dehydration. Liquid diet (Nutrison, Nutricia, France) was available ad libitum 48 h after surgery. The IR jejuno-colonic rats were under oral nutrition without or with PN through a catheter implanted in the jugular vein. PN diet, prepared aseptically, was perfused 48 h after surgery via a syringe pump (Precidor-Infors AG Basel) at 1.5 ml/h during 8 h daily, providing 12 kcal/day. Free access to food and PN administration were restored 48 hours after surgery and total calorie intake was calculated daily.
Previous studies have reported maximal intestinal morphological changes between day 4 and 12 after the surgery11,27.Thus, we set 7 days as the experimental period for this study. Body weight was monitored daily and food intake was determined from day 2 to 7 post-surgery. At day 7, overnight fasted rats were anesthetized; blood was collected in chilled tubes containing heparin or heparin and dipeptidyl peptidase IV (DPP-IV) inhibitor (Roche Applied Science). Plasma was aliquoted and stored in −80 °C until analyses. Then the rats were sacrificed, hypothalamus and intestinal mucosa scrappings were collected and rapidly frozen until extraction of total ARN for RTq-PCR analyses. Other intestinal segments were formalin-fixed overnight and embedded in paraffin for classical histology and immunohistochemistry studies (see Supplementary Material).
Quantification of gene expression in colon mucosa and hypothalamus
Total RNA was extracted from unfrozen colon mucosa scrappings and from hypothalamus tissue with Trizol reagent (Invitrogen, Saint Aubin, France). After reverse transcription with 8 μg total RNA, RTq-PCR real-time qPCR was performed in duplicate for each cDNA, using specific primers and the ABI PRISM 7000 sequence detection system and TaqMan universal PCR technology as described in details in Supplementary Material and compared to mean target gene expression of sham operated rats as calibrator samples. Fold induction was calculated using the comparative 2−Δct Method.
Clinical study
Human subjects
Nine adult patients with SBS were recruited from our tertiary care center for intestinal failure (Beaujon Hospital). They gave informed consent for participation to the study. The protocol was approved (No. 15–046) by the Institutional Review Board-IRB-00006477- of HUPNVS, Paris 7 University, APHP.
The patients were in a steady state, with their last surgery realized more than 6 months before the blood test. They all were on a non-restricted oral diet and 6 of them had PN dependence. Criteria for inclusion were extensive small bowel resection, with a remnant small bowel length <150 cm, with some colon in continuity (jeuno-colonic anastomosis) or with ileum in continuity (jeuno-ileal anastomosis). Criteria for exclusion were upper gastrointestinal tract surgery, small bowel lesions in the remnant gut, organ failure other than digestive, evolutive neoplasia, intestinal fistulae and treatment by growth hormone, GLP-2, steroids or immunosuppressive therapy in the 4 months preceding the study. The patients had no signs of sepsis and no antibiotherapy.
Five adult healthy subjects with normal BMI (between 18.5–24.9 kg/m2) who gave their informed consent for the study were also recruited (ANSM authorization B131095–81). Procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 1983.
Study design
The blood samples were collected in SBS and control subjects during their routine monitoring after an overnight fast (SBS on PN receiving saline solution to avoid dehydration), before (T0), 30 and 90 min after consumption of a standardized breakfast of 725 kcal (100 g of bread, 20 g of butter, 20 g of jam, 30 g of French emmental, 20 g of powder skimmed milk, 10 g of sugar, 250 ml of tea or coffee and 500 ml of Vichy St-Yorre water). Blood samples were drawn in EDTA tube Vacutainer (Becton Dickinson, France) containing DPP-IV inhibitor (DPP-IV-010, Millipore) and serine protease inhibitor, 4-(Aminoethyl)-benzenesulfolnyl fluoride hydrochloride or Pefabloc (Roche Applied Science). Samples were centrifuged between 863 to 2000 g for 10 min. Aliquoted plasma was stored in −80 °C until analyses.
Plasma hormone analyses
Human and rat plasma concentrations of PYY, GLP-1, GLP-2 and ghrelin were quantified as described in details (Supplementary Material). The hormone assessment results were expressed as absolute value and incremental area under the curve (iAUC) calculated by subtracting baseline levels from all subsequent readings or total area under the curve (tAUC) for ghrelin.
Statistical analyses
All values are expressed as the mean ± S.E.M. Non-parametric tests were used: Mann-Whitney test to compare two groups and Kruskal-Wallis test followed by Dunn’s adjusted multiple comparisons to compare more than two groups were performed using GraphPad Prism version 6.0 for Windows (GraphPad Software, San Diego, CA, USA). A value of P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Gillard, L. et al. Enhanced Ghrelin Levels and Hypothalamic Orexigenic AgRP and NPY Neuropeptide Expression in Models of Jejuno-Colonic Short Bowel Syndrome. Sci. Rep. 6, 28345; doi: 10.1038/srep28345 (2016).
Supplementary Material
Acknowledgments
The authors thank Nathalie Ialy-Rado from Core Animal Facilities and Isabelle Pingenot from the Department of Gastroenterology and Nutritional Support at Beaujon Hospital for their technical supports. They thank also Robert Ducroc and Henri Duboc for helpful criticisms and suggestions. This research was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), Assistance Publique des Hôpitaux de Paris (APHP), a BIOCODEX grant to FJ and a LACTALIS grant through the Société Francophone de Nutrition Clinique et Métabolisme to LG. LG and JBC received Ph.D. fellowships from the French Ministry of Education and Research; LB received grants from Fonds d’Etudes et de Recherche du Corps Médical (FERCM) and from DHU UNITY; LRP received a grant from Fondation pour la Recherche Médicale. Thanks to Société Française de Nutrition for travel award to LG for DDW.
Footnotes
Author Contributions L.G., F.J., J.L.B. and A.B. initiated and designed the study, analyzed and interpreted the data and wrote the manuscript; F.J. recruited the humans subjects for the clinical study; L.G., L.B., A.C.J., B.S.I., F.C., J.B.C., C.M., M.L.G., A.B., J.L.B. and I.P. contributed to the acquisition of data; B.S.I. and L.R.P. performed the surgeries for the preclinical model; M.L.G., J.B.C., J.N.F., M.T. and J.M.L. have critically read the manuscript. All authors approved the final manuscript.
References
- Nordgaard I., Hansen B. S. & Mortensen P. B. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am. J. Clin. Nutr. 64, 222–231 (1996). [DOI] [PubMed] [Google Scholar]
- Crenn P. et al. Net digestive absorption and adaptive hyperphagia in adult short bowel patients. Gut 53, 1279–1286 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. B. et al. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut 47, 370–376 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale J. M. et al. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the ‘colonic brake’ to gastric emptying. Gut 39, 267–272 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing B. et al. Intestinal absorption of free oral hyperalimentation in the very short bowel syndrome. Gastroenterology 100, 1502–1508 (1991). [DOI] [PubMed] [Google Scholar]
- Joly F. et al. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie 92, 753–761 (2010). [DOI] [PubMed] [Google Scholar]
- Mayeur C. et al. Faecal D/L lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PloS One 8, e54335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeur C. et al. Extensive Intestinal Resection Triggers Behavioral Adaptation, Intestinal Remodeling and Microbiota Transition in Short Bowel Syndrome. Microorganisms 4, 16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman I., Cheetham B. C., Rosenthal J. & Levine G. M. Effect of fiber and its fermentation on colonic adaptation after cecal resection in the rat. JPEN J. Parenter. Enteral Nutr. 19, 100–106 (1995). [DOI] [PubMed] [Google Scholar]
- Jeppesen P. B. et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120, 806–815 (2001). [DOI] [PubMed] [Google Scholar]
- Koopmann M. C. et al. Colonic GLP-2 is not sufficient to promote jejunal adaptation in a PN-dependent rat model of human short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 33, 629- 638–639 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.-R., Beck P.-L. & Sigalet D.-L. Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation. World J. Gastroenterol. WJG 12, 4117–4129 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. B. et al. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut 45, 559–563 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino Crespo C., Perianes Cachero A., Puebla Jiménez L., Barrios V. & Arilla Ferreiro E. Peptides and food intake. Front. Endocrinol. 5, 58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J. et al. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 137, 997–1005, 1005–4 (2009). [DOI] [PubMed] [Google Scholar]
- Cheeseman C. I. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am. J. Physiol. 273, R1965–1971 (1997). [DOI] [PubMed] [Google Scholar]
- Baggio L. L. & Drucker D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007). [DOI] [PubMed] [Google Scholar]
- Drucker D. J. & Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu. Rev. Physiol. 76, 561–583 (2014). [DOI] [PubMed] [Google Scholar]
- Maljaars P. W. J., Peters H. P. F., Mela D. J. & Masclee, A. a. M. Ileal brake: a sensible food target for appetite control. A review. Physiol. Behav. 95, 271–281 (2008). [DOI] [PubMed] [Google Scholar]
- Messing B. et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 117, 1043–1050 (1999). [DOI] [PubMed] [Google Scholar]
- Cummings D. E. et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 (2001). [DOI] [PubMed] [Google Scholar]
- Cummings D. E., Frayo R. S., Marmonier C., Aubert R. & Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am. J. Physiol. Endocrinol. Metab. 287, E297–304 (2004). [DOI] [PubMed] [Google Scholar]
- van der Klaauw A. A. & Farooqi I. S. The hunger genes: pathways to obesity. Cell 161, 119–132 (2015). [DOI] [PubMed] [Google Scholar]
- Chen H. Y. et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145, 2607–2612 (2004). [DOI] [PubMed] [Google Scholar]
- Compher C. W., Kinosian B. P. & Metz D. C. Ghrelin does not predict adaptive hyperphagia in patients with short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 33, 428–432 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey K. L. et al. Morphological and functional changes in the colon after massive small bowel resection. J. Pediatr. Surg. 45, 1581–1590 (2010). [DOI] [PubMed] [Google Scholar]
- Ljungmann K. et al. Time-dependent intestinal adaptation and GLP-2 alterations after small bowel resection in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G779–785 (2001). [DOI] [PubMed] [Google Scholar]
- Martin G. R. et al. Nutrient-stimulated GLP-2 release and crypt cell proliferation in experimental short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G431–438 (2005). [DOI] [PubMed] [Google Scholar]
- Joly F. et al. Morphological adaptation with preserved proliferation/transporter content in the colon of patients with short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G116–123 (2009). [DOI] [PubMed] [Google Scholar]
- Neary N. M., Druce M. R., Small C. J. & Bloom S. R. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut 55, 135 (2006). [PMC free article] [PubMed] [Google Scholar]
- Mason B. L., Wang Q. & Zigman J. M. The central nervous system sites mediating the orexigenic actions of ghrelin. Annu. Rev. Physiol. 76, 519–533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst P. J., Janssen S., Tack J. & Depoortere I. Role of the AMP-activated protein kinase (AMPK) signaling pathway in the orexigenic effects of endogenous ghrelin. Regul. Pept. 173, 27–35 (2012). [DOI] [PubMed] [Google Scholar]
- Cowley M. A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 (2003). [DOI] [PubMed] [Google Scholar]
- van der Klaauw A. A. & Farooqi I. S. The hunger genes: pathways to obesity. Cell 161, 119–132 (2015). [DOI] [PubMed] [Google Scholar]
- Molina A. et al. Serum leptin concentrations in patients with short-bowel syndrome. Clin. Nutr. Edinb. Scotl. 19, 333–338 (2000). [DOI] [PubMed] [Google Scholar]
- Goldstein J. L. et al. Surviving starvation: essential role of the ghrelin-growth hormone axis. Cold Spring Harb. Symp. Quant. Biol. 76, 121–127 (2011). [DOI] [PubMed] [Google Scholar]
- Fetissov S. O. et al. Alterations of arcuate nucleus neuropeptidergic development in contactin-deficient mice: comparison with anorexia and food-deprived mice. Eur. J. Neurosci. 22, 3217–3228 (2005). [DOI] [PubMed] [Google Scholar]
- Wichmann A. et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14, 582–590 (2013). [DOI] [PubMed] [Google Scholar]
- Kunkel D. et al. Efficacy of the glucagon-like peptide-1 agonist exenatide in the treatment of short bowel syndrome. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 23, 739–e328 (2011). [DOI] [PubMed] [Google Scholar]
- Jeppesen P. B. et al. Short bowel patients treated for two years with glucagon-like Peptide 2: effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol. Res. Pract. 2009, 616054 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno M. et al. Peptide YY enhances NaCl and water absorption in the rat colon in vivo. Experientia 48, 47–50 (1992). [DOI] [PubMed] [Google Scholar]
- Wewer Albrechtsen N. J., Kuhre R. E., Toräng S. & Holst J. J. The intestinal distribution pattern of appetite- and glucose regulatory peptides in mice, rats and pigs. BMC Res. Notes 9, 60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardy H. et al. Changes induced in colonocytes by extensive intestinal resection in rats. Dig. Dis. Sci. 51, 326–332 (2006). [DOI] [PubMed] [Google Scholar]
- Tolhurst G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 295, E1160–1166 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzenne N. M., Cani P. D., Daubioul C. & Neyrinck A. M. Impact of inulin and oligofructose on gastrointestinal peptides. Br. J. Nutr. 93 Suppl 1, S157–161 (2005). [DOI] [PubMed] [Google Scholar]
- Wu T. et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes. Metab. 15, 474–477 (2013). [DOI] [PubMed] [Google Scholar]
- Onaga T., Zabielski R. & Kato S. Multiple regulation of peptide YY secretion in the digestive tract. Peptides 23, 279–290 (2002). [DOI] [PubMed] [Google Scholar]
- Dossa A. Y. et al. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G81–92 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.