Abstract
Background:
Thyroid hormones play an important role in lipid metabolism. Overt hypothyroidism is known to be associated with increased lipid profiles, but the effect of subclinical hypothyroidism (SCH) on lipid profile remains controversial.
Objectives:
The aim of this study was to assess the association between thyroid disorders and serum lipid levels.
Materials and Methods:
The present study was conducted within the framework of Tehran lipid and glucose study (TLGS). Serum concentrations of TSH and FT4, cholesterol, triglycerides and HDL-C were measured in 5786 randomly selected subjects. Serum LDL was calculated according to the Friedwald formula.
Results:
The study assessed 5154 subjects including 42.5% males and 57.5% females, with a mean age of 39.71 ± 14.2 years (ranged 20 - 90 years). Serum cholesterol was significantly higher in overt hypothyroidism in comparison to subclinical hypothyroidism (P = 0.003). Serum cholesterol, HDL –C, LDL-C and TG did not differ between subclinical hypothyroid and control groups. Among euthyroid men, serum FT4 levels were inversely correlated with serum cholesterol and TG. In euthyroid women, serum FT4 levels were correlated positively with serum HDL-C and negatively correlated with TG and TG/HDL-C ratio and TSH levels were associated negatively with, HDL-C.
Conclusions:
No differences existed in lipid profiles between subclinical hypothyroidism and euthyroid subjects. There are correlations between serum FT4 and TSH and lipid profiles.
Keywords: Hypothyroidism, Lipid Profile, Tehran Thyroid Study (TTS), Thyroid
1. Background
Thyroid hormones play an important role in synthesis, mobilization and metabolism of lipids (1). Therefore, hypothyroidism is a major cause of secondary dyslipidemia. Investigations report elevated levels of TC and LDL-C in patients with overt hypothyroidism. These patients may also present elevated to normal levels of TG and HDL-C (2, 3). In subclinical hypothyroidism (SCH), there is an elevation in thyroid stimulating hormone (TSH) with normal levels of thyroxine (T4) and triiodothyronine (T3) (4). This condition which is more common in women and older populations may progress to overt hypothyroidism (5, 6). There is growing evidence that SCH is a risk factor for cardiovascular diseases, particularly in elderly women (4, 7, 8).
Hyperlipidemia is a well-known cardiovascular risk factor, despite the absence of any hepatic adverse effect using statins in these patients (9), it is important to discriminate the roll of subclinical thyroid dysfunction as a secondary cause of hyperlipidemia. Studies of lipid profiles in patients with SCH reported conflicting results. Most studies suggest that patients with SCH had higher levels of TC, LDL-C and possibly TG compared with euthyroid controls (10-12); whereas, levels of HDL-C were similar in the two groups (2-4, 13). Some other studies reported no significant differences between lipid profiles in patients with SCH and euthyroid controls when adjusting for potential confounders (1, 6). To our knowledge, very few community based studies were performed to investigate the association between thyroid disorders and lipid levels.
2. Objectives
The aim of our study was to determine the association between thyroid disorders and lipid profiles in a Tehranian population.
3. Materials and Methods
3.1. Study Population
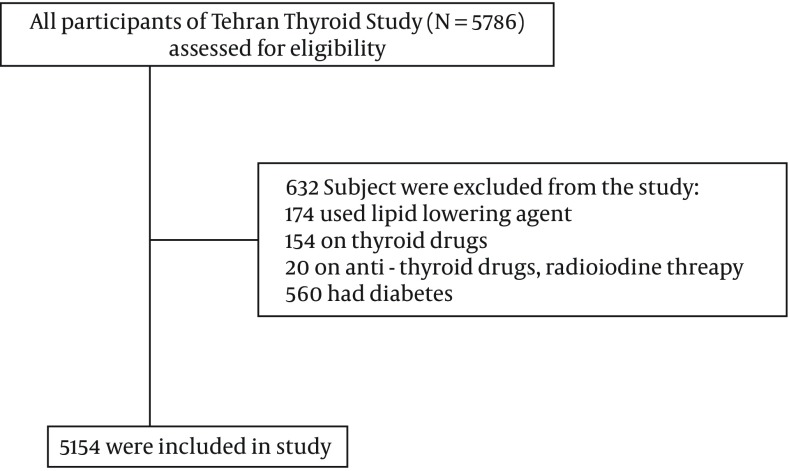
The present study was conducted within the framework of Tehran Lipid and Glucose Study (TLGS), a prospective population-based cohort study performed on over 15000 residents of district-13 of Tehran to determine the prevalence and incidence of risk factors for non-communicable disease. From baseline study of TLGS in 1999 - 2000, 5786 individuals aged 39.7 ±14.2 years were randomly selected. We excluded subjects with diabetes (defined as using oral hypoglycemic agents or insulin, baseline FPG ≥ 126 mg/dL or 2-h post-challenge plasma glucose (2h-PCPG) ≥ 200 mg/dL (n = 560), present thyroxine users (n = 154), antithyroid drugs users (n = 20) or lipid lowering medications (n = 174) , finally 5154 individuals were studied (Figure 1). The study was approved by the human research committee of Shahid Beheshti university of medical sciences.
Figure 1. Sampling Framework of Study.
Anthropometric measurements were taken with shoes removed and wearing light clothing. Weight and height were measured according to the standard protocol. Waist circumference was measured at the level of umbilicus and hip circumference was measured at the widest girth of the hip. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. WHR was calculated as WC divided by hip circumference.
3.2. Laboratory Analysis
Fasting blood samples was drawn from all participants between 7:00 and 9: 00 AM into vacutainer tubes at baseline and at each reassessment. Serum total cholesterol (TC) and triglycerides (TGs) were measured using enzymatic calorimetric tests with cholesterol esterase and cholesterol oxidase and glycerol phosphate oxidase, respectively. High density lipoprotein-cholesterol (HDL-C) was measured after precipitation of apolipoprotein B containing lipoproteins with phosphotungstic acid. Low density lipoprotein-cholesterol (LDL-C) was calculated from serum TC, TGs and HDL-C concentrations expressed in mg/dL using the Friedwald formula (14), if TGs concentration was lower than 400 mg/dL. Assay performance was monitored at every 20 tests interval using the lipid control serum, Precinorm (normal range) and Precipath (pathologic range) wherever applicable (Boehringer Mannheim, Germany) and TruLab N and TruLab P (Pars Azmon Inc., Iran). All these biochemical tests were performed on the day of sampling using commercial kits (Pars Azmon Inc., Iran) by the Selectra 2 auto-analyser (Vital Scientific, Spankeren, The Netherlands). All samples were analyzed when quality control met the acceptable criteria. Inter- and intra-assay coefficients of variations were less than 2.1 % for TG, less than 2% for TC and less than 3% for HDL- C. Free thyroxine (FT4) and thyroid stimulating hormone (TSH) were determined on -70ºC stored serum samples by electrochemiluminescence immunoassay (ECLIA) method, using Roche Diagnostics kits and Roche/Hitachi Cobas e- 411 analyzer (GmbH, Mannheim, Germany). Lyophilized quality control material (Lyphochek Immunoassay plus Control, Bio-Rad Laboratories) was used to monitor the accuracy of assay; intra- and inter-assay CVs were 1.3% and 3.7% for FT4 and 1.5% and 4.5% for TSH determinations, respectively.
3.3. Definitions
We defined SCH and overt hypothyroidism, according to our national reference ranges obtained in a population free of thyroid diseases. The 2.5th and 97.5th percentiles TSH were 0.32 mU/L and 5.06 mU/L, respectively. The mean ± SD and median (IQR) for FT4 for negative TPOAb individuals were 1.19 ± 0.16 and 1.18 (1.08 - 1.31) ng/dL, respectively. We defined subclinical hypothyroidism as TSH levels > 5.06 mU/L in the presence of normal FT4 level. Overt hypothyroidism was defined as TSH levels > 5.06 mU/L and decreased FT4 levels (< 1.08 ng/dL) (15).
3.4. Statistical Analysis
Data with normal distribution were expressed as mean ± SD or as median and range for Non-normal variables. Analysis of variance (ANOVA; normal distribution), Kruskal-Wallis H (Non-normal distributed variables) were used to compare mean values and proportions of baseline variables between subclinical hypothyroidism, overt hypothyroidism and controls. Relationship between variables was assessed by Pearson correlation coefficient (normal variables) and Spearman correlation coefficient (Non-normal variables). (Stepwise) Linear regression models were used to determine whether the association between total cholesterol, TG, LDL-C and HDL-C with each unit of TSH and free T4 remained significant after adjusting for age, sex, BMI, waist, W/H ratio and smoking. Interactions of these covariates were explored. TG and TSH were log-transformed to improve the fit of the linear regression models. Statistical significance level was assigned for P < 0.05. Statistical analysis was performed using SPSS (version 21.0, SPSS Inc).
4. Results
The study assessed 5154 subjects including 2193 (42.5%) males and 2961 (57.5%) females, with a mean age of 39.71 ± 14.2 years (ranged 20 - 90 years); the prevalence of hypothyroidism was as follows: subclinical hypothyroidism 5.6% and hypothyroidism 1.9%. Demographics, anthropometrics and hormonal profile of patients with subclinical hypothyroidism, overt hypothyroidism and euthyroid controls are shown in Table 1.
Table 1. Demographic, Anthropometrics and Hormonal Profiles of Patients With Subclinical and Overt Hypothyroidism and Euthyroid Controls a,b.
| Controls | Subclinical hypothyroidism | Overt Hypothyroidism | P Value | |
|---|---|---|---|---|
| Gender | < 0.01 | |||
| Female | 2961 (57.5) | 221 (76.7) | 83 (83) | |
| Male | 2193 (42.5) | 67 (23.3) | 17 (17) | |
| Age, y | 39.69 ± 14.23 | 38.50 ± 14.51 | 44.14 ± 13.77 | 0.003 |
| BMI, kg/m 2 | 26.34 ± 4.80 | 26.4 ± 5.04 | 28.85 ± 4.76 | 0.001 |
| Weigh, kg | 70.2 ± 12.9 | 67.7 ± 12.9 | 73.1 ± 13.6 | 0.001 |
| Waist, cm | 87.3 ± 12.0 | 85.7 ± 13.0 | 92.3 ± 11.7 | 0.001 |
| W/H ratio | 0.87 ± 0.09 | 0.87 ± 0.08 | 0.84 ± 0.09 | 0.001 |
| SBP, mmHg | 116 ± 17 | 116 ± 17 | 116 ± 18 | 0.952 |
| DBP, mmHg | 76.0 ± 10.6 | 75.2 ± 10.4 | 77.1 ± 9.5 | 0.281 |
| TSH, mU/L | 1.59 (1.37) | 6.60 (2.72) | 13.03 (29.01) | 0.001 |
| FT4, ng/dl | 1.22 ± 0.18 | 1.11 ± 0.14 | 0.72 ± 0.20 | 0.001 |
| TC, mg/dl | 200 ± 45 | 199 ± 46 | 216 ± 41 | 0.001 |
| HDLC, mg/dl | 41.7 ± 10.9 | 42 ± 11 | 42 ± 11 | 0.251 |
| LDLC, mg/dl | 127.5 ± 36 | 127.96 ± 38 | 140 ± 35 | 0.003 |
| TG, mg/dl | 133 (104) | 117 (116) | 157 (126) | 0.007 |
| TG/HDL | 4.26 ± 3.63 | 4.16 ± 4.01 | 5.06 ± 4.15 | 0.086 |
a Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FT4, free thyroxine; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, Total cholesterol; TGs, triglycerides, SBP, systolic blood pressure; TSH, thyrotropin; WC, waist circumference; W/H, waist to hip.
b All values represent mean ± SD except for TSH and TG ( median and IQR are shown).
A total of 39 subjects with subclinical hypothyroidism had TSH levels more than 10 mU/L and 39 patients with overt hypothyroidism had TSH levels below 10 mU/L.
In 288 subjects with SCH, 140 (48.6%) had positive results for thyroid antibodies. In 100 subjects with overt hypothyroidism, 72 had positive titers. Total cholesterol level was significantly higher in overt hypothyroidism in comparison to subclinical hypothyroidism (P = 0.003). BMI, weight and serum levels of LDL cholesterol and TG were significantly higher in overt hypothyroidism in comparison to subclinical hypothyroidism. Mean ± SD of HDL level was 37.6 ± 9.1 and 44.7 ± 11.1 mg/dL in men and women, respectively. There was a weak correlation between serum TSH and HDL levels in both male (r = -0.04 P< 0.01) and female (r = -0.04 P < 0.01). FT4 was weakly correlated with HDL level only in females (r = 0.04 P < 0.001). Serum levels of total cholesterol, HDL-C, LDL-C and TG did not differ between subclinical hypothyroid group and controls (Table 1). There were significant positive correlations between age and serum total cholesterol level (r = 0.0.4, P = 0.00), LDL-C (r = 0.4, P = 0.00), triglycerides (r = 0.2, P = 0.00), SBP (r = 0.5, P = 0.00), DBP (r = 0.3, P = 0.00) but not with HDL-C. In total population, there were no significant correlations between serum TSH levels and age, BMI, WC, W/H ratio or between TSH and total cholesterol, LDL-C, HDL-C and triglycerides. There was a relatively weak negative correlation between TSH and TG/HDL (r = -0.02, P = 0.04). There were no correlations between TSH and lipid profiles in individuals with subclinical hypothyroid, but there was a weak negative correlation between serum concentrations of FT4 and LDL-C, triglycerides, total cholesterol and HDL-C (Table 2 in the supplementary appendix, available with the full text of this article at www.endometabol.com). In normal population, we found evidence of interaction between TSH levels and smoking for serum levels of HDL-C and TG. There was a statistically significant interaction between TSH levels and gender on the serum levels of TC, LDL-C and TG and also a there was a statistically significant interaction between TSH and age on serum levels of LDL-C. There was a statistically significant interaction between smoking status and FT4 in HDL-C and total cholesterol; we also found an interaction between FT4 and gender.
Table 2. Correlations Between TSH and FT4 With Serum Lipid Concentrations.
| TSH, mU/L | FT4, ng/dL | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Subclinical Hypothyroidism | Overt Hypothyroidism | Controls | All | Subclinical Hypothyroidism | Overt Hypothyroidism | Controls | |
| TC, mg/dL | -0.021a (0.109) | 0.092 (0.117) | 0.278 (0.005) | -0.038 (0.03) | -0.174 (0.000) | -0.210 (0.000) | -0.336 (0.001) | -0.164 (0.00) |
| HDLC, mg/dL | 0.028 (0.047) | 0.042 (0.474) | -0.036 (0.727) | 0.023 (0.113) | -0.062 (0.000) | -0.064 (0.279) | 0.070 (0.49) | -0.066 (0.00) |
| LDLC, mg/dL | -0.25 (0.073) | 0.066 (0.284) | 0.21 (0.046) | -0.043 (0.004) | -0.174 (0.000) | -0.184 (0.003) | -0.381 (0.00) | -0.133 (0.00) |
| TG, mg/dL | -0.022 (0.109) | 0.074 (0.209) | 0.117 (0.244) | -0.031 (0.03) | -0.089 (.000) | -0.045 (0.443) | -0.146 (0.14) | -0.81 (0.00) |
| TG/HDL | -0.028 (0.042) | 0.058 (0.327) | 0.086 (0.399) | -0.033 (0.024) | -0.043 (0.002) | -0.019 (0.751) | -0.122 (.023) | -0.035 (.017) |
a Spearman’s correlation coefficient between TSH and lipid levels. Pearson correlation coefficient between FT4 and lipid levels.
Among euthyroid men, FT4 levels were inversely associated with total cholesterol and TG, but were not associated with LDL-C and HDL-C and TG/HDL-C. In euthyroid women, FT4 levels were associated with HDL-C and negatively associated with TG and TG/HDL-C, but there were not associated with TC and LDL-C (Table 2 in the supplementary appendix, available with the full text of this article at www.endometabol.com). Among euthyroid men, TSH levels were associated negatively with HDL-C, but we found no association between TSH and total cholesterol, LDL-C, TG or TG/HDL (Table 3 in the supplementary appendix, available with the full text of this article at www.endometabol.com). The association between lipid profiles and anthropometric factors and smoking is shown in data. In euthyroid men, waist/hip ratio was associated with total cholesterol, LDL-C TG and TG/HDL. In women, waist/hip ratio was associated with TC, TG, TG/HDL-C and, but not associated with LDL -C (Table 4 in the supplementary appendix, available with the full text of this article at www.endometabol.com).
Table 3. Linear Regression of FT4 With Lipid Profiles in Normal Population in Men and Women a,b.
| TC | HDL | LDL | TG | TG/HDL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Men | |||||||||||||||
| Age, y | 0.54 | 0.06 | <0.01 | 0.05 | 0.01 | < 0.01 | 0.54 | 0.05 | < 0.01 | - | - | -0.01 | < 0.01 | < 0.01 | |
| BMI, Kg/m2 | 3.73 | 0.56 | <0.01 | - | - | - | 2.79 | 0.49 | < 0.01 | 0.05 | < 0.01 | < 0.01 | 0.17 | 0.02 | < 0.01 |
| Weight, kg | -13.55 | 5.04 | <0.01 | - | - | - | - | - | - | -0.17 | 0.06 | < 0.01 | - | - | - |
| Waist, cm | -1.07 | 0.27 | <0.01 | -0.21 | 0.01 | < 0.01 | -0.70 | 0.24 | < 0.01 | -0.10 | < 0.01 | < 0.01 | - | - | - |
| W/H ratio | 117.309 | 25.07 | <0.01 | - | - | - | 44.35 | 21.88 | 0.04 | 2.51 | 0.30 | < 0.01 | 12.01 | 1.63 | 0.04 |
| Smoking | 8.266 | 1.83 | <0.01 | 1 | 0.40 | 0.01 | 5.16 | 1.60 | 0.01 | - | - | - | - | - | - |
| Women | |||||||||||||||
| Age, y | 1.40 | 0.06 | <0.01 | 0.10 | 0.02 | < 0.01 | 1.06 | 0.05 | < 0.01 | 0.01 | 0.005 | < 0.01 | 0.01 | 0.00 | < 0.01 |
| BMI, Kg/m2 | 1.24 | 0.17 | <0.01 | - | - | - | 0.91 | 0.14 | < 0.01 | 0.02 | 0.002 | < 0.01 | 0.09 | 0.01 | < 0.01 |
| Weight, kg | - | - | 2.78 | 1.28 | < 0.01 | - | - | - | -0.11 | 0.05 | 0.04 | -0.82 | 0.32 | < 0.01 | |
| Waist, cm | - | - | -.09 | 0.02 | < 0.01 | - | - | - | - | - | - | - | |||
| W/H ratio | 32.13 | 11.27 | <0.01 | -8.59 | 4.32 | 0.03 | - | - | - | 1.23 | 0.14 | < 0.01 | 6.79 | 0.81 | < 0.01 |
| Smoking | 15.90 | 1.77 | <0.01 | 2.05 | 0.51 | 0.04 | 10.07 | 1.54 | < 0.01 | 0.11 | 0.002 | < 0.01 | 0.29 | 0.12 | 0.02 |
a Abbreviations: B, reported as unstandardized B; P, P-value; SE, standard Error of estimated B.
b TG and TSH were transformed into logarithmic scale because of non-normality.
Table 4. Linear Regression of TSH With Lipid Profiles in Normal Population in Men and Women.
| TC | HDL | LDL | TG | TG/HDL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | P | B | SE | P | B | SE | P | B | SE | P | B | SE | P | |
| Men | |||||||||||||||
| Age, y | 0.58 | 0.06 | < 0.01 | 0.05 | 0.01 | < 0.01 | 0.54 | 0.05 | < 0.01 | - | - | -0.01 | 0.00 | 0.04 | |
| BMI, kg/m2 | 3.87 | 0.56 | < 0.01 | - | - | - | 2.79 | 0.49 | < 0.01 | 0.05 | 0.002 | < 0.01 | 0.17 | 0.02 | < 0.01 |
| Weight, kg | - | - | - | -0.77 | 0.35 | 0.02 | - | - | - | - | - | - | - | - | |
| Waist, cm | -1.11 | 0.27 | < 0.01 | -0.21 | 0.01 | < 0.01 | -0.70 | 0.24 | < 0.01 | -0.01 | 0.005 | < 0.01 | - | - | - |
| W/H ratio | 117.87 | 25.11 | < 0.01 | - | - | - | 44.35 | 21.88 | 0.04 | 2.60 | 0.3 | < 0.01 | 12.01 | 1.63 | < 0.01 |
| Smoking | 8.70 | 1.82 | < 0.01 | 1.00 | 0.40 | 0.01 | 5.16 | 1.60 | < 0.01 | - | - | - | - | - | - |
| Women | |||||||||||||||
| Age, y | 1.40 | 0.06 | < 0.01 | 0.08 | 0.01 | < 0.01 | 1.06 | 0.05 | < 0.01 | 0.001 | 0.006 | < 0.01 | 0.01 | 0.004 | < 0.01 |
| BMI, Kg/m2 | 1.24 | 0.17 | < 0.01 | - | - | - | 0.91 | 0.14 | < 0.01 | 0.02 | 0.001 | < 0.01 | 0.10 | 0.01 | < 0.01 |
| Weight, kg | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Waist, cm | - | - | - | -0.14 | .02 | < 0.01 | - | - | - | - | - | - | - | - | - |
| W/H ratio | 32.14 | 11.27 | < 0.01 | - | - | - | - | - | - | 1.23 | 0.14 | < 0.01 | 6.78 | 0.81 | < 0.01 |
| Smoking | 15.93 | 1.77 | < 0.01 | 2.03 | 0.50 | < 0.01 | 10.07 | 1.54 | < 0.01 | 0.11 | 0.02 | < 0.01 | 0.32 | 0.12 | < 0.01 |
5. Discussion
In this study, lipid profiles did not differ between subclinical hypothyroid and normal individuals. In the euthyroid cohort, FT4 levels among men were inversely associated with total cholesterol and TG among women; they were positively associated with HDL-C level and negatively with TG and TG/HDL-C levels.
One consequence of overt hypothyroidism is elevated lipid levels; however, we found no rising level of lipid profile in subclinical hypothyroidism compared to euthyroid subjects. The results of observational studies of serum lipid levels in patients with subclinical hypothyroidism have been inconsistent. The results of our study, contrary to the result of the Colorado thyroid study, showed modest elevation of TSH accompanied by higher mean total cholesterol level than normal TSH (10). Although serum total cholesterol was not adjusted for age or sex; our results were also contrary to those of Walsh et al. investigation, which showed increased level of both serums TC and LDL-C in individuals with subclinical hypothyroid even after adjustment for age and sex (16). The result of EPIC-Norfolk study showed higher total and LDL cholesterol in women with subclinical hypothyroidism (17). Concordant to our results, Pirich et al. revealed no differences in lipid profiles and other cardiovascular risks in subclinical hypothyroid group in comparison to those with normal TSH levels (18). The National Health and Nutrition Examination Survey III, after adjustment for age, race, sex and use of lipid-lowering drugs, found that subclinical hypothyroidism was not associated with alterations in serum levels of total cholesterol, LDL-C, triglycerides or HDL-C (6). In the Rotterdam study, Hak et al. did not find that total cholesterol level was higher in women with subclinical hypothyroidism than euthyroid women (7). The differences between the results of various studies are probably due to different cut points used for the definition of subclinical hypothyroidism, differences in the prevalence of this disorder, and ethnicity, age and gender of participants.
Our observation regarding the association between TSH and lipid levels in euthyroid cohort was first examined in the EPIC -Norfolk, a population based study showing that even in euthyroid population, there are associations between TSH levels and lipid profiles and they found that TSH was associated with total cholesterol, LDL-C and HDL-C in euthyroid men and only with HDL-C in euthyroid women (17). Similar to their findings we found an interaction between TSH levels and gender on serum levels of TC, LDL-C and TG. Results of the Busselton Thyroid study showed that after adjustment for age and sex, the association between TSH and total cholesterol, LDL-C remained significant (16). Not all the studies mentioned reached the same conclusion regarding the presence/lack of associations between lipid profiles and TSH levels. The NHANES III found no significant association between serum levels of total cholesterol, LDL-C, HDL-C and subclinical hypothyroidism (6).
This study showed a negative correlation between FT4 with total cholesterol and TG in euthyroid men and a negative correlation between FT4 and TG levels in euthyroid women. Blood lipids had no difference between sub-clinically hypothyroid patients and controls. The relationship between FT4 and lipid profile was confirmed in many studies, but the direction of this association was different. Some studies showed that FT4 was significantly and independently associated with serum levels of TC, HDL-C and LDL-C (19, 20), but other studies found a negative associated between FT4 level, TC and LDL-C levels (21, 22). The reason for this inconsistency between studies is not clear. It may be partly because the association between TSH and total cholesterol is relatively weak or might be due to differences in study population or ethnicity. Another point for conflicting results might be different criteria for recruitment of individuals according to the normal reference ranges of FT4 and TSH in different studies; for instance, the results of our study differ from the study of Walsh et al. (16) as a community-based study, in which the reference range of TSH was 0.4 to 4.0 mU/L and FT4 0.7 to 1.8 ng/dL.
The strength of this study was that it is a community based study. It does however have some limitations; measurements of TSH and FT4 and lipid profiles were performed just once. Our results must be cautiously interpreted for the general population because we excluded patients receiving thyroid medications. This study is not a prospective study and therefore it demonstrates only association and not causation of various variables.
The present study showed no differences in lipid profiles as a potential cardiometabolic risk between subclinical hypothyroidism and euthyroid subjects.
Acknowledgments
We would like to acknowledge personnel of research institute for endocrine science laboratory for their collaboration and assistance. We also thank Ms. Niloofar Shiva for language editing of the manuscript.
References
- 1.Velkoska Nakova V, Krstevska B, Bosevski M, Dimitrovski C, Serafimoski V. Dyslipidaemia and hypertension in patients with subclinical hypothyroidism. Prilozi. 2009;30(2):93–102. [PubMed] [Google Scholar]
- 2.Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep. 2004;6(6):451–6. doi: 10.1007/s11886-004-0054-3. [DOI] [PubMed] [Google Scholar]
- 3.Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J. 2011;5:76–84. doi: 10.2174/1874192401105010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efstathiadou Z, Bitsis S, Milionis HJ, Kukuvitis A, Bairaktari ET, Elisaf MS, et al. Lipid profile in subclinical hypothyroidism: is L-thyroxine substitution beneficial? Eur J Endocrinol. 2001;145(6):705–10. doi: 10.1530/eje.0.1450705. [DOI] [PubMed] [Google Scholar]
- 5.Bell RJ, Rivera-Woll L, Davison SL, Topliss DJ, Donath S, Davis SR. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease - a community-based study. Clin Endocrinol. 2007;66(4):548–56. doi: 10.1111/j.1365-2265.2007.02771.x. [DOI] [PubMed] [Google Scholar]
- 6.Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia. Ann Fam Med. 2004;2(4):351–5. doi: 10.1370/afm.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270–8. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Neves C, Alves M, Medina JL, Delgado JL. Thyroid diseases, dyslipidemia and cardiovascular pathology. Rev Port Cardiol. 2008;27(10):1211–36. [PubMed] [Google Scholar]
- 9.Kalantari S, Naghipour M. Statin therapy and hepatotoxicity: Appraisal of the safety profile of atorvastatin in hyperlipidemic patients. Adv Biomed Res. 2014;3:168. doi: 10.4103/2277-9175.139133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 11.Jung CH, Sung KC, Shin HS, Rhee EJ, Lee WY, Kim BS, et al. Thyroid dysfunction and their relation to cardiovascular risk factors such as lipid profile, hsCRP, and waist hip ratio in Korea. Korean J Intern Med. 2003;18(3):146–53. doi: 10.3904/kjim.2003.18.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalantari S, Heidarzadeh A. Thyroxine Therapy Improves Serum Lipoproteins and Some Clinical Findings In Patients With Subclinical Hypothyroidism. Int J Endocrinol Metabol. 2006;4(2):106–11. [Google Scholar]
- 13.Mansourian AR. The state of serum lipids profiles in sub-clinical hypothyroidism: a review of the literature. Pak J Biol Sci. 2010;13(11):556–62. doi: 10.3923/pjbs.2010.556.562. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 15.Amouzegar A, Delshad H, Mehran L, Tohidi M, Khafaji F, Azizi F. Reference limit of thyrotropin (TSH) and free thyroxine (FT4) in thyroperoxidase positive and negative subjects: a population based study. J Endocrinol Invest. 2013;36(11):950–4. doi: 10.3275/9033. [DOI] [PubMed] [Google Scholar]
- 16.Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, et al. Thyroid dysfunction and serum lipids: a community-based study. Clin Endocrinol (Oxf). 2005;63(6):670–5. doi: 10.1111/j.1365-2265.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 17.Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf). 2010;72(3):404–10. doi: 10.1111/j.1365-2265.2009.03640.x. [DOI] [PubMed] [Google Scholar]
- 18.Pirich C, Mullner M, Sinzinger H. Prevalence and relevance of thyroid dysfunction in 1922 cholesterol screening participants. J Clin Epidemiol. 2000;53(6):623–9. doi: 10.1016/s0895-4356(99)00187-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, et al. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf). 2009;70(1):152–60. doi: 10.1111/j.1365-2265.2008.03304.x. [DOI] [PubMed] [Google Scholar]
- 20.Chin KY, Ima-Nirwana S, Mohamed IN, Aminuddin A, Johari MH, Ngah WZ. The relationships between thyroid hormones and thyroid-stimulating hormone with lipid profile in euthyroid men. Int J Med Sci. 2014;11(4):349–55. doi: 10.7150/ijms.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92(2):491–6. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 22.Garduno-Garcia Jde J, Alvirde-Garcia U, Lopez-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163(2):273–8. doi: 10.1530/EJE-10-0312. [DOI] [PubMed] [Google Scholar]