Abstract
Complex associations exist among socioeconomic status (SES) in early life, beliefs about oral health care (held by individuals and their parents), and oral health–related behaviors. The pathways to poor adult oral health are difficult to model and describe, especially due to a lack of longitudinal data. The study aim was to explore possible pathways of oral health from birth to adulthood (age 38 y). We hypothesized that higher socioeconomic position in childhood would predict favorable oral health beliefs in adolescence and early adulthood, which in turn would predict favorable self-care and dental attendance behaviors; those would lead to lower dental caries experience and better self-reported oral health by age 38 y. A generalized structural equation modeling approach was used to investigate the relationship among oral health–related beliefs, behaviors in early adulthood, and dental health outcomes and quality of life in adulthood (age, 38 y), based on longitudinal data from a population-based birth cohort. The current investigation utilized prospectively collected data on early (up to 15 y) and adult (26 and 32 y) SES, oral health–related beliefs (15, 26, and 32 y), self-care behaviors (15, 28, and 32 y), oral health outcomes (e.g., number of carious and missing tooth surfaces), and oral health–related quality of life (38 y). Early SES and parental oral health–related beliefs were associated with the study members’ oral health–related beliefs, which in turn predicted toothbrushing and dental service use. Toothbrushing and dental service use were associated with the number of untreated carious and missing tooth surfaces in adulthood. The number of untreated carious and missing tooth surfaces were associated with oral health–related quality of life. Oral health toward the end of the fourth decade of life is associated with intergenerational factors and various aspects of people’s beliefs, SES, dental attendance, and self-care operating since the childhood years.
Keywords: epidemiology, quality of life, growth/development, dental public health, access to care, caries treatment
Introduction
Oral conditions can affect quality of life, with consequences including dysfunction, pain, discomfort, and disability. They consistently rank among the most frequently reported illness episodes (Spencer 2001) yet have been described as the “silent epidemic” (Satcher 2000) in that dental health issues are often overlooked in the wider health discourse. Chronic oral conditions (e.g., dental caries and periodontitis) are largely irreversible and cumulative (Locker 1988), and so life course epidemiology readily lends itself to their investigation. During childhood, the onset and progression of these conditions are driven by structural and behavioral factors (Fisher-Owens et al. 2007). Structural factors include socioeconomic position (Delgado-Angulo and Bernabé 2015), social capital (Rouxel et al. 2015), and social and economic policies (Thomson et al. 2002). Behavioral factors include diet (Moynihan and Petersen 2004), self-care (Walsh et al. 2010; Broadbent et al. 2011), and the use of dental care (Thomson et al. 2010; Aldossary et al. 2015).
Exposures that affect risk for the occurrence of chronic oral conditions may occur at any point in life, but investigating the interplay among those is challenging, and the effects of prior exposures are invariably modified by current circumstances (Hertzman et al. 2001). A number of life course models have been proposed, including the critical period model, the critical period model with later-life effect modifiers, the “accumulation of risk” model, and the “chains of risk” model (Nicolau et al 2007; Mishra et al. 2010). Structural equation modeling (SEM) has been proposed as a valid approach to investigating these life course models, but its satisfactory deployment in oral health research has been hindered by a lack of suitable longitudinal data (Newton and Bower 2005; Baker 2007; Baker and Gibson 2014). Application of SEM to life course data may provide support for these life course theories about how social factors may shape a person’s beliefs and behaviors throughout life and ultimately affect oral health and oral health–related quality of life (OHRQoL). There have been only 3 previous reports from oral health studies based on an SEM approach with longitudinal data. One was of Singaporean preschoolers, which used a 1-y follow-up (Gao et al. 2010); another was of Malaysian 12- to 13-y-olds, which used a 6-mo follow-up (Baker et al. 2010); and the third was of Thai 10- to 14-y-olds, which used follow-ups at 3, 6, and 9 mo (Gururatana et al. 2014). Such short follow-up times carry the risk of spurious associations because there may be insufficient variation in the observed disease incidence (or increment) and the true effects of putative determinants may remain obscure. Thus, our understanding of how unfavorable oral health behaviors originate, persist, and affect health is limited by a lack of available longitudinal life course data.
The aim of this study was to develop a model of oral health from birth to adulthood (age, 38 y) using longitudinal data from a birth cohort study. Because most of our measures are categorical, we used generalized SEM to assess the associations among the variables, instead of adopting a SEM approach. We hypothesized that higher socioeconomic position in childhood would predict favorable oral health beliefs in adolescence and early adulthood, which in turn would predict favorable self-care and dental attendance behaviors; those would lead to less untreated dental caries, fewer teeth lost due to caries, and better self-reported oral health by age 38 y.
Materials and Methods
Participants were members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members were born in Dunedin, New Zealand, between April 1972 and March 1973, and 1,037 (91% of eligible births; 52% male) participated in the first follow-up at age 3 y; these constituted the base sample for the remainder of the study. Cohort families represented the full range of socioeconomic status (SES) in New Zealand’s South Island. Over 90% of cohort members identified as New Zealand European or “white,” while 7.5% self-identified as being Māori. This matches the ethnic distribution of the South Island of New Zealand. Follow-ups were done at ages 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and 38 y, when we assessed 961 (95.4%) of the surviving 1,007 study members. The Otago Research Ethics Committee, Dunedin, New Zealand, granted ethics approval for each assessment phase. Study members gave informed consent before participating (Poulton et al. 2015).
The indicator of SES used in this analysis was occupation. Childhood SES was calculated as the average of the highest SES level of either parent of each study member, assessed repeatedly from birth to 15 y. This method was used because measurement of SES at a single point early in life does not describe cumulative exposure to low SES during childhood. SES during adulthood was based on individually assessed occupation during the age 26- and 32-y interviews. Standard New Zealand occupationally based indices were used to classify SES (Irving and Elley 1977; Elley and Irving 1985). These classifications use a 6-interval scoring system (e.g., a doctor scores 1 and a laborer scores 6). The resulting scores were used to assign individuals to 1 of 3 SES groups based on predetermined thresholds: scores of 1 and 2 were allocated to the high SES group; those scoring 3 or 4 were allocated to the medium SES group; and the remainder (scores 5 or 6) were categorized as low SES.
Data on parental oral health–related beliefs were collected when study members were aged 5 y. Parents were asked whether they believed that diet has a significant influence on tooth decay and whether they believed that certain foods and drinks (specifically, milk, honey, fluoridated water, apples, sweet biscuits, peanuts, potato crisps, and dried raisins) help to “build strong teeth or keep them healthy.” A score was derived as the percentage of questions answered correctly.
Data on study members’ oral health–related beliefs were collected at ages 15, 18, and 26 y. The beliefs referred to the benefit for oral health of 1) avoiding a lot of sweet foods, 2) using fluoride toothpaste, 3) visiting the dentist regularly, 4) keeping the teeth and gums very clean, 5) drinking fluoridated water, and 6) using dental floss. They were asked to rate each item on a 4-point scale as extremely important, fairly important, doesn’t matter much/not very important, or not at all important. These were coded such that the higher the score, the more positive the oral health beliefs.
At ages 26 and 32 y, study members were asked about their usual reason for visiting the dentist. Dental attendance was reported as “regular” (usually attends for dental checkups) or “nonregular” (attends the dentist only when a problem occurs). Study members were asked about their frequency of toothbrushing at ages 15, 26, and 32 y with the question “When do you brush your teeth?” Response options included more than once a day, once a day, not every day, less than once a week, and never. For the current analyses, response options were recoded to at least once a day and less than once a day.
Dental caries experience by age 38 y was assessed by 3 calibrated examiners, and the methodology used has been reported (Broadbent et al. 2013). Untreated dental caries and tooth loss are reported as counts of decayed and missing tooth surfaces. The number of filled tooth surfaces is not included in the model, because a majority of dental restorations were placed prior to adulthood and do not affect OHRQoL in the same way as missing teeth and untreated caries.
The short-form Oral Health Impact Profile (OHIP-14; Slade 1997) was used to assess study members’ OHRQoL at age 38 y. The OHIP-14 questionnaire has 14 items corresponding to the 7 domains of functional limitation, physical pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap. Study members’ experience of OHRQoL impacts during the 4 wk prior to their age 38 interview were coded as very often (score 4), fairly often (3), occasionally (2), hardly ever (1), or never (0). A total OHIP-14 score was calculated by summing responses over all 14 items, with possible scores ranging from 0 to 56. Item weights were not used.
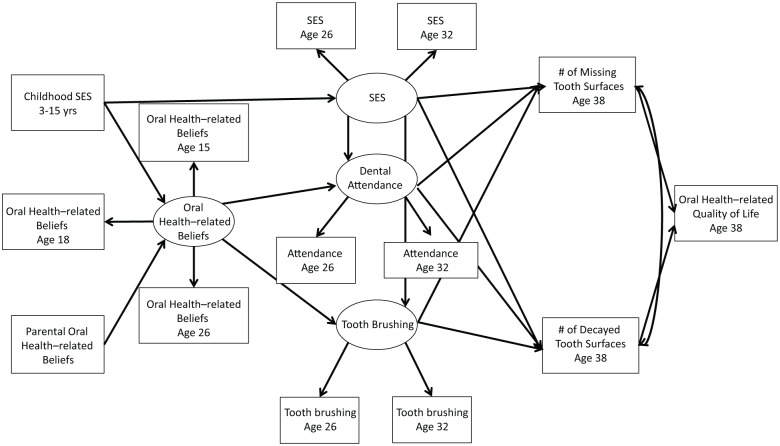
Generalized SEM was used to assess the relationships among early oral health influences, oral health–related beliefs, oral health–related behaviors, clinical health outcomes, and OHRQoL. The hypothesized model of the relationships is presented in the Figure. All statistical analyses were performed in Mplus 5.12. Probit regression was used for categorical dependent variables. Both unstandardized and standardized effects are given, along with the bias-corrected bootstrap confidence intervals for the unstandardized results. A standardized effect can be interpreted as the proportion of a standard deviation increase in 1 variable due to a 1–standard deviation increase in another. Latent variables were constructed for oral health–related beliefs according to the participants’ oral health–related beliefs at ages 15, 18, and 26 y and for overall adulthood SES through adult SES status at ages 26 and 32 y. Similarly, the latent variables for describing adult dental health–related behaviors were based on dental attendance and toothbrushing frequency at ages 26 and 32 y.
Figure.
Life course model of oral health–related beliefs, behaviors, and health outcomes. SES, socioeconomic status.
Results
The generalized structural equation model was undertaken on the basis of data for 878 of the original 1,037 study members. Mplus used all the available data in the analysis but excluded 139 participants with missing data on either childhood or parental oral health–related beliefs and a further 20 participants who had either childhood or parental oral health–related beliefs information but were missing data for other variables. The model fit was good (root mean square error of approximation <0.01). At age 38 y, OHIP-14 data were available for 848 participants, among whom the mean OHIP-14 score was 8.0 (SD 8.2); ≥1 impacts were experienced frequently or always by 189 (22.3%).
The participants who were excluded from the analysis did not differ from those included in respect of childhood SES (χ2 = 4.66, P = 0.10). Of those included, 20.6% were in the low-SES group, 64.1% in the medium-SES group, and 15.3% in the high-SES group. These proportions were 22.2%, 56.2% and 21.6% (respectively) for the participants who were excluded.
The measurement part of the model is presented in Table 1, where the loadings of the observed variables on the latent variables are provided. Standardized loadings provide the size of the correlation between the observed variables and the latent variable. All the loadings for adult SES, dental attendance, and toothbrushing frequency were statistically significant and substantially high, indicating that the constructed latent variables successfully summarized the information on the observed variables. Although the loadings for early oral health–related beliefs were relatively lower than the loadings for the other latent variables, they were still reasonable.
Table 1.
Latent Variable Loadings for the Observed Variables in the Model.
| Latent Variable | Std Estimate | Estimate | 95% CI |
|---|---|---|---|
| Early oral health–related beliefs | |||
| At age 15 y | 0.568 | — | — |
| At age 18 y | 0.463 | 0.855 | 0.616 to 1.152 |
| At age 26 y | 0.750 | 1.278 | 0.969 to 1.798 |
| Adult socioeconomic status | |||
| At age 26 y | 0.741 | — | — |
| At age 32 y | 0.614 | 0.817 | 0.572 to 1.131 |
| Dental attendance | |||
| At age 26 y | 0.868 | — | — |
| At age 32 y | 0.889 | 1.025 | 0.783 to 1.364 |
| Toothbrushing frequency | |||
| At age 26 y | 0.980 | — | — |
| At age 32 y | 0.871 | 0.883 | 0.715 to 1.049 |
95% CI, 95% confidence interval.
In accordance with the study hypothesis, childhood SES was associated with participants’ early adulthood oral beliefs at ages 15, 18, and 26 y, as were parental oral health–related beliefs. Positive dental beliefs at early adulthood then predicted better dental self-care behaviors at ages 26 and 32 y, such as attending for routine dental checkups and brushing the teeth frequently. Furthermore, the model suggested that adult SES (at ages 26 and 32 y) is also a strong predictor of the dental self-care behaviors at ages 26 and 32 y.
Favorable dental self-care behaviors were more frequently observed among participants of higher adult SES. In terms of dental outcomes by age 38 y, the number of missing tooth surfaces was negatively associated with adult SES and dental attendance at ages 26 and 32 y. The number of decayed surfaces was negatively associated with all 3 variables: fewer decayed tooth surfaces were observed among participants with higher SES and among those with better self-care behaviors at ages 26 and 32 y. Finally, more decayed and missing tooth surfaces led to worse OHRQoL (Table 2; Fig.).
Table 2.
Direct Effects among the Variables.
| Pathways | Std Estimate | Estimate | 95% CI |
|---|---|---|---|
| Oral health–related beliefs (from ages 15 to 26 y) on | |||
| Childhood SES (from ages 3 to 15 y) | 0.119 | 0.311 | 0.083 to 0.577 |
| Parental oral health–related beliefs | 0.147 | 1.817 | 0.725 to 3.100 |
| Adult SES (at ages 26 and 32 y) on | |||
| Childhood SES (from ages 3 to 15 y) | 0.389 | 0.504 | 0.385 to 0.647 |
| Dental attendance (at ages 26 and 32 y) on | |||
| Adult SES (at ages 26 and 32 y) | 0.236 | 0.267 | 0.129 to 0.443 |
| Oral health–related beliefs (from age 15 to 26 y) | 0.362 | 0.203 | 0.138 to 0.288 |
| Toothbrushing frequency (at ages 26 and 32 y) on | |||
| Adult SES (at ages 26 and 32 y) | 0.421 | 0.549 | 0.328 to 0.849 |
| Oral health–related beliefs (from age 15 to 26 y) | 0.486 | 0.314 | 0.228 to 0.415 |
| Missing tooth surfaces (at ages 26 and 32 y) on | |||
| Adult SES (at ages 26 and 32 y) | −0.203 | −4.036 | −9.231 to −0.175 |
| Dental attendance (at ages 26 and 32 y) | −0.334 | −5.883 | −10.330 to −2.811 |
| Toothbrushing frequency (at ages 26 and 32 y) | −0.034 | −0.517 | −2.390 to 2.205 |
| Decayed tooth surfaces (at ages 26 and 32 y) on | |||
| Adult SES (at ages 26 and 32 y) | −0.189 | −1.186 | −2.143 to −0.367 |
| Dental attendance (at ages 26 and 32 y) | −0.214 | −1.184 | −2.286 to −0.395 |
| Toothbrushing frequency (at ages 26 and 32 y) | −0.225 | −1.082 | −2.013 to −0.427 |
| OHRQoL (age 38 y) on | |||
| Missing tooth surfaces (at ages 26 and 32 y) | 0.303 | 0.157 | 0.091 to 0.225 |
| Decayed tooth surfaces (at ages 26 and 32 y) | 0.284 | 0.467 | 0.297 to 0.674 |
95% CI, 95% confidence interval; OHRQoL, oral health–related quality of life; SES, socioeconomic status.
Discussion
This study set out to identify important antecedents—from childhood through adolescence and early adulthood—in OHRQoL at the age of 38 y, using generalized SEM applied to a longitudinal data set from a life course study. It found important roles for oral beliefs, SES in childhood and adulthood, dental attendance, self-care, and accumulated dental caries experience.
A major strength of the current study is that it is the first SEM analysis to use oral health information collected prospectively over almost 4 decades; previous such investigations with SEM have used shorter follow-up periods of ≤1 y (Baker et al. 2010; Gao et al. 2010; Gururatana et al. 2014). Moreover, the current study used no exposure variables, which were nearer than 6 y previously to the measurement of the dependent variables. The long period over which the exposure data were collected means that there can be few doubts about either the directionality of the observed associations or the variation in the dependent variables. The findings of the study are likely to be generalizable to populations of developed nations similar to New Zealand. The study has some weaknesses; for example, the aggregated form of the OHIP-14 was used to represent OHRQoL. Some have suggested that the GOHAI (Geriatric Oral Health Assessment Index) may be a more appropriate measure (Locker et al. 2001); nevertheless, the OHIP-14 is a validated and valuable epidemiologic tool that correlates strongly with the GOHAI (Locker et al. 2001; Rodakowska et al. 2014). Difficulties may also arise with the use of occupation as an indicator of SES, since it is a proxy for educational attainment and income—for instance, an individual may be well educated or have a high income but be unemployed, or one may be unemployed and share a household with an employed person. Finally, a drawback of the generalized SEM approach used in this study is that it does not allow us to calculate indirect effects in the same way as with SEM, because the models are nonlinear; however, this approach was necessary because of the categorical nature of many of the variables used in the model.
The findings help validate inferences drawn from recent applications of SEM in cross-sectional samples (e.g., Donaldson et al. 2008; Polk et al. 2010; Tolvanen et al. 2012; Duijster et al. 2014), as well as those from recent longitudinal research (e.g., Baker et al. 2010; Gao et al. 2010; Gururatana et al. 2014). As with those earlier studies, SES was of central importance: childhood SES shaped beliefs and directly influenced subsequent adult SES. Oral health–related beliefs were shown to be crucial in determining dental service utilization and self-care (supporting earlier observations of the same birth cohort; Broadbent et al. 2006) and were substantially influenced by parental oral health–related beliefs. The latter finding suggests a continuity in oral health beliefs about a range of preventive behaviors and (by extension) oral health, which was highlighted earlier in findings from the same cohort (Shearer et al. 2011). Our data offer support for the central importance of the “accumulation of risk” model (Mishra et al. 2010) in the occurrence of oral disease and ill health. That is, there is no strong evidence for a critical or sensitive period; rather, it is a balance of the ongoing adverse and beneficial exposures—along with contemporary influences (Hertzman et al. 2001)—that determine overall oral health and OHRQoL in midlife.
Dental self-care (toothbrushing) in adulthood influenced all 3 aspects of dental caries experience by age 38 y, consistent with earlier observations relating to caries experience by age 32 y (Broadbent et al. 2011). In turn, the number of tooth surfaces that were either decayed or missing due to dental caries was associated with OHRQoL at age 38 y. The model that we present demonstrates a path of associations, linking factors acting in childhood, adolescence, and early adulthood with their effects on dental health and OHRQoL in midlife. This path of associations illustrates how factors acting in childhood can affect an individual’s OHRQoL in adulthood.
In conclusion, the findings from this investigation of the determinants of dental caries experience and self-reported oral health by the age of 38 y show that what we become toward the end of our fourth decade of life is influenced by intergenerational factors and various aspects of our beliefs, socioeconomic position, dental attendance, and self-care, which operate over the years since childhood.
Author Contributions
J.M. Broadbent, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J. Zeng, S.R. Baker, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; L.A. Foster Page, contributed to conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript; S. Ramrakha, contributed to data interpretation, critically revised the manuscript; W.M. Thomson, contributed to design, data acquisition, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank the study members for their continuing participation in the Dunedin Study, the study founder, Dr. Phil Silva, and Director Professor Richie Poulton. The authors thank Associate Professor Sheila Williams and Professor Rob McGee for their contribution to the development of the model.
Footnotes
The age 26 dental data collection was supported by the New Zealand Dental Association Research Foundation (Auckland, New Zealand) and the University of Otago (Dunedin, New Zealand). The age 32 dental data collection was supported by grant R01 DE-015260-01A1 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, USA, and a program grant from the New Zealand Health Research Council (Dunedin, New Zealand). The age 38 data collection was supported by a program grant from the New Zealand Health Research Council. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council and Ministry of Business, Innovation and Employment.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aldossary A, Harrison VE, Bernabe E. 2015. Long-term patterns of dental attendance and caries experience among British adults. Eur J Oral Sci. 123(1):39–45. [DOI] [PubMed] [Google Scholar]
- Baker SR. 2007. Testing a conceptual model of oral health: a structural equation modelling approach. J Dent Res. 86(8):708–712. [DOI] [PubMed] [Google Scholar]
- Baker SR, Gibson B. 2014. Social oral epidemic(olog)²y where next: one small step or one giant leap? Community Dent Oral Epidemiol. 42(6):481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SR, Mat A, Robinson PG. 2010. What psychosocial factors influence adolescents’ oral health? J Dent Res. 89(11):1230–1235. [DOI] [PubMed] [Google Scholar]
- Broadbent JM, Page LA, Thomson WM, Poulton R. 2013. Permanent dentition caries through the first half of life. Br Dent J. 215(7):E12. [DOI] [PubMed] [Google Scholar]
- Broadbent JM, Thomson WM, Boyens JV, Poulton R. 2011. Dental plaque and oral health during the first 30 years of life. J Am Dent Assoc. 142(4):415–426. [DOI] [PubMed] [Google Scholar]
- Broadbent JM, Thomson WM, Poulton R. 2006. Oral health beliefs in adolescence and oral health in young adulthood. J Dent Res. 85(4):339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Angulo EK, Bernabé E. 2015. Intergenerational mobility and adult oral health in a British cohort. Community Dent Oral Epidemiol. 43(3):255–261. [DOI] [PubMed] [Google Scholar]
- Donaldson AN, Everitt B, Newton JT, Steele J, Sherriff M, Bower E. 2008. The effects of social class and dental attendance on oral health. J Dent Res. 87(1):60–64. [DOI] [PubMed] [Google Scholar]
- Duijster D, van Loveren C, Dusseldorp E, Verrips GH. 2014. Modelling community, family, and individual determinants of child dental caries. Eur J Oral Sci. 122(2):125–133. [DOI] [PubMed] [Google Scholar]
- Elley WB, Irving JC. 1985. The Elley-Irving Socio-economic Index: 1981 census revision. NZ J Educ Stud. 20(2):115–128. [Google Scholar]
- Fisher-Owens SA, Gansky SA, Platt LJ, Weintraub JA, Soobader MJ, Bramlett MD, Newacheck PW. 2007. Influences on children’s oral health: a conceptual model. Pediatrics. 120(3):e510–e520. [DOI] [PubMed] [Google Scholar]
- Gao XL, Hsu CY, Xu YC, Loh T, Koh D, Hwarng HB. 2010. Behavioral pathways explaining oral health disparity in children. J Dent Res. 89(9):985–990. [DOI] [PubMed] [Google Scholar]
- Gururatana O, Baker SR, Robinson PG. 2014. Determinants of children’s oral-health-related quality of life over time. Community Dent Oral Epidemiol. 42(3):206–215. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Power C, Matthews S, Manor O. 2001. Using an interactive framework of society and lifecourse to explain self-rated health in early adulthood. Soc Sci Med. 53(12):1575–1585. [DOI] [PubMed] [Google Scholar]
- Irving JC, Elley WB. 1977. A socio-economic index for the female labour force in New Zealand. NZ J Educ Stud. 12:154–163. [Google Scholar]
- Locker D. 1988. Measuring oral health: a conceptual framework. Community Dent Health. 5(1):3–18. [PubMed] [Google Scholar]
- Locker D, Matear D, Stephens M, Lawrence H, Payne B. 2001. Comparison of the GOHAI and OHIP-14 as measures of the oral health-related quality of life of the elderly. Community Dent Oral Epidemiol. 29(5):373–381. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Cooper R, Kuh D. 2010. A life course approach to reproductive health: theory and methods. Maturitas. 65(2):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan P, Petersen PE. 2004. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 7(1A):201–226. [DOI] [PubMed] [Google Scholar]
- Newton JT, Bower EJ. 2005. The social determinants of oral health: new approaches to conceptualising and researching complex causal networks. Community Dent Oral Epidemiol. 33(1):25–34. [DOI] [PubMed] [Google Scholar]
- Nicolau B, Thomson WM, Allison PJ, Steele JG. 2007. Life-course epidemiology: concepts and theoretical models with particular reference to oral chronic conditions. Community Dent Oral Epidemiol. 35(4):241–249. [DOI] [PubMed] [Google Scholar]
- Polk DE, Weyant RJ, Manz MC. 2010. Socio-economic factors in adolescents’ oral health: are they mediated by oral hygiene behaviours or preventive interventions? Community Dent Oral Epidemiol. 38(1):1–9. [DOI] [PubMed] [Google Scholar]
- Poulton R, Moffitt TE, Silva PA. 2015. The Dunedin Multidisciplinary Health and Development Study: overview of the first. 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 50(5):679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodakowska E, Mierzyńska K, Bagińska J, Jamiołkowski J. 2014. Quality of life measured by OHIP-14 and GOHAI in elderly people from Bialystok, north-east Poland. BMC Oral Health. 14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouxel PL, Heilmann, Aida J, Tsakos G, Watt RG. 2015. Social capital: theory, evidence, and implications for oral health. Community Dent Oral Epidemiol. 43(2):97–105. [DOI] [PubMed] [Google Scholar]
- Satcher D. 2000. Oral health in America: a report of the Surgeon General. Washington (DC): U.S. Department of Health and Human Services. [Google Scholar]
- Shearer DM, Thomson WM, Broadbent JM, Poulton R. 2011. Maternal oral health predicts their children’s caries experience in adulthood. J Dent Res. 90(5):672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD. 1997. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 25(4):284–290. [DOI] [PubMed] [Google Scholar]
- Spencer AJ. 2001. What options do we have for organising, providing and funding better public dental care? Commissioned paper series 2001/02. University of Sydney, Australian Health Policy Institute; [accessed 2016 Feb 3]. http://www.adelaide.edu.au/arcpoh/downloads/publications/reports/miscellaneous/spencer-options-paper.pdf. [Google Scholar]
- Thomson WM, Williams SM, Broadbent JM, Poulton R, Locker D. 2010. Long-term dental visiting patterns and adult oral health. J Dent Res. 89(3):307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WM, Williams SM, Dennison PJ, Peacock DW. 2002. Were New Zealand’s structural changes to the Welfare State in the early 1990s associated with a measurable increase in oral health inequalities among children? Aust N Z J Public Health. 26(6):525–530. [DOI] [PubMed] [Google Scholar]
- Tolvanen M, Lahti S, Miettunen J, Hausen H. 2012. Relationship between oral-health-related knowledge, attitudes and behaviour among 15-16-year-old adolescents: a structural equation modeling approach. Acta Odontol Scand. 70(2):169–176. [DOI] [PubMed] [Google Scholar]
- Walsh T, Worthington HV, Glenny AM, Appelbe P, Marinho VC, Shi X. 2010. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 1:CD007868. [DOI] [PubMed] [Google Scholar]